Abstract
Background
Alzheimer's disease (AD) is a chronic and irreversible neurodegenerative disease. Oxidative stress emerges at the early AD stage. As a non-invasive therapy with few adverse reactions, transcutaneous electrical acupoint stimulation (TEAS) combines acupuncture points of traditional Chinese medicine (TCM) and electrical stimulation. This study aimed to investigate the amelioration effects of preventive TEAS treatment (P-TEAS) on cognitive impairment and oxidative stress in AD model rats.
Methods
The AD model was established via subcutaneous injections of D-galactose (D-gal, 120 mg/kg/d) into the back of neck for 9 weeks in Sprague Dawley (SD) rats to simulate the oxidative stress in the early AD stage. On the first day of the 10th week, Aβ1–42 (1 μg/μl) was injected into the CA1 regions of the bilateral hippocampus. P-TEAS was synchronized from the first day of subcutaneous D-gal injections for 9 weeks.
Results
Empirical measurements showed that P-TEAS can improve the spatial memory ability of AD model rats in the Morris water maze. Superoxide dismutase (SOD) was upregulated in the P-TEAS group. Through the detection of the anti-oxidative stress signaling pathway, namely, Kelch-like ECH-associated protein 1 (Keap1)/ NFE2-related factor 2 (Nrf2), it was found that P-TEAS could promote Nrf2 entering into the nucleus and upregulating the production of protective factors heme oxygenase 1 (HO-1) and NADPH quinone oxidoreductase 1 (NQO1). It was also found that P-TEAS could downregulate the expressions of BCL2-associated X-protein (Bax), caspase 3, and caspase 9 to inhibit neuronal apoptosis.
Conclusions
P-TEAS has similar efficacy to electroacupuncture in preventing AD occurrence and development. P-TEAS is a new non-invasive intervention therapy for the prevention of AD.
Keywords: Alzheimer's disease, Transcutaneous electrical acupoint stimulation, Neuronal apoptosis, Oxidative stress
Graphical abstract
1. Introduction
As a neurodegenerative disease, Alzheimer's disease (AD) has an increasingly high occurrence with the aging process.1 The main pathological features of Alzheimer's disease include neuron loss, extracellular amyloid beta peptide aggregation to senile plaques (SP), intracellular tau hyperphosphorylation to form neurofibrillary tangles (NFTs), and synaptic degeneration in the cerebral cortex and hippocampus.2,3 Brain tissues consume a large amount of oxygen and have very low antioxidant levels,4 as shown in studies indicating that oxidative stress exists at the early AD stage, even prior to the formation of SP and NFTs.5 Oxidative stress is considered a contributing factor to aging and neurodegenerative disease progression.6 When oxidative stress occurs, B-cell lymphoma-2 (Bcl2) on the mitochondrial membrane is downregulated. The pro-apoptotic protein Bcl2-associated X-protein (Bax) makes the mitochondrial permeability transition pore (MPTP) irreversibly open and the mitochondrial membrane potential decrease.7 Cytochrome C (Cytc) is released into the cytoplasm and polymerizes with apoptotic protease activator 1 (Apaf-1) to activate caspase 9, which in turn activates caspase 3 and initiates apoptotic processes concomitantly.8 NFE2-related factor 2 (Nrf2) is a major regulator that neutralizes reactive oxygen species (ROS) and restores redox balance. Kelch-like ECH-associated protein 1 (Keap1) is localized to the mitochondrial surface and interacts with Nrf2 to immobilize Nrf2 to the mitochondria, thereby reducing its transcriptional activity.9 As an essential anti-oxidative stress factor, Nrf2-Keap1 induces the gene expressions of antioxidants such as heme oxygenase 1 (HO-1), NADPH quinone oxidoreductase 1 (NQO1), and superoxide dismutase (SOD) to reduce oxidative stress injury.10
At present, the main AD treatment drugs include acetylcholinesterase inhibitors (AChEIs) and N-methyl-D-aspartate receptor (NMDAR) inhibitors.11 However, AChEIs and NMDAR only improve disease symptoms, without significant effects on the functional capacity of patients from moderate AD stages.12 Recently, the US Food and Drug Administration (FDA) approved Aduhelm, the first antibody for AD treatment, after tests on limited numbers of individuals with MCI or early AD.13 Unlike AChEIs and NMDAR, Aduhelm is designed to clear protein clumps called amyloid in the brain.14 However, researchers remain skeptical about Aduhelm, owing to the AD pathogenesis complexity,15 not to mention the fact that Aduhelm was approved through the accelerated approval pathway of the FDA, with serious clinical benefit uncertainties.16
In recent years, more attention has been paid to nonpharmacologic therapies for AD,17 whose aims focus on AD occurrence prevention and development deterrence.18 As an innovative and practical technology for external application in traditional Chinese medicine, transcutaneous electrical acupoint stimulation (TEAS) combines the meridian theoretical principles and transcutaneous electrical nerve stimulation (TENS).19 Different from electroacupuncture (EA), TEAS stimulates acupoint areas with certain current intensities, being non-invasive and painless.20 At present, TEAS is mainly used to improve postoperative cognitive impairment in elderly patients.21
This study focused on the anti-oxidative stress effects of preventive TEAS (P-TEAS) treatments on cognitive learning ability amelioration in AD model rats.
2. Methods
2.1. Animals and grouping
Forty-eight male Sprague Dawley (SD) rats (200±20 g) were obtained from Shanghai SLAC Laboratory Animal Company and raised in the Animal Experimental Center of Nanjing University of Chinese Medicine. All animals were randomly divided into four groups using a random number table method: the control group (Ctrl, n = 12), the AD group (AD, n = 12), the preventive electroacupuncture group (P-EA, n = 12), and the preventive transcutaneous electrical acupoint stimulation group (P-TEAS, n = 12). They were housed in specific pathogen-free grade chambers. The housing conditions were thermostatically set at 24±1 °C and a relative humidity of 55±5% on a 12 h reverse light–dark cycle with food and water ad libitum (n = 4 each cage). The animal experiment protocol was carried out following the Ethics Review Committee of Nanjing University of Chinese Medicine (Approval No. 202109A033).
2.2. AD model rat establishment
The AD model was established in this study combining D-galactose (D-gal) and Aβ1–42. D-gal (≥99%, Sigma, 59–23–4) was dissolved in sterile saline (120 mg/kg/d) and filtered with a sterile membrane filter (0.22 μm, Millipore).22 Under aseptic conditions, Aβ1–42 (Sigma, SCP0038–1 mg) was dissolved in sterile saline to prepare a stock solution with a final concentration of 1 μg/μl and incubated at 37 °C for 7 days to prepare Aβ1–42 oligomers.23 After acclimatization for 1 week, all rats, except the rats in the Ctrl group, were subcutaneously injected with D-gal (120 mg/kg/d) at the back of neck for 9 weeks. On the first day of the 10th week, 5 μl Aβ1–42 (1 μg/μl) was injected into the CA1 regions of bilateral hippocampus at a speed of 1 μl/min by a microinjection device (posterior to the bregma: −3.8 mm, lateral to the midline: ±3.2 mm, below the dura: 2.7 mm). After injection, the needle was retained for 2 min to ensure Aβ1–42 absorption, followed by a slow withdraw of the needle.24 The Ctrl group was subcutaneously injected with equivalent sterile saline at the back of neck for 9 weeks, and then sterile saline was injected into the CA1 region on the same day as the rest of the rats. Rats with brain surgery were anesthetized using isoflurane (oxygen flow rate: 2.5 l/min, induction concentration: 4%, maintenance concentration: 2.5%).
2.3. Experimental design and treatments
Rats in the Ctrl group and AD group were fixed in the homemade fixator for 20 min each time for 9 weeks without any therapeutic intervention. The interventions of the P-EA group and P-TEAS group were synchronized from the first day of subcutaneous injection of D-gal, performed at acupoints “Baihui” (GV 20) and “Shenshu” (BL 23) six times per week for 9 weeks for 20 min each time.25 The 9-week intervention was followed by Aβ1–42 injection. After recovery for one week, all animals were tested using the Morris water maze (MWM) task to evaluate their learning and memory abilities.
