Summary
Background
Dengue is a global problem that seems to be worsening, as hyper-urbanization associated with climate change has led to a significant increase in the abundance and geographical spread of its principal vector, the Aedes aegypti mosquito. Currently available solutions have not been able to stop the spread of dengue which shows the urgent need to implement alternative technologies as practical solutions. In a previous pilot trial, we demonstrated the efficacy and safety of the method ‘Natural Vector Control’ (NVC) in suppressing the Ae. aegypti vector population and in blocking the occurrence of an outbreak of dengue in the treated areas. Here, we expand the use of the NVC program in a large-scale 20 months intervention period in an entire city in southern Brazil.
Methods
Sterile male mosquitoes were produced from locally sourced Ae. aegypti mosquitoes by using a treatment that includes double-stranded RNA and thiotepa. Weekly massive releases of sterile male mosquitoes were performed in predefined areas of Ortigueira city from November 2020 to July 2022. Mosquito monitoring was performed by using ovitraps during the entire intervention period. Dengue incidence data was obtained from the Brazilian National Disease Surveillance System.
Findings
During the two epidemiological seasons, the intervention in Ortigueira resulted in up to 98.7% suppression of live progeny of field Ae. aegypti mosquitoes recorded over time. More importantly, when comparing the 2020 and 2022 dengue outbreaks that occurred in the region, the post-intervention dengue incidence in Ortigueira was 97% lower compared to the control cities.
Interpretation
The NVC method was confirmed to be a safe and efficient way to suppress Ae. aegypti field populations and prevent the occurrence of a dengue outbreak. Importantly, it has been shown to be applicable in large-scale, real-world conditions.
Funding
This study was funded by Klabin S/A and Forrest Innovations Ltd.
Keywords: Sterile Insect Technology, Natural vector control, Arboviruses, Dengue, Mosquito-borne diseases
Research in context.
Evidence before this study
Previously, several Sterile Insect Technology (SIT) methodologies were shown to successfully suppress Aedes (Ae.) aegypti mosquito populations relative to control areas. These include the use of bacterially (Wolbachia) infected male mosquitoes leading to incompatibility with wild females; genetically modified male mosquitoes carrying a dominant lethal gene that leads to female larval mortality, and, more recently, our new methodology Natural Vector Control (NVC). NVC is a SIT-based method that uses massive releases of sterile male mosquitoes produced from the combined treatment with dsRNA and thiotepa that was shown to be highly effective at suppressing mosquito populations and preventing dengue even when treatment and control areas were interchanged.
Added value of this study
Although the present study was not randomized, its design enabled prospective comparison (before and after the intervention), as well as comparison with neighboring cities. As a result, strong evidence was produced regarding the impact of the NVC intervention on mosquito control. More importantly, the reduction in dengue cases resulting from the intervention supports its potential use with short-term effects on public health regarding dengue and other arboviruses. The present study also provided real-life evidence that NVC can be implemented on a large scale for an entire city.
Implications of all the available evidence
NVC represents an accessible, effective, reliable, scalable, and reproducible system for preventing dengue and potentially other mosquito-borne illnesses. The evidence produced by this study, combined with the existing body of evidence on NVC and other SITs, will be essential in supporting evidence-based public policies and implementation programs in regions endemic to dengue.
Introduction
Dengue is a mosquito-borne viral disease that represents a massive and growing problem worldwide. Globally, its main vectors are mosquitoes of the specie Aedes aegypti (Ae. aegypti). This mosquito is also the principal global vector of chikungunya, yellow fever and Zika.1 Historically, the incidence of arboviruses, especially dengue, was concentrated throughout the tropics.1 However, hyper-urbanization and climate change resulting from anthropological activities, has prompted the expansion of this mosquito into regions that until recently were not infested.2,3 Subsequently, dengue and other Ae. aegypti-transmitted arboviruses are becoming a concern to these regions.2, 3, 4
Along with the global increase in the incidence of dengue, cases of severe dengue have also increased proportionally, with subsequent impact on the morbidity and mortality of affected populations and important social and economic implications.2,5 To date, interventions such as vaccination, have failed to control the spread of these arboviruses (other than yellow fever), and it is becoming clear that effective and safe vector elimination is the key to reaching the WHO goals for dengue, i.e. reducing its global incidence by 75% until 2027.6,7
Sterile Insect Technology (SIT) has emerged in the last decade as a highly effective and ecologically viable method to constrain the spread of disease-carrying mosquitoes. This technique, in its various manifestations, is based on the massive release of sterile male mosquitoes in the intervention area, which mate with wild females and leads to a progressive reduction of mosquito numbers in subsequent generations.8 The techniques used to date to reduce mosquito populations include the sterilization of mosquitoes by irradiation,8 the use of genetically modified mosquitoes carrying a dominant lethal gene,9 and incompatible insect technology, which utilizes mosquitoes infected with Wolbachia bacteria.10, 11, 12, 13
We previously provided direct evidence of prevention of dengue using a technique called Natural Vector Control (NVC), a method that uses massive releases of sterile male mosquitoes produced from the combined treatment of dsRNA and thiotepa.14 We demonstrated that reversal of treatment and control areas in two neighborhoods in a Brazilian city, over two epidemiological seasons, brought >90% reduction of dengue cases in the treatment areas in the midst of a dengue epidemic in both seasons.14,15 Herein we present the results over two seasons of an intervention with large-scale deployment of NVC in Ortigueira, a city located in the southern region of Brazil.
