Abstract
The tick-transmitted hemoparasite Babesia bovis causes an acute infection that results in persistence and immunity against challenge infection in cattle that control the initial parasitemia. Resolution of acute infection with this protozoal pathogen is believed to be dependent on products of activated macrophages (Mφ), including inflammatory cytokines and nitric oxide (NO) and its derivatives. B. bovis stimulates inducible nitric oxide synthase (iNOS) and production of NO in bovine Mφ, and chemical donors of NO inhibit the growth of B. bovis in vitro. However, the induction of inflammatory cytokines in Mφ by babesial parasites has not been described, and the antiparasitic activity of NO produced by B. bovis-stimulated Mφ has not been definitively demonstrated. We report that monocyte-derived Mφ activated by B. bovis expressed enhanced levels of inflammatory cytokines interleukin-1β (IL-1β), IL-12, and tumor necrosis factor alpha that are important for stimulating innate and acquired immunity against protozoal pathogens. Furthermore, a lipid fraction of B. bovis-infected erythrocytes stimulated iNOS expression and NO production by Mφ. Cocultures of Mφ and B. bovis-infected erythrocytes either in contact or physically separated resulted in reduced parasite viability. However, NO produced by bovine Mφ in response to B. bovis-infected erythrocytes was only partially responsible for parasite growth inhibition, suggesting that additional factors contribute to the inhibition of B. bovis replication. These findings demonstrate that B. bovis induces an innate immune response that is capable of controlling parasite replication and that could potentially result in host survival and parasite persistence.
Understanding the cellular and molecular basis for immunity to hemoparasitic diseases, such as babesiosis and malaria, is central to devising safe and effective therapeutics and vaccines. Innate immune mechanisms are hypothesized to be important for the resolution of acute infection with these parasites, whereas acquired immunity is likely more important for resistance to homologous and heterologous parasite strain challenge (9, 14). Parasite-activated Mφ inhibit parasite growth during acute infection and contribute to the development of acquired T-cell-mediated and humoral immunity by presenting antigen and directing a type 1 immune response through the production of certain cytokines. The mammalian stages of some protozoa, such as Toxoplasma gondii, Trypanosoma cruzi, and Trypanosoma brucei, activate Mφ to secrete oxygen radicals, nitric oxide (NO), and inflammatory cytokines, including interleukin-1 (IL-1), tumor necrosis factor alpha (TNF-α), and IL-12 (18, 32, 34, 45). Plasmodium falciparum also stimulates Mφ to produce NO (32) and human peripheral blood mononuclear cells (PBMCs) to produce enhanced levels of TNF-α, IL-12, and gamma interferon (IFN-γ) (35). In contrast, the promastigote stage of Leishmania parasites fails to activate these responses in murine Mφ (18). Thus, the ability of specific parasites or parasitic stages to evade or induce Mφ activation may be a critical determinant in the outcome of acute infection and the development of acquired immunity. We recently determined that Babesia bovis-infected erythrocytes and a membrane-enriched fraction of merozoites stimulated inducible nitric oxide synthase (iNOS) transcription and NO production (37) by peripheral blood monocyte-derived Mφ of cattle. However, induction of inflammatory cytokines by B. bovis has not been demonstrated.
Cytokines, including IL-12 and TNF-α produced by Mφ and other antigen-presenting cells, are critical for generating and regulating innate and acquired immune responses against many pathogens. IL-12 activates natural killer (NK) cells to produce IFN-γ and contributes to the development of acquired immunity through its ability to promote the differentiation of IFN-γ-producing Th cells and to enhance IFN-γ production by differentiated Th cells (30, 41). IFN-γ and TNF-α are also important for activating effector functions of phagocytic cells. For example, TNF-α enhanced neutrophil-mediated killing of mouse malarial parasites (24) and, in concert with IFN-γ, stimulated the production of NO by murine and bovine Mφ (16). Because IFN-γ activates Mφ, it is hypothesized to be a key cytokine in the protective immune response to Babesia parasites (9). Consistent with this, Babesia-specific CD4+ T-cell lines and clones derived from cattle protected against challenge secreted IFN-γ (5, 43). In addition, supernatants from B. bovis-stimulated CD4+ T-cell lines that contained IFN-γ and TNF-α induced NO production by bovine Mφ (37). These observations raise the question of whether B. bovis merozoites can stimulate the induction of cytokines in Mφ that participate in inflammatory responses and prime for type 1 CD4+ T cells.
In response to acute infection with B. bovis, activated Mφ are believed to kill parasites by phagocytosis and through production of soluble toxic mediators, including NO, peroxynitrite, and superoxide. Evidence for nonphagocytic inhibition of B. bovis includes in vitro growth inhibition by soluble factors from cultured Mφ (28) and babesiacidal activity of chemical donors of NO (23). Similar results were reported for related malarial parasites (31, 40). While these results strongly suggest that Mφ-derived NO produced in response to B. bovis controls parasite growth, this has not been definitively demonstrated.
Although both TNF-α and NO likely function as elements of protective immunity against hemoprotozoan parasites, overproduction of these molecules has been implicated in the pathological sequelae of disease (21, 45). Therefore, rational vaccine design is critically dependent on characterizing Mφ cytokine induction by B. bovis, which may result in either severe pathology or resolution of acute infection and development of a long-lasting protective immunity. The studies reported here were undertaken to identify the cytokines induced by B. bovis-infected erythrocytes and to determine whether parasite lipids activate Mφ. In addition, we have attempted to define the contribution of NO to parasite growth inhibition by B. bovis-activated Mφ.
MATERIALS AND METHODS
Culture of B. bovis and lipid extraction.
The Mexico strain of B. bovis was cultured in bovine erythrocytes obtained from Babesia-negative donors (7, 37). All parasite cultures tested negative for endotoxin (<6 pg/ml) by using the Limulus amebocyte lysate assay (Whittaker M.A. Bioproducts, Walkersville, Md.), and all were negative for Mycoplasma when tested by PCR with a kit from Stratagene (La Jolla, Calif.) as previously described (37). Lipids were extracted from B. bovis-infected erythrocytes as previously described (3). Briefly, a chloroform-methanol extraction, yielding a final ratio of 1:1:0.9 (chloroform:methanol:water [vol/vol/vol]), was performed. The organic fraction was collected, evaporated under nitrogen, and quantified. The same extraction procedure was conducted with uninfected erythrocytes as a control.