For the P-EA group, sterile disposable stainless steel acupuncture needles (0.18 mm in diameter, 13 mm in length) were inserted horizontally at GV 20 (the midpoint of both ears of rats) at a depth of 5 mm and inward and perpendicularly at BL 23 (either side of the second lumbar vertebra) with a 5 mm depth. BL 23 was needled unilaterally each time but alternated daily between left and right.26 For the P-TEAS group, hydrogel electrode sheets (5 mm in width, 5 mm in length) were adhered at GV 20 and BL 23 (alternated daily between left and right). Acupuncture needles or hydrogel electrode sheets were connected with an electronic acupuncture treatment instrument (Hwato, SDZ-V) at a range of 1–1.5 mA, a frequency of 20 Hz, and a continuous wave for 20 min/d six times per week for 9 weeks.27 Neither acupuncture needles nor hydrogel electrode sheets crossed the heart to prevent electric current from passing through the heart and injuring the rats.
2.4. Morris water maze test
The MWM was used to observe rats’ behavior and detect their learning and memory ability through a small animal behavior tracking system.28 The main body of the Morris water maze was a blue circular pool (120 cm in diameter, 80 cm in depth). The pool was filled with water to a depth of 35 cm, the water temperature was adjusted to 22±2 °C, and the pool was divided into four quadrants. In a four-day hidden platform trial, a blue circular platform (10 cm in diameter) was placed in the middle of the fourth quadrant (the target quadrant) and hidden about 1.5 cm below the water surface. Each rat was placed in the pool from the four different quadrants, facing the tank wall. If the platform was not found within 120 s, the rat was guided to the platform with a glass rod. The latency period was recorded as 120 s, and after a rest on the platform for 30 s, the next quadrant test was continued. In a one-day probe trial, the platform was removed, and a spatial exploration test was performed with rats placed at the opposite position to the target quadrant. The number of times rats crossed the original platform and the amount of time rats stayed in the target quadrant in 120 s were recorded. Finally, one rat was killed after P-EA treatment, two rats in the AD group were killed after brain surgery, and two rats in the AD group and four rats in the Ctrl group were killed after the MWM test. After the rats with poor vital signs were eliminated, there were a total of 32 rats (Ctrl: n = 8, AD: n = 8, P-EA: n = 8, P-TEAS: n = 8).
2.5. Perfusion and tissue preparation
After the MWM test, rats (n = 4 per group) were anesthetized with isoflurane (oxygen flow rate: 2.5 L/min, induction concentration: 4%, maintenance concentration: 3%), placed in a supine position, and underwent cardiac perfusion with ice-cold phosphate-buffered saline (PBS). The brains were removed and fixed with 4% paraformaldehyde for 24 h, and frozen sectioning was performed after sucrose gradient dehydration (10%, 20%, 30% w/v, respectively). Brain coronal sections (20 mm) from the hippocampal region were collected on glass slides and stored at −80 °C until use.29 Blood samples were collected from the abdominal aorta of the remaining rats before sacrificing the rats by cervical decapitation. Brains were removed, and cortical and hippocampal brain tissues were immediately isolated, flash-frozen in liquid nitrogen, and stored at −80 °C.
2.6. Nissl staining
The brain slices were rinsed three times with PBS for 5 min each time and one final time with pure water for 10 min. The sections were immersed in tar violet staining solution, stained in a temperature chamber at 37 °C for 30 min, rinsed with water, differentiated in color separation solution for 10 s, and again rinsed with water. A gradient ethanol series (90–100% w/v, 90 s) was performed to dehydrate the tissues; then xylene was used to make slices transparent, and neutral resin was used to seal the slices. One whole brain from each group was reserved for later use or as a potential spare sample. Three brain slices were randomly taken from each group to assess neuronal loss under a microscope (DM2500, LEICA). Brain slices with three random visual fields were used for statistical analysis to count the Nissl bodies in the hippocampal CA1, CA3, and DG regions.
2.7. ELISA for oxidative stress products
Rat serum and cortical tissues were collected, and SOD and malondialdehyde (MDA) levels in serum and cortical tissues were detected according to the instructions of the ELISA kit (Shanghai Jiechun Industrial Co., Ltd.).
2.8. Quantitative real-time polymerase chain reaction (qPCR) analysis
qPCR detection was conducted to measure the mRNA expressions of Bax, Bcl2, caspase 9, caspase 3, Nrf2, Keap1, Ho1, and NQO1 in the cortex and hippocampus. β-action was adopted as an internal reference gene. The cortical and hippocampal brain tissues (20 mg from each rat) were collected, and total RNAs were extracted using a silica gel-based membrane purification method with the Cell/Tissue Total RNA Isolation Kit (RC101, Vazyme Biotech). A NanoDrop One was used to measure mRNA concentration and purity (A260/A280 values). The RNA was reverse transcribed into cDNA and stored at −20 °C, according to the Evo M-MLV reverse transcription premixed kit (AG11728, Accurate Biology), with the Applied Biosystems Veriti 96-Well Fast Thermal Cycler (Thermo Fisher Scientific Inc., USA). Applied Biosystems StepOnePlus Real-Time fluorescent quantitative PCR instrument was used to perform a two-stage RT-PCR amplification reaction under the following conditions: 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s, and at 60 °C for 30 s, according to the SYBR Green Premix Pro-Taq HS qPCR Kit (AG 11701, Accurate Biology). The primer sequences were designed by Accurate Biology (Table 1). The formula for relative mRNA expression was 2−△△Ct.
Table 1.
Primer sequences.
| Primer name | Forward primer sequence (5′→3′) | Reversed primer sequence (3′→5′) |
|---|---|---|
| rat Bax | GCGATGAACTGGACAACAAC | CAAAGTAGAAAAGGGCAACCAC |
| rat Bcl2 | CAAGAATGCAAAGCACATCCAA | TGCAGGTACCAATAGCACTTCG |
| rat Caspase3 | GCCGAAACTCTTCATCATTCAGG | TGCCATATCATCGTCAGTTCCAC |
| rat Caspase9 | ACATGATCGAGGATATTCAGC | TTGGCCTGTGTCCTCTAAGCA |
| rat Nrf2 | TGGATATTCCCAGCCACGTTG | CTTAAATCAGTCATGGCCGTCT |
| rat Keap1 | CATTCTACTCATCTAGGGCATC | ATTGGCTTCTAATACCCCGAAA |
| rat HO-1 | GTTATTTCCCCAGTTCTACCAG | AAAGACAGCCCTACTTGGTT |
| rat NQO1 | TCAGTTTTCGCCTTTGTTCCAC | GCCCCTAATCTGACCTCGTTC |
| rat β-actin | GGAGATTACTGCCCTGGCTCCTA | GACTCGTACTCCTGCTTGCTG |
2.9. Western blot (WB) assay
Protein samples were extracted from the cortex and hippocampus, followed by the expression level detections of Bax, caspase 9, Nrf2, and Keap1 by WB. Total proteins were extracted from the tissues (20 mg per sample) using lysis buffer (200 μl RIPA) and protease inhibitor (2 μl cocktail, 2 μl 1 mM PMSE) and then sonicated six times for 5 s each time on ice and centrifuged at 12,000 rpm for 15 min at 4 °C. The supernatant was collected, and the bicinchoninic acid (BCA) method was used to detect the protein concentration. Then, 5 × loading buffer was added in proportion to the denaturation in a metal bath at 100 °C for 10 min. Nuclear proteins were extracted from the tissues using the Nuclear and Cytoplasmic Protein Extraction Kit (KGP/150, KeyGEN Biotech) (50 mg per sample). Nuclear proteins and denaturation detections were performed as described above. Fifty micrograms of protein lysate were electrophoresed into SDS-PAGE gels and transferred to PVDF membranes (Millipore), and then the membranes were blocked with 5% skim milk (5 mg of skimmed milk powder dissolving in 100 ml of TBST) for 2 h at 24±1 °C. After washing three times for 10 min each time with TBST, the primary antibodies were incubated with the membranes overnight at 4 °C. Primary antibodies in the study included the following: rabbit anti-Bax antibody (1:1000, ab32503, Abcam), rabbit anti-caspase-9 antibody (1:1000, ab184786, Abcam), rabbit anti-NRF2 antibody (1:1000, 16396–1-AP, Proteintech), rabbit anti-KEAP1 antibody (1:1000, D6B12, Cell Signaling Technology), mouse beta actin antibody (1:30,000, 66009–1-lg, Proteintech), and mouse lamin B1 antibody (1:30,000, 66095–1-lg, Proteintech). The next day, membranes were rewarmed in primary antibodies for 1 h at 24±1 °C, and after washing three times for 10 min each time with TBST, while secondary antibodies (Goat anti-mouse IgG/ Goat anti-rabbit IgG, 1:5000, Proteintech) were incubated at 24±1 °C for 1 h. The membranes were washed three times for 10 min each time with TBST and in full contact with ECL hypersensitive luminescence solution (PE0010, Solarbio). The target proteins were visualized after exposure with a Bio-Rad gel imaging system, and the gray value of protein bands was detected using ImageJ software.
