Abstract
PURPOSE:
Compared to the general population, suicide is more common in the elderly and in patients with cancer. We sought to examine the incidence of suicide in patients with bladder cancer and evaluate the impact of radical cystectomy in this high-risk population.
METHODS:
Patients diagnosed with urothelial carcinoma from 1988 to 2010 were identified in the Survey, Epidemiology, and End Results (SEER) database. Contingency tables of suicide rates and standardized mortality ratios (SMRs) and 95% confidence intervals were calculated. Multivariable logistic regression models were performed to generate odds ratios (ORs) for the identification of factors associated with suicide in this population.
RESULTS:
There were 439 suicides among patients with bladder cancer observed for 1,178,000 person-years (Standard Morbidity Ratio [SMR] = 2.71). All demographic variables analyzed had a higher SMR for suicide compared to the general population, in particular age ≥80 years (SMR = 3.12), unmarried status (SMR = 3.41), and white race (SMR = 2.60). The incidence of suicide was higher in the general population for patients who underwent radical cystectomy compared to those who did not (SMR = 3.54 vs SMR = 2.66). On multivariate analysis, the strongest predictors of suicide were male gender (vs female; OR = 6.63) and distant disease (vs localized; OR = 5.43).
CONCLUSIONS:
Clinicians should be aware of risk factors for suicide in patients diagnosed with bladder cancer, particularly older, white, unmarried patients with distant disease, and/or those who have undergone radical cystectomy. A multidisciplinary team-based approach, including wound ostomy care trained nursing staff and mental health care providers, may be essential to provide care required to decrease suicide rates in this at-risk population.
Keywords: Bladder cancer, Marital status, Ostomy, Race, SEER, Suicide, Urinary diversion, Wound and ostomy nursing
Introduction
In 2015, approximately 74,000 new cases of bladder cancer and a 16,000 mortalities will occur in the United States. 1 Radical cystectomy with urinary diversion is the standard of care for muscle invasive bladder cancer; it is an inherently morbid procedure, given the age and comorbid conditions frequently associated with this patient population. 2,3 Beyond the health-related impact, bladder cancer treatment may have a significant effect on sexual and psychosocial well-being. Patients undergoing radical cystectomy often have body image and self-esteem concerns and implications. 4
Suicide in patients with a medical condition, in particular a cancer diagnosis, accounts for approximately 70% of all suicides in the United States. 5 In a comprehensive population-based study, Misono and colleagues 5 reported that patients with cancer have nearly twice the incidence of suicide compared to the general population, more common in males, whites, and older patients. Previous studies have suggested higher suicide rates for cancer patients with multiple forms of cancer associated with dismal prognoses including lung, pancreatic, oropharyngeal, and breast malignancies. 5–8
Although the incidence of suicide is higher in patients with genitourinary malignancies compared to the general population, 5,9 the factors associated with suicide among patients with bladder cancer, and especially the impact of patients undergoing radical cystectomy, have not been thoroughly evaluated. Thus, we sought to use a population-based cohort to evaluate the suicide incidence in patients with bladder cancer and assess the impact of radical cystectomy on suicide rates.
Methods
Patients diagnosed with bladder urothelial carcinoma were identified from the Surveillance, Epidemiology, and End Results (SEER) database (1988–2010). The SEER database reports cancer-specific outcomes from specific geographic areas representing 28% of the US population. 10 Patients were identified in the SEER database utilizing the bladder cancer primary site code (C67.0-C67.9) and International Classification of Diseases for Oncology (ICD-O) codes (8120/3, 8122/3, 8123/3, 8130/3, 8131/3) for urothelial carcinoma. Comparisons to the general United States population were based on data from the National Center for Injury Prevention and Control (1999–2010). 11
Demographic variables of interest included age at diagnosis (by decade), gender, race (African American vs white vs other), and marital status (married vs single/divorced/widowed). Clinical variables included receipt of surgery (radical cystectomy vs none) and SEER stage (localized vs regional vs distant). Patients were considered to have committed suicide as previously described. 5 Overall survival was calculated for those dead of suicide and those dead of causes other than suicide.