Culture of bovine monocyte-derived Mφ.
Monocyte-derived Mφ were isolated from PBMCs from two Babesia-naïve donor cattle by plastic adherence and culturing for 6 days as previously described (37). After 6 days of culture, Mφ were harvested and used for NO and cytokine induction assays and parasite growth inhibition assays.
Analysis of iNOS and cytokine mRNA by RT-PCR.
Mφ were cultured for 6 h in 24-well plates at a concentration of 5 × 105 cells per well in 0.5 ml of complete RPMI 1640 medium and infected red blood cells (IRBCs) at a final concentration of 10% packed cell volume (PCV) and 10% parasitized erythrocytes (PE) in the presence or absence of 50 U of recombinant bovine IFN-γ (Ciba-Geigy; kindly provided by Lorne Babiuk, Veterinary Infectious Disease Organization [VIDO], Saskatoon, Saskatchewan, Canada) per ml. As a negative control, equivalent numbers of uninfected RBCs (URBCs) from the same donor were added to the Mφ cultures. As a positive control, Mφ were similarly incubated with 100 ng of lipopolysaccharide (LPS) per ml from Escherichia coli O55:B5 (Sigma Chemical Co., St. Louis, Mo.) plus 50 U of IFN-γ per ml. RNA was isolated, treated with DNase (Ambion, Inc., Austin, Tex.), and analyzed for iNOS and cytokine expression by reverse transcription-PCR (RT-PCR) as previously described (36). The primers for bovine IL-1β, IL-10, IL-12 p40, IL-12 p35, IL-18, iNOS, TNF-α, and β-actin are listed in Table 1. The cycle number chosen for each primer set was empirically determined for each set of samples, based on the positive control (i.e., LPS plus IFN-γ-treated sample), and was selected to fall within the linear range of amplification. Samples were compared by normalizing the target signal to the β-actin signal from each sample and then comparing the normalized values.
TABLE 1.
Source, sequences, and annealing temperature for primers used in RT-PCR analysis
| Target | Primer
|
Annealing temp (°C) | Product size (bp) | GenBank accession no. | |
|---|---|---|---|---|---|
| Sense (5′-3′) | Antisense (5′-3′) | ||||
| IL-1β | ATGGCAACCGTACCTGAACCC | AGAGAGGGTTTCCATTCTGAAGTC | 60 | 795 | M37211 |
| IL-10 | GTTGCCTGGTCTTCCTGGCTG | TATGTAGTTGATGAAGATGTC | 60 | 482 | U00799 |
| IL-12 p35 | CACCTCAGTTTGGGCAGGAGCCTC | CTCAGATAGCTCATCATTCTGTCG | 65 | 596 | U14416 |
| IL-12 p40 | GTGGCTGACAGCAATCAGTACTG | ACTGCAGGACACAGATGCCCATTC | 60 | 553 | U11815 |
| IL-18 | AGACCTGGAATCAGATCAC | CATCATGTCCTGGAACAC | 50 | 347 | AF124789 |
| iNOS | TAGAGGAACATCTGGCCAGG | TGGCAGGGTCCCCTCTGATG | 60 | 372 | U14640 |
| TNF-α | ATGAGCACCAAAAGCATGATCCGG | CCAAAGTAGACCTGCCCAGACTC | 60 | 689 | Z14137 |
| β-actin | ACCAACTGGGACGACATGGAG | GCATTTGCGGTGGACAATGGA | 60 | 890 | K00622/K00623 |
NO2− detection by the Griess reaction.
Mφ were cultured for 2 days at a concentration of 105 cells per well of 96-well flat-bottom plates with 5 to 125 μg of lipid per ml prepared from URBCs or IRBCs, without or with 50 U of IFN-γ per ml, 10 μg of polymyxin B (Sigma) per ml, or 250 μM l-arginine competitor, NG-monomethyl-l-arginine (l-NMMA; Calbiochem, La Jolla, Calif.). Culture supernatants were transferred (50 μl per well) to new 96-well, flat-bottom plates, and 50 μl of 1% sulfanilamide (Sigma) in 2.5% H3PO4 per well followed by 50 μl 0.1% (wt/vol) naphthylethylenediamine dihydrochloride (Sigma) in 2.5% H3PO4 per well were added to the supernatants; the A540 was compared to a NaNO2 standard curve. Results are presented as the mean micromolar concentration of nitrite (NO2−) in quadruplicate cultures ± 1 standard deviation (SD). The Student one-tailed t test was used to determine statistically significant differences in NO2− production.
TNF-α detection by ELISA.
Mφ were cultured for 24 h with URBCs or IRBCs (10% PCV; 10% PE) or with 100 ng of LPS per ml, with or without 50 U of IFN-γ per ml. Supernatants were serially diluted (twofold up to 1:128) and compared by enzyme-linked immunosorbent assay (ELISA) with recombinant bovine TNF-α diluted from 0.04 to 10 ng per ml as a standard. A capture ELISA for bovine TNF-α was used as previously described (12) with the following modifications. Immulon II ELISA plates (Dynax Technologies, Chantilly, Va.) were coated with 100 μl of anti-bovine TNF-α monoclonal antibody 1D11-13 (VIDO) diluted 1:1,000 in carbonate buffer (pH 9.5) overnight at 4°C. Plates were washed six times with TBST (10 mM Tris, 150 mM NaCl, 0.05% Tween-20, pH 7.6). Samples serially diluted in TBST-g (TBST containing 0.5% gelatin) were added to the plates and incubated for 2 h at room temperature or overnight at 4°C. Plates were washed with TBST. Rabbit anti-TNF-α serum (VIDO) diluted 1:1,500 in PBS-g (PBS containing 0.5% gelatin) was added for 1 h at room temperature. Plates were washed with TBST, and biotinylated goat anti-rabbit IgG (H+L chains; Zymed Laboratories, San Francisco, Calif.) diluted 1:10,000 in PBS-g was added for 1 h at room temperature. Plates were washed in TBST, and strepavidin-alkaline phosphatase (GIBCO, Rockville, Md.) diluted 1:2,000 in PBST-g was added for 1 h at room temperature. Plates were washed, and substrate p-nitrophenyl phosphate di(Tris) salt crystalline (PNPP) diluted to 1 mg per ml in 1% diethanolamine with 0.5 mM MgCl2 (pH 9.8) was added. The reaction was stopped by addition of 30 μl of 0.3 M EDTA (pH 8.0) per well, and the optical density at 405 nm was determined with an ELISA plate reader. The Student one-tailed t test was used to determine statistically significant differences in TNF-α production.