2.10. Immunofluorescence (IF) analysis
The brain slices were rinsed three times with PBS for 5 min each and blocked in 5% BSA for 1 h. The primary antibody (rabbit anti-NRF2 antibody, 1:500,16396–1-AP, Proteintech) was incubated with the slices overnight at 4 °C. The next day, the slices were rewarmed in the primary antibody for 1 h at 24±1 °C and then washed three times for 5 min each time with 0.3% PBST. The secondary antibody (goat anti-rabbit IgG, 1:500, SA00013–4, Proteintech) was incubated at room temperature for 1 h in a wet box. Then, slices were washed in 0.3% PBST three times for 5 min each time and stained with 2–4-Amidinophenyl-6-indolecarbamidine dihydrochloride (DAPI, P0131, Beyotime) for 5 min. Fluorescence was captured on a LEICA DMI8 inverted microscope system.
2.11. Statistical analyses
The experimental data were analyzed using GraphPad Prism 9.3.1 and expressed as mean ± standard deviation (SD). Normality and lognormality tests were used to test whether quantitative data were normally distributed. Two-way ANOVA was used for Morris water maze positioning and navigation experiment data, and the other experimental data were analyzed via one-way ANOVA. The Brown-Forsythe and Welch ANOVA tests were used to analyze the data with significantly unequal variances. Ordinary one-way ANOVA was performed for homogenous variances. A p value <0.05 was considered statistically significant.
3. Results
3.1. Spatial learning and memory improvements
As shown in Fig. 1, the MWM was used to investigate the P-TEAS effects on AD rats’ spatial memory and learning abilities. Fig. 1a shows the escape latency time (ELT) over 4 days of the hidden platform trial after 9-week pre-treatment. All groups demonstrated an ELT reduction in the 4-day training, without any significant difference in their time to find the platform. All groups except the AD group spent less time finding the hidden platform. Compared with the Ctrl group, the AD group's platform crossing times were significantly reduced (p<0.05), as shown in Fig. 1b. The platform crossing times in P-TEAS and P-EA were significantly higher than that in the AD group (p<0.05), and there was no significant difference from the Ctrl group (p>0.05). Fig. 1c illustrates a significant residence time difference in the target quadrant of rats in the AD group compared with the Ctrl group (p<0.001). P-TEAS and P-EA spent significantly more time in the target quadrant than the AD group did (p<0.001). The P-TEAS and P-EA groups did not differ significantly from the Ctrl group (p>0.05). As shown in Fig. 1d, there was a significant difference between the Ctrl group and the AD group in the time to find the virtual platform (p<0.01). P-TEAS and P-EA spent less time finding the virtual platform compared to the AD group (p<0.01) and did not differ significantly from the Ctrl group (p>0.05). As seen in Fig. 1e, there was no significant difference in the swimming speed of rats in each group (p>0.05).
Fig. 1.
Spatial learning and memory improvements (n = 8). (a) In the days 1–4 hidden platform trials, the time it took the rats in each group to find the platform. (b) In the day 5 probe trial, the number of platform crossings in the space exploration experiment in each group. (c) In the day 5 probe trial, the time spent in the target quadrant of the spatial exploration experiment in each group. (d) In the day 5 probe trial, the time to find the platform of rats in each group. (e) In the day 5 probe trial, the swimming speed of rats in each group. (f) In the day 5 probe trial, trajectories to find the platform first in each group. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 vs. AD group, #p<0.05, ##p<0.01, ###p<0.001 vs. Ctrl group.
3.2. Antioxidant enzyme activity SOD increase and oxidative damage product MDA decrease
The results from the ELISA of SOD and MDA in serum are shown in Fig. 2a and Fig. 2b. Fig. 2a shows that, compared to Ctrl group, there were significant SOD activity differences among the AD, P-EA, and P-TEAS groups (p<0.0001). However, the SOD activity was higher in the P-EA and P-TEAS groups than in the AD group (p<0.0001). There was a significant difference between the P-TEAS group and the P-EA group (p<0.0001). Compared with the Ctrl group, MDA in the AD and P-EA groups was significantly increased (p<0.01), as shown in Fig. 1b. MDA was also significantly reduced (p<0.01) in the P-TEAS group compared with the AD group.
Fig. 2.
Antioxidant enzyme activity of superoxide dismutase (SOD) increase and oxidative damage product malondialdehyde (MDA) decrease (n = 7). (a) The contents of SOD in serum. (b) The contents of MDA in serum. (c) The contents of SOD in the cortex. (d) The contents of MDA in the cortex. **p<0.01, ***p<0.001, ****p<0.0001 vs. AD group, ##p<0.01, ###p<0.001, ####p<0.0001 vs. Ctrl group, &p<0.05, &&&&p<0.0001 vs. P-EA group.
The cortex was examined with an ELISA kit. Fig. 2c shows that, compared with the Ctrl group, SOD activities in the AD and P-EA groups were significantly reduced (p<0.001). SOD activities in the P-EA and P-TEAS groups were significantly higher than that in the AD group (p<0.001). Furthermore, the P-TEAS group showed a better effect on SOD activity than the P-EA group (p<0.05). As shown in Fig. 2d, compared with the Ctrl group, the MDA in the AD and P-EA groups was significantly increased (p<0.01). However, the content of MDA in the P-TEAS and P-EA groups was lower than that in the AD group (p<0.0001).
3.3. Activated Keap-1/Nrf2 signaling pathway
The mRNA expressions of oxidative stress-related genes were analyzed via qPCR, including Nrf2, Keap1, HO-1, and NQO1 in the cortex and hippocampus. Compared with the Ctrl group, the gene expressions of Nrf2, HO-1, and NQO-1 were downregulated (p<0.05), but a significant upregulation of Keap1 gene expression was observed in the AD group (p<0.05) (Fig. 3). The anti-oxidative stress genes (Nrf2, HO-1, and NQO-1) of the P-EA and P-TEAS groups were increased very significantly compared with the AD group (p<0.05), and the Nrf2 inhibitor gene (Keap1) was decreased (p<0.01), but there were no significant differences compared with the Ctrl group (p>0.05).
Fig. 3.
Activated Kelch-like ECH-associated protein 1 (Keap1)/ NFE2-related factor 2 (Nrf2) signaling pathway: Quantitative real-time polymerase chain reaction (qPCR) (n = 4). (a) Nrf2, Keap1, heme oxygenase 1 (HO-1), NADPH quinone oxidoreductase 1 (NQO1) mRNA expressions in the cortex. (b) Nrf2, Keap1, HO-1, NQO1 mRNA expressions in the hippocampus. *p<0.05, **p<0.01 vs. AD group, #p<0.05, ##p<0.01, ###p<0.001 vs. Ctrl group.