Methods
Production of NVC sterile male mosquitoes
The Ae. aegypti colony was established from eggs collected in the northern region of Paraná, Brazil (2017), using ovitraps.16 Mosquitoes were reared as described previously.14
The NVC sterile male mosquitoes’ large-scale production process was performed essentially as described in de Castro Poncio et al., 2021.14 Daily batches of millions of pathogen-free Ae. aegypti eggs were hatched and incubated for 1 h in a PTB2-dsRNA solution.14 Then, larvae were rinsed and fed in rearing trays until they reached the pupal phase. At this stage, males and females were mechanically separated using an appropriate device (Larval-Pupal Separator, Model 5412, John W. Hock Company, Florida, USA). Male pupae were soaked in a thiotepa solution (0.6% W/V) for 1 h, then washed to remove and inactivate any remnants of thiotepa solution.17 After emerging as adults, NVC sterile male mosquitoes were fed 10% sugar solution. All the batches of NVC sterile male mosquitoes underwent quality control to detect the potential presence of contaminating females and verify the sterility of the males.
NVC sterile male mosquito releases and monitoring field Ae. aegypti mosquitoes
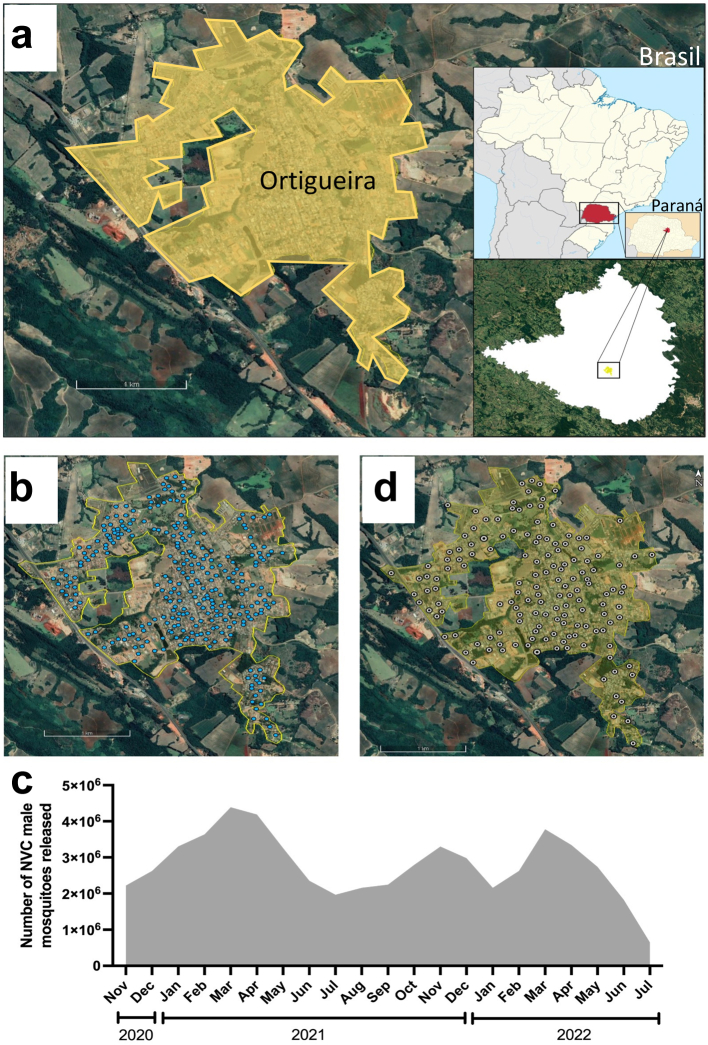
Ortigueira is a city in the northern region of Parana state, southern Brazil, with a population of 21 thousand inhabitants (source: 2021 extrapolation from the 2010 Brazilian census) (Fig. 1a). It has a subtropical climate, with an average temperature of approximately 20 °C over the year. Releases of NVC mosquitoes were performed on a weekly basis from November 2020 to July 2022. NVC male mosquitoes were packed in plastic containers and manually released from a car driving along the streets of the city, as previously described.14 The number of NVC sterile male mosquitoes released in Ortigueira was based on the size of the area, number of eggs collected and the number of viable larvae collected at the release sites, with an initial proportion of 200 sterile male mosquitoes per hectare x number of viable larvae. Fig. 1b shows the points of NVC mosquito releases, and the total number of mosquitoes released per month is shown in Fig. 1c.
Fig. 1.
Satellite image showing the location of Ortigueira in Parana, South of Brazil (Google Earth v.7.3.6.9, 2022). (a) Satellite image of Ortigueira urban area and surroundings. The right-upper map shows the position of Parana state within Brazil and right-lower inset shows the location of Ortigueira within Parana state. (b) Map of Ortigueira showing the points where NVC mosquitoes were released (blue marks). (c) Number of NVC sterile male mosquitoes monthly released in Ortigueira during the intervention period. (d) Map of Ortigueira showing the points where ovitraps were installed (white marks).
Monitoring of local Ae. aegypti abundance was performed by weekly installation of 159 ovitraps in predefined houses or in the peri domiciliary area of the residences in Ortigueira (Fig. 1d).14,18,19 Eggs from each ovitrap were counted and hatched for a 48-h period. The mean number of larvae/ovitrap that hatched in this period were considered viable progeny. The mosquito monitoring started one month before the beginning of NVC releases and continued until one month after the NVC intervention period.
Estimation of the mosquito field population suppression was performed as previously described.14,20 Londrina city was used as a control city for Ae. aegypti monitoring by using ovitraps, similarly as described for Ortigueira. Briefly, monthly moving averages relative to the same period of the control area were calculated according to equation M = (Ta/Ca)/(Tb/Cb)−1, where M is the viable progeny change, Ta is mean larvae per point in the intervention area (Ortigueira) after release, Ca is mean larvae per point in the control area (Londrina) after release, Tb is mean larvae per point in the treated area before release, and Cb is mean larvae per point in the control area before release. This was done by comparing monthly data against baseline data obtained across the 2 months prior to the beginning of releases. The corresponding 95% confidence intervals (CIs) were calculated by a 10,000-loop bootstrap20 for the entire period of releases and for each period of 4 weeks, to follow the effect along the project and considering the similar effect in coming weeks. The CIs were calculated using R version 4.2.1 (R version 4.2.1–2022-06-23 ucrt–Copyright © 2022 The R Foundation for Statistical Computing).