IL-12 detection by bioassay.
IL-12 activity was evaluated based on its ability to stimulate IFN-γ production in normal bovine PBMCs (4, 36). Mφ culture supernatants (1:2) or recombinant human IL-12 (rHuIL-12) (0.001 to 1.0 ng per ml; kindly provided by Genetics Institute, Inc., Cambridge, Mass.) was added to PBMCs stimulated with 1 μg of phytohemagglutinin (PHA; Sigma) per ml and cultured at 2 × 106 cells per ml in 48-well plates. PBMC supernatants were collected after 48 h and stored at −70°C until analysis. IFN-γ production by PBMCs was measured using a commercial ELISA according to the manufacturer's instructions (CSL Limited, Parkville, Victoria, Australia). IFN-γ activity was determined from a standard curve derived with a T-cell supernatant estimated, by the vesicular stomatitus virus cytopathic effect reduction assay, to contain 440 U of IFN-γ per ml. The limit of sensitivity was 0.275 U per ml. Mφ supernatants were also evaluated for residual exogenous IFN-γ, as some Mφ cultures received a final concentration of 50 U of recombinant bovine IFN-γ (rBoIFN-γ) per ml. The Student one-tailed t test was used to determine statistically significant differences in IFN-γ-inducing activity.
IL-1 detection by bioassay.
IL-1 activity was assessed by the ability to enhance the proliferative response of mitogen-stimulated mouse thymocytes. Briefly, thymocytes from two 4- to 6-week-old C3H/HeJ mice were harvested, pooled, and plated at 106 cells per well in 96-well flat-bottom plates. Mφ supernatants (1:4, 1:8, 1:16) or rHuIL-1β (Peprotech, Inc., Rocky Hill, N.J.) were added with an optimal concentration (8 μg per ml) of PHA and incubated for 72 h. [3H]thymidine (0.25 μCi) was added to each well during the last 6 h of culture, and incorporation of radioactivity was determined on a beta plate reader (Wallac, Gaithersburg, Md.).
Parasite growth inhibition assays.
Three different approaches were used to measure the effect of NO on inhibition of B. bovis replication. First, sodium nitroprusside (SNP; Sigma) was used as a chemical donor of NO. Quadruplicate wells of B. bovis-infected erythrocytes (10% PCV, 10% PE) were established with various concentrations of SNP (1 to 1,000 μM) in 96-well flat-bottom plates and cultured for 2 days. For the last 6 h of culture, 50 μCi of [3H]hypoxanthine (Amersham, Cleveland, Ohio) was added to each well to measure parasite replication (20). Cells were harvested, and incorporation of radioactivity was determined by liquid scintillation counting. In parallel experiments, NO production by SNP was assessed after 2 days by using the Griess assay to analyze NO2−.
Second, Mφ were cultured in quadruplicate wells of 96-well plates at 105 cells per well with B. bovis (10% PCV; 10% PE) without and with 250 μM l-NMMA, and growth inhibition was determined by incorporation of 50 μCi of [3H]hypoxanthine added during the last 6 h of culture. Inhibition of B. bovis growth by bovine Mφ was determined as the difference between incorporation by B. bovis alone and incorporation by B. bovis in the presence of bovine Mφ. Controls included URBCs or Mφ cultured alone. Uptake of radioactivity by Mφ was low (<2,000 cpm per well containing 105 cells), and uptake by URBCs was negative (<500 cpm per well for 10% PCV). Inhibition by Mφ was compared with inhibition by Mφ plus l-NMMA. The general formula for parasite growth inhibition was as follows:
 |
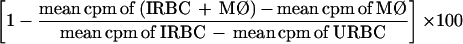 |
Since percent inhibition was calculated as a function of average populations, the standard deviation for percent inhibition was calculated according to the following general formula:
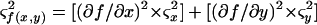 |
Third, a two-compartment culture system was also employed to measure the effect of soluble Mφ products on parasite replication, essentially as described by Quakyi et al. (29). B. bovis-infected erythrocytes (10% PCV; 10% PE) were cultured in 24-well plates and were physically separated from bovine Mφ (105 cells in 100 μl) cultured on a 0.4-μm-pore-size membrane in a cell culture insert (Costar, Cambridge, Mass.). After 2 days of culture, B. bovis-infected erythrocytes were transferred to quadruplicate wells of a 96-well flat-bottom plate, radiolabeled with [3H]hypoxanthine, harvested, and counted. The Student one-tailed t test was used to compare parasite growth in the presence and absence of l-NMMA.
RESULTS AND DISCUSSION
Induction of inflammatory cytokines in Mφ by B. bovis.
We examined cytokines produced by activated Mφ that are known to regulate NO production and to participate in the acquisition of type 1 immune responses. As observed previously for LPS-activated and LPS plus IFN-γ-activated Mφ (32), IL-18 was constitutively expressed and not upregulated upon B. bovis stimulation (data not shown). However, B. bovis did induce transcriptional upregulation of IL-12 p40 and IL-12 p35 in the absence or presence of IFN-γ (Fig. 1A). The inflammatory cytokines IL-1β and TNF-α were also induced upon Mφ exposure to B. bovis in the absence (Fig. 1B) or presence (Fig. 1C) of IFN-γ. As depicted in Fig. 1B and 1C, the requirement for IFN-γ was not absolute but varied by Mφ preparation. Both patterns were repeatedly observed. The induction of these cytokine transcripts paralleled B. bovis-induced upregulation of iNOS mRNA (37). IL-10, which has been shown to downregulate bovine IFN-γ expression (4), was not upregulated in response to B. bovis (data not shown). URBCs had no effect on cytokine expression.