Contingency tables of suicide rates, standardized mortality ratios (SMRs), and 95% confidence intervals (CIs) were calculated using the Mid-P exact test as previously described. 5,12 Suicide rates (number of suicides divided by person-years of survival) were calculated to allow comparison between patients with bladder cancer and the general population. 5,11 Matched suicide rates were gender and race specific. Calculated SMRs for specific age groups were compared to the same age group in the US population, otherwise matched suicide rates were based on the age range 63 to 80 years. 11
Multivariable logistic regression models were performed to generate odds ratios (ORs) for the identification of factors associated with suicide among persons with bladder cancer. The models were constructed and analyses were performed using backward selection, removing all insignificant variables until the best-fi t model was achieved. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, North Carolina). All tests were 2-sided and with a statistical significance set at P <.05.
Results
There were 439 suicides among patients with bladder cancer observed for 1,178,000 person-years (SMR = 2.71, 95% CI = 2.02–3.62). Median overall survival among patients with bladder cancer was 21 months (dead of suicide) and 16 months (dead of causes other than suicide). All demographics variables analyzed had a higher SMR for suicide compared to the general population (Table 1), in particular age ≤ 39 years (SMR = 2.90, 95% CI = 1.99–4.15), age ≥ 80 years (SMR = 3.12, 95% CI = 2.43–3.97), single/divorced/widowed status (SMR = 3.41, 95% CI = 2.57–4.38), and white race (SMR = 2.60, 95% CI = 1.91–3.40).
TABLE 1.
Incidence of Suicide Among Patients With Bladder Cancer by Demographics
| Variable | Suicide, n | Person-Years | Suicide Rate per 100,000 Person-Years | SMRa | 95% CI |
|---|---|---|---|---|---|
|
| |||||
| Population | 439 | 1,178,000 | 37 | 2.71 | 2.02–3.62 |
| Age, y | |||||
| ≤39 | 6 | 26,748 | 22 | 2.90 | 1.99–4.15 |
| 40–49 | 17 | 74,136 | 23 | 1.39 | 0.93–1.95 |
| 50–59 | 44 | 193,785 | 23 | 1.41 | 0.95–1.99 |
| 60–69 | 105 | 338,328 | 31 | 2.39 | 1.75–3.30 |
| 70–79 | 170 | 369,801 | 46 | 3.14 | 2.36–4.04 |
| 80 + | 97 | 175,203 | 55 | 3.12 | 2.43–3.97 |
| Gender | |||||
| Female | 24 | 286,001 | 8 | 1.97 | 1.12–3.38 |
| Male | 415 | 891,999 | 47 | 1.84 | 1.40–2.38 |
| Marital status | |||||
| Married | 271 | 796,772 | 34 | 2.47 | 1.83–3.37 |
| SDW | 152 | 323,898 | 47 | 3.41 | 2.57–4.38 |
| Race | |||||
| African American | 8 | 46,764 | 17 | 3.73 | 2.49–5.66 |
| White | 420 | 1,077,530 | 39 | 2.60 | 1.91–3.40 |
Abbreviations: CI, confidence interval; SDW, single/divorced/widowed; SMR, standardized mortality ratio.
Compared to suicide rates of the United States according to Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Web-based Injury Statistics Query and Reporting System (WISQARS) (1999–2010).
The incidence of suicide was higher in patients who underwent radical cystectomy when compared to the general population and patients who did not undergo radical cystectomy (SMR = 3.54, 95% CI = 2.70–4.54 vs SMR = 2.66, 95% CI = 1.95–3.54) (Table 2). In addition, patients with regional (SMR = 5.90, 95% CI = 4.80–7.24) and distant disease (SMR = 8.28, 95% CI = 6.90–9.79) had a significantly increased SMR compared to the general population.
TABLE 2.
Incidence of Suicide Among Patients With Bladder Cancer by Intervention and Stage
| Variable | Suicide, n | Person-Years | Suicide Rate per 100,000 Person-Years | SMRa | 95% CI |
|---|---|---|---|---|---|
|
| |||||
| Intervention | |||||
| Cystectomy | 39 | 80,062 | 49 | 3.54 | 2.70–4.54 |
| No cystectomy | 400 | 1,093,986 | 37 | 2.66 | 1.95–3.54 |
| Stage | |||||
| Localized | 318 | 1,013,265 | 31 | 2.28 | 1.65–3.11 |
| Regional | 106 | 130,529 | 81 | 5.90 | 4.80–7.24 |
| Distant | 7 | 6,147 | 114 | 8.28 | 6.90–9.79 |
Abbreviations: CI, confidence interval; SMR, standardized mortality ratio.