Epidemiological data
Subsequent to the completion of the NVC mosquito releases (at the end of the second epidemiological year), data regarding dengue cases was obtained from the Brazilian National Disease Surveillance system (SINAN). The dataset considered the neighbourhood of primary residence and date of onset of illness. Authoctone and alloctone cases were not differentiated, since the percentage of imported cases was very low (ranging from 0 to 0.5% of all reported cases).21 Data on dengue is available on SINAN since 2000. Suspected cases of dengue are reported according to a case definition of fever plus at least two of the following additional symptoms: malaise, headache, myalgia, nausea, vomiting, cutaneous rash and arthralgia.22 PCR testing is performed only for severe and fatal cases, pregnant women and young children.23
To assess the impact of the NVC intervention on the incidence of dengue in Ortigueira, epidemiological data on dengue in Ortigueira and the neighboring cities (Marilândia do Sul, Tamarana, Telêmaco Borba, Mauá da Serra, Apucarana, Imbaú, Londrina, Grandes Rios and Tibagi) were also collected. Dengue incidence in indicated periods was calculated as (number of dengue cases/number of inhabitants) × 100,000. The number of inhabitants in each city was based on demographic data from the Brazilian Institute of Geography and Statistics (IBGE). To evaluate the protective effect of NVC intervention on dengue incidence, the incidence rate ratio (IRR) was calculated (IRR = IDT/IDC). Where IDT is the incidence of dengue cases in the treated area (Ortigueira) and IDC is the dengue incidence of control cities during the period of intervention. Values of IRR < 1 indicate that the NVC treatment is protective against dengue; 95% CIs were calculated using R software.
Estimation of dengue suppression after NVC intervention was performed using the 10,000 Loop Bootstrap simulation using 3-month period of comparison, starting in January 2020, one year before the intervention.14,20 Telêmaco Borba, Grandes Rios, Imbaú and Tibagi were used as control cities for dengue incidence in the same period of NVC intervention performed in Ortigueira. Monthly moving averages relative to the same period at control cities were calculated according to the equation M = (Ta/Ca)/(Tb/Cb)−1, where M is the dengue incidence change, Ta is dengue incidence in Ortigueira after NVC releases, Ca is the incidence in the control cities after release, Tb is dengue incidence in Ortigueira before NVC releases, and Cb is dengue incidence in the control cities before NVC period releases. This was done by comparing 3-months data intervals against baseline data obtained across the 12 months prior to the beginning of releases. The corresponding 95% confidence intervals (CIs) were calculated by a 10,000-loop bootstrap20 for the entire period of releases and for each period of 3 months, to follow the effect along the project and considering the similar effect in the coming months. The CIs were calculated using R version 4.2.1 (R version 4.2.1–2022-06-23 ucrt–Copyright © 2022 The R Foundation for Statistical Computing).
Ethics and trial registration
An Environmental License from the Environmental Institute of Paraná was obtained (license number 36127) to perform the NVC intervention in Ortigueira. In addition, consent to perform the intervention was given by the Sanitary and Epidemiological Surveillance service the Health Secretariat and the Ortigueira City Hall (consent letter 20201109_101236 and transport authorization 197/2020).
Role of the funding source
This study was funded by Klabin S/A and Forrest Innovations Ltd. Forrest Innovations Ltd. convened and led the study implementation. Klabin S/A did not have any involvement in the study design, in the collection, analysis, and interpretation of data, nor in the writing of the report or in the decision to submit the manuscript for publication.
Results
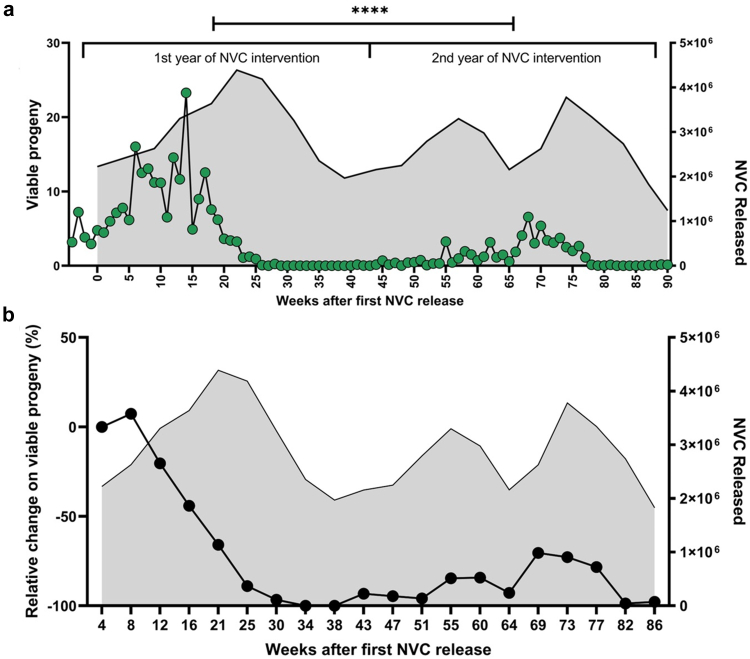
To demonstrate the feasibility of implementing NVC in an entire city and the impact of this intervention on the prevention of dengue, a disease that has great social, economic, and public health implications, the city of Ortigueira, in Paraná, was chosen to receive a 20 months intervention plan. A total of 59 million sterile male mosquitoes (NVC) were released during the period from November 2020 to July 2022, in 200 points in the city, and the monitoring of mosquito infestation was carried out with the placement of ovitraps in 159 points throughout the city (Fig. 1). The intervention in Ortigueira resulted in a reduction in viable progeny of Ae. aegypti from the 18th week of intervention (Fig. 2a). From then on, the viable progeny of mosquitoes droped to close to zero and remained low throughout the second year of the intervention.