FIG. 1.
B. bovis enhances transcription of cytokine mRNA in bovine Mφ. Mφ were cultured for 6 h with URBCs or IRBCs in the presence or absence of 50 U of IFN-γ per ml or with LPS plus IFN-γ. RNA was isolated, subjected to DNase treatment, and analyzed by RT-PCR. (A) Analysis of IL-12 p40, IL-12 p35, and β-actin. (B and C) Analysis of IL-1β, TNF-α, and β-actin. The data in each panel are representative of three independent experiments.
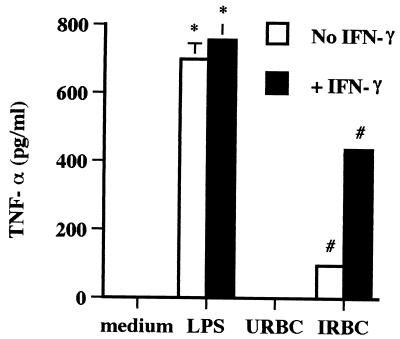
An ELISA specific for bovine TNF-α was employed to verify the stimulation of TNF-α protein production by B. bovis. Mφ cultured with B. bovis in the absence or presence of IFN-γ produced TNF-α, whereas control supernatants from Mφ cultured with URBC did not (Fig. 2). When B. bovis-infected erythrocytes induced TNF-α production, IFN-γ potentiated the effect. However, similar to the RT-PCR data, some Mφ preparations did not respond to B. bovis alone but required the presence of exogenous IFN-γ (data not shown).
FIG. 2.
TNF-α production by Mφ stimulated with B. bovis. Mφ were cultured for 24 h with URBCs, IRBCs, or LPS in the presence or absence of 50 U of IFN-γ per ml. Supernatants were tested by ELISA and compared with recombinant bovine TNF-α as a standard. Results are presented as the mean ± 1 SD of duplicate determinations and are representative of three independent experiments performed with Mφ from different cattle. ∗, P < 0.05, for Mφ cultured with LPS alone or plus IFN-γ compared to Mφ cultured with medium or IFN-γ alone, respectively. #, P < 0.05, for Mφ cultured with IRBCs alone or plus IFN-γ compared to Mφ cultured with URBCs alone or URBCs plus IFN-γ, respectively.
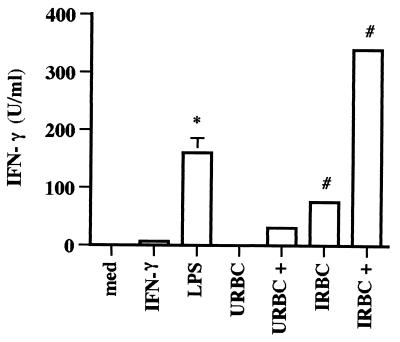
To evaluate functional IL-12 production in response to B. bovis, a bioassay was employed based on the ability of IL-12 to induce IFN-γ production by bovine PBMCs costimulated with PHA (4, 36). Supernatants from Mφ cultured with B. bovis in the absence or presence of IFN-γ were capable of inducing significant amounts of IFN-γ by PBMCs, whereas supernatants from Mφ cultured with URBCs contained little or no IFN-γ-inducing activity (Fig. 3). IFN-γ levels in the macrophage supernatants were all <3 U per ml (data not shown). While these data strongly indicate that the supernatants contained IL-12, the possibility that IL-18 contributed to the observed effect cannot be ruled out.
FIG. 3.
IL-12-like activity is present in supernatants from Mφ treated with IRBCs in the absence or presence of IFN-γ. Bovine Mφ were cultured for 24 h with URBCs or IRBCs (10% PE, 10% PCV) in the absence or presence (+) of 50 U of IFN-γ per ml. Mφ were cultured with medium or IFN-γ alone as negative controls or with 0.1 μg of LPS per ml as a positive control. To assay IFN-γ induction by these supernatants, bovine PBMCs were cultured for 48 h with PHA and either Mφ supernatants diluted 1:2 or rHuIL-12 (0.001 to 1.0 ng per ml) to create a standard curve. Supernatants from PBMCs cultured with medium or PHA alone served as negative controls. All supernatants were analyzed for IFN-γ production by ELISA. Results are presented as the mean ± 1 SD of duplicate determinations. The data are representative of two independent experiments. ∗, P < 0.01, for Mφ cultured with LPS compared to Mφ cultured with medium. #, P < 0.01, for Mφ cultured with IRBCs compared to Mφ cultured with URBCs or URBCs plus IFN-γ.
The presence of biologically active IL-1 in Mφ supernatants was also evaluated based on the ability of IL-1 to enhance PHA-driven proliferation of mouse thymocytes. Supernatants from Mφ cultured with URBCs in the absence or presence of IFN-γ and Mφ cultured with B. bovis-infected erythrocytes in the absence of IFN-γ failed to enhance PHA-driven thymocyte proliferation (data not shown). However, IL-1 activity was detected in supernatants diluted 1:4 from Mφ cultured with B. bovis-infected erythrocytes in the presence of IFN-γ (3,162 ± 800 [mean ± SD] cpm incorporated compared to the response to PHA alone [1,097 ± 235 cpm]). A 1:2 dilution was inhibitory for mouse thymocytes. As a positive control, 1 ng of HuIL-1β per ml resulted in optimal proliferation of 2,058 ± 206 (mean ± SD) cpm. These results suggested that although bovine IL-1 was detectable, the mouse thymocyte costimulation assay was not very sensitive in our hands. This may also account for the failure to detect IL-1 activity in cultures of Mφ and B. bovis in the absence of exogenous IFN-γ.
Together these data indicate that B. bovis not only stimulates iNOS and NO production by Mφ (37) but also stimulates the production of inflammatory cytokines. At the transcript and protein levels, the requirement for exogenously added IFN-γ was not absolute but varied with each Mφ preparation. We interpret this to indicate that the apparent requirement for IFN-γ in some experiments may reflect a threshold effect, in which the activation state of the Mφ at the time of the assay determines whether IFN-γ is needed as a cofactor with B. bovis to stimulate cytokine expression.
