Abstract
Isogenic flagellum-negative mutants of Helicobacter pylori and Helicobacter mustelae were screened for their ability to adhere to primary human and ferret gastric epithelial cells, respectively. We also evaluated the adherence of an H. pylori strain with a mutation in the flbA gene, a homologue of the flbF/lcrD family of genes known to be involved in the regulation of H. pylori flagellar biosynthesis. H. pylori and H. mustelae mutants deficient in production of FlaA or FlaB and mutants deficient in the production of both FlaA and FlaB showed no reduction in adherence to primary human or ferret gastric epithelial cells compared with the wild-type parental strains. However, adherence of the H. pylori flbA mutant to human gastric cells was significantly reduced compared to the adherence of the wild-type strain. These results show that flagella do not play a direct role in promoting adherence of H. pylori or H. mustelae to gastric epithelial cells. However, genes involved in the regulation of H. pylori flagellar biosynthesis may also regulate the production of an adhesin.
Adherence of Helicobacter pylori to the gastric mucosa is thought to be an important virulence determinant of the organism. Helicobacter mustelae infects ferrets naturally and is the only Helicobacter species other than H. pylori known to be associated with both gastritis and peptic ulcer disease in its natural host (8, 9). H. mustelae has been shown to adhere tightly to the gastric mucosa of ferrets (20). H. mustelae infection of the ferret may prove to be a very useful natural animal model of H. pylori infection in humans and for the study of adherence of bacteria to the gastric mucosa and their role in peptic ulceration.
Motility and the production of an active urease enzyme are two factors which have been shown to be essential for the colonization of experimental animals by either H. pylori or H. mustelae (1, 2, 4, 5, 6, 7, 25). Motility is conferred on both organisms by the possession of flagella. H. pylori possesses a bundle of unipolar flagella, while H. mustelae exhibits both polar and lateral flagella. The flagellar filaments of H. pylori and H. mustelae are composed of two constituents. FlaA, encoded by flaA, is the major component and has a molecular mass of 53 kDa (16). FlaB, encoded by flaB, has a molecular mass of 54 kDa and seems to be located mainly at the proximal part of the filament (15). H. pylori and H. mustelae FlaA are 72% identical (14). The FlaB proteins of H. pylori and H. mustelae share 81.7% identical amino acids (24). H. pylori FlaA has 58% homology with H. pylori FlaB, and H. mustelae FlaA and FlaB have 56% amino acid similarities (14). The H. pylori flaA and flaB genes are unlinked on the chromosome, and their products share only limited amino acid sequence similarity. It seems likely that both genes can be regulated differentially by environmental conditions. The flagellar filament is linked to the flagellar basal body by means of the hook which itself is a polymer of the flagellar hook protein FlgE (21). Mutations in FlgE do not prevent the synthesis of either FlaA or FlaB. Each flagellum is enveloped by a sheath that is thought to serve as a protective shield against gastric acidity for the acid-labile flagellar filament (11).
Isogenic flaA-, flaB-, and flaA flaB-negative mutants of both H. pylori and H. mustelae have been constructed (14, 24). FlaA mutants produce truncated flagella and are only weakly motile, whereas FlaB mutants produce normal flagella but have diminished motility in a soft-agar assay. Mutants lacking both FlaA and FlaB are aflagellate and nonmotile. By using aflagellate flaA flaB double mutants it has been shown that motility is essential for the ability of H. pylori and H. mustelae to colonize the stomachs of gnotobiotic piglets (7) or specific-pathogen-free ferrets (1), respectively. In the piglet model, mutations in a single flagellin gene were sufficient to prevent persistent colonization. Single-gene mutants of H. mustelae were still able to colonize ferrets, although only at a low density. Whether flagella per se are involved solely in motility or also in attachment of the organisms to the gastric mucosa is an important question. To answer this question we have screened isogenic flaA-, flaB-, and flaA flaB-negative mutants of H. pylori and H. mustelae for adherence to primary gastric cells isolated from humans and ferrets respectively. Adherence of all mutants to Kato III cells was assessed for the purpose of comparison. We have also looked at the adherence of an H. pylori strain with a mutation in the flbA gene, a homologue of the flbF/lcrD family of genes (23). This mutant strain expresses neither the FlaA nor the FlaB flagellin protein, and expression of the FlgE hook protein is reduced in comparison with that of the wild-type strain. The flbA mutants are aflagellate and completely nonmotile.
