Abstract
Introduction
Lipocalin‐2 (LCN2) is an acute‐phase protein that could mediate neuroinflammation after brain injury. We aimed to evaluate if LCN2 level was associated with early neurological deterioration (END) in acute ischemic stroke patients, thus hindering clinical recovery.
Methods
We conducted a prospective study of acute ischemic stroke patients between June 2021 and February 2022. Serum LCN2 concentration was measured after admission using an enzyme‐linked immunosorbent assay. Outcomes included END and 90‐day poor functional outcome (modified Rankin Scale 3‐6). The National Institutes of Health Stroke Scale increment ≥4 points within 72 h after admission was defined as END.
Results
A total of 253 acute ischemic stroke patients (mean age, 65.2 ± 13.4 years; 64.0% male) were recruited. In the multivariate adjustment, increased serum LCN2 levels (per 1‐SD increase of LCN2) were associated with a higher risk of END (odds ratio [OR], 1.64; 95% confidence interval [CI], 1.20–2.25; p = .002) and 90‐day poor outcome (OR, 1.73; 95% CI, 1.22–2.45; p = .002). Restricted cubic splines found a linear relationship between LCN2 level and 90‐day unfavorable outcome (END, p = .001 for linearity; 90‐day poor outcome, p = .013 for linearity). Subgroup analysis further confirmed the significant association of LCN2 with clinical outcomes.
Conclusions
This study demonstrated that higher circulating LCN2 level was associated with an increased risk of early clinical worsening and 90‐day unfavorable outcomes in ischemic stroke patients.
Keywords: biomarker, clinical worsening, functional outcome, ischemic stroke, LCN2
1. END was a common complication after ischemic stroke; 2. Higher LCN2 levels were found in patients with END and 90 days unfavorable outcome? 3. LCN2 may involve in the pathophysiology of clinical worsening after stroke.

1. INTRODUCTION
Stroke is the second leading cause of adult disability and mortality worldwide (GBD 2019 Stroke Collaborators, 2021). Despite advances in disease prevention and acute management in China, the stroke burden is expected to increase in the past 7 years (2013–2019) (Tu et al., 2022). Some patients experience neurological worsening during the acute phase, namely, early neurological deterioration (END), with a prevalence ranging from 5% to 40% (Siegler & Martin‐Schild, 2011; Thanvi et al., 2008; Zhang et al., 2016). Several studies demonstrated early or subacute neurological deterioration might have a deleterious impact on functional recovery (Liu et al., 2020; Mori et al., 2012). Therefore, rapid prediction of clinical outcomes at the acute phase of ischemic stroke is of vital importance for prognosis improvement.
Lipocalin‐2 (LCN2) is an acute‐phase protein of the lipocalin family and is highly expressed in response to brain injury and inflammatory stimuli (Dekens et al., 2018; Naudé et al., 2012; Xiao et al., 2017; Zhao et al., 2019). The LCN2 protein was primarily expressed in astrocytes and endothelial cells after focal cortical ischemia in mice (Wan et al., 2022). In a previous animal study of the transient middle cerebral artery occlusion model, LCN2 inhibition could alleviate ischemic brain damage, including amelioration of neuroinflammation and blood–brain barrier disruption (Jin et al., 2014). Moreover, in vitro studies demonstrated that LCN2 deficiency in astrocytes could alleviate direct neurotoxic effects on neurons under oxygen and glucose‐deprived conditions (Suk, 2016). A previous clinical study found that LCN2/MMP‐9 complex concentrations could be used to identify unstable atherosclerotic plaques and major adverse cardiovascular events (Cheng et al., 2014; Hemdahl et al., 2006). However, few data are available to date regarding the prognostic value of serum LCN2 in ischemic stroke patients. Herein, we performed a prospective study and aimed to evaluate whether circulating LCN2 levels were correlated with clinical outcomes in patients with ischemic stroke.
2. METHODS
2.1. Study population
Patients diagnosed with first‐ever ischemic stroke and hospitalized within 72 h after symptoms onset were prospectively enrolled in Jinling Hospital during June 2021 and February 2022. The exclusion criteria of this study were as follows: (1) age <18 years; (2) early discharged within 3 days after admission; (3) pre‐stroke modified Rankin Scale (mRS) score >2; (4) had severe pulmonary disease, renal and liver failure, and active malignant. This study protocol was reviewed and approved by the ethics committee of Jinling Hospital (2021DZGZR‐YBB‐115). All subjects or their legally authorized representatives signed informed consent before entering the study.
2.2. Baseline data collection
Baseline data were collected after admission by trained neurologic clinicians. These data included age, sex, vascular risk factors, baseline stroke severity, stroke subtype, infarct volume, and white matter lesions (WMLs). Stroke severity was evaluated by the National Institutes of Health Stroke Scale (NIHSS) (Brott et al., 1989). Pretreatment infarction core was assessed by the Alberta stroke program early computerized tomography (ASPECT) score (Barber et al., 2000). Stroke etiology was defined according to the criteria of Trial of Org 10172 in Acute Stroke Treatment (TOAST) (Adams et al., 1993). We measured the WMLs in the hemisphere contralateral to acute stroke using the Fazekas scale (Fazekas et al., 1987). The total WMLs score was calculated by summing up the scores for subcortical WMLs and periventricular WMLs, ranging from 0 to 6. According to previous studies, severe WMLs were defined as a total WMLs score ≥3 (Kim et al., 2014; Yakushiji et al., 2014).