Induction of NO production and iNOS by a lipid fraction of B. bovis-infected erythrocytes.
Because a membrane-enriched fraction of B. bovis merozoites stimulated the highest level of NO production by Mφ (37), we hypothesized that a lipid component was responsible for this activity. Lipids extracted from B. bovis-infected erythrocytes induced significantly greater (P < 0.05) NO production by Mφ when compared to lipids from URBCs (Fig. 4A). The positive control, LPS plus IFN-γ, yielded 82.6 ± 4.0 (mean ± SD) μM NO2−. The induced NO production was resistant to the addition of polymyxin B, indicating that endotoxin contamination was not a contributing factor. l-NMMA blocked lipid-induced NO production, demonstrating that NO production was iNOS dependent. The lack of NO induction by lipids from URBCs suggests that the active molecules are of parasite origin. In support of this, all lipid modifications observed in B. bovis-infected erythrocyte membranes were shown to be produced through the biosynthetic activity of the parasite (15). Together, these data indicate that a B. bovis lipid component is capable of activating Mφ.
FIG. 4.
Lipids from B. bovis-infected erythrocytes induce NO production and transcription of iNOS mRNA by bovine Mφ. (A) Lipids from URBCs and IRBCs were cultured for 2 days with Mφ at the indicated concentrations. NO production was assessed using the Griess assay. Results are presented as the mean ± 1 SD of two independent experiments. ∗, P < 0.05, for Mφ cultured with IRBCs compared to Mφ cultured with lipid from URBCs; #, P < 0.05, for Mφ cultured with IRBCs compared to Mφ cultured with B. bovis lipid, but without l-NMMA. (B) Bovine Mφ were cultured for 6 h with lipids from URBCs and IRBCs in the presence or absence of IFN-γ (50 U per ml). RNA was isolated, subjected to DNase treatment, and analyzed by RT-PCR.
The ability of parasite lipids to induce inflammatory cytokine and iNOS transcripts was also determined. Surprisingly, we did not observe enhanced transcription of either TNF-α or IL-12 p35 or p40 mRNA upon exposure to B. bovis lipids, whereas iNOS transcription was induced following exposure to IRBC lipids in the absence or presence of IFN-γ, relative to the URBC controls (Fig. 4B). While the B. bovis lipid preparation was clearly capable of activating NO production by Mφ, the effect was observed only when using 125 μg of lipid per ml, indicating that the stimulatory component comprised a minor fraction of the total preparation. Among protozoal lipids known to activate Mφ are parasite membrane-derived glycolipids, including glycosylphosphatidylinositol (GPI) moieties. In murine malaria, purified Plasmodium toxin, identified as a GPI molecule, was sufficient to induce iNOS expression and production of NO, TNF-α, and IL-1 by Mφ (2, 33, 38). Furthermore, GPI-associated lipid molecules from T. cruzi and protein-associated glycolipids from T. gondii induced IL-12 production by murine Mφ (18). In contrast, Leishmania promastigotes and lipophosphoglycan molecules derived therefrom failed to stimulate Mφ (reviewed in reference 18). With B. bovis, it is possible that the lipid extract had an inhibitory effect on cytokine expression and that other parasite molecules, such as DNA (6), induce cytokine expression.
Role of NO in inhibition of B. bovis growth by B. bovis-activated Mφ.
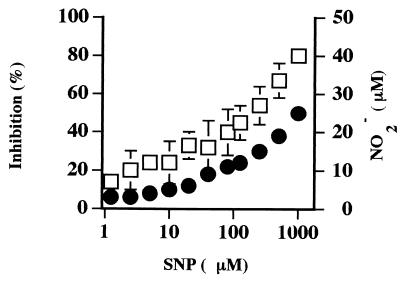
SNP was used as a chemical donor of NO to confirm the ability of NO to inhibit the growth of B. bovis in vitro (19). A dose-dependent growth inhibition was observed, for which a maximal inhibition of approximately 80% was observed with 1,000 μM SNP (Fig. 5). SNP-derived NO2− levels in the parasite cultures fell within the biologically relevant range of 10 to 25 μM that is routinely produced by Mφ stimulated with B. bovis components (37) (Fig. 4).
FIG. 5.
SNP, a chemical donor of NO, inhibits growth of B. bovis at concentrations that are biologically relevant. Inhibition of B. bovis growth was assessed in quadruplicate cultures by measuring incorporation of [3H]hypoxanthine (squares). SNP-derived NO production from quadruplicate wells was assessed using the Griess assay (circles). Results for both experiments are shown as the sample means ± 1 SD and are representative of three independent experiments.
Next, B. bovis growth inhibition by bovine Mφ was measured under conditions that permitted phagocytosis of the IRBCs. When B. bovis-infected erythrocytes were cocultured with Mφ, growth of the parasite was reproducibly inhibited relative to growth in the absence of Mφ (Table 2). The addition of 250 μM l-NMMA partially, but significantly (P < 0.05), restored parasite growth, indicating that Mφ-induced inhibition of B. bovis growth is only partially dependent on NO. Addition of IFN-γ did not significantly enhance growth inhibition of B. bovis by Mφ (data not shown), demonstrating that B. bovis-infected erythrocytes were sufficient to induce phagocytosis and NO production by bovine Mφ. Furthermore, IFN-γ had no direct inhibitory activity on B. bovis (data not shown).
TABLE 2.
Inhibition of B. bovis by bovine Mφ in a phagocytosis-permissive system is partially reversible by the addition of l-NMMA
| Expt no. | % Inhibitiona
|
|
|---|---|---|
| Without l-NMMA | With l-NMMA | |
| 1 | 88 ± 1 | 77 ± 1b |
| 2 | 57 ± 2 | 44 ± 5b |
| 3 | 75 ± 3 | 62 ± 4b |
| 4 | 89 ± 1 | 57 ± 1c |
Inhibition of B. bovis replication in the absence or presence of 250 μM l-NMMA was determined as described in the text. The data are presented as the means ± 1 SD of quadruplicate determinations for experiments 1 to 3 and duplicate determinations for experiment 4. Experiments were performed on four occasions and used Mφ derived from two cattle, with an individual animal per experiment.