In addition, A western blot was used to determine the total protein (TP) expression of Nrf2 and Keap1 and the nuclear protein (NP) and the cytoplasmic proteins (CP) expression of Nrf2. Fig. 4 shows that, compared with the Ctrl group, there was no significant difference in the TP expression levels of Nrf2 in the AD group (p>0.05, Fig. 4b, g). However, the downregulation of Nrf2 (TP) in P-EA and P-TEAS was significantly different in the cortex (p<0.05, Fig. 4b), while there was no significant difference in the hippocampus (p>0.05, Fig. 4g), compared with the AD group. Compared with the Ctrl group, the AD group had upregulation of Keap1 (p<0.05, Fig. 4c, h). The protein expression of Keap1 in the P-EA and P-TEAS groups was significantly downregulated (p<0.05, Fig. 4c, h) compared with the AD group. There were no significant differences in the expression of Nrf2 (NP) between the AD group and the Ctrl group (p>0.05, Fig. 4d, i). In the P-EA and P-TEAS groups, a significant upregulation was observed (p<0.01, Fig. 4d, i) compared with both the Ctrl group and the AD group. There were no significant differences in the expression of Nrf2 (CP) between the AD group and the Ctrl group (p>0.05, Fig. 4e, j). In the P-EA and P-TEAS groups, a significant downregulation was observed (p<0.01, Fig. 4e, j) compared with both the Ctrl group and the AD group. Fig. 5 shows nuclei stained with DAPI; compared with the Ctrl group and AD group, Nrf2 levels in the nucleus were significantly increased in the P-EA and P-TEAS groups (p<0.05).
Fig. 4.
Activated Keap1/Nrf2 signaling pathway: western blot (WB) (n = 4). (a) Representative western blot bands of Nrf2 (TP), Keap1, Nrf2 (NP), and Nrf2 (CP) in the cortex. (b, c) β-actin was used as a loading control in the cortex. (d, e) Lamin B1 was used as a loading control in the cortex. (f) Representative western blot bands of Nrf2 (TP), Keap1, Nrf2 (NP), and Nrf2 (CP) in the hippocampus. (g, h) β-actin was used as a loading control in the hippocampus. (i, j) Lamin B1 was used as a loading control in the hippocampus. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 vs. AD group, # p<0.05, ##p<0.01, ###p<0.001, ####p<0.0001 vs. Ctrl group.
Fig. 5.
Activated Keap1/Nrf2 signaling pathway: Immunofluorescence (IF) (× 20).
3.4. Improvement in neuronal activity and hindrance of apoptosis
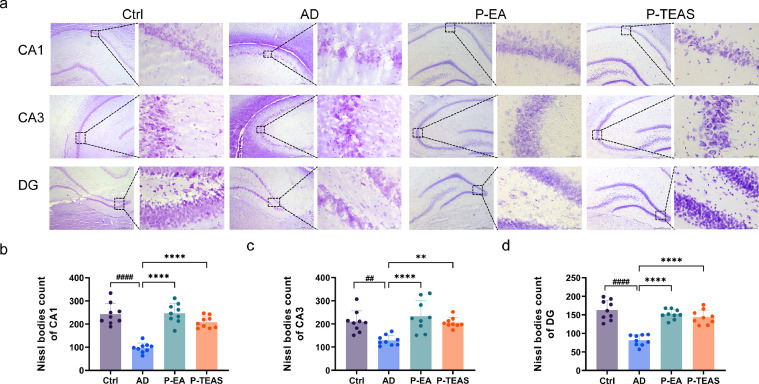
Nissl staining was used to observe the changes in Nissl bodies to determine liable nerve cell damage. Fig. 6A shows that, compared with the AD group, the pyramidal neurons in the Ctrl group in the hippocampal CA1, CA3, and DG regions were clearly discernible, intact with a neat arrangement. Fig. 6B indicates that, compared with the Ctrl group, Nissl bodies were significantly decreased in the AD group in the cytoplasm (p<0.01, Fig. 6B). Compared with the AD group, the pyramidal neurons were neatly arranged in the hippocampal CA1, CA3, and DG regions of the P-EA and P-TEAS groups, without vacuole changes (Fig. 6A). The numbers of Nissl bodies were significantly increased (p<0.01, Fig. 2B).
Fig. 6.
Improvement in neuronal activity and hindrance of apoptosis: Nissl staining (n = 3). (a) Nissl staining for the hippocampal CA1, CA3, and DG regions of rats. (b) Quantitative analysis of Nissl body positive cells in the CA1 region. (c) Quantitative analysis of Nissl body positive cells in the CA3 region. (d) Quantitative analysis of Nissl body positive cells in the DG region. **p<0.01, ****p<0.0001 vs. AD group, ##p<0.01, #### p<0.0001 vs. Ctrl group.
The cortex and hippocampus were analyzed to test the mRNA expressions of apoptosis-related genes (Bax, Bcl2, caspase 3 and caspase 9) via qPCR. Fig. 7 illustrates that, compared with the Ctrl group, the gene expression of Bcl2 was downregulated (p<0.01), but a significant upregulation of Bax, caspase 3, and caspase 9 gene expressions was observed in the AD group (p<0.05). There was a significant decrease in the pro-apoptotic genes (Bax, caspase 3, and caspase 9) of the P-EA and P-TEAS groups compared with the AD group (p<0.05), and the antiapoptotic gene (Bcl2) was increased (p<0.05), without significant differences compared with the Ctrl group (p>0.05). The gene expression level of caspase 9 in the cortex was higher in the P-TEAS group than in the P-EA group, reaching a significant difference (p<0.01), while there was no significant difference in the Ctrl group (p>0.05).
Fig. 7.
Improvement in neuronal activity and hindrance of apoptosis: qPCR (n = 4). (a) B-cell lymphoma-2 (Bcl2), Bcl2 associated X-protein (Bax), caspase 3, and caspase 9 mRNA expressions in the cortex. (b) Bax, Bcl2, caspase 3, and caspase 9 mRNA expressions in the hippocampus. *p<0.05, **p<0.01, ***p<0.001 vs. AD group, #p<0.05, ##p<0.01, ###p<0.001 vs. Ctrl group, &&p<0.01 vs. P-EA group.
Additionally, a western blot was used to determine Bax and caspase 9 expressions. As shown in Fig. 8, compared with the Ctrl group, the protein expression levels of Bax and caspase 9 were significantly upregulated (p<0.01) in the cortex and hippocampus of the AD group. Furthermore, compared with the AD group, the P-EA and P-TEAS groups had downregulation (p<0.05), though there were no significant differences compared with the Ctrl group (p>0.05).
Fig. 8.
Improvement in neuronal activity and hindrance of apoptosis: WB (n = 4). (a) Representative western blot bands of Bax and caspase 9 in the cortex. (b, c) β-actin was used as a loading control in the cortex. (d) Representative western blot bands of Bax and caspase 9 in the hippocampus. (e, f) β-actin was used as a loading control in the hippocampus. *p<0.05, ***p<0.001, ****p<0.0001 vs. AD group, ##p<0.01, ###p<0.001, ####p<0.0001 vs. Ctrl group.
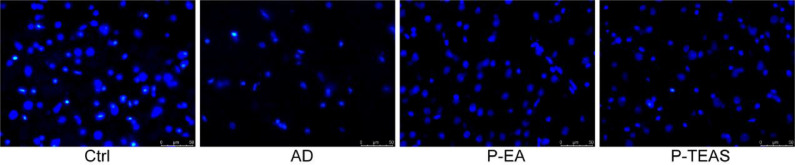
The morphology of the nuclei in the hippocampus was observed by DAPI staining. As shown in Fig. 9, the nuclei in the Ctrl group appeared fully round or oval, without nuclear shrinkage. Those in the AD group, were deformed, shrunken, and fragmented. Most of the nuclei in the P-EA and P-TEAS groups, were round or oval, although a few irregularly shaped nuclei did appear in the visual field.
Fig. 9.
Improvement in neuronal activity and hindrance of apoptosis: DAPI staining (× 40).
4. Discussion
4.1. Selection of acupoints
As a chronic progressive and irreversible neurodegenerative disorder, AD pathogenesis in traditional Chinese medicine (TCM) has been differentiated as kidney essence deficiency, qi and blood deficiency, and blood stasis in syndromes.30,31 This account of early AD stages is consistent with the mitophagy pathogenesis, which leads to impaired mitochondrial redox activity and induces ROS generation and oxidative stress.32 In TCM clinical practice, acupuncture and moxibustion against GV 20, BL 23 are frequently used in AD treatments.33,34 GV 20 is the intersection point of the governor vessel and all yang meridians. It is good at invigorating qi and increasing yang, and can clear dizziness, invigorate the brain, and increase vitality.35 BL 23 is an acupoint on the Bladder Meridian of Foot Tai Yang with functions including tonifying the kidneys, warming yang, strengthening the waist, and promoting fluidity.36Therefore, GV 20 and BL 23 acupoints were used for stimulation in this study as a preventive AD treatment for rats, with AD induced by Aβ1–42 and D-gal.