Fig. 2.
Suppression of a field Ae. aegypti mosquito population in Ortigueira during the 1st and 2nd years of NVC intervention. The grey shadows in each graph indicate the number of NVC sterile male mosquitoes released monthly in Ortigueira during the NVC period of intervention (right axis). (a) Releases of NVC sterile male mosquitoes occurred weekly between November 2020 and July 2022 (Weeks 0–90). The city was monitored with egg collection by using ovitraps during the entire period of NVC intervention. The collected eggs were transferred to the laboratory, where they were hatched. The mean number of larvae hatched from eggs of each ovitrap (159 points) over the 90 weeks was defined as viable progeny (Left axis). Statistical analysis: Paired t test provided a P value of <0.0001 for the difference between 1st and 2nd year of NVC intervention. (b) Suppression of the Ae. aegypti wild population in Ortigueira during the NVC program. Monthly moving averages showing percentage change in Ae. aegypti abundance in Ortigueira, measured by mean number of larvae per trap relative to control area (Londrina). In weeks 30–34, there was a 98.7% (95% CI, 98.3%–100%) reduction in the number of mosquitoes compared to week 4.
To quantify the reduction in viable progeny of Ae. aegypti in Ortigueira during the NVC intervention period, we measured monthly moving averages of viable larvae hatched from eggs collected during field surveillances in both Ortigueira (intervention area) and Londrina (control area), a city near Ortigueira, as previously described.14,20 Over the 2 seasons of intervention, NVC dramatically reduced the Ae. aegypti mosquito field population in Ortigueira, reaching up to 98.7% (CI 98.3%–100%) reduction in the number of viable larvae during the entire period of intervention (Fig. 2b and Supplementary Figure S1).
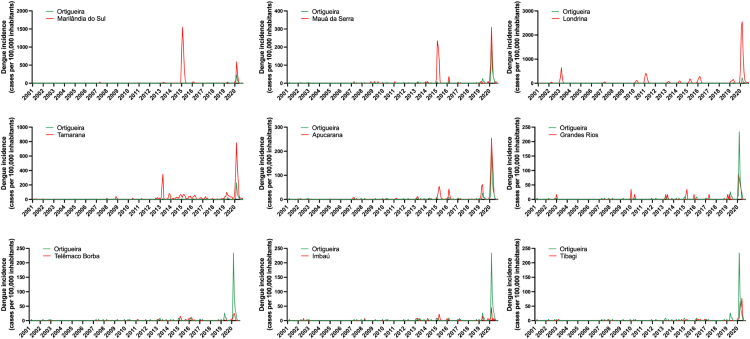
Next, we evaluated the incidence of dengue in Ortigueira throughout the intervention period compared to the occurrence of dengue in other cities in the region. Ortigueira is surrounded by several other cities that have sociodemographic and climatic similarities (Supplementary Table S2). We compared neighboring cities as controls, based mainly on the historical similarity of the occurrence of dengue outbreaks/or epidemics between the years 2000 and mid-2020, a period prior to the start of our NVC intervention.22 We did not consider the history of other arboviruses (Zika and chikungunya) as the incidence of these diseases in the region has been very low since they began to be reported in Brazil (Supplementary Figure S3). As can be seen in Fig. 3, among the neighboring cities evaluated, Marilândia do Sul, Mauá da Serra, Londrina, Tamarana and Apucarana presented different dengue incidence peaks compared with the dengue peaks that occurred in Ortigueira in the same period. On the other hand, the cities Grandes Rios, Telêmaco Borba, Imbaú and Tibagi showed similar patterns of dengue incidence over the last 2 decades when compared to Ortigueira, and therefore were chosen to serve as controls of dengue occurrence during and after the NVC intervention period.
Fig. 3.
Historical dengue incidence in Ortigueira vs neighboring cities. Each graph shows the dengue incidence from 2001 to 2020 (displayed as cases per 100,000 inhabitants) in Ortigueira (green line) compared with neighboring cities (red lines), as indicated (Marilândia do Sul, Mauá da Serra, Londrina, Tamarana, Apucarana, Grandes Rios, Telêmaco Borba, Imbaú, and Tibagi). Data source: Brazilian National Disease Surveillance System (SINAN).
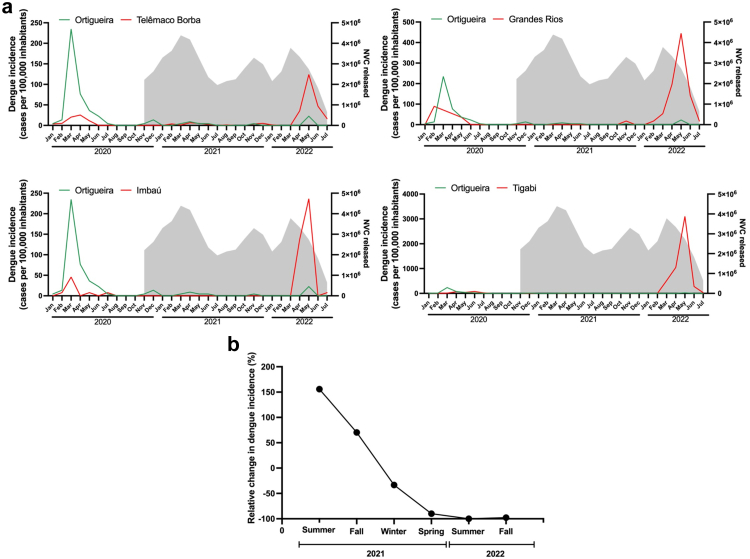
We had previously demonstrated the effectiveness of the NVC intervention in reducing dengue cases by almost 95%, by alternating the NVC-treated and control areas in 2 different epidemiological seasons.14 Herein we expanded the NVC intervention to an entire city. As we can see in Fig. 4a, which shows the dengue incidence from January 2020 to July 2022, the first peak of incidence in the cities of the region (including Ortigueira) occurred in March 2020, before the NVC intervention period. The second and more important peak of dengue incidence occurred in May 2022. This period includes the NVC intervention performed in Ortigueira. The other cities nearby did not receive NVC intervention. The incidence of dengue in the neighboring cities ranged from 120 cases per 100,000 inhabitants (Telêmaco Borba) to 3090 cases per 100,000 inhabitants (Tibagi). In contrast, Ortigueira, presented a much lower incidence (20 cases per 100,000 inhabitants) in the same period. Compared to the neighboring cities, the incidence of dengue in Ortigueira was between 6 and 150 times lower.