P < 0.05 for comparison with inhibition in the absence of l-NMMA, determined by the Student one-tailed t test.
Statistical analysis was not performed because the samples were tested in duplicate.
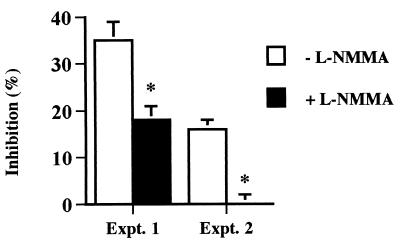
Finally, to eliminate the effects of phagocytosis on parasite growth inhibition, a two-compartment system separating parasites from Mφ was employed. Under these conditions, soluble factors released by Mφ inhibited the growth of B. bovis by 16 to 35% (Fig. 6). Addition of l-NMMA resulted in significant, but not always complete, restoration of parasite growth (Fig. 6), supporting the conclusion that growth inhibitory molecules in addition to NO are produced in response to B. bovis.
FIG. 6.
Inhibition of B. bovis by bovine Mφ in a system that does not permit phagocytosis is partially NO dependent. Bovine Mφ were plated in cell culture inserts and separated from IRBCs in the wells of a 24-well plate by a 0.4-μm-pore-size membrane without (white bars) or with (black bars) l-NMMA. Quadruplicate aliquots of IRBCs were transferred to a 96-well plate. Inhibition of parasite growth was assessed by measuring parasite incorporation of [3H]hypoxanthine. Results from two separate experiments performed with Mφ from different cattle are shown as the sample means ± 1 SD for each experiment. ∗, P < 0.05, for B. bovis cultured with l-NMMA compared to B. bovis cultured with Mφ but without l-NMMA.
Concluding remarks.
This study demonstrates for the first time that B. bovis induces Mφ inflammatory and regulatory cytokines IL-1β, IL-12, and TNF-α that are hypothesized to be important for both innate and acquired immune responses against this parasite (9). In cattle, TNF-α is an important cofactor with IFN-γ for NO production by Mφ (1, 19, 26), although it does not exhibit direct babesiacidal activity (reference 39 and data not shown). IL-12 stimulates NK cells to produce IFN-γ (41), and in cattle it was shown to stimulate IFN-γ production during priming (44) and to enhance IFN-γ production by memory/effector CD4+ T cells specific for B. bovis (4, 42). Ex vivo production of IFN-γ and TNF-α by PBMCs correlated with the resolution of acute infection in calves vaccinated with a recombinant B. bovis antigen and subsequently challenged (11). In addition to activating Mφ, IFN-γ enhances production of opsonizing immunoglobulin G2 antibody in cattle (8, 13, 27), which is believed to facilitate removal of parasitized erythrocytes (9). IL-1 enhances the expression of IL-2 receptors on antigen-specific helper T cells, thereby promoting their expansion in response to autocrine IL-2 (10).
We also report for the first time that a lipid fraction of B. bovis-infected erythrocytes induces NO production by bovine Mφ. Previous studies showed that a membrane-enriched fraction of B. bovis merozoites stimulated NO production (37). Together, these results are consistent with the potential for GPI molecules, believed to anchor certain membrane proteins of B. bovis (22), to activate Mφ. Further studies are needed to verify the identities of the lipids involved.
NO, induced in Mφ by B. bovis, was only partially growth inhibitory for this parasite, indicating that NO is not solely responsible for control of Babesia replication. When parasites and Mφ were physically separated, l-NMMA reversed growth inhibition by more than 50%, whereas when parasites were allowed to be phagocytosed, the effect of l-NMMA was less striking. These observations support the in vivo data of Gale et al. (17), who found that administration of an iNOS inhibitor ameliorated some of the symptoms of acute B. bovis infection, but had a limited and inconsistent effect on parasitemia. These results suggested that NO was produced during acute infection but that it might not be sufficient to limit parasite replication.
B. bovis paradoxically stimulates an innate immune response that could result in the control of acute infection and survival of the host. However, for this parasite, which can cause a virulent and often fatal cerebral form of disease, success is measured by the ability to maintain a persistent infection that results when cattle naturally survive acute infection (25). Persistently infected animals provide a reservoir for subsequent transmission by ticks to susceptible animals. The ability to stimulate protective, innate immune responses used naturally by the pathogen may be a key factor in designing vaccine adjuvants and delivery systems for the prevention of babesiosis.
ACKNOWLEDGMENTS
We thank Deb Alperin and Kim Kegerreis for excellent technical assistance.
This research was supported by NIH NIAID grant R01-AI30136 (W.C.B.), USDA NRICGP grants 96-35204-3667 (G.H.P.) and 98-35204-6737 (L.K.M.S.), and by the Organization of American States Fellowship Program (J.F.-C.).