2.3. LCN2 level assessment
The blood samples were collected within 24 h after admission and processed under standard laboratory procedure. Serum samples were stored at −80°C for further analysis. The serum LCN2 levels were measured using the ELISA Kit (Cat. EK0853; Boster Biological Technology, Wuhan, China). The intra‐ and inter‐assay coefficients of variation were <7.5% and <7.5%, respectively. The minimum detectable concentration was 10.0 pg/mL. All samples were measured by a laboratory technician who was blinded to any clinical information of the study participants.
2.4. Clinical outcome assessment
The clinical outcomes included END and functional outcomes at 90 days. The neurological deficit was evaluated using the NIHSS at baseline and continued 1–3 times a day for 72 h by a certified neurologist, who was blind to clinical information. In our study, END was defined as a total NIHSS score ≥4 points deterioration within 72 h after admission (Alawneh et al., 2009; Sun et al., 2014). The 3‐month follow‐up was conducted via telephone or outpatient clinic using the mRS. The functional outcome was dichotomized as favorable (mRS 0–2) and unfavorable (mRS 3–6).
2.5. Statistical analysis
Data were presented as the mean ± standard deviations (SD) or median with interquartile range (IQR) for continuous variables and as percentages for categorical variables. Univariate analyses were utilized using the Fisher exact test or χ 2 test for qualitative variables, and the t‐test or Mann–Whitney U test for quantitative variables, where appropriate. Logistic regression analyses were used to assess the associations of LCN2 levels with END and functional outcomes. Then, age, sex, and variables with a p value <.1 in univariate analysis were adjusted in the multivariate logistic regression analysis. Subgroup analysis was conducted to test the robustness of our findings. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. We further evaluated the pattern and magnitude of the association between LCN2 level and clinical outcomes using the restricted cubic splines with 3 kn (at 5th, 50th, and 95th percentiles) adjusted for covariates (Durrleman & Simon, 1989). All analyses were conducted using statistical software SPSS version 24.0 (IBM, New York, NY, USA) and R statistical software (R, version 4.1; R Project). A 2‐sided p value <.05 was considered to be statistically significant.
3. RESULTS
In this study, 273 ischemic patients hospitalized within 72 h after symptoms onset were screened for analysis. We excluded four patients early discharged within 72 h, three patients had a pre‐stroke mRS score >2, seven patients diagnosed with active malignant, and six patients lost in the follow‐up. Finally, a total of 253 patients were enrolled. Finally, a total of 253 patients were analyzed, among whom 162 (64.0%) patients were male. The mean age was 65.2 ± 13.4 years. Hypertension was present in 176 (69.6%), diabetes mellitus in 87 (34.4%), hyperlipidemia in 34 (13.4%), and severe WMLs in 129 (51.0%) patients. Fifty‐one (20.2%) patients received reperfusion therapy after admission. The median NIHSS score at admission was 4.0 points. The median serum LCN2 concentration was 330.0 ng/mL (IQR, 258.6–500.4 ng/mL). The difference in clinical data stratified by the quartile of LCN2 was demonstrated in Table 1. Increased LCN2 levels showed a significant correlation with severe WMLs (p = .021) and hypersensitive C‐reactive protein (p = .024).
TABLE 1.
Comparison of baseline data stratified by the lipocalin‐2 (LCN2) quartile
| Variable | 1st quartile (n = 63) | 2nd quartile (n = 62) | 3rd quartile (n = 64) | 4th quartile (n = 64) | p Value |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age (years) | 63.5 ± 15.5 | 64.1 ± 13.9 | 65.4 ± 11.4 | 68.0 ± 11.5 | .491 |
| Male, n (%) | 40 (63.5) | 40 (64.5) | 44 (68.8) | 38 (59.4) | .745 |
| Body mass index (kg/m2) | 24.9 ± 3.2 | 24.6 ± 3.5 | 25.2 ± 3.5 | 25.0 ± 3.1 | .837 |