4.2. TEAS intervention
Compared with the efficacy of current pharmacologic therapies, nonpharmacologic intervention has a better improvement effect on memory and impairments in daily activities, with relatively reduced adverse reactions.37 As a relatively safe procedure, EA is one of the nonpharmacologic therapies and has been widely used to treat neurological diseases in TCM with impressive therapeutic effects.38 Studies have shown that acupuncture can act on multiple targets and offer substantial benefits to alleviate the occurrence and development of Alzheimer's disease.39 EA is one form of acupuncture therapy, and it has been widely used in clinical and experimental research into Alzheimer's disease and vascular dementia.40 Studies have shown that EA can alleviate cognitive deficits in the hippocampus and enhance the number of pyramidal neurons. It plays an important role in protecting neuroinflammation and inhibiting pro-inflammatory cytokines, and even has great efficiency in improving intellectual ability.41, 42, 43 EA is a reliable nonpharmacological treatment for Alzheimer's disease.44 AD is an insidious disease with chronic progressive course. The early stages of AD manifest is mild cognitive decline without any clinical symptoms.45 A report from the EU/US/CTAD task force states that AD should be treated at its early stages before irreversible neurological damage occurs.46 Studies show that early AD patients can be accurately screened by saliva,47 peripheral blood,48 and Magnetic Resonance Imaging (MRI).49 Preventive interventions for the high-risk group can improve brain health and ultimately slow cognitive decline.50,51 TCM focuses on the alleviation of chronic disease and preventive treatment.52 Previous studies have shown that P-EA can alleviate cognitive impairment by preventing oxidative stress and neuronal apoptosis.53 Experimental studies have shown that P-EA at GV 20 and BL 23 can alleviate cognitive impairment in model rats with D-gal-induced Alzheimer's disease by inhibiting GSK-3β.26,54 Clinical trials have shown that scalp electroacupuncture can increase SOD activity and reduce lipid peroxides (LPO) and NO content to mitigate cognitive impairment. It is concluded that one possible mechanism by which scalp electroacupuncture improves cognitive impairment is to improve the body's ability to clear ROS and mitigate anti-oxidative stress.55 Prophylactic acupuncture stimulation of D-gal combined with Aβ1–40 at DU 24 and GB 13 has also been found to reduce oxidative stress and neuronal apoptosis in AD model rats.56 However, EA is an invasive treatment, so it involves certain adverse reactions such as pain, bruising, bleeding, dizziness, anxiety, and infection. Furthermore, EA treatment requires practitioners with the specific skill needed for accurate positioning and deep acupuncture. It is also extremely time-consuming.57, 58, 59 For these reasons, in this study, P-EA served as the positive control group, and the effects of P-TEAS and P-EA were tested on the premise of the effective treatment of therapeutic EA, to promote the non-invasive, painless, and superficial stimulation of TEAS.
EA and TEAS are both types of electrical acupoint stimulation, and both can serve as alternatives to manual acupuncture60; the mechanism of action involves peripheral neuromodulation, which activates nerve endings to produce action potentials.61 Studies have shown that TEAS and EA have similar effects in the treatment of pain, inflammatory response, and reproductive issues, and patients often prefer TEAS due to its non-invasive and painless nature.62,63 One study used functional magnetic resonance imaging (fMRI) to find the default mode network (DMN) and sensorimotor network (SMN). TEAS may enhance functional connectivity in the DMN. TEAS is also more sensitive to SMN than manual acupuncture and EA are. TEAS may be effective in treating such sensory dysfunctions as pain, drug abuse, and Alzheimer's disease.64
TEAS treatment is justified as an alternative due to being non-invasive and painless, with shallow surface stimulation sites. With an easy procedure, patients can be treated at home by themselves.65 At present, TEAS is mostly used in elderly patients with postoperative cognitive decline and impairment.66 The present study investigated whether TEAS has similar efficacy to EA in preventing or slowing AD development. If they have similar efficacy, TEAS could be used instead of EA and could be extended to home care for AD patients after further clinical trials.
4.3. Major research findings
In this study, we continually intervened in AD rats from the first day of D-gal induction for 9 weeks and then injected Aβ1–42 bilaterally into the hippocampal CA1 region of rats to complete the modeling and preventive intervention. After 1 week of rest, the MWM test was performed to examine the rats’ spatial learning and memory abilities. It was observed that P-TEAS had the same curative effect as P-EA in improving AD rats’ learning and memory abilities. SOD and MDA detection in serum and the cerebral cortex revealed that, although they could not reach the levels of the Ctrl group, both P-EA and P-TEAS had upregulated SOD expression, and MDA expression was decreased. Furthermore, P-TEAS had a better effect than P-EA. It has been reported that SOD and MDA can be used to indicate the activation of the Keap1/Nrf2 signaling pathway, which plays an essential role in anti-oxidative stress and can induce the transcription of protective genes including SOD, HO-1, and NOQ1.57,67 The results showed that both P-EA and P-TEAS could downregulate the expression of the Keap1 protein, which has the ability to stabilize Nrf2, and Nrf2 protein was upregulated in the nucleus to upregulate the expression level of HO-1 and NQO1 genes. Oxidative stress can aggravate ROS production, which leads to over-accumulation of intracellular ROS, then leading to mitochondrial apoptosis.68 The number and morphology of Nissl bodies can reflect the functional neuronal activity and neuronal apoptosis.69 The hippocampal CA1, CA3, and DG regions play an important role in spatial learning, memory, and cognitive function.70 The hippocampal CA1 region is involved in information processing, as the hippocampal CA3 region plays a role in associative memory because it contains N-methyl-D-aspartate NMDA receptors,71 and the DG region can give rise to new neurons involved in cognitive tasks.72 Therefore, in this study, Nissl staining was used to detect the Nissl bodies in the hippocampal CA1, CA3, and DG regions to observe the structure and function of neurons. The results showed that P-TEAS had a protective effect on the neurons of AD model rats, and its effect was similar to that of P-EA. In addition, after mitophagy, the pro-apoptotic factor Bax is upregulated, and the anti-apoptotic factor Bcl2 is downregulated.73 Cytc forms a complex with APAF-1, activates caspase 9, and then activates caspase 3, initiating the apoptotic cascade.74 In this study, it was found that P-TEAS upregulated the expression of the Bcl2 protective gene and downregulated the expression of Bax, caspase 3, and caspase 9 pro-apoptotic genes, which showed a protective effect on neurons for AD model rats, with similar efficacy to P-EA.
The stimulation of GV 20 and BL 23 acupoints by P-TEAS can activate the Keap1/Nrf2 signaling pathway in AD model rats and lead to Nrf2 entering the nucleus, thereby activating the downstream protective factors HO-1, NQO1, and SOD. In addition, the pro-apoptotic factors Bax, caspase 3, and caspase 9 were downregulated and the anti-apoptotic factor Bcl2 was upregulated to protect the normal function and structure of neurons and reduce apoptosis.
Thus, P-TEAS improves cognitive impairment, activates the Keap1/Nrf2 antioxidant stress pathway to reduce oxidative stress injury, and prevents neuronal damage and neuronal apoptosis. It is a new non-invasive intervention therapy to prevent AD occurrence and development.