Compared to suicide rates of the United States according to Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Web-based Injury Statistics Query and Reporting System (WISQARS) (1999–2010).
Patients with bladder cancer had a significantly increased incidence of suicide over all time periods since diagnosis compared to the general population, with the highest incidence in the first 5 years after diagnosis (SMR = 3.05, 95% CI = 2.26–3.96; > 15 years after diagnosis SMR = 2.18, 95% CI = 1.53–2.94). Multivariable analysis revealed that the strongest predictors of suicide were male gender (vs female; OR = 6.63, 95% CI = 4.29–10.25) and distant disease (vs localized; OR = 5.43, 95% CI = 2.51–11.76) (Table 3). African American race was a protective of factor for suicide (vs white; OR = 0.46, 95% CI = 0.23–0.92).
TABLE 3.
Factors Associated With Suicide Among Patients With Bladder Cancer a
| Variable | OR | 95% CI | P |
|---|---|---|---|
|
| |||
| Age | 1.03 | 1.03–1.04 | <.001 |
| Male vs female | 6.63 | 4.29–10.25 | <.001 |
| SDW vs married | 1.65 | 1.34–2.03 | <.001 |
| Race | |||
| African American vs white | 0.46 | 0.23–0.92 | .03 |
| Other vs white | 0.50 | 0.26–0.96 | .04 |
| No cystectomy vs cystectomy | 1.72 | 1.17–2.53 | .006 |
| Stage of disease | |||
| Regional vs localized | 3.67 | 2.77–4.86 | < .001 |
| Distant vs localized | 5.43 | 2.51–11.76 | < .001 |
Abbreviations: CI, confidence interval; OR, odds ratio; SDW, single/ divorced/widowed.
Adjusted for age, gender, marital status, race, grade, intervention, and stage of disease.
Discussion
We found that a diagnosis of bladder cancer increases the incidence of suicide compared to the general population (SMR = 2.71). Unlike other malignancies, we found that those treated with definitive surgery (radical cystectomy) had a higher incidence of suicide compared to those who did not undergo surgical treatment. Although suicide risk is highest during the first 5 years after diagnosis, it remains significantly elevated compared to the general population at all time periods after diagnosis. On multivariable analysis, older age, male gender, white race, unmarried status, nonoperative management, and aggressive disease were all independent predictors for suicide in this cohort of bladder cancer patients.
Well-established risk factors for suicide include a history of psychiatric illness or abuse, genetic diseases, increasing age, male gender, white race, unmarried status, and any medical illness. 5,13–15 Risk factors for bladder cancer include older age and male gender; tobacco use, a major risk factor for bladder cancer, has also been associated with an increased suicide risk and may play a role in the increased suicide rates for patients diagnosed with bladder cancer. 15 Furthermore, prior studies demonstrate that unmarried status and lower socioeconomic status are associated with advanced disease at diagnosis, with later stage disease possibly increasing the risk of suicide in these patients. 9,16,17
Surgical intervention carries a risk of morbidity/mortality and is stressful to patients and their families. The specifi c health-related quality-of-life (HRQOL) implications of surgical treatment options for bladder cancer patients are particularly disruptive, as the morbidity for radical cystectomy with urinary diversion is high (40%-46.7%). 18,19 Up to 60% of patients will experience stoma-related complications and 27% will be readmitted to the hospital within 90 days of their operation. 19–21 Urinary diversion may include an incontinent stoma (ileal conduit), continent stoma/catheterizable urinary reservoir, and orthotopic neobladder. 19 Up to 75% of patients who have undergone radical cystectomy with urinary diversion report difficulty adjusting to their urinary diversion. 22 While oncologic outcomes and morbidity and mortality rates between diversion types are similar, patients with continent diversions may have an improved HRQOL. 23 Patients with an incontinent diversion describe concerns regarding peristomal skin problems, difficulty managing urinary diversion appliances, fear of cancer recurrence, financial concerns, and family distress up to > 9 years after surgery. 22
Secondary to the invasive and physically altering consequences of a radical cystectomy, regardless of diversion modality, patient HRQOL and well-being must be assessed in a number of different settings: physically, psychologically, socially, and spiritually (Figure 1). 24 Physical well-being is not only altered by the surgical procedure itself, but patients may also endure urine leakage, odor, peristomal skin damage, fatigue, and weakness. 22 Psychological disturbances include poor quality and adequacy of sleep, contributing to risk factors for depression, anxiety, and altered pain perception. 25 Socially, patients may experience embarrassment of their disease and body dysmorphia, thus restricting social interaction, travel, and intimacy. 22 Among patients undergoing radical cystectomy, up to 37.5% will discontinue sexual activity after surgery. 22 Urinary incontinence may also be a cause of social embarrassment and is a risk factor for postoperative depression. 22 Given the potentially aggressive nature of bladder cancer and the need for careful surveillance, patient psychological health should be judiciously assessed at follow-up clinic visits.26
FIGURE 1.