| Vascular risk factors, n (%) | |||||
| Hypertension | 45 (71.4) | 43 (69.4) | 42 (65.6) | 46 (71.9) | .865 |
| Diabetes mellitus | 20 (31.7) | 23 (37.1) | 19 (29.7) | 25 (39.1) | .650 |
| Hyperlipidemia | 8 (12.7) | 8 (12.9) | 9 (14.3) | 9 (14.1) | .993 |
| Coronary heart disease | 9 (14.3) | 6 (9.7) | 9 (14.3) | 3 (4.7) | .243 |
| Smoking | 21 (33.3) | 20 (32.3) | 28 (43.8) | 22 (34.4) | .510 |
| Clinical data | |||||
| Previous statin therapy, n (%) | 9 (14.3) | 17 (27.4) | 12 (18.8) | 10 (5.6) | .236 |
| Previous antiplatelet therapy, n (%) | 10 (15.9) | 16 (25.8) | 14 (21.9) | 14 (21.9) | .598 |
| Systolic blood pressure (mmHg) | 149.3 ± 26.8 | 144.7 ± 23.0 | 143.1 ± 19.6 | 147.6 ± 26.0 | .344 |
| Diastolic blood pressure (mmHg) | 81.9 ± 15.0 | 82.4 ± 14.4 | 83.5 ± 12.0 | 84.8 ± 15.0 | .556 |
| Baseline NIHSS, score | 3.0 (2.0, 10.0) | 3.0 (2.0, 8.0) | 4.0 (2.0, 8.0) | 4.0 (2.0, 9.0) | .754 |
| Severe white matter lesions | 23 (36.5) | 32 (51.6) | 33 (51.6) | 41 (64.1) | .021 |
| Site of infarction, n (%) | .346 | ||||
| Anterior circulation | 50 (79.4) | 46 (24.2) | 56 (87.5) | 54 (84.4) | .236 |
| Posterior circulation | 14 (22.2) | 16 (25.8) | 9 (14.1) | 10 (15.6) | .295 |
| Cause of stroke, n (%) | .968 | ||||
| Atherosclerotic | 21 (33.3) | 23 (37.1) | 26 (40.6) | 26 (40.6) | |
| Cardioembolic | 10 (15.9) | 12 (19.4) | 11 (17.2) | 8 (12.5) | |
| Small vessel occlusion | 22 (34.9) | 18 (29.0) | 17 (26.6) | 22 (34.4) | |
| Others | 10 (15.9) | 9 (14.5) | 10 (15.6) | 8 (12.5) | |
| Laboratory data | |||||
| Blood urea nitrogen/creatinine | 13.9 ± 4.7 | 14.0 ± 4.5 | 12.8 ± 4.0 | 13.7 ± 4.4 | .432 |
| Homocysteine (mmol/L) | 11.5 (10.0, 14.9) | 12.9 (9.7, 15.4) | 12.7 (9.8, 16.4) | 12.4 (10.2, 16.9) | .980 |
| Baseline blood glucose (mmol/L) | 5.7 (4.9, 7.7) | 6.0 (5.0, 9.3) | 6.1 (5.0, 7.9) | 6.3 (5.0, 8.2) | .576 |
| Hs‐CRP (mg/L) | 3.2 (1.2, 7.5) | 4.3 (1.5, 10.3) | 5.3 (1.9, 9.3) | 8.8 (1.6, 14.5) | .024 |
Abbreviations: Hs‐CRP, hypersensitive C‐reactive protein; NIHSS, National Institutes of Health Stroke Scale.
Table 2 demonstrates the baseline data of the study population stratified by the clinical outcomes. During the hospitalization, 41 (16.2%) subjects experienced END, and 18 (7.2%) patients died. As compared to patients without END, patients with END were more likely to develop diabetes (p = .034) and had higher baseline NIHSS score (p = .009) and LCN2 levels (p = .021). During the 90‐day follow‐up, 116 (45.8%) patients experience unfavorable outcomes (mRS score of 3–6). Age (p = .044), baseline NIHSS score (p = .001), severe WMLs (p = .025), and LCN2 levels (p = .010) differed significantly between patients with and without poor outcome.
TABLE 2.
Comparison of baseline data stratified by clinical outcomes
| Variable | All patients (n = 253) | With END (n = 41) | Without END (n = 212) | p Value | Unfavorable outcome (n = 116) | Favorable outcome (n = 137) | p Value |
|---|---|---|---|---|---|---|---|
| Demographic characteristics | |||||||
| Age (years) | 65.2 ± 13.4 | 67.7 ± 11.8 | 64.7 ± 13.7 | .182 | 67.0 ± 12.6 | 63.6 ± 14.0 | .044 |
| Male, n (%) | 162 (64.0) | 29 (70.7) | 133 (62.7) | .954 | 76 (65.6) | 86 (62.8) | .650 |
| Body mass index (kg/m2) | 24.9 ± 3.3 | 24.9 ± 4.1 | 24.9 ± 3.2 | .951 | 25.0 ± 3.7 | 24.9 ± 3.0 | .731 |
| Vascular risk factors, n (%) | |||||||
| Hypertension | 176 (69.6) | 29 (70.7) | 147 (69.3) | .859 | 79 (68.1) | 97 (70.8) | .642 |
| Diabetes mellitus | 87 (34.4) | 20 (48.8) | 67 (31.6) | .034 | 40 (34.5) | 47 (34.3) | .977 |
| Hyperlipidemia | 34 (13.4) | 5 (12.2) | 29 (13.7) | .799 | 13 (11.2) | 21 (15.3) | .338 |
| Coronary heart disease | 27 (10.7) | 3 (7.3) | 24 (11.4) | .442 | 13 (11.2) | 14 (10.2) | .781 |
| Smoking | 91 (36.0) | 16 (39.0) | 75 (35.4) | .656 | 41 (35.3) | 50 (36.5) | .849 |
| Clinical data | |||||||