REFERENCES
- 1.Adler H, Peterhans E, Nicolet J, Jungi T W. Inducible l-arginine-dependent nitric oxide synthase activity in bovine bone marrow-derived macrophages. Biochem Biophys Res Commun. 1994;198:510–515. doi: 10.1006/bbrc.1994.1075. [DOI] [PubMed] [Google Scholar]
- 2.Bate C A W, Taverne J, Roman E, Moreno C, Playfair J H L. Tumor necrosis factor induction by malaria exoantigens depends upon phospholipid. Immunology. 1992;75:129–135. [PMC free article] [PubMed] [Google Scholar]
- 3.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 4.Brown W C, Davis W C, Tuo W. Human interleukin-12 upregulates proliferation and interferon-γ production by parasite antigen-stimulated Th cell clones and γ/δ T cells of cattle. Annu NY Acad Sci. 1996;795:321–324. doi: 10.1111/j.1749-6632.1996.tb52682.x. [DOI] [PubMed] [Google Scholar]
- 5.Brown W C, Estes D M. Type 1 and type 2 responses in cattle and their regulation. In: Schijns V E C J, Horzinek M C, editors. Cytokines in veterinary medicine. New York, N.Y: CAB International; 1997. pp. 15–33. [Google Scholar]
- 6.Brown W C, Estes D M, Chantler S E, Kegerreis K A, Suarez C E. DNA and a CpG oligonucleotide derived from Babesia bovis are mitogenic for bovine B cells. Infect Immun. 1998;66:5423–5432. doi: 10.1128/iai.66.11.5423-5432.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown W C, Logan K S, Wagner G G, Tetzlaff C L. Cell-mediated immune responses to Babesia bovis merozoite antigens in cattle following infection with tick-derived or cultured parasites. Infect Immun. 1991;59:2418–2426. doi: 10.1128/iai.59.7.2418-2426.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown W C, McElwain T F, Palmer G H, Chantler S E, Estes D M. Bovine CD4+ T-lymphocyte clones specific for rhoptry-associated protein 1 of Babesia bigemina stimulate enhanced immunoglobulin G1 (IgG1) and IgG2 synthesis. Infect Immun. 1999;67:155–164. doi: 10.1128/iai.67.1.155-164.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown W C, Palmer G H. Designing blood-stage vaccines against Babesia bovis and B. bigemina. Parasitol Today. 1999;15(7):275–281. doi: 10.1016/s0169-4758(99)01471-4. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello C A. Role of interleukin-1 in infectious diseases. Immunol Rev. 1992;127:120–146. doi: 10.1111/j.1600-065x.1992.tb01411.x. [DOI] [PubMed] [Google Scholar]
- 11.East I J, Zakrzewski H, Gale K R, Leatch G, Dimmock C M, Thomas M B, Waltisbuhl D J. Vaccination against Babesia bovis: T cells from protected and unprotected animals show different cytokine profiles. Int J Parasitol. 1997;27:1537–1545. doi: 10.1016/s0020-7519(97)00141-0. [DOI] [PubMed] [Google Scholar]
- 12.Ellis J A, Godson D, Campos M, Sileghem M, Babiuk L A. Capture immunoassay for ruminant tumor necrosis factor-α: comparison with bioassay. Vet Immunol Immunopathol. 1993;35:289–300. doi: 10.1016/0165-2427(93)90040-b. [DOI] [PubMed] [Google Scholar]
- 13.Estes D M, Closser N M, Allen G K. IFN-γ stimulates IgG2 production from bovine B cells costimulated with anti-μ and mitogen. Cell Immunol. 1994;154:287–295. doi: 10.1006/cimm.1994.1078. [DOI] [PubMed] [Google Scholar]
- 14.Fell A, Smith N. Immunity to asexual blood stages of Plasmodium: is resistance to acute malaria adaptive or innate? Parasitol Today. 1998;14(9):364–369. doi: 10.1016/s0169-4758(98)01298-8. [DOI] [PubMed] [Google Scholar]
- 15.Florin-Christensen J, Suarez C E, Florin-Christensen M, Hines S A, Mcelwain T F, Palmer G H. Phosphatidylcholine formation is the predominant lipid biosynthetic event in the hemoparasite Babesia bovis. Mol Biochem Parasitol. 2000;106:147–156. doi: 10.1016/s0166-6851(99)00209-1. [DOI] [PubMed] [Google Scholar]
- 16.Frankova D, Zidek Z. IFN-γ-induced TNF-α is a prerequisite for in vitro production of nitric oxide generated in murine peritoneal macrophages by IFN-γ. Eur J Immunol. 1998;28:838–843. doi: 10.1002/(SICI)1521-4141(199803)28:03<838::AID-IMMU838>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 17.Gale K R, Waltisbuhl D J, Bowden J M, Jorgensen W K, Matheson J, East I J, Zakrzewski H, Leatch G. Amelioration of virulent Babesia bovis infection in calves by administration of the nitric oxide synthase inhibitor aminoguanidine. Parasite Immunol. 1998;20:441–445. doi: 10.1046/j.1365-3024.1998.00169.x. [DOI] [PubMed] [Google Scholar]
- 18.Gazzinelli R T, Camargo M M, Almeida I C, Morita Y S, Giraldo M, Acosta-Serrano A, Hieny S, Englund P T, Ferguson M A J, Travassos L R, Sher A. Identification and characterization of protozoan products that trigger the synthesis of IL-12 by inflammatory macrophages. Chem Immunol. 1997;68:136–152. doi: 10.1159/000058689. [DOI] [PubMed] [Google Scholar]
- 19.Goff W L, Johnson W C, Wyatt C R, Cluff C W. Assessment of bovine mononuclear phagocytes and neutrophils for induced l-arginine-dependent nitric oxide production. Vet Immunol Immunopathol. 1996;55:45–62. doi: 10.1016/s0165-2427(96)05629-2. [DOI] [PubMed] [Google Scholar]
- 20.Goff W L, Yunker C E. Babesia bovis: increased percentage of parasitized erythrocytes in cultures and assessment of growth by incorporation of [3H]hypoxanthine. Exp Parasitol. 1986;62:202–210. doi: 10.1016/0014-4894(86)90024-x. [DOI] [PubMed] [Google Scholar]
- 21.Grau G E, Piguet P-F, Vassalli P, Lambert P-H. Tumor necrosis factor and other cytokines in cerebral malaria: experimental and clinical data. Immunol Rev. 1989;112:49–70. doi: 10.1111/j.1600-065x.1989.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 22.Hines S A, McElwain T F, Buening G M, Palmer G H. Molecular characterization of Babesia bovis merozoite surface proteins bearing epitopes immunodominant in protected cattle. Mol Biochem Parasitol. 1989;37:1–10. doi: 10.1016/0166-6851(89)90096-0. [DOI] [PubMed] [Google Scholar]