H. pylori parental wild-type strain N6 produces both FlaA and FlaB and is fully motile. Four isogenic mutants of H. pylori strain N6 were used in this study (Table 1). For H. mustelae, the parental wild-type strain F1 and three isogenic mutants were used in this study (Table 1). The isogenic mutant strains of H. pylori and H. mustelae were constructed by electroporation-mediated allelic exchange (14, 23, 24). H. pylori and H. mustelae strains were grown on Columbia blood agar (Gibco) plates containing 7% (vol/vol) defibrinated horse blood for 3 days at 37°C in an atmosphere of 10% CO2 and 5% O2. Strains with isogenic mutations in flaA, flaB, and flbA were grown on blood agar plates containing kanamycin (20 mg/liter), and double mutants were grown on blood agar plates containing both kanamycin (25 mg/liter) and chloramphenicol (25 mg/liter). Quantitation of bacteria in suspension was accomplished by optical density measurements at 450 nm and by viable counts. Appropriate dilutions of the bacterial suspension were spread on Columbia blood agar plates and after incubation of plates at 37°C under microaerobic conditions for 5 days, CFU per milliliter were enumerated.
TABLE 1.
Wild-type and isogenic flagellum-negative mutants of H. pylori and H. mustelae used in this study
| Bacterium | FlaA | FlaB | Motility | Flagellation | Reference |
|---|---|---|---|---|---|
| H. pylori | |||||
| N6 (wild type) | + | + | Motile | Normal | 23 |
| N6 flaA::Km | − | + | Weakly motile | Short truncated flagella | 14, 23 |
| N6 flaB::Km | + | − | 30–40% reduced motility | Normal | 23 |
| N6 flaA::cat/flab::Km | − | − | Nonmotile | Aflagellate | 14 |
| N6 flbA::Km | − | − | Nonmotile | Aflagellate | 23 |
| H. mustelae | |||||
| F1 (wild type) | + | + | Motile | Normal | 14 |
| F1 flaA::Km | − | + | Weakly motile | Short truncated flagella | 14 |
| F1 flaB::Km | + | − | 30–40% reduced motility | Normal | 14 |
| F1 flaA::cat/flaB::Km | − | − | Nonmotile | Aflagellate | 14 |
Kato III cells are gastric adenocarcinoma cells obtained from the American Type Culture Collection. They were grown in RPMI 1640 medium (BioWhittaker) containing 20 mM HEPES buffer and 12 mM sodium bicarbonate supplemented with 5% (vol/vol) fetal calf serum. For adherence assays cells were scraped from the base of the flask and centrifuged at 200 × g for 5 min. Cells were resuspended in RPMI 1640 medium and counted by microscopy using a hemocytometer.
Primary human gastric epithelial cells were isolated from biopsy tissue by digestion with 0.1 mM EDTA and 0.1 mM dithiothreitol followed by digestion with 0.05% (wt/vol) collagenase, as previously described (3). Cells were resuspended in RPMI 1640 tissue culture medium and counted using a hemocytometer. Viability was determined using the trypan blue exclusion assay and ranged from 65 to 85% on a day-to-day basis. Cells were used immediately in the adherence assays.
Ferret gastric tissue was obtained by sacrificing 10- to 12-week-old ferrets and removing the stomach. All ferrets were negative for H. mustelae by culture and by urease activity of biopsy specimens taken from the antrum, fundus, and duodenum (three samples from each site). The serosa was separated from the tissue by injecting RPMI 1640 tissue culture medium containing 10% (vol/vol) fetal calf serum underneath the serosa and peeling it away. The remaining tissue was minced using scalpels, and epithelial cells were isolated as described above. Cells were either used immediately in the adherence assay or suspended in a solution of RPMI 1640 medium containing 50% (vol/vol) fetal calf serum and 10% (vol/vol) dimethyl sulfoxide (DMSO), frozen using a Planar Kryo 10 controlled-rate freezer, and stored in liquid nitrogen. Vials of cells were then thawed as required. Viability of cells was assessed using the trypan blue exclusion assay prior to use in the adherence assays. Viability ranged from 60 to 80% on a day-to-day basis.