| Previous statin therapy, n (%) | 48 (19.0) | 5 (12.2) | 43 (20.3) | .227 | 18 (15.5) | 30 (21.9) | .197 |
| Previous antiplatelet therapy, n (%) | 54 (12.3) | 9 (22.0) | 45 (21.2) | .917 | 23 (19.8) | 31 (22.6) | .588 |
| Systolic blood pressure (mmHg) | 146.2 ± 24.0 | 148.6 ± 20.6 | 145.6 ± 24.6 | .476 | 145.5 ± 23.1 | 146.7 ± 24.8 | .666 |
| Diastolic blood pressure (mmHg) | 83.2 ± 14.1 | 85.6 ± 13.2 | 82.7 ± 14.2 | .224 | 82.4 ± 13.6 | 83.8 ± 14.6 | .458 |
| Baseline NIHSS, score | 4.0 (2.0, 9.0) | 7.0 (3.5, 10.0) | 3.0 (2.0, 8.0) | .009 | 8.0 (4.0, 12.0) | 2.0 (1.0, 4.0) | .001 |
| Severe white matter lesions | 129 (51.0) | 26 (63.4) | 103 (48.6) | .082 | 68 (58.6) | 61 (44.5) | .025 |
| Site of infarction, n (%) | |||||||
| Anterior circulation | 206 (81.4) | 32 (78.0) | 174 (82.1) | .544 | 94 (81.0) | 112 (81.8) | .848 |
| Posterior circulation | 49 (19.4) | 9 (22.0) | 40 (18.9) | .647 | 24 (20.7) | 25 (18.2) | .624 |
| Cause of stroke, n (%) | .481 | .130 | |||||
| Atherosclerotic | 96 (37.9) | 14 (34.1) | 82 (38.7) | 47 (40.5) | 49 (35.8) | ||
| Cardioembolic | 41 (16.2) | 10 (24.4) | 31 (14.6) | 24 (20.7) | 17 (12.4) | ||
| Small vessel occlusion | 79 (31.2) | 12 (29.3) | 67 (31.6) | 32 (27.6) | 47 (34.3) | ||
| Others | 37 (14.6) | 5 (12.2) | 32 (15.1) | 13 (11.2) | 24 (17.5) | ||
| Laboratory data | |||||||
| Blood urea nitrogen/creatinine | 13.6 ± 4.4 | 14.6 ± 5.6 | 13.4 ± 4.1 | .124 | 13.9 ± 4.6 | 13.3 ± 4.2 | .261 |
| Homocysteine (mmol/L) | 12.5 (10.0, 15.4) | 11.6 (9.7, 14.1) | 12.8 (10.0, 15.5) | .229 | 12.5 (10.0, 15.5) | 12.8 (10.1, 15.4) | .661 |
| Baseline blood glucose (mmol/L) | 6.0 (5.0, 8.1) | 7.0 (4.9, 8.7) | 6.0 (5.0, 7.8) | .501 | 6.6 (5.0, 8.6) | 5.7 (4.9, 7.3) | .102 |
| Hs‐CRP (mg/L) | 5.0 (1.5, 10.4) | 6.2 (1.0, 13.4) | 5.0 (1.5, 10.3) | .494 | 6.3 (1.4, 14.2) | 4.6 (1.5, 8.6) | .177 |
| LCN2 (ng/mL) | 330.0 (258.6, 500.4) | 414.4 (296.0, 571.6) | 316.2 (248.3, 494.3) | .021 | 382.6 (281.5, 545.3) | 314.7 (237.0, 466.3) | .010 |
| LCN2 quartiles, n (%) | .089 | .154 | |||||
| First | 63 (24.9) | 4 (9.8) | 59 (27.8) | 24 (20.7) | 39 (28.5) | ||
| Second | 62 (24.5) | 12 (29.3) | 50 (23.6) | 25 (21.6) | 37 (27.0) | ||
| Third | 64 (25.3) | 11 (26.8) | 53 (25.0) | 31 (26.7) | 33 (24.1) | ||
| Fourth | 64 (25.3) | 14 (34.1) | 50 (23.6) | 36 (31.0) | 28 (20.4) |
Abbreviations: END, early neurological deterioration; Hs‐CRP, hypersensitive C‐reactive protein; LCN2, lipocalin‐2; NIHSS, National Institutes of Health Stroke Scale.
After adjusting for age, sex, and variables with a p value <.1 in univariate analysis, the multivariate regression analysis model showed that higher LCN2 levels were associated with an increased risk of END (per 1‐SD increase, OR, 1.64; 95% CI, 1.20–2.25; p = .002) and 90‐day poor outcome (per 1‐SD increase, OR, 1.73; 95% CI, 1.22–2.45; p = .000) (Table 3). In addition, the association of serum LCN2 with risk of END and the 90‐day unfavorable prognosis was similar across subgroups stratified according to age, sex, admission NIHSS score, and receiving reperfusion therapy (p > .05 for interaction for all; Figure 1).
TABLE 3.
Multivariate analysis of the association between lipocalin‐2 (LCN2) levels and clinical outcome
| Variables | Adjusted OR (95% CI) for END* | p Value | Adjusted OR (95% CI) for unfavorable outcome* | p Value |
|---|---|---|---|---|
| Per 1‐SD increase in LCN2 | 1.64 (1.20–2.25) | .002 | 1.73 (1.22–2.45) | .002 |
| LCN2 quartiles | ||||
| First | Reference | Reference | ||
| Second | 4.35 (1.22–15.44) | .023 | 1.59 (0.64–3.93) | .317 |
| Third | 3.89 (1.09–13.89) | .037 | 2.19 (0.90–5.30) | .082 |
| Fourth | 5.16 (1.46–18.23) | .011 | 3.07 (1.25–7.56) | .015 |
Abbreviations: CI, confidence interval; END, early neurological deterioration; OR, odds ratio.
*Multivariate logistic regression analysis was adjusted for age, sex, reperfusion therapy, and variables with a p value <.1 in the univariate analysis.
FIGURE 1.