4.4. Limitations
There are some limitations to this study. First, the AD model was established using D-gal combined with Aβ1–42 to simulate the early oxidative stress state of AD patients and the pathological phenomena of senile plaque formation in the hippocampus. The therapeutic effects need to be verified further in a transgenic model. Moreover, this study was a preliminary experiment to find the Keap1/Nrf2 signaling pathway after detecting SOD and MDA content using ELISA. In future experiments, an Nrf2 inhibitor group should be further added to expand the functional significance understanding of Nrf2 activation by P-TEAS. In addition, the aim of this study was to verify the efficacy of P-TEAS compared with P-EA, and an NMDAR inhibitor group was not added. In a subsequent study, an NMDAR inhibitor group will be used to detect the efficacies of nonpharmacologic treatment and pharmacologic treatment. This study did not include sham acupuncture, so there may be non-specific factors affecting efficacy. Sham acupuncture is commonly used in clinical studies of pain, anesthesia, stroke, depression, obesity, and primary dysmenorrhea, and it aims to render the subjects unaware of which group they are in, thereby avoiding non-specific effects.75 However, in the experiment in which acupuncture was tested on an Alzheimer's disease animal model, non-specific factors were found to have little influence on the efficacy of acupuncture. One meta-analysis included 12 studies of acupuncture or EA in mouse models of Alzheimer's disease. True acupuncture was compared with sham acupuncture, and the results showed that true acupuncture could effectively improve cognitive impairment in Alzheimer's disease model mice, and these results differed significantly from those of sham surgery.76 The main purpose of this study was to determine whether P-TEAS and P-EA have similar efficacy, so no sham acupuncture group was established. Finally, both the optimal electrical stimulation intensity and frequency need further confirmation in similar refined studies.
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding
This research was supported by the National Natural Science Foundation of China (No. 72174095) and the Jiangsu Graduate Training Innovation Program Project (KYCX22_2049).
Ethical statement
This research was approved by the institutional animal care and use committee (the Ethics Review Committee of Nanjing University of Chinese Medicine, approval No. 202109A033).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Heng Weng: Methodology, Software, Validation, Investigation, Data curation, Writing – original draft, Writing – review & editing. Qing Wang: Methodology, Software, Investigation, Writing – review & editing, Funding acquisition. Ran Ye: Methodology, Formal analysis. Yamei Bai: Resources, Funding acquisition. Hui Yang: Methodology. Guihua Xu: Conceptualization, Resources, Supervision, Project administration, Funding acquisition. Qiuqin Wang: Conceptualization, Supervision.
Acknowledgements
The authors would like to thank LetPub (www.letpub.com), for its linguistic assistance during the preparation of this manuscript.
Contributor Information
Guihua Xu, Email: 7115@njucm.edu.cn.
Qiuqin Wang, Email: qiuqin.wang@njucm.edu.cn.
References
- 1.2022 Alzheimer's disease facts and figures. Alzheimer's Dementia. 2022;18(4):700–789. doi: 10.1002/alz.12638. [DOI] [PubMed] [Google Scholar]
- 2.Pan X.J., Misrani A., Tabassum S., Yang L. Mitophagy pathways and Alzheimer's disease: from pathogenesis to treatment. Mitochondrion. 2021;59:37–47. doi: 10.1016/j.mito.2021.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Scheltens P., De Strooper B., Kivipelto M., Holstege H., Chételat G., Teunissen C.E., et al. Alzheimer's disease. Lancet. 2021;397(10284):1577–1590. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai R., Guo J., Ye X.Y., Xie Y., Xie T. Oxidative stress: the core pathogenesis and mechanism of Alzheimer's disease. Ageing Res. Rev. 2022;77 doi: 10.1016/j.arr.2022.101619. [DOI] [PubMed] [Google Scholar]
- 5.Staurenghi E., Giannelli S., Testa G., Sottero B., Leonarduzzi G., Gamba P. Cholesterol dysmetabolism in Alzheimer's disease: a starring role for astrocytes? Antioxidants (Basel, Switzerland) 2021;10(12) doi: 10.3390/antiox10121890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dupré C., Bongue B., Helmer C., Dartigues J.F., Hupin D., Roche F., et al. Physical activity types and risk of dementia in community-dwelling older people: the Three-City cohort. BMC Geriatr. 2020;20(1):132. doi: 10.1186/s12877-020-01538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y., Feng Y., Ye X., Peng H., Du J., Yao X., et al. Endogenous SO(2) Controls Cell Apoptosis: the State-of-the-Art. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.729728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y., Yang X., Ge X., Zhang F. Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid hemorrhage mice. Biomed. Pharmacother. 2019;109:726–733. doi: 10.1016/j.biopha.2018.10.161. [DOI] [PubMed] [Google Scholar]
- 9.Tsai T.F., Chen P.C., Lin Y.C., Chou K.Y., Chen H.E., Ho C.Y., et al. Miconazole Contributes to NRF2 Activation by Noncanonical P62-KEAP1 Pathway in Bladder Cancer Cells. Drug Des. Devel. Ther. 2020;14:1209–1218. doi: 10.2147/DDDT.S227892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Q., Xie K., Yi J., Song Z., Zhang H., He X. The effects of magnolol supplementation on growth performance, meat quality, oxidative capacity, and intestinal microbiota in broilers. Poult. Sci. 2022;101(4) doi: 10.1016/j.psj.2022.101722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu T.W., Lane H.Y., Lin C.H. Novel therapeutic approaches for Alzheimer's disease: an updated review. Int. J. Mol. Sci. 2021;22(15) doi: 10.3390/ijms22158208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsunaga S., Kishi T., Nomura I., Sakuma K., Okuya M., Ikuta T., et al. The efficacy and safety of memantine for the treatment of Alzheimer's disease. Expert. Opin. Drug Saf. 2018;17(10):1053–1061. doi: 10.1080/14740338.2018.1524870. [DOI] [PubMed] [Google Scholar]
- 13.Mukhopadhyay S., Banerjee D. A primer on the evolution of aducanumab: the first antibody approved for treatment of Alzheimer's disease. J. Alzheimer's Dis. 2021;83(4):1537–1552. doi: 10.3233/JAD-215065. [DOI] [PubMed] [Google Scholar]
- 14.Mullard A. Landmark Alzheimer's drug approval confounds research community. Nature. 2021;594(7863):309–310. doi: 10.1038/d41586-021-01546-2. [DOI] [PubMed] [Google Scholar]
- 15.Rabinovici G.D. Controversy and progress in Alzheimer's disease - FDA approval of Aducanumab. New Engl. J. Med. 2021;385(9):771–774. doi: 10.1056/NEJMp2111320. [DOI] [PubMed] [Google Scholar]
- 16.the U.S. Food and Drug Administration. Aducanumab (marketed as Aduhelm) Information.; Accessed 14 Sep.