The adherence assay used in these experiments has been previously described (3). Briefly, gastric cells and bacteria were incubated together at 37°C for 30 min under microaerobic conditions. Cells were stained with whole-cell H. pylori or H. mustelae antibody raised in rabbits (1/200 dilution); then they were washed and subsequently stained with fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G (Sigma). Adherence was assessed by phase-contrast microscopy, fluoresence microscopy, and flow cytometry.
By using a dot plot display of forward- and right-angle scatter, the flow cytometer (Becton Dickinson) was gated to include single cells and to exclude cell debris, clumps of cells, and nonadherent bacteria. A total of 10,000 gated events were collected, and analysis of the data was performed by using the Lysis II software program from Becton Dickinson. This program produces histograms of each cell sample and calculates the mean channel fluorescence (mean channel number) of the cell population, which is directly related to the surface density of fluorescently labeled H. pylori adhering to the cell. Mean channel numbers of cells with adherent bacteria and without bacteria were compared. Results are also expressed as the percent fluorescent cells (i.e., the percent positive events or the percentage of cells with bacteria attached); this value is calculated from the fluoresence frequency distribution histograms and is based on the relative number of cells versus the relative fluoresence intensity. The threshold for positivity was set for each experiment by flow cytometric analysis of cells without adherent bacteria which had been stained with anti-H. pylori or anti-H. mustelae whole-cell antibody and the fluorescein isothiocyanate-labeled second antibody. Unless otherwise stated, results are expressed as the mean results of at least three separate experiments ± the standard error of the means. Results were analyzed using the Student t-test.
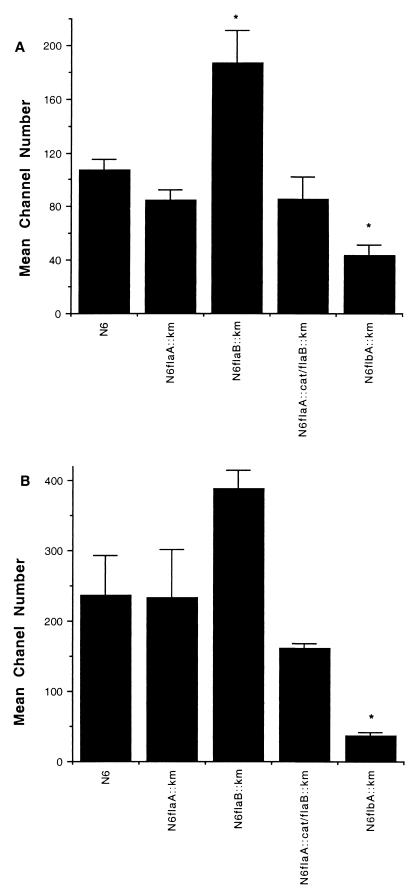
H. pylori strain N6 and the mutants N6 flaA::Km, N6 flaB::Km, and N6 flaA::cat/flaB::Km all bound to Kato III cells and to primary human gastric cells (Fig. 1). These results were confirmed by phase-contrast microscopy (results not shown). Strain N6 flaB::Km consistently bound better than the parental strain, N6, to both Kato III and primary human gastric cells. This difference reached statistical significance (P < 0.05) for Kato III cells but not for primary human gastric cells. In contrast, adherence of H. pylori strain N6 flbA::Km to Kato III and to primary human gastric cells (Fig. 1) was significantly reduced compared to that of N6. This result was confirmed by immunofluorescent microscopy (Fig. 2).
FIG. 1.
Binding of H. pylori strain N6 and isogenic flagellar negative mutants to Kato III cells (A) and to primary human gastric cells (B). Bacteria and cells were incubated together at a ratio of 200:1, and adherence was assessed by flow cytometry as outlined in the text. The results are means ± standard errors of the means for three experiments. ∗, statistically significantly different result from that obtained with H. pylori strain N6 (P < 0.05).
FIG. 2.