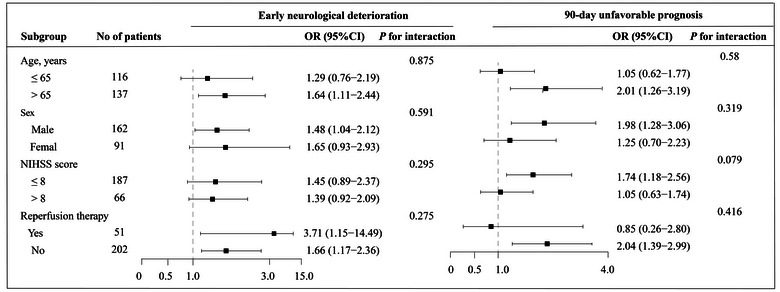
Subgroup analyses of the association between serum lipocalin‐2 (LCN2) and outcomes after ischemic stroke. Odds ratios (ORs) were calculated for each standard deviation (SD) increase in serum LCN2 levels after adjustment for age, sex, and variables with a p value <.1 in the univariate analysis, except for the stratified variable.
The pattern and magnitude of the relationship between LCN2 levels and clinical outcomes are shown in Figure 2. The multiple‐adjusted spline regression model displayed a linear association of LCN2 with risk of END (p = .001 for linearity) and 90‐day poor outcome (p = .013 for linearity).
FIGURE 2.
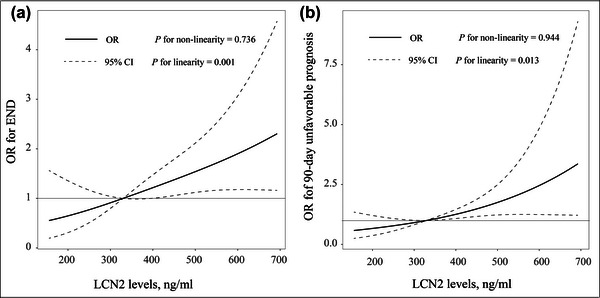
Association of serum lipocalin‐2 (LCN2) levels with risk of early neurological deterioration (END) (A) and 90‐day unfavorable outcome (B). Odds ratios and 95% confidence intervals were derived from restricted cubic spline regression, with knots placed at the 5th, 50th, and 95th percentiles of the distribution of LCN2. The reference point for serum LCN2 is the midpoint of the reference group from the categorical analysis. Odds ratios were adjusted for age, sex, and variables with a p value <.1 in the univariate analysis.
4. DISCUSSION
In this cohort study of 253 subjects with ischemic stroke, we demonstrated that baseline circulating LCN2 levels were positively associated with the development of END and 90‐day poor functional outcome, which remained statistically significant after adjustment for important prognostic covariates of stroke.
Previous investigations on END used different definitions, leading to a discrepancy in the incidence rates. In this study, we defined the END as an NIHSS increment ≥4 points within 72 h after admission, which is the most commonly used definition (Siegler & Martin‐Schild, 2011; Sun et al., 2014; Thanvi et al., 2008). As a result, 16.2% of patients were diagnosed with END, which was similar to previous data (Siegler & Martin‐Schild, 2011; Thanvi et al., 2008; Zhang et al., 2016). In addition, the inhospital mortality rate was 7.2%, which is slightly higher than that previously reported in a study from the big data observatory platform for stroke of China (death/discharge against medical advice: 6.2%) (Tu et al., 2021). This discrepancy might due to the difference in study sample and methods.
During the past few years, LCN2 was considered an attractive blood‐based biomarker of inflammation and ischemia. Clinical studies have confirmed an increased level of LCN2 in the serum of patients with mild cognitive impairment (Choi et al., 2011) and in the cerebrospinal fluid of patients with multiple sclerosis (Al Nimer et al., 2016). Furthermore, elevated circulating LCN2 levels have been linked to an increased risk of cardiovascular disease (Cheng et al., 2014; Wu et al., 2014). Although the above studies are supportive of the role of LCN2 in the pathogenesis of central nervous system disease, few data are available detecting the LCN2 in association with secondary brain injury and stroke morbidity in ischemic stroke patients. In our study, we were able to support the assumption that LCN2 has the potential to predict early outcomes after ischemic stroke. The mechanisms underlying the association between LCN2 and neurological deterioration after stroke are incompletely clear. However, some hypothetical causes might lead to functional disability. First, LCN2 could regulate the blood–brain barrier integrity. LCN2 was reported to reduce MMP‐9 degradation and prolong its activity, thereby augmenting the deleterious effects of MMP‐9 on the blood–brain barrier (Turner & Sharp, 2016). LCN2 also induces the expression of vascular endothelial growth factors, which could affect vascular permeability either directly or via astrocytes (Kim et al., 2017). During the acute phase after stroke, immune cells could infiltrate into the ischemic hemisphere through the blood–brain barrier and promote brain tissue damage (Wang et al., 2020). Second, LCN2 was involved in the expression of pro‐inflammatory cytokines, chemokines, and adhesion molecules after ischemic stroke (Jin et al., 2014; Wang et al., 2015). LCN2 could activate its receptor of 24p3R and promote the cellular release of the high mobility group box 1, which could subsequently aggravate oxidative stress and NLRP3 inflammasome activation (Mondal et al., 2020). Therefore, these findings suggest that LCN2 may induce neurological deterioration by mediating oxidative stress and neuroinflammation after stroke. Finally, iron overload after stroke may induce perihematomal edema and brain injury (Keep et al., 2012). LCN2 may function as a mediator of iron homeostasis as it is capable of transporting iron into cells through the siderophore (Devireddy et al., 2005). However, there are some controversies regarding the regulation of intracellular iron concentration by LCN2. For example, in the sepsis model of LCN2‐deficient mice, intracellular labile iron was elevated (Srinivasan et al., 2012). The inconsistency between these data may be due to differences in methodologies. The role of LCN2 in iron transport after ischemic stroke requires further study.