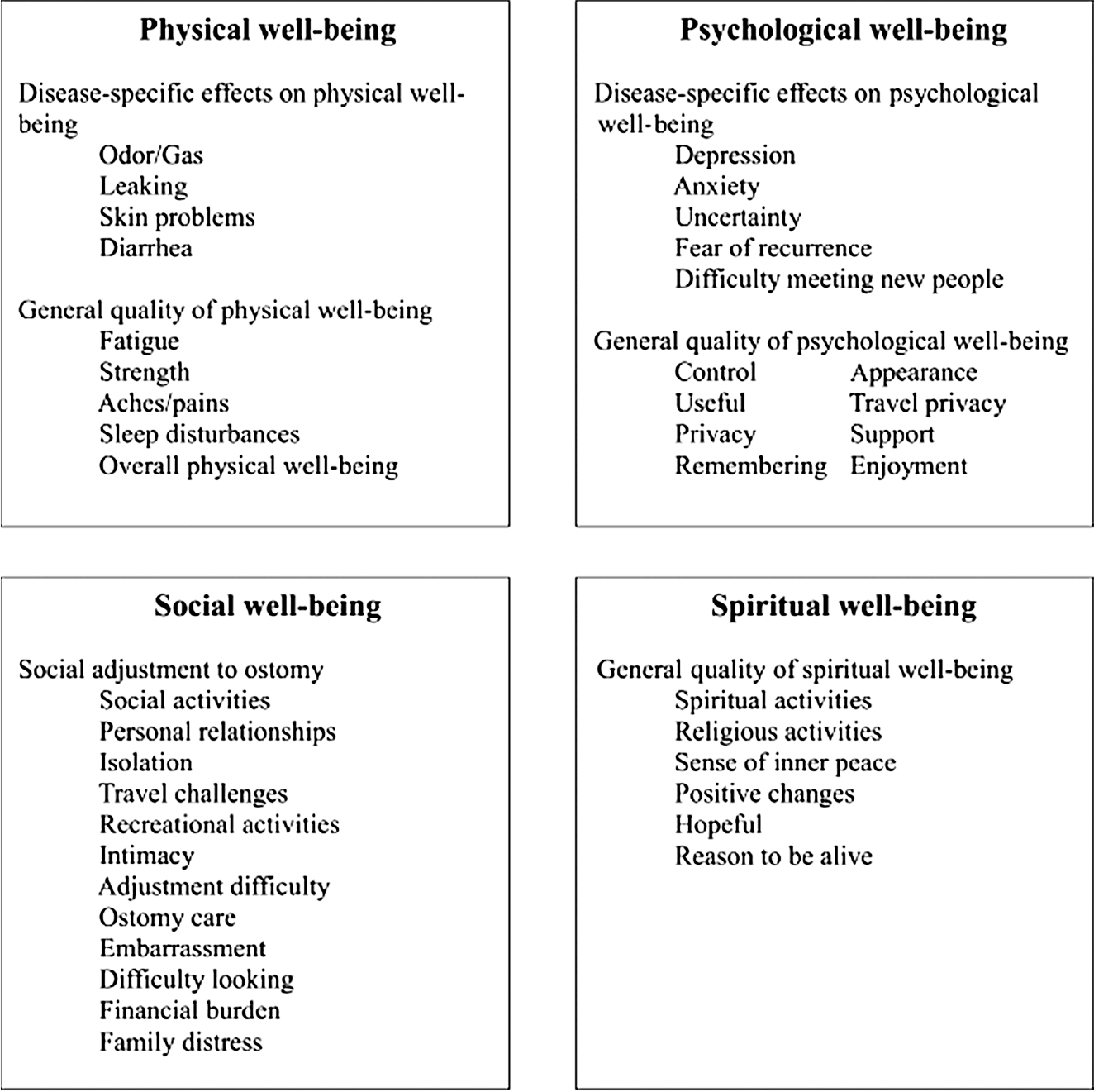
Quality-of-life model for ostomy patients. 24 Reprinted with permission from Springer.