Adherence of H. pylori strain N6 (A) and strain N6 flbA::Km (B) to primary human gastric cells isolated from a gastric biopsy specimen. Bacteria and cells were incubated together at a ratio of 200:1, and adherence was assessed by fluorescent microscopy using a 50× water immersion lens.
H. mustelae strain F1 and the isogenic flagellum-negative mutant strains F1 flaA::Km, F1 flaB::Km and F1 flaA::cat/flaB::Km were tested for binding to Kato III cells and to primary ferret gastric epithelial cells at a range of different ratios of bacteria to cells. All strains bound to both cell types. This result was confirmed by phase-contrast microscopy (results not shown). Adherence of the bacteria to Kato III cells revealed that strain F1 flaA::Km showed a decrease in adherence at the lower bacterium/cell ratio (P < 0.05) (Fig. 3). However, all mutants showed similar levels of adherence to ferret gastric epithelial cells and at the lower bacterium/cell ratio of 50:1 they showed greater adherence than the wild type, but this result was not statistically significantly different (P > 0.05) (Fig. 3).
FIG. 3.
Adherence of H. mustelae strain F1 and isogenic flagellar negative mutants to Kato III cells (A) and to primary ferret gastric cells (B). Different concentrations of bacteria were incubated with the cells, and adherence was assessed by flow cytometry as outlined in the text. The results are means ± standard errors of the means for three experiments. ∗, statistically significantly different result from that obtained with H. mustelae strain F1 (P < 0.05).
It has previously been speculated in the literature that the flagellar sheath of H. pylori plays a role in promoting adherence of the organism to the gastric mucosa (11, 17). Flagellin has also been proposed as an adhesin in the binding of Campylobacter jejuni to cultured cells (19). However, the role of flagella in the interactions of C. jejuni with nonpolarized and polarized epithelial cells was examined with flagellar mutants. There was no statistical difference in binding of the parental wild type strain and the aflagellate, nonmotile mutants to the cells indicating that flagella are not involved in adherence to epithelial cells (12). Likewise, the present study has shown that flagella of H. pylori and H. mustelae do not play a direct role in promoting adherence of the organisms to gastric cells of their respective natural hosts, i.e., humans and ferrets. Wild-type strains, mutants deficient in the production of single flagellin species, and aflagellate double flagellin mutants were able to adhere to Kato III and to primary gastric cells. The fact that the flaA flaB double mutants were able to adhere to gastric cells as well as the wild-type strains did show that the flagellar sheath is unlikely to play a role in mediating adherence of the bacteria to the gastric mucosa.
Surprisingly, strain N6 flaB::Km consistently adhered better than the wild-type strain or the other mutants to both Kato III and primary human gastric cells. However, this difference in binding failed to reach statistical significance when we used primary human gastric cells. Strain N6 flaB::Km did grow more rapidly than the wild type and other mutant strains on blood agar plates, and although we used the same number of organisms in each experiment, it is possible that this strain was in a slightly different phase of growth. It is unlikely that mutation of flaB induces upregulation of an adhesin since the double mutant strain N6 flaA::cat/flaB::Km did not display increased binding compared to the wild-type parental strain.
Strain N6 flbA::Km, which is mutated in the regulatory gene flbA, behaved very differently from the other mutants. This mutant showed a marked reduction in adherence to primary gastric cells which was very different from that seen with strain N6 flaA::cat/flaB::Km, despite the fact that phenotypically the two mutant strains were very similar (i.e., aflagellate and nonmotile). FlbA is a membrane protein involved in the expression of flagellin genes encoded by the flbA gene which is a member of the lcrD/flbF/invA gene family (23). These proteins are components of type III secretion systems which are involved in the export of flagellar proteins or the injection of virulence factors into eukaryotic target cells. The FlbA protein itself, being a cytoplasmic membrane protein, is unlikely to function as an adhesin. This suggests that flbA is involved in the regulation of genes which play a role in mediating adherence of the organism to gastric cells. It has recently been shown that the flbA gene of H. pylori can play a role in modulating urease activity in E. coli. An E. coli strain which expressed both H. pylori urease and the NixA nickel transporter was constructed. Almost no urease activity could be detected in this strain when it contained the subcloned flbA gene. Thus, flagellar biosynthesis and urease activity may be linked in H. pylori (18). A number of other flagellar regulatory genes have been described. One of these genes is fliQ, which encodes FliQ, a protein with homology to the FliQ protein found in Salmonella enterica serovar Typhimurium and shown to be membrane bound. FliQ is required for flagellation, but fliQ does not encode any structural or regulatory component (10). An isogenic fliQ mutant of H. pylori showed a reduced level of adherence to AGS cells, a gastric cell line, compared with the parental wild-type strain. FliI is an ATPase protein found to be necessary for flagellar assembly in H. pylori (13). An H. pylori fliI knockout mutant showed reduced expression of OMP4, an outer membrane protein (22). The results of those studies combined with the results of the present study suggest that genes involved in the regulation of H. pylori flagellar biosynthesis can also play a role in regulating the production of adhesins and other potential virulence factors in Helicobacter organisms.