Several caveats should be considered when explaining the present results. First, this was a single‐center study with a relatively small sample size, which limits the generalization to other groups of subjects. Second, the circulating LCN2 level was measured only once after admission, we therefore unable to evaluate the dynamic changes of LCN2 after stroke. Third, although several confounders in the multivariate analysis were controlled, there is also a possibility of residual confounding in our study. Finally, data were observational, which cannot establish causation. We, therefore, recommend a prospective validation in a larger cohort to verify our findings.
5. CONCLUSION
In summary, this study performed in the Chinese population demonstrated that increased serum LCN2 may be an independent predictor of END and 90‐day poor outcome after ischemic stroke. Further studies are warranted to determine the pathophysiological role of LCN2 in mediating stroke outcomes.
AUTHOR CONTRIBUTIONS
Yi Xie, Xingfeng Zhuo, and Kai Xing designed the research and wrote the manuscript. Zhenqian Huang, Hongquan Guo, and Pengyu Gong carried out the data collection and follow‐up. Xingfeng Zhuo performed the data curation and review. Xingfeng Zhuo and Yun Li supervised the study. All authors have made an intellectual contribution to the manuscript and approved the submission.
CONFLICT OF INTEREST STATEMENT
All the authors declare that there is no conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.2979.
Xie, Y. , Zhuo, X. , Xing, K. , Huang, Z. , Guo, H. , Gong, P. , Zhang, X. , & Li, Y. (2023). Circulating lipocalin‐2 as a novel biomarker for early neurological deterioration and unfavorable prognosis after acute ischemic stroke. Brain and Behavior, 13, e2979. 10.1002/brb3.2979
Yi Xie, Xingfeng Zhuo, and Kai Xing contributed equally to this work.
Contributor Information
Xiaohao Zhang, Email: zxh_neurology@126.com.
Yun Li, Email: yun801026@sina.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- Adams, H. P. , Bendixen, B. H. , Kappelle, L. J. , Biller, J. , Love, B. B. , Gordon, D. L. , & Marsh, E. E. (1993). Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke; A Journal of Cerebral Circulation, 24(1), 35–41. 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- Alawneh, J. A. , Moustafa, R. R. , & Baron, J.‐C. (2009). Hemodynamic factors and perfusion abnormalities in early neurological deterioration. Stroke; A Journal of Cerebral Circulation, 40(6), e443–e450. 10.1161/STROKEAHA.108.532465 [DOI] [PubMed] [Google Scholar]
- Al Nimer, F. , Elliott, C. , Bergman, J. , Khademi, M. , Dring, A. M. , Aeinehband, S. , Bergenheim, T. , Romme Christensen, J. , Sellebjerg, F. , Svenningsson, A. , Linington, C. , Olsson, T. , & Piehl, F. (2016). Lipocalin‐2 is increased in progressive multiple sclerosis and inhibits remyelination. Neurology Neuroimmunology & Neuroinflammation, 3(1), e191. 10.1212/NXI.0000000000000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber, P. , Demchuk, A. , Zhang, J. , & Buchan, A. (2000). Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet, 355(9216), 1670–1674. [DOI] [PubMed] [Google Scholar]
- Brott, T. , Adams, H. P. , Olinger, C. P. , Marler, J. R. , Barsan, W. G. , Biller, J. , Spilker, J. , Holleran, R. , Eberle, R. , & Hertzberg, V. (1989). Measurements of acute cerebral infarction: A clinical examination scale. Stroke; A Journal of Cerebral Circulation, 20(7), 864–870. 10.1161/01.STR.20.7.864 [DOI] [PubMed] [Google Scholar]
- Cheng, J. M. , Akkerhuis, K. M. , Meilhac, O. , Oemrawsingh, R. M. , Garcia‐Garcia, H. M. , Van Geuns, R.‐J. , Piquer, D. , Merle, D. , Du Paty, E. , Galéa, P. , Jaisser, F. , Rossignol, P. , Serruys, P. W. , Boersma, E. , Fareh, J. , & Kardys, I. (2014). Circulating osteoglycin and NGAL/MMP9 complex concentrations predict 1‐year major adverse cardiovascular events after coronary angiography. Arteriosclerosis, Thrombosis, and Vascular Biology, 34(5), 1078–1084. 10.1161/ATVBAHA.114.303486 [DOI] [PubMed] [Google Scholar]
- Choi, J. , Lee, H.‐W. , & Suk, K. (2011). Increased plasma levels of lipocalin 2 in mild cognitive impairment. Journal of the Neurological Sciences, 305(1–2), 28–33. 10.1016/j.jns.2011.03.023 [DOI] [PubMed] [Google Scholar]
- Dekens, D. W. , Naudé, P. J. W. , Keijser, J. N. , Boerema, A. S. , De Deyn, P. P. , & Eisel, U. L. M. (2018). Lipocalin 2 contributes to brain iron dysregulation but does not affect cognition, plaque load, and glial activation in the J20 Alzheimer mouse model. Journal of Neuroinflammation, 15(1), 330. 10.1186/s12974-018-1372-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devireddy, L. R. , Gazin, C. , Zhu, X. , & Green, M. R. (2005). A cell‐surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell, 123(7), 1293–1305. 10.1016/j.cell.2005.10.027 [DOI] [PubMed] [Google Scholar]