Since the prototypical patient with bladder cancer is similar to the at-risk patient for suicide in the general population, the burden of identifying avenues to improve psychosocial outcomes in these patients lies with the multidisciplinary health care team. Proper urostomy care for patients undergoing radical cystectomy with an ileal conduit requires not only physical skills but also emotional resiliency to handle the lifestyle changes that result from urinary diversion. 27 These patients require education and training regarding ostomy care, whereas patients with continent diversions and orthotopic neobladders require education and competency to self-catheterize. Due to the complexities involved with urinary diversion care, one study found that only ~50% of urostomy patients are able to care for their stoma independently. 27 Additionally, up to 30% of patients feel they receive inadequate postoperative stoma care from visiting nurses. 19 The need for preoperative patient training and education, use of standardized assessment tools, as well as ongoing training with WOC nurses, have been recommended to improve patient outcomes. 22 The Urostomy Education Scale, the first validated urostomy self-care assessment, is the recommended tool when evaluating a patient’s ability to perform necessary self-care. 28, 29 Opportunities for education include during preoperative planning, perioperative hospitalization, as well as meeting the discharge criteria of basic ostomy care, diet, fluid management, recognizing signs of complications, ability to order ostomy supplies, and transitioning care to the home setting. 30
The importance of identifying patients at high risk for suicide may help in developing and utilizing mental health intervention strategies in order to treat patients effectively and improve quality of life. 31, 32 The National Comprehensive Cancer Network provides a guideline for identifying and explaining depression risk factors in oncology patients. They define distress as a multifactorial emotional experience of psychological, social, and/or spiritual nature that may interfere with the ability to cope effectively with the diagnosis. 33 Distress can range from sadness and fear to even more disabling symptoms such as anxiety and depression. Since the National Comprehensive Cancer Network recognizes that patients are at risk at all time periods from diagnosis to follow-up surveillance, they recommend routine, repetitive screening of all patients for distress in order to recognize early signs and improve psychological treatment of these patients. 33 Specific to bladder cancer patients and those undergoing radical cystectomy, the importance of the WOC nursing staff in transitioning bladder cancer patients postoperatively is essential. These reasons include: (i) educating patients on proper ostomy care technique, 20 (ii) troubleshooting ostomy care after the immediate postoperative period, (iii) assisting in obtaining and ordering ostomy supplies for home use, and (iv) providing an avenue for communication and assessing symptoms of depression that may not be communicated to the physician (ie, white coat syndrome, clinic visit time constraints, etc). Secondary to the long-term risk of suicide for patients with bladder cancer, developing an extended regimen, specifically during the at-risk time period in the first 5 years, may be appropriate. This information may be useful for clinicians counseling patients with bladder cancer pre- and post–radical cystectomy, as well as with screening for emotional distress and depression after surgery.
Limitations
Limitations of the SEER database include the lack of smoking history, medical, family, and/or social history information to identify comorbid conditions, previous mental health conditions, and genetic/social risks for placing patients at risk for suicide. Psychiatric pharmacotherapy and financial burden are difficult to ascertain in a population-based database study of this nature, as well as inability to obtain data for suicidal ideation or attempts. We were also unable to identify the exact surgical technique or type of urinary diversion utilized. Further analysis may be completed to understand the specific suicide risk of each diversion modality and perhaps guide surgical procedures based on patients deemed to be at high risk. Strengths of the current study include the large sample size with sufficient data and follow-up to explore multivariable analysis for factors associated with suicide.