- 17.Watt J.A., Goodarzi Z., Veroniki A.A., Nincic V., Khan P.A., Ghassemi M., et al. Comparative Efficacy of Interventions for Aggressive and Agitated Behaviors in Dementia: a Systematic Review and Network Meta-analysis. Ann. Intern. Med. 2019;171(9):633–642. doi: 10.7326/M19-0993. [DOI] [PubMed] [Google Scholar]
- 18.García-Morales V., González-Acedo A., Melguizo-Rodríguez L., Pardo-Moreno T., Costela-Ruiz V.J., Montiel-Troya M., et al. Current Understanding of the Physiopathology, Diagnosis and Therapeutic Approach to Alzheimer's Disease. Biomedicines. 2021;9(12) doi: 10.3390/biomedicines9121910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xi L., Fang F., Yuan H., Wang D. Transcutaneous electrical acupoint stimulation for postoperative cognitive dysfunction in geriatric patients with gastrointestinal tumor: a randomized controlled trial. Trials. 2021;22(1):563. doi: 10.1186/s13063-021-05534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Z., Zhang Y., Zhang H., Zhao X., Wei H., He S., et al. Effects of Transcutaneous Electrical Acupoint Stimulation on Stress Response during Intubation and Extubation in Patients Undergoing Video-Assisted Thoracoscopic Surgery: a Prospective, Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. 2021;2021 doi: 10.1155/2021/1098915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X., Kong D., Du J., Ban Y., Xu H. Transcutaneous electrical acupoint stimulation affects older adults' cognition after general anesthesia: a meta-analysis. Geriatr. Nurs. (Minneap) 2022;46:144–156. doi: 10.1016/j.gerinurse.2022.05.010. New York, NY. [DOI] [PubMed] [Google Scholar]
- 22.Ali A., Shah S.A., Zaman N., Uddin M.N., Khan W., Ali A., et al. Vitamin D exerts neuroprotection via SIRT1/nrf-2/NF-kB signaling pathways against D-galactose-induced memory impairment in adult mice. Neurochem. Int. 2021;142 doi: 10.1016/j.neuint.2020.104893. [DOI] [PubMed] [Google Scholar]
- 23.Guo H.D., Zhu J., Tian J.X., Shao S.J., Xu Y.W., Mou F.F., et al. Electroacupuncture improves memory and protects neurons by regulation of the autophagy pathway in a rat model of Alzheimer's disease. Acupunct. Med. 2016;34(6):449–456. doi: 10.1136/acupmed-2015-010894. [DOI] [PubMed] [Google Scholar]
- 24.Li W., Kong L.H., Wang H., Shen F., Wang Y.W., Zhou H., et al. High-frequency electroacupuncture evidently reinforces hippocampal synaptic transmission in Alzheimer's disease rats. Neural Regen. Res. 2016;11(5):801–806. doi: 10.4103/1673-5374.182708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang M., Xv G.H., Wang W.X., Meng D.J., Ji Y. Electroacupuncture improves cognitive deficits and activates PPAR-γ in a rat model of Alzheimer's disease. Acupunct. Med. 2017;35(1):44–51. doi: 10.1136/acupmed-2015-010972. [DOI] [PubMed] [Google Scholar]
- 26.Yu C.C., He C., Du Y.J., Gao S., Lin Y.F., Wang S.Q., et al. Preventive electroacupuncture reduces cognitive deficits in a rat model of D-galactose-induced aging. Neural Regen. Res. 2021;16(5):916–923. doi: 10.4103/1673-5374.297090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hao M., Dou Z., Xu L., Shao Z., Sun H., Li Z. RNA sequencing analysis of gene expression by electroacupuncture in guinea pig gallstone models. Evid.-Based Complement. Altern. Med. 2022;2022 doi: 10.1155/2022/3793946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vorhees C.V., Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mi X., Zeng G.R., Liu J.Q., Luo Z.S., Zhang L., Dai X.M., et al. Ganoderma Lucidum triterpenoids improve maternal separation-induced anxiety- and depression-like behaviors in mice by mitigating inflammation in the periphery and brain. Nutrients. 2022;14(11) doi: 10.3390/nu14112268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu B., Zhou C., Zhang J., Ling Y., Hu Q., Wang Y., et al. Latest study on the relationship between pathological process of inflammatory injury and the syndrome of spleen deficiency and fluid retention in Alzheimer's disease. Evid.-Based Complement. Altern. Med. 2014;2014 doi: 10.1155/2014/743541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W., Min L., Qiu X., Wu X., Liu C., Ma J., et al. Biological function of long non-coding RNA (LncRNA) Xist. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.645647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song M., Zhao X., Song F. Aging-dependent mitophagy dysfunction in Alzheimer's disease. Mol. Neurobiol. 2021;58(5):2362–2378. doi: 10.1007/s12035-020-02248-y. [DOI] [PubMed] [Google Scholar]
- 33.Yu C., Wang L., Kong L., Shen F., Du Y., Kong L., et al. Acupoint combinations used for treatment of Alzheimer's disease: a data mining analysis. J. Tradit. Chin. Med. = Chung i tsa chih ying wen pan. 2018;38(6):943–952. [PubMed] [Google Scholar]
- 34.Song Y.Y., Xu W.T., Zhang X.C., Ni G.X. Mechanisms of electroacupuncture on Alzheimer's disease: a review of animal studies. Chin. J. Integr. Med. 2020;26(6):473–480. doi: 10.1007/s11655-020-3092-9. [DOI] [PubMed] [Google Scholar]
- 35.Shu J., Ren W., Chen S., Li L., Zhu H., Jin A. Effect of somatosensory interaction transcutaneous electrical acupoint stimulation on cancer-related fatigue and immunity: a randomized controlled trial. Am. J. Clin. Oncol. 2022;45(7):316–324. doi: 10.1097/COC.0000000000000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng H.P., Huang C.M., Ho W.C., Lee Y.C. Acupuncture differentially affects the high-frequency spectral energy in radial pulse positions depending on type of lower back pain. Evid.-Based Complement. Altern. Med. 2019;2019 doi: 10.1155/2019/4024501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kishita N., Backhouse T., Mioshi E. Nonpharmacological Interventions to Improve Depression, Anxiety, and Quality of Life (QoL) in People With Dementia: an Overview of Systematic Reviews. J. Geriatr. Psychiatry Neurol. 2020;33(1):28–41. doi: 10.1177/0891988719856690. [DOI] [PubMed] [Google Scholar]
- 38.Su X.T., Sun N., Zhang N., Wang L.Q., Zou X., Li J.L., et al. Effectiveness and safety of acupuncture for vascular cognitive impairment: a systematic review and meta-analysis. Front. Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.692508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu C.C., Du Y.J., Wang S.Q., Liu L.B., Shen F., Wang L., et al. Experimental evidence of the benefits of acupuncture for Alzheimer's Disease: an updated review. Front. Neurosci. 2020;14 doi: 10.3389/fnins.2020.549772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng P.P., Deng P., Liu L.H., Ai Q., Yin J., Liu Z., et al. Electroacupuncture alleviates postoperative cognitive dysfunction in aged rats by inhibiting hippocampal neuroinflammation activated via microglia/TLRs pathway. Evid.-Based Complement. Altern. Med. 2017;2017 doi: 10.1155/2017/6421260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y., Hu S., Lin H., He J., Tang C. Electroacupuncture at GV24 and bilateral GB13 improves cognitive ability via influences the levels of Aβ, p-tau (s396) and p-tau (s404) in the hippocampus of Alzheimer's disease model rats. Neuroreport. 2020;31(15):1072–1083. doi: 10.1097/WNR.0000000000001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai M., Lee J.H., Yang E.J. Electroacupuncture attenuates cognition impairment via anti-neuroinflammation in an Alzheimer's disease animal model. J. Neuroinflammation. 2019;16(1):264. doi: 10.1186/s12974-019-1665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin D., Zhang J., Zhuang W., Yan X., Yang X., Lin S., et al. The effect of electroacupuncture versus manual acupuncture through the expression of TrkB/NF-κB in the subgranular zone of the dentate gyrus of telomerase-deficient mice. Evid.-Based Complement. Altern. Med. 2018;2018 doi: 10.1155/2018/1013978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu S., Li S., Xia Y., Zhang H., Tian J., Shan C., et al. Effects of multi-mode physical stimulation on APP/PS1 Alzheimer's disease model mice. Heliyon. 2022;8(12):e12366. doi: 10.1016/j.heliyon.2022.e12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ribarič S. Detecting early cognitive decline in Alzheimer's disease with brain synaptic structural and functional evaluation. Biomedicines. 2023;11(2) doi: 10.3390/biomedicines11020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vellas B., Bateman R., Blennow K., Frisoni G., Johnson K., Katz R., et al. Endpoints for Pre-Dementia AD Trials: a Report from the EU/US/CTAD Task Force. J. Prev. Alzheimers Dis. 2015;2(2):128–135. doi: 10.14283/jpad.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McNicholas K., François M., Liu J.W., Doecke J.D., Hecker J., Faunt J., et al. Salivary inflammatory biomarkers are predictive of mild cognitive impairment and Alzheimer's disease in a feasibility study. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.1019296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xue W., Li J., Fu K., Teng W. Differential expression of mRNAs in peripheral blood related to prodrome and progression of Alzheimer's disease. Biomed. Res. Int. 2020;2020 doi: 10.1155/2020/4505720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Z., Chuah J.H., Lai K.W., Chow C.O., Gochoo M., Dhanalakshmi S., et al. Conventional machine learning and deep learning in Alzheimer's disease diagnosis using neuroimaging: a review. Front. Comput. Neurosci. 2023;17 doi: 10.3389/fncom.2023.1038636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Svenningsson A.L., Stomrud E., Palmqvist S., Hansson O., Ossenkoppele R. Axonal degeneration and amyloid pathology predict cognitive decline beyond cortical atrophy. Alzheimers Res. Ther. 2022;14(1):144. doi: 10.1186/s13195-022-01081-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres S., Alexander A., O'Bryant S., Medina L.D. Cognition and the predictive utility of three risk scores in an ethnically diverse sample. J. Alzheimer's Dis. 2020;75(3):1049–1059. doi: 10.3233/JAD-191284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan X., Meng F., Wang D., Guo Q., Ji Z., Yang L., et al. Perceptions of traditional Chinese medicine for chronic disease care and prevention: a cross-sectional study of Chinese hospital-based health care professionals. BMC Complement. Altern. Med. 2018;18(1):209. doi: 10.1186/s12906-018-2273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Z., Chen L., Guo Y., Li D., Zhang J., Liu L., et al. The neuroprotective and neural circuit mechanisms of acupoint stimulation for cognitive impairment. Chin. Med. 2023;18(1):8. doi: 10.1186/s13020-023-00707-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu C.C., Wang J., Ye S.S., Gao S., Li J., Wang L., et al. Preventive Electroacupuncture Ameliorates D-Galactose-Induced Alzheimer's Disease-Like Pathology and Memory Deficits Probably via Inhibition of GSK3β/mTOR Signaling Pathway. Evid.-Based Complement. Altern. Med. 2020;2020 doi: 10.1155/2020/1428752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yue A., Han X., Mao E., Wu G., Gao J., Huang L., et al. The effect of scalp electroacupuncture combined with Memantine in patients with vascular dementia: a retrospective study. Medicine (Baltimore) 2020;99(33):e21242. doi: 10.1097/MD.0000000000021242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang J., Tang C., Liao W., Zhu M., Liu M., Sun N. The antiapoptotic and antioxidative stress effects of Zhisanzhen in the Alzheimer's disease model rat. Neuroreport. 2019;30(9):628–636. doi: 10.1097/WNR.0000000000001243. [DOI] [PubMed] [Google Scholar]
- 57.Li Y., Chandra T.P., Song X., Nie L., Liu M., Yi J., et al. H2S improves doxorubicin-induced myocardial fibrosis by inhibiting oxidative stress and apoptosis via Keap1-Nrf2. Technol. Health Care. 2021;29(S1):195–209. doi: 10.3233/THC-218020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y., Zhang Z., Cui F., Liu J., Wang Y., Jiang J., et al. Traditional Chinese Medicine Bionic Tiger Bone Powder for the Treatment of AI-Associated Musculoskeletal Symptoms. Evid.-Based Complement. Altern. Med. 2017;2017 doi: 10.1155/2017/2478565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee S., Nam D., Kwon M., Park W.S., Park S.J. Electroacupuncture to alleviate postoperative pain after a laparoscopic appendectomy: study protocol for a three-arm, randomised, controlled trial. BMJ Open. 2017;7(8) doi: 10.1136/bmjopen-2016-015286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu Z.H., Dong H.L., Huang-Fu J.W., Fan X.J., Zhao W.X., Min S., et al. Effect of dual-acupoint and single-acupoint electric stimulation on postoperative outcomes in elderly patients subjected to gastrointestinal surgery: study protocol for a randomized controlled trial. Trials. 2018;19(1):669. doi: 10.1186/s13063-018-3052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ao L., Shi J., Bai Y., Zhang S., Gan J. Effects of transcutaneous electrical acupoint stimulation on perioperative immune function and postoperative analgesia in patients undergoing radical mastectomy: a randomized controlled trial. Exp. Ther. Med. 2021;21(3):184. doi: 10.3892/etm.2021.9615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang J., Li D., Tang W., Guo J., Chen W., Yong Y., et al. Pretreatment with transcutaneous electrical acupoint stimulation to prevent postoperative ileus in patients undergoing laparoscopic colon surgery: study protocol for a randomised controlled trial. BMJ Open. 2020;10(8) doi: 10.1136/bmjopen-2019-030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qu F., Li R., Sun W., Lin G., Zhang R., Yang J., et al. Use of electroacupuncture and transcutaneous electrical acupoint stimulation in reproductive medicine: a group consensus. J. Zhejiang Univ. Sci. B. 2017;18(3):186–193. doi: 10.1631/jzus.B1600437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang Y., Wang H., Liu Z., Dong Y., Dong Y., Xiang X., et al. Manipulation of and sustained effects on the human brain induced by different modalities of acupuncture: an fMRI study. PLoS ONE. 2013;8(6):e66815. doi: 10.1371/journal.pone.0066815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tu J.F., Kang S.B., Wang L.Q., Yan S.Y., Yan C.Q., Su X.T., et al. Smart phone-based transcutaneous electrical acupoint stimulation as adjunctive therapy for hypertension (STAT-H trial): protocol for a cluster randomised controlled trial. BMJ Open. 2022;12(7) doi: 10.1136/bmjopen-2021-058172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang T., Ou L., Chen Z., Li J., Shang Y., Hu G. Transcutaneous electrical acupoint stimulation for the prevention of postoperative cognitive dysfunction: a systematic review and meta-analysis. Front. Med. (Lausanne) 2021;8 doi: 10.3389/fmed.2021.756366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu X., Li Y., Mu X. Effect of Quercetin on PC12 Alzheimer's Disease Cell Model Induced by Aβ (25-35) and its mechanism based on Sirtuin1/Nrf2/HO-1 pathway. Biomed. Res. Int. 2020;2020 doi: 10.1155/2020/8210578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang X., Wang Y., Li X., Yang A., Li Z., Wang D. The anti-carcinogenesis properties of erianin in the modulation of oxidative stress-mediated apoptosis and immune response in liver cancer. Aging. 2019;11(22):10284–10300. doi: 10.18632/aging.102456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang L., Hu X. Positive effect of α-asaronol on the incidence of post-stroke epilepsy for rat with cerebral ischemia-reperfusion injury. Molecules. 2022;27(6) doi: 10.3390/molecules27061984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Z., Bai H., Ma X., Shen M., Li R., Qiu D., et al. Blockade of the NLRP3/caspase-1 axis attenuates ketamine-induced hippocampus pyroptosis and cognitive impairment in neonatal rats. J. Neuroinflammation. 2021;18(1):239. doi: 10.1186/s12974-021-02295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trabolsi C., Takash Chamoun W., Hijazi A., Nicoletti C., Maresca M., Nasser M. Study of Neuroprotection by a Combination of the Biological Antioxidant (Eucalyptus Extract) and the Antihypertensive Drug Candesartan against Chronic Cerebral Ischemia in Rats. Molecules. 2021;26(4) doi: 10.3390/molecules26040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adachi N., Sakhri F.Z., Ikemoto H., Ohashi Y., Kato M., Inoue T., et al. Kamikihito rescued depressive-like behaviors and hippocampus neurogenesis in chronic restraint stress rats. J. Tradit. Complement. Med. 2022;12(2):172–179. doi: 10.1016/j.jtcme.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai J., Gao L., Wang Y., Li Y., Ye Z., Tong S., et al. TMBIM1 promotes proliferation and attenuates apoptosis in glioblastoma cells by targeting the p38 MAPK signalling pathway. Transl. Oncol. 2022;19 doi: 10.1016/j.tranon.2022.101391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiang F., Ma S.Y., Lv Y.L., Zhang D.X., Song H.P., Huang Y.S. Tumor necrosis factor receptor-associated protein 1 regulates hypoxia-induced apoptosis through a mitochondria-dependent pathway mediated by cytochrome c oxidase subunit II. Burns Trauma. 2019;7:16. doi: 10.1186/s41038-019-0154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Z.X., Li Y., Zhang X.G., Chen S., Yang W.T., Zheng X.W., et al. Sham electroacupuncture methods in randomized controlled trials. Sci. Rep. 2017;7:40837. doi: 10.1038/srep40837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao F.Y., Fu Q.Q., Zheng Z., Lao L.X., Song H.L., Shi Z. Verum- versus sham-acupuncture on Alzheimer's disease (AD) in animal models: a preclinical systematic review and meta-analysis. Biomed. Res. Int. 2020;2020 doi: 10.1155/2020/5901573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.