Fig. 4.
Dengue incidence in the NVC intervention area (Ortigueira) and control neighboring cities from January 2020 to July 2022. (a) Dengue incidence in Ortigueira and the indicated control cities Telemaco Borba, Grandes Rios, Imbau and Tibagi is shown in the left axis, expressed as the number of dengue cases per 100,000 inhabitants. The grey shadows in each graph indicate the number of NVC sterile male mosquitoes released monthly in Ortigueira during the NVC intervention period. The incidence of dengue in Ortigueira in May 2022 was 20 cases per 100,000 inhabitants (95% CI, 10–20), while the dengue incidence in control cities was 120 cases (95% CI, 100–150) in Telêmaco Borba; 440 cases (95% CI, 300–660) in Grandes Rios; 240 cases (95% CI, 170–340) in Imbaú; and 3090 cases (95% CI, 2870–3340) in Tibagi. (b) Monthly moving averages of dengue incidence in Ortigueira and control cities. A 10,000 bootstrap simulation was performed to show the percentage change in dengue incidence in Ortigueira, using the dengue incidence of the four neighboring cities as controls. In the spring of 2021, there was 89.9% reduction in the dengue incidence in Ortigueira compared to winter 2021 (95% CI, 79.7%–100%).
The potential protective effect of NVC was confirmed calculating the dengue ratio between areas. The IRR showed a reduction of 89.1% in the incidence of dengue in Ortigueira compared to the other four control cities (CI 95.5%–82.3%) during the two years of intervention. We also performed the measurement of monthly moving averages of dengue incidence in both Ortigueira (intervention area) and control cities. As can be seen in Fig. 4b, using the monthly moving averages method, the number of dengue cases was reduced by up to 97.7% (CI 95.7%–99.7%) over the intervention period.
Discussion
Mosquito transmitted diseases are a massive global burden whose geographical area is expanding due to climate change and urbanization.24 Dengue incidence has grown dramatically in the last two decades. Globally, the number of dengue cases reported to the World Health Organization has increased from 505,430 cases in 2000, to over 2.4 million in 2010 and 4.2 million in 2019.25 In Brazil, dengue has increased in magnitude and frequency in recent years, inflicting a significant economic and social burden.26 In the state of Parana in Brazil, the epidemiological years of 2020 and 2022 were by far the two worst dengue epidemic years in its history.15,21
The Sterile Insect Technology (SIT) has been demonstrated to be effective in reducing incidence of disease or damage in different insect species.27 Natural Vector Control (NVC)-based SIT was previously shown to be highly effective in preventing dengue by successfully suppressing the Ae. aegypti mosquito population.14 In a previous controlled field trial, we described the production method of NVC sterile male mosquitoes, as well as the safety and efficacy of the method in suppressing Ae. aegypti mosquito population. In that study we performed two intervention periods (INT1 and INT2), in which the treatment and control areas were alternated between the INT1 and INT2 periods. In both intervention periods, the incidence of dengue was drastically reduced in the areas where the NVC was implemented, which showed that the NVC program is robust and not biased.14 Herein we extended the program to a large-scale intervention in an entire city in the State of Paraná, southern Brazil. Our objective was to demonstrate the feasibility of a large-scale NVC deployment in real-life conditions.
Herein we show a clear reduction in viable progeny of Ae. aegypti throughout the intervention period in Ortigueira reaching a mosquito population of less than 1% of the pre-intervention mosquito population, as compared with the same calendar period before the start of the intervention (Fig. 2). The effective suppression of the mosquito population started approximately 15 weeks after the beginning of the NVC intervention. The likely reason for the relatively long period before effective suppression was evident is that the releases of NVC sterile mosquitoes started only at the end of November 2020, which is already summer in Ortigueira. This delay in the start of releases was due to bureaucratic reasons (authorizations and local regulatory approvals). Such a long period before effective suppression is achieved (due to starting releases in the summer when mosquito populations already start growing exponentially), underscore the importance to begin the intervention as early as possible in the mosquito season. This has also already been demonstrated in other SIT studies.9 In addition, our results indicate that an intervention period of at least 2 years, associated with continuous monitoring and releases during mosquito peak periods, is necessary to avoid a rebound in the increase in the number of mosquitoes and consequent higher risk of dengue outbreaks, as has already been observed in previous studies.14 The risk of rebounds in the mosquito population after effective suppression may be due to the fact that in Ae. aegypti eggs may lie dormant for over a year.28
It is important to note that during the period of intervention with NVC in Ortigueira, no other method of controlling the mosquito vector, such as the use of insecticides, was used. In contrast, a neighboring city, Tibagi, implemented the use of Ultra-Low Volume insecticide spraying in public areas in an attempt to contain the spread of dengue in the city. This reactive insecticide intervention failed to control the spread of dengue in the city, which was the worst among the control cities in the region (Fig. 4a).29
Another important aspect to consider after a large-scale NVC intervention is the possibility of mosquito reintroduction after suppression has been achieved. It is well known that the distribution of Ae. aegypti is largely driven by human movement.30 Ortigueira is not an isolated city, and there is daily movement of vehicles and people between Ortigueira and the neighboring cities. This represents the reality of the global economy, and therefore, to be effective, mosquito population suppression projects need to be performed on a large-scale. The solution to control the mosquito reintroduction in the area previously treated with NVC lies in maintaining entomological surveillance, particularly around high-risk introduction routes such as highways,30 and subsequently carrying out punctual releases of NVC sterile male mosquitoes in areas at greatest risk of mosquito reintroduction.