- Durrleman, S. , & Simon, R. (1989). Flexible regression models with cubic splines. Statistics in Medicine, 8(5), 551–561. 10.1002/sim.4780080504 [DOI] [PubMed] [Google Scholar]
- Fazekas, F. , Chawluk, J. B. , Alavi, A. , Hurtig, H. I. , & Zimmerman, R. A. (1987). MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR – American Journal of Roentgenology, 149(2), 351–356. 10.2214/ajr.149.2.351 [DOI] [PubMed] [Google Scholar]
- GBD 2019 Stroke Collaborators . (2021). Global, regional, and national burden of stroke and its risk factors, 1990‐2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurology, 20, 795–820. 10.1016/S1474-4422(21)00252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemdahl, A.‐L. , Gabrielsen, A. , Zhu, C. , Eriksson, P. , Hedin, U. , Kastrup, J. , Thorén, P. , & Hansson, G. K. (2006). Expression of neutrophil gelatinase‐associated lipocalin in atherosclerosis and myocardial infarction. Arteriosclerosis, Thrombosis, and Vascular Biology, 26(1), 136–142. 10.1161/01.ATV.0000193567.88685.f4 [DOI] [PubMed] [Google Scholar]
- Jin, M. , Kim, J.‐H. , Jang, E. , Lee, Y. M. , Han, H. S. , Woo, D. K. , Park, D. H. , Kook, H. , & Suk, K. (2014). Lipocalin‐2 deficiency attenuates neuroinflammation and brain injury after transient middle cerebral artery occlusion in mice. Journal of Cerebral Blood Flow and Metabolism, 34(8), 1306–1314. 10.1038/jcbfm.2014.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keep, R. F. , Hua, Y. , & Xi, G. (2012). Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurology, 11(8), 720–731. 10.1016/S1474-4422(12)70104-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, G.‐M. , Park, K.‐Y. , Avery, R. , Helenius, J. , Rost, N. , Rosand, J. , Rosen, B. , & Ay, H. (2014). Extensive leukoaraiosis is associated with high early risk of recurrence after ischemic stroke. Stroke; A Journal of Cerebral Circulation, 45(2), 479–485. 10.1161/STROKEAHA.113.003004 [DOI] [PubMed] [Google Scholar]
- Kim, J.‐H. , Ko, P.‐W. , Lee, H.‐W. , Jeong, J.‐Y. , Lee, M.‐G. , Kim, J.‐H. , Lee, W.‐H. , Yu, R. , Oh, W.‐J. , & Suk, K. (2017). Astrocyte‐derived lipocalin‐2 mediates hippocampal damage and cognitive deficits in experimental models of vascular dementia. Glia, 65(9), 1471–1490. 10.1002/glia.23174 [DOI] [PubMed] [Google Scholar]
- Liu, P. , Liu, S. , Feng, N. , Wang, Y. , Gao, Y. , & Wu, J. (2020). Association between neurological deterioration and outcomes in patients with stroke. Annals of Translational Medicine, 8(1), 4. 10.21037/atm.2019.12.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal, A. , Bose, D. , Saha, P. , Sarkar, S. , Seth, R. , Kimono, D. , Albadrani, M. , Nagarkatti, M. , Nagarkatti, P. , & Chatterjee, S. (2020). Lipocalin 2 induces neuroinflammation and blood‐brain barrier dysfunction through liver‐brain axis in murine model of nonalcoholic steatohepatitis. Journal of Neuroinflammation, 17(1), 201. 10.1186/s12974-020-01876-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, M. , Naganuma, M. , Okada, Y. , Hasegawa, Y. , Shiokawa, Y. , Nakagawara, J. , Furui, E. , Kimura, K. , Yamagami, H. , Kario, K. , Okuda, S. , Koga, M. , Minematsu, K. , & Toyoda, K. (2012). Early neurological deterioration within 24 hours after intravenous rt‐PA therapy for stroke patients: The Stroke Acute Management with Urgent Risk Factor Assessment and Improvement rt‐PA Registry. Cerebrovascular Diseases, 34(2), 140–146. 10.1159/000339759 [DOI] [PubMed] [Google Scholar]
- Naudé, P. J. W. , Nyakas, C. , Eiden, L. E. , Ait‐Ali, D. , Heide, R. , Engelborghs, S. , Luiten, P. G. M. , De Deyn, P. P. , Boer, J. A. , & Eisel, U. L. M. (2012). Lipocalin 2: Novel component of proinflammatory signaling in Alzheimer's disease. The FASEB Journal, 26(7), 2811–2823. 10.1096/fj.11-202457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler, J. E. , & Martin‐Schild, S. (2011). Early Neurological Deterioration (END) after stroke: The END depends on the definition. International Journal of Stroke, 6(3), 211–212. 10.1111/j.1747-4949.2011.00596.x [DOI] [PubMed] [Google Scholar]
- Srinivasan, G. , Aitken, J. D. , Zhang, B. , Carvalho, F. A. , Chassaing, B. , Shashidharamurthy, R. , Borregaard, N. , Jones, D. P. , Gewirtz, A. T. , & Vijay‐Kumar, M. (2012). Lipocalin 2 deficiency dysregulates iron homeostasis and exacerbates endotoxin‐induced sepsis. Journal of Immunology, 189(4), 1911–1919. 10.4049/jimmunol.1200892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk, K. (2016). Lipocalin‐2 as a therapeutic target for brain injury: An astrocentric perspective. Progress in Neurobiology, 144, 158–172. 10.1016/j.pneurobio.2016.08.001 [DOI] [PubMed] [Google Scholar]