- 23.Johnson W C, Cluff C W, Goff W L, Wyatt C R. Reactive oxygen and nitrogen intermediates and products from polyamine degradation are babesiacidal in vitro. Annu NY Acad Sci. 1996;791:136–147. doi: 10.1111/j.1749-6632.1996.tb53520.x. [DOI] [PubMed] [Google Scholar]
- 24.Kumaratilake L M, Ferrante A, Rzepcayk C M. Tumor necrosis factor enhances neutrophil-mediated killing of Plasmodium falciparum. Infect Immun. 1990;58:788–793. doi: 10.1128/iai.58.3.788-793.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahoney D F, Wright I G, Mirre G B. Bovine babesiosis: the persistence of immunity to Babesia argentine and B. bigemina in calves (Bos taurus) after naturally acquired infection. Ann Trop Med Parasitol. 1973;67:197–203. doi: 10.1080/00034983.1973.11686877. [DOI] [PubMed] [Google Scholar]
- 26.Mason G L, Yang Z, Olchowy T W J, Jian Z, Bochsler P N. Nitric oxide production and expression of inducible nitric oxide synthase by bovine alveolar macrophages. Vet Immunol Immunopathol. 1996;53:15–27. doi: 10.1016/0165-2427(96)05557-2. [DOI] [PubMed] [Google Scholar]
- 27.McGuire T C, Musoke A J, Kurtti T. Functional properties of bovine IgG1 and IgG2: interaction with complement, macrophages, neutrophils and skin. Immunology. 1979;38:249–256. [PMC free article] [PubMed] [Google Scholar]
- 28.Montealegre F, Levy M G, Ristic M, James M A. Growth inhibition of Babesia bovis in culture by secretions from bovine mononuclear phagocytes. Infect Immun. 1985;50:523–526. doi: 10.1128/iai.50.2.523-526.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quakyi I A, Currier J, Fell A, Taylor D W, Roberts T, Houghten R A, England R D, Berzofsky J A, Miller L H, Good M F. Analysis of human T cell clones specific for conserved peptide sequences within malaria proteins. J Immunol. 1994;153:2082–2092. [PubMed] [Google Scholar]
- 30.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley S B, Menon S, Kastelein R, Bazan F, O'Garra A. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-γ production and activates IRAK and NFκB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 31.Rockett K A, Awburn M M, Cowden W B, Clark I A. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun. 1991;59:3280–3283. doi: 10.1128/iai.59.9.3280-3283.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rockett K A, Kwiatkowski D, Bate C A W, Awburn M M, Rockett E J, Clark I A. In vitro induction of nitric oxide by an extract of Plasmodium falciparum. J Infect. 1996;32:187–196. doi: 10.1016/s0163-4453(96)80018-1. [DOI] [PubMed] [Google Scholar]
- 33.Schofield L, Hackett F. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J Exp Med. 1993;177:145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schofield L, Novakovic S, Gerold P, Schwarz R T, McConville M J, Tachado S D. Glycosylphosphatidylinositol toxin of Plasmodium upregulates intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin expression in vascular endothelial cells and increases leukocyte and parasite cytoadherence via tyrosine kinase-dependent signal transduction. J Immunol. 1996;156:1886–1896. [PubMed] [Google Scholar]
- 35.Scragg I G, Hensmann M, Bate C A, Kwiatkowski D. Early cytokine induction by Plasmodium falciparum is not a classical endotoxin-like process. Eur J Immunol. 1999;29:2636–2644. doi: 10.1002/(SICI)1521-4141(199908)29:08<2636::AID-IMMU2636>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 36.Shoda L K M, Zarlenga D S, Hirano A, Brown W C. Cloning of a cDNA encoding bovine IL-18. J Interferon Cytokine Res. 1999;19:1169–1177. doi: 10.1089/107999099313118. [DOI] [PubMed] [Google Scholar]
- 37.Stich R W, Shoda L K M, Dreewes M, Adler B, Jungi T W, Brown W C. Stimulation of nitric oxide production in macrophages by Babesia bovis. Infect Immun. 1998;66:4130–4136. doi: 10.1128/iai.66.9.4130-4136.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tachado S D, Gerold P, McConville M J, Baldwin T, Quilici D, Schwarz R T, Schofield L. Glycosylphosphatidylinositol toxin of Plasmodium induces nitric oxide synthase expression in macrophages and vascular endothelial cells by a protein tyrosine kinase-dependent and protein kinase C-dependent signaling pathway. J Immunol. 1996;156:1897–1907. [PubMed] [Google Scholar]
- 39.Tambrallo L J, Buening G M, McLaughlin R M. The effect of neutrophils, tumor necrosis factor, and granulocyte macrophage/colony stimulating factor on Babesia bovis and Babesia bigemina in culture. Vet Parasitol. 1992;43:177–188. doi: 10.1016/0304-4017(92)90159-7. [DOI] [PubMed] [Google Scholar]
- 40.Taylor-Robinson A W. Antimalarial activity of nitric oxide: cytostasis and cytotoxicity towards Plasmodium falciparum. Biochem Soc Trans. 1997;25:262S. doi: 10.1042/bst025262s. [DOI] [PubMed] [Google Scholar]
- 41.Trinchieri G. Interleukin 12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 42.Tuo W, Estes D M, Brown W C. Comparative effects of IL-12 and IL-4 on cytokine expression by antigen-stimulated memory CD4+ T cells of cattle: IL-12 enhances IFN-γ production, but IL-4 has marginal effects on cytokine expression. J Interferon Cytokine Res. 1999;19:743–751. doi: 10.1089/107999099313587. [DOI] [PubMed] [Google Scholar]
- 43.Tuo W, Macmillan H, Gunter N, Bazer F W, Brown W C. Upregulation of interleukin-4 and IFN-γ expression by IFN-τ, a member of the type 1 IFN family. J Interferon Cytokine Res. 1999;19:179–187. doi: 10.1089/107999099314324. [DOI] [PubMed] [Google Scholar]
- 44.Tuo W, Palmer G H, McGuire T C, Zhu D, Brown W C. Interleukin-12 as an adjuvant promotes immunoglobulin G and type 1 cytokine recall responses to major surface protein 2 of the ehrlichial pathogen Anaplasma marginale. Infect Immun. 2000;68:270–280. doi: 10.1128/iai.68.1.270-280.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright I G, Goodger B V, Clark I A. Immunopathophysiology of Babesia bovis and Plasmodium falciparum infections. Parasitol Today. 1988;4:214–218. doi: 10.1016/0169-4758(88)90161-5. [DOI] [PubMed] [Google Scholar]