Acknowledgments
This work was supported by grants from The Childrens Research Centre, Dublin 12, and The Health Research Board, Ireland.
REFERENCES
- 1.Andrutis K A, Fox J G, Schauer D B, Marini R P, Li X, Yan L, Josenhans C, Suerbaum S. Infection of the ferret stomach by isogenic flagellar mutant strains of Helicobacter mustelae. Infect Immun. 1997;65:1962–1966. doi: 10.1128/iai.65.5.1962-1966.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrutis K A, Fox J G, Schauer D B, Marini R P, Murphy J C, Yan L, Solnick J V. Inability of an isogenic urease-negative mutant strain of Helicobacter mustelae to colonize the ferret stomach. Infect Immun. 1995;63:3722–3725. doi: 10.1128/iai.63.9.3722-3725.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clyne M, Drumm B. Adherence of Helicobacter pylori to primary human gastrointestinal cells. Infect Immun. 1993;61:4051–4057. doi: 10.1128/iai.61.10.4051-4057.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eaton K A, Brooks C L, Morgan D R, Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991;59:2470–2475. doi: 10.1128/iai.59.7.2470-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eaton K A, Krakowka S. Effect of gastric pH on urease-dependent colonization of gnotobiotic piglets by Helicobacter pylori. Infect Immun. 1994;62:3604–3607. doi: 10.1128/iai.62.9.3604-3607.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eaton K A, Morgan D R, Krakowka S. Motility as a factor in the colonisation of gnotobiotic piglets by Helicobacter pylori. J Med Microbiol. 1992;37:123–127. doi: 10.1099/00222615-37-2-123. [DOI] [PubMed] [Google Scholar]
- 7.Eaton K A, Suerbaum S, Josenhans C, Krakowka S. Colonization of gnotobiotic piglets by Helicobacter pylori deficient in two flagellin genes. Infect Immun. 1996;64:2445–2448. doi: 10.1128/iai.64.7.2445-2448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox J G, Correa P, Taylor N S, Lee A, Otto G, Murphy J C, Rose R. Helicobacter mustelae-associated gastritis in ferrets. An animal model of Helicobacter pylori gastritis in humans. Gastroenterology. 1990;99:352–361. doi: 10.1016/0016-5085(90)91016-y. [DOI] [PubMed] [Google Scholar]
- 9.Fox J G, Edrise B M, Cabot E B, Beaucage C, Murphy J C, Prostak K S. Campylobacter-like organisms isolated from gastric mucosa of ferrets. Am J Vet Res. 1986;47:236–239. [PubMed] [Google Scholar]
- 10.Foynes S, Dorrell N, Ward S J, Zhang Z Z, McColm A A, Farthing M, Wren B W. Functional analysis of the roles of FliQ and FlhB in flagellar expression in Helicobacter pylori. FEMS Microbiol Lett. 1999;174:33–39. doi: 10.1111/j.1574-6968.1999.tb13546.x. [DOI] [PubMed] [Google Scholar]
- 11.Geis G, Suerbaum S, Forsthoff B, Leying H, Opferkuch W. Ultrastructure and biochemical properties of the flagellar sheath of Helicobacter pylori. J Med Microbiol. 1993;38:371–377. doi: 10.1099/00222615-38-5-371. [DOI] [PubMed] [Google Scholar]
- 12.Grant C C R, Konkel M E, Cieplak W, Jr, Tompkins L S. Role of flagella in adherence, internalization, and translocation of Campylobacter jejuni in nonpolarized and polarized epithelial cell cultures. Infect Immun. 1993;61:1764–1771. doi: 10.1128/iai.61.5.1764-1771.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenks P W, Foynes S, Ward S J, Constantinidou C, Penn C W, Wren B W. A flagellar-specific ATPase (FliI) is necessary for flagellar export in Helicobacter pylori. FEMS Microbiol Lett. 1997;152:205–211. doi: 10.1111/j.1574-6968.1997.tb10429.x. [DOI] [PubMed] [Google Scholar]