It is stating the obvious that total or almost total absence of a mosquito vector precludes transmission of dengue or any other arbovirus.31 Indeed, the entire northern region of Paraná experienced dengue epidemics in 2020 and 2022, including several cities surrounding Ortigueira (Fig. 4 and Supplementary Figure S4). In contrast, subsequent to our NVC intervention in Ortigueira, the population of mosquitoes was simply so low during the 2021–2022 seasons (Fig. 2) that dengue transmission was an extremely unlikely event. It is true that the dynamics of transmission and incidence of dengue can be unpredictable and varied over the years.32 Therefore, we cannot completely exclude the possibility that the almost total absence of dengue cases in Ortigueira was due to chance. Notwithstanding, this variability of dengue distribution didn't seem to happen in Paraná between the years of 2019–2022, since the vast majority of cities that had extensive dengue epidemics in 2019 and 2020 presented similar patterns of dengue outbreaks in 2022 (Supplementary Figure S4). In addition, to reduce the potential of a biased comparison, we compared incidence of dengue to all the neighboring cities with similar dengue epidemiological profiles, as well as a general evaluation of the epidemiological profile of dengue in the State of Paraná. Most importantly, we observed a 99% reduction in the Ae. aegypti mosquito population in Ortigueira when comparing pre and post NVC intervention of the same calendar period (Fig. 2). Therefore, our results suggest that the reduction of up to 150 times in the dengue incidence compared to other cities is a direct result of the NVC intervention.
The mosquito suppression SIT methodologies, such as genetic modification to introduce a dominant lethal gene,9 cytoplasmatic incompatibility IIT33,34 and more recently NVC14 have all shown reduction in the Ae. aegypti mosquito population. However, despite these numerous demonstrations of efficacy and safety, SIT is still not being routinely used as a real-world strategy to combat the vector.35 Among the challenges to be overcome so that such technologies (including NVC) are widely implemented worldwide, we can mention the high costs related to the production of sterile mosquitoes (inputs and process), storage and logistics for transporting and releasing mosquitoes, the infrastructure required for on-site molecular surveillance, regulatory and legislation framework (authorizations for monitoring and releases) and public engagement.36 The current NVC intervention program has achieved economies of scale that can effectively and economically provide long-term dengue prevention programs. It is important to emphasize that not only NVC, but any SIT-based interventions are likely to be used in conjunction with other vector control programs and strategies, rather than as standalone measures.
Traditionally, new treatments, vaccines and prevention methods must go through several stages of testing and regulatory approvals, ranging from pre-clinical trials to commercial approvals before being widely implemented.37 In the pre-COVID-19 pandemic period, these processes could take more than 15 years to complete.37,38 The COVID-19 pandemic demonstrated that this process can be optimized and accelerated, and still prove safe and effective for the human population.39 By joining of forces, the different stakeholders involved in global health, which included scientists, investors, regulatory authorities and the industry, promoted the de-bureaucratization of several processes—most notably vaccines and drug treatments, that resulted in the faster relative containment of the COVID-19 pandemic.
Dengue and other arboviruses cause massive global human and economic burden. In 2017, the WHO proposed a target goal of a 75% reduction in global dengue cases by 2027.1 In the current trajectory, this goal will probably not be achieved. On the contrary, an increase in the incidence of dengue has been observed over the last few years,40 and human-induced global warming phenomena and its direct consequences, such as flooding and heatwaves, will likely further exasperate arboviral disease transmission.41 Therefore, new safe and effective vector control tools like NVC, a SIT-based method, are urgently needed to reduce the burden of vector-borne diseases and mitigate threats to existing methods, such as ineffectiveness and resistance development to insecticides.1 The potential suffering resulting from dengue and other mosquito trasnmitted diseases can and should be averted.
Conclusions
Herein we provided additional evidence of the effectiveness of the NVC strategy to control dengue outbreaks in endemic/high incidence areas. By successfully reducing the Ae. aegypti mosquito population by over 99%, dengue incidence was up to 150 times lower in Ortigueira city, compared to several neighboring cities during the same period. Currently, the plethora of successful SIT interventions for Aedes mosquito suppression support their global implementation as part of integrated vector management programs. We call on WHO and other stakeholders involved in prevention and treatment of arbovirus disease to consider massive implementation of such existing technologies in endemic and highly affected areas in order to mitigate human suffering.
Contributors
All authors contributed substantially to the overall design of the study, as well as to the analysis and interpretation of the data collected. All authors reviewed and approved the final version of the manuscript. LCP, FAA, DAO, AOR, BPS, DR, JMP, DACF, CS, and NP planned the study, performed the experiments, and collected the data. All authors (LCP, FAA, DAO, AOR, BPS, DR, JMP, DACF, CS, and NP) accessed and verified the data. LCP, FAA, and NP wrote the manuscript and prepared tables and figures.
Data sharing statement
All data generated and analyzed during this study are included in this published article [and its supplementary information files].