- Sun, W. , Liu, W. , Zhang, Z. , Xiao, L. , Duan, Z. , Liu, D. , Xiong, Y. , Zhu, W. , Lu, G. , & Liu, X. (2014). Asymmetrical cortical vessel sign on susceptibility‐weighted imaging: A novel imaging marker for early neurological deterioration and unfavorable prognosis. European Journal of Neurology, 21(11), 1411–1418. 10.1111/ene.12510 [DOI] [PubMed] [Google Scholar]
- Thanvi, B. , Treadwell, S. , & Robinson, T. (2008). Early neurological deterioration in acute ischaemic stroke: Predictors, mechanisms and management. Postgraduate Medical Journal, 84(994), 412–417. 10.1136/pgmj.2007.066118 [DOI] [PubMed] [Google Scholar]
- Tu, W.‐J. , Chao, B.‐H. , Ma, L. , Yan, F. , Cao, L. , Qiu, H. , Ji, X.‐M. , & Wang, L.‐D. (2021). Case‐fatality, disability and recurrence rates after first‐ever stroke: A study from bigdata observatory platform for stroke of China. Brain Research Bulletin, 175, 130–135. 10.1016/j.brainresbull.2021.07.020 [DOI] [PubMed] [Google Scholar]
- Tu, W.‐J. , Hua, Y. , Yan, F. , Bian, H. , Yang, Y. , Lou, M. , Kang, D. , He, L. , Chu, L. , Zeng, J. , Wu, J. , Chen, H. , Han, J. , Ma, L. , Cao, L. , & Wang, L. (2022). Prevalence of stroke in China, 2013‐2019: A population‐based study. The Lancet Regional Health: Western Pacific, 28, 100550. 10.1016/j.lanwpc.2022.100550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, R. J. , & Sharp, F. R. (2016). Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Frontiers in Cellular Neuroscience, 10, 56. 10.3389/fncel.2016.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, T. , Zhu, W. , Zhao, Y. , Zhang, X. , Ye, R. , Zuo, M. , Xu, P. , Huang, Z. , Zhang, C. , Xie, Y. , & Liu, X. (2022). Astrocytic phagocytosis contributes to demyelination after focal cortical ischemia in mice. Nature Communications, 13(1), 1134. 10.1038/s41467-022-28777-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G. , Weng, Y.‐C. , Han, X. , Whaley, J. D. , McCrae, K. R. , & Chou, W.‐H. (2015). Lipocalin‐2 released in response to cerebral ischaemia mediates reperfusion injury in mice. Journal of Cellular and Molecular Medicine, 19(7), 1637–1645. 10.1111/jcmm.12538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , Higashikawa, K. , Yasui, H. , Kuge, Y. , Ohno, Y. , Kihara, A. , Midori, Y. A. , Houkin, K. , & Kawabori, M. (2020). FTY720 protects against ischemia‐reperfusion injury by preventing the redistribution of tight junction proteins and decreases inflammation in the subacute phase in an experimental stroke model. Translational Stroke Research, 11(5), 1103–1116. 10.1007/s12975-020-00789-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, G. , Li, H. , Fang, Q. , Jiang, S. , Zhang, L. , Zhang, J. , Hou, X. , Lu, J. , Bao, Y. , Xu, A. , & Jia, W. (2014). Elevated circulating lipocalin‐2 levels independently predict incident cardiovascular events in men in a population‐based cohort. Arteriosclerosis, Thrombosis, and Vascular Biology, 34(11), 2457–2464. 10.1161/ATVBAHA.114.303718 [DOI] [PubMed] [Google Scholar]
- Xiao, X. , Yeoh, B. S. , & Vijay‐Kumar, M. (2017). Lipocalin 2: An emerging player in iron homeostasis and inflammation. Annual Review of Nutrition, 37, 103–130. 10.1146/annurev-nutr-071816-064559 [DOI] [PubMed] [Google Scholar]
- Yakushiji, Y. , Charidimou, A. , Hara, M. , Noguchi, T. , Nishihara, M. , Eriguchi, M. , Nanri, Y. , Nishiyama, M. , Werring, D. J. , & Hara, H. (2014). Topography and associations of perivascular spaces in healthy adults: The Kashima scan study. Neurology, 83(23), 2116–2123. 10.1212/WNL.0000000000001054 [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Sun, Z. , Ding, C. , Tang, Y. , Jiang, X. , Xie, Y. , Li, C. , Zhang, L. , Hu, D. , Li, T. , Xu, G. , & Sheng, L. (2016). Metabolic syndrome augments the risk of early neurological deterioration in acute ischemic stroke patients independent of inflammatory mediators: A hospital‐based prospective study. Oxidative Medicine and Cellular Longevity, 2016, 8346301. 10.1155/2016/8346301<./bib> [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, N. , Xu, X. , Jiang, Y. , Gao, J. , Wang, F. , Xu, X. , Wen, Z. , Xie, Y. , Li, J. , Li, R. , Lv, Q. , Liu, Q. , Dai, Q. , Liu, X. , & Xu, G. (2019). Lipocalin‐2 may produce damaging effect after cerebral ischemia by inducing astrocytes classical activation. Journal of Neuroinflammation, 16(1), 168. 10.1186/s12974-019-1556-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