Conclusions
Clinicians at all levels of health care should be aware of risk factors for suicide in patients diagnosed with bladder cancer, particularly older, white, unmarried patients with distant disease and/or those who have undergone radical cystectomy and urinary diversion. A multifaceted team-based approach with a wound ostomy care trained nursing staff is necessary to provide the care required to potentially decrease rates of suicide in patients treated with radical cystectomy. There should also be a low threshold for the involvement of a mental health care provider in caring for these patients. This approach may help manage the aspects of treatment that disrupt preoperative functionality, HRQOL, and body image.
Footnotes
The authors declare no conflictsof interest.
Contributor Information
Zachary Klaassen, Department of Surgery, Section of Urology, Medical College of Georgia, Georgia Regents University, Augusta..
John M. DiBianco, Department of Surgery, Section of Urology, Medical College of Georgia, Georgia Regents University, Augusta..
Rita P. Jen, Department of Surgery, Section of Urology, Medical College of Georgia, Georgia Regents University, Augusta..
Benjamin Harper, Department of Surgery, Section of Urology, Medical College of Georgia, Georgia Regents University, Augusta..
Grace Yaguchi, Department of Surgery, Section of Urology, Medical College of Georgia, Georgia Regents University, Augusta..
Lael Reinstatler, Department of Surgery, Section of Urology, Medical College of Georgia, Georgia Regents University, Augusta..
Cynthia Woodard, Department of Surgery, Section of Urology, Veterans Affairs Medical Center, Augusta, Georgia..
Kelvin A. Moses, Department of Urology, Vanderbilt University, Nashville, Tennessee..
Martha K. Terris, Department of Surgery, Section of Urology, Medical College of Georgia, Georgia Regents University, Augusta..
Rabii Madi, Department of Surgery, Section of Urology, Medical College of Georgia, Georgia Regents University, Augusta..
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 2.Park JC, Citrin DE, Agarwal PK, Apolo AB. Multimodal management of muscle-invasive bladder cancer. Curr Probl Cancer. 2014; 38: 80–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sagalowsky AI, Jarrett TW, Flanigan RD. Urothelial tumors of the upper urinary tract and ureter. In: Wein AJ, Kavoussi LR, Novick AC, eds. Campbell-Walsh Urology. 10th ed. Philadelphia, PA: Elsevier Saunders; 2012: 1516–1553. [Google Scholar]
- 4.Gilbert SM, Miller DC, Hollenbeck BK, Montie JE, Wei JT. Cancer survivorship: challenges and changing paradigms. J Urol. 2008; 179: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misono S, Weiss NS, Fann JR, Redman M, Yueh B. Incidence of suicide in persons with cancer. J Clin Oncol. 2008; 26: 4731–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjorkenstam C, Edberg A, Ayoubi S, Rosen M. Are cancer patients at higher suicide risk than the general population? Scan J Public Health. 2005; 33: 208–214. [DOI] [PubMed] [Google Scholar]
- 7.Yousaf U, Christensen ML, Engholm G, Storm HH. Suicides among Danish cancer patients 1971–1999. Br J Cancer. 2005; 92: 995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hem E, Loge JH, Haldorsen T, Ekeberg O. Suicide risk in cancer patients from 1960 to 1999. J Clin Oncol. 2004; 22: 4209–4216. [DOI] [PubMed] [Google Scholar]
- 9.Klaassen Z, Jen RP, DiBianco JM, et al. Factors associated with suicide in patients with genitourinary malignancies. Cancer. 2015;121:1864–1872. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER). http://seer.cancer.gov. Published 2013. Accessed December 29, 2014.