Dengue incidence data analyzed during the current study are available in the Brazilian Notifiable Diseases Information System (SINAN) repository, at https://datasus.saude.gov.br/acesso-a-informacao/doencas-e-agravos-de-notificacao-de-2007-em-diante-sinan/.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
LCP, FAA, DAO, AOR, BPS, DR and JMP are employees of Forrest Brasil Tecnologia Ltda. NP is an employee of Forrest Innovations Ltd. DACF and CS are not related to Forrest or Klabin companies, and participate in all research process with access to the full dataset. NP is the cofounder and CEO of Forrest Innovations Ltd, and declared shares from this company. All the authors have declared no financial, professional, or personal competing interests that might have influenced the performance or presentation of the work described in this manuscript.
Acknowledgements
We thank Uilson Paiva, Marilu Mazurechen, Rafael de Araújo Ribeiro, and Priscila Basile from Klabin S/A for their assistance in facilitating public engagement and community education initiatives during the implementation of the project in Ortigueira. We also thank Dr. Marise Lopes Fermino for providing scientific and writing support.
This study was funded by Klabin S/A and Forrest Innovations Ltd.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100498.
Appendix A. Supplementary data
References
- 1.World Health Organization . Special programme for research and training in tropical diseases. New ed. TDR: World Health Organization; Geneva: 2017. Global vector control response 2017-2030; p. 53. [Google Scholar]
- 2.Lorenz C., Azevedo T.S., Virginio F., Aguiar B.S., Chiaravalloti-Neto F., Suesdek L. Impact of environmental factors on neglected emerging arboviral diseases. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson M.F., Juarez J.G., Kraemer M.U.G., Messina J.P., Hamer G.L. Global patterns of aegyptism without arbovirus. PLoS Negl Trop Dis. 2021;15 doi: 10.1371/journal.pntd.0009397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert M.A., Stewart-Ibarra A.M., Estallo E.L. Climate change and viral emergence: evidence from Aedes-borne arboviruses. Curr Opin Virol. 2020;40:41–47. doi: 10.1016/j.coviro.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laserna A., Barahona-Correa J., Baquero L., Castañeda-Cardona C., Rosselli D. Economic impact of dengue fever in Latin America and the Caribbean: a systematic review. Rev Panam Salud Publica. 2018;42 doi: 10.26633/RPSP.2018.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan American Health Organization . Organización Panamericana de la Salud; 2020. Integrated management strategy for arboviral disease prevention and control in the Americas.https://iris.paho.org/handle/10665.2/52492 Available from: [Google Scholar]
- 7.Achee N.L., Gould F., Perkins T.A., et al. A critical assessment of vector control for dengue prevention. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benelli G., Jeffries C.L., Walker T. Biological control of mosquito vectors: past, present, and future. Insects. 2016;7:52. doi: 10.3390/insects7040052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvalho D.O., McKemey A.R., Garziera L., et al. Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng X., Zhang D., Li Y., et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature. 2019;572:56–61. doi: 10.1038/s41586-019-1407-9. [DOI] [PubMed] [Google Scholar]
- 11.Crawford J.E., Clarke D.W., Criswell V., et al. Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nat Biotechnol. 2020;38:482–492. doi: 10.1038/s41587-020-0471-x. [DOI] [PubMed] [Google Scholar]
- 12.Ritchie S.A., van den Hurk A.F., Smout M.J., Staunton K.M., Hoffmann A.A. Mission accomplished? We need a guide to the ‘post release’ world of Wolbachia for Aedes -borne disease control. Trends Parasitol. 2018;34:217–226. doi: 10.1016/j.pt.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Calvitti M., Marini F., Desiderio A., Puggioli A., Moretti R. Wolbachia density and cytoplasmic incompatibility in Aedes albopictus: concerns with using artificial Wolbachia infection as a vector suppression tool. PLoS One. 2015;10 doi: 10.1371/journal.pone.0121813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Castro Poncio L., dos Anjos F.A., de Oliveira D.A., et al. Novel sterile insect technology program results in suppression of a field mosquito population and subsequently to reduced incidence of dengue. J Infect Dis. 2021;224:1005–1014. doi: 10.1093/infdis/jiab049. [DOI] [PubMed] [Google Scholar]
- 15.Secretaria de Estado da Saúde D de A e V em S. Boletins epidemiológicos: situação da Dengue, Chikungunya e Zika vírus no Parana 2019/2020. 2020. https://www.dengue.pr.gov.br/sites/dengue/arquivos_restritos/files/documento/2020-11/boletimdengue43_2020.pdf Available from:
- 16.Dibo M.R., Chiaravalloti-Neto F., Battigaglia M., et al. Identification of the best ovitrap installation sites for gravid Aedes (Stegomyia) aegypti in residences in Mirassol, state of São Paulo, Brazil. Mem Inst Oswaldo Cruz. 2005;100:339–343. doi: 10.1590/s0074-02762005000400001. [DOI] [PubMed] [Google Scholar]
- 17.Sharma V.P., Patterson R.S., Grover K.K., LaBrecque G.C. Chemosterilization of the tropical house mosquito Culex pipiens fatigans Wied: laboratory and field cage studies. Bull World Health Organ. 1973;48:45–48. [PMC free article] [PubMed] [Google Scholar]
- 18.Catteruccia F., Crisanti A., Wimmer E.A. Transgenic technologies to induce sterility. Malar J. 2009;8(Suppl 2):S7. doi: 10.1186/1475-2875-8-S2-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atkinson M.P., Su Z., Alphey N., Alphey L.S., Coleman P.G., Wein L.M. Analyzing the control of mosquito-borne diseases by a dominant lethal genetic system. Proc Natl Acad Sci U S A. 2007;104:9540–9545. doi: 10.1073/pnas.0610685104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorman K., Young J., Pineda L., et al. Short-term suppression of Aedes aegypti using genetic control does not facilitate Aedes albopictus. Pest Manag Sci. 2016;72:618–628. doi: 10.1002/ps.4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Secretaria de Saúde do Paraná . 2022. Boletins da Dengue.https://www.dengue.pr.gov.br/Pagina/Boletins-da-Dengue Available from: [Google Scholar]