- 14.Josenhans C, Labigne A, Suerbaum S. Comparative ultrastructural and functional studies of Helicobacter pylori and Helicobacter mustelae flagellin mutants: both flagellin subunits are necessary for full motility in Helicobacter species. J Bacteriol. 1995;177:3010–3020. doi: 10.1128/jb.177.11.3010-3020.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kostrzynska M, Betts J D, Austin J W, Trust T J. Identification, characterization, and spatial localization of two flagellin species in Helicobacter pylori flagella. J Bacteriol. 1991;173:937–946. doi: 10.1128/jb.173.3.937-946.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leying H, Suerbaum S, Geis G, Haas R. Cloning and genetic characterisation of a Helicobacter pylori flagellin gene. Mol Microbiol. 1992;6:2863–2874. doi: 10.1111/j.1365-2958.1992.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 17.Luke C J, Penn C W. Identification of a 29kDa flagellar sheath protein in Helicobacter pylori using a murine monoclonal antibody. Microbiology. 1995;141:597–604. doi: 10.1099/13500872-141-3-597. [DOI] [PubMed] [Google Scholar]
- 18.McGee D J, May C A, Garner R M, Himpsl J M, Mobley H L T. Isolation of Helicobacter pylori genes that modulate urease activity. J Bacteriol. 1999;181:2477–2484. doi: 10.1128/jb.181.8.2477-2484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McSweegan E, Walker R I. Identification and characterization of two Campylobacter jejuni adhesins for cellular and mucous substrates. Infect Immun. 1986;53:141–148. doi: 10.1128/iai.53.1.141-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Rourke J, Lee A, Fox J G. An ultrastructural study of Helicobacter mustelae: evidence of a specific association with gastric mucosa. J Med Microbiol. 1992;36:420–427. doi: 10.1099/00222615-36-6-420. [DOI] [PubMed] [Google Scholar]
- 21.O'Toole P W, Kostrzynska M, Trust T J. Non-motile mutants of Helicobacter pylori and Helicobacter mustelae defective in flagellar hook protein. Mol Microbiol. 1994;14:691–703. doi: 10.1111/j.1365-2958.1994.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 22.Porwollik S, Noonan B, O'Toole P W. Molecular characterization of a flagellar export locus of Helicobacter pylori. Infect Immun. 1999;67:2060–2070. doi: 10.1128/iai.67.5.2060-2070.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitz A, Josenhans C, Suerbaum S. Cloning and genetic characterization of the Helicobacter pylori flbA gene which codes for a membrane protein involved in coordinated expression of flagellar genes. J Bacteriol. 1997;179:987–997. doi: 10.1128/jb.179.4.987-997.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suerbaum S, Josenhans C, Labigne A. Cloning and genetic characterization of the Helicobacter pylori and Helicobacter mustelae flaB flagellin genes and construction of H. pylori flaA- and flaB-negative mutants by electroporation-mediated allelic exchange. J Bacteriol. 1993;175:3278–3288. doi: 10.1128/jb.175.11.3278-3288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuda M, Karita M, Morshed M G, Okita K, Nakazawa T. A urease-negative mutant of Helicobacter pylori constructed by allelic exchange mutagenesis lacks the ability to colonize the nude mouse stomach. Infect Immun. 1994;62:3586–3589. doi: 10.1128/iai.62.8.3586-3589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]