- 11.Hoyert DL, Heron MP, Murphy SL, Kung HC. Deaths: final data for 2003. Natl Vital Stat Rep. 2006; 54: 1–120. [PubMed] [Google Scholar]
- 12.Ury HK, Wiggins AD. A nother shortcut method for calculating the confidence interval of a Poisson variable (or of a standardized mortality ratio). Am J Epidemiol. 1985; 122: 197–198. [DOI] [PubMed] [Google Scholar]
- 13.Schneider RK. Chapter 229. The Suicidal Patient. In: McKean SC, Ross JJ, Dressler DD, Brotman DJ, Ginsberg JS. eds. Principles and Practice of Hospital Medicine. New York, NY: McGraw-Hill; 2012. http://accessmedicine.mhmedical.com/content.aspx?bookid=496&Sectionid=41304220. Accessed April 4, 2015. [Google Scholar]
- 14.O’Connell H, Chin AV, Cunningham C, Lawlor BA. Recent developments: suicide in older people. BMJ. 2004; 329: 895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller M, Azrael D, Hemenway D. The epidemiology of case fatality rates for suicide in the northeast. Ann Emerg Med.2004; 43: 723–730. [DOI] [PubMed] [Google Scholar]
- 16.Kendal WS. Suicide and cancer: a gender-comparative study. Ann Oncol. 2007;18:381–387. [DOI] [PubMed] [Google Scholar]
- 17.Nasseri K, Mills PK, Mirshahidi HR, Moulton LH. Suicide in cancer patients in California, 1997–2006. Arch Suicide Res. 2012; 16: 324–333. [DOI] [PubMed] [Google Scholar]
- 18.Thong AE, Gonzalgo ML. Learning from our patients: complications and the future of radical cystectomy for bladder cancer. J Urol. 2012; 187: 2018. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed NE, Chaoprang Herrera P, Hudson S, et al. Muscle invasive bladder cancer: examining survivor burden and unmet needs. J Urol. 2014; 191: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salvadalena GD. The incidence of stoma and peristomal complications during the first 3 months after ostomy creation. J Wound Ostomy Continence Nurs. 2013; 40: 400–406. [DOI] [PubMed] [Google Scholar]
- 21.Stimson CJ, Chang SS, Barocas DA, et al. Early and late perioperative outcomes following radical cystectomy: 90-day readmissions, morbidity and mortality in a contemporary series. J Urol. 2010; 184: 1296–1300. [DOI] [PubMed] [Google Scholar]
- 22.Gemmill R, Sun V, Ferrell B, Krouse RS, Grant M. Going with the flow: quality-of-life outcomes of cancer survivors with urinary diversion. J Wound Ostomy Continence Nurs. 2010; 37: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gore JL, Saigal CS, Hanley JM, Schonlau M, Litwin MS; Urologic Diseases in America Project. Variations in reconstruction after radical cystectomy. Cancer. 2006; 107: 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant M, Ferrell B, Dean G, Uman G, Chu D, Krouse R. Revision and psychometric testing of the City of Hope Quality of Life-Ostomy Questionnaire. Qual Life Res. 2004; 13: 1445–1457. [DOI] [PubMed] [Google Scholar]
- 25.Hegadoren KM. Assessment and treatment of depression: a review in high-risk populations. J Wound Ostomy Continence Nurs. 2004; 31: 259–272. [DOI] [PubMed] [Google Scholar]
- 26.Witjes JA, Comperat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014; 65: 778–792. [DOI] [PubMed] [Google Scholar]
- 27.Tal R, Cohen MM, Yossepowitch O, et al. An ileal conduit—who takes care of the stoma? J Urol. 2012; 187: 1707–1712. [DOI] [PubMed] [Google Scholar]
- 28.Jensen BT, de Blok W, Kiesbye B, Kristensen SA. Validation of the urostomy education scale: the European experience. Urol Nurs. 2013; 33: 219–229. [PubMed] [Google Scholar]
- 29.Kristensen SA, Laustsen S, Kiesbye B, Jensen BT. The Urostomy Education Scale: a reliable and valid tool to evaluate urostomy self-care skills among cystectomy patients. J Wound Ostomy Continence Nurs. 2013; 40: 611–617. [DOI] [PubMed] [Google Scholar]
- 30.Prinz A, Colwell JC, Cross HH, Mantel J, Perkins J, Walker CA. Discharge planning for a patient with a new ostomy: best practice for clinicians. J Wound Ostomy Continence Nurs. 2015;4 2:79–82. [DOI] [PubMed] [Google Scholar]
- 31.Rehse B, Pukrop R. Effects of psychosocial interventions on quality of life in adult cancer patients: meta analysis of 37 published controlled outcome studies. Patient Educ Couns. 2003; 50: 179–186. [DOI] [PubMed] [Google Scholar]
- 32.Jayadevappa R, Malkowicz SB, Chhatre S, Johnson JC, Gallo JJ. The burden of depression in prostate cancer. Psychooncology. 2012; 21: 1338–1345. [DOI] [PubMed] [Google Scholar]
- 33.National Comprehensive Cancer Network. Distress management. NCCN Guidelines. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Published 2014. Accessed December 23, 2014.