- 22.Durovni B., Saraceni V., Eppinghaus A., et al. The impact of large-scale deployment of Wolbachia mosquitoes on dengue and other Aedes-borne diseases in Rio de Janeiro and Niterói, Brazil: study protocol for a controlled interrupted time series analysis using routine disease surveillance data. F1000Res. 2020;8:1328. doi: 10.12688/f1000research.19859.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ministério da Saúde S de V em S . Departamento de Vigilância Epidemiológica; 2009. Guia de vigilância epidemiológica.https://bvsms.saude.gov.br/bvs/publicacoes/guia_vigilancia_epidemiologica_7ed.pdf Available from: [Google Scholar]
- 24.Messina J.P., Brady O.J., Golding N., et al. The current and future global distribution and population at risk of dengue. Nat Microbiol. 2019;4:1508–1515. doi: 10.1038/s41564-019-0476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng Z., Zhan J., Chen L., Chen H., Cheng S. Global, regional, and national dengue burden from 1990 to 2017: a systematic analysis based on the global burden of disease study 2017. eClinicalMedicine. 2021;32 doi: 10.1016/j.eclinm.2020.100712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Junior J.B.S., Massad E., Lobao-Neto A., Kastner R., Oliver L., Gallagher E. Epidemiology and costs of dengue in Brazil: a systematic literature review. Int J Infect Dis. 2022;122:521–528. doi: 10.1016/j.ijid.2022.06.050. [DOI] [PubMed] [Google Scholar]
- 27.Bourtzis K., Vreysen M.J.B. Sterile insect technique (SIT) and its applications. Insects. 2021;12:638. doi: 10.3390/insects12070638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faull K.J., Williams C.R. Intraspecific variation in desiccation survival time of Aedes aegypti (L.) mosquito eggs of Australian origin. J Vector Ecol. 2015;40:292–300. doi: 10.1111/jvec.12167. [DOI] [PubMed] [Google Scholar]
- 29.Marini G., Guzzetta G., Marques Toledo C.A., Teixeira M., Rosà R., Merler S. Effectiveness of Ultra-Low volume insecticide spraying to prevent dengue in a non-endemic metropolitan area of Brazil. PLoS Comput Biol. 2019;15 doi: 10.1371/journal.pcbi.1006831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraemer M.U.G., Reiner R.C., Brady O.J., et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol. 2019;4:854–863. doi: 10.1038/s41564-019-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization . World Health Organization; Geneva: 2017. UNICEF/UNDP/World Bank/WHO special programme for research and training in tropical diseases. Global vector control response 2017-2030; p. 51.https://apps.who.int/iris/handle/10665/259205 Available from: [Google Scholar]
- 32.Gurevitz J.M., Antman J.G., Laneri K., Morales J.M. Temperature, traveling, slums, and housing drive dengue transmission in a non-endemic metropolis. PLoS Negl Trop Dis. 2021;15 doi: 10.1371/journal.pntd.0009465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slatko B.E., Luck A.N., Dobson S.L., Foster J.M. Wolbachia endosymbionts and human disease control. Mol Biochem Parasitol. 2014;195:88–95. doi: 10.1016/j.molbiopara.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 34.O'Neill S.L. In: Hilgenfeld R., Vasudevan S.G., editors. vol. 1062. Springer Singapore; Singapore: 2018. The use of Wolbachia by the world mosquito program to interrupt transmission of Aedes aegypti transmitted viruses; pp. 355–360. (Dengue and Zika: control and antiviral treatment strategies). Advances in Experimental Medicine and Biology. Available from: http://link.springer.com/10.1007/978-981-10-8727-1_24. [DOI] [PubMed] [Google Scholar]
- 35.Schairer C.E., Najera J., James A.A., Akbari O.S., Bloss C.S. Oxitec and MosquitoMate in the United States: lessons for the future of gene drive mosquito control. Pathog Glob Health. 2021;115:365–376. doi: 10.1080/20477724.2021.1919378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliva C.F., Benedict M.Q., Collins C.M., et al. Sterile insect technique (SIT) against Aedes species mosquitoes: a roadmap and good practice framework for designing, implementing and evaluating pilot field trials. Insects. 2021;12:191. doi: 10.3390/insects12030191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris Z.S., Wooding S., Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104:510–520. doi: 10.1258/jrsm.2011.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanney S.R., Wooding S., Sussex J., Grant J. From COVID-19 research to vaccine application: why might it take 17 months not 17 years and what are the wider lessons? Health Res Policy Syst. 2020;18:61. doi: 10.1186/s12961-020-00571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bok K., Sitar S., Graham B.S., Mascola J.R. Accelerated COVID-19 vaccine development: milestones, lessons, and prospects. Immunity. 2021;54:1636–1651. doi: 10.1016/j.immuni.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosseini S., Oliva-Ramírez J., Vázquez-Villegas P., et al. Dengue fever: a worldwide threat an overview of the infection process, environmental factors for a global outbreak, diagnostic platforms and vaccine developments. Curr Top Med Chem. 2018;18:1531–1549. doi: 10.2174/1568026618666181105130000. [DOI] [PubMed] [Google Scholar]
- 41.Nava A., Shimabukuro J.S., Chmura A.A., Luz S.L.B. The impact of global environmental changes on infectious disease emergence with a focus on risks for Brazil. ILAR J. 2017;58:393–400. doi: 10.1093/ilar/ilx034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.