Abstract
Objective: TURKBIO registry, established in 2011, is the first nationwide biological database in Turkey. This study aimed to provide an overview of TURKBIO data collected by June 2018.
Methods: The registry included adult patients with rheumatoid arthritis (RA), ankylosing spondylitis (AS), nonradiographic axial spondyloarthritis (nr-AxSpA), and psoriatic arthritis (PsA). Demographic and clinical features, disease activity markers, and other follow-up parameters, current and previous treatments, and adverse events were registered electronically at each visit using open-source software. The registration of patient-reported outcome measures was carried out electronically by the patients using touch screens.
Results: TURKBIO registry included a total of 41,145 treatment series with biologicals. There were 2,588 patients with axSpA (2,459 AS and 129 nr-axSpA), 2,036 with RA, and 428 with PsA. The total number of patients, including those with other diagnoses, was 5,718. In the follow-up period, the number of patients and also visits steadily increased by years. The yearly mean number of visits per patient was found to be 2.3. Significant improvements in disease activity and health assessment parameters were observed following the biological treatments. Biologics were often given in combination with a conventional synthetic disease-modifying antirheumatic drug in patients with RA. Infections were the most commonly seen adverse events, followed by allergic reactions. Tuberculosis was observed in 12 patients, malignancy in 18, and treatment-related mortality in 31.
Conclusion: TURKBIO provided a valuable real-life experience with the use of biologics in rheumatic diseases in Turkey.
Keywords: Biologic registry, rheumatic disease, Turkey
Main Points
TURKBIO provided a valuable real-life experience with the use of biologics in rheumatic diseases in Turkey.
Significant improvements in disease activity and health assessment parameters were observed with the biologics.
Infections were the most commonly seen adverse events, followed by allergic reactions.
Data obtained by the registry also allowed to determine drug survivals and influencing factors.
Introduction
The rapid developments in the treatment of rheumatologic diseases are considered satisfactory. New treatment agents are undoubtedly approved after their effectiveness, and safety were proved in several randomized controlled trials with high qualities. Unfortunately, this type of clinical trial cannot provide sufficient data on real-life usage as the patients with some concomitant situations have been excluded from the study, and the duration is not as long as for the occurring of some adverse events. In that case, observational studies intervene to obtain the needed data. Several biological registries investigated the efficacy and safety of treatment in rheumatologic diseases across the world.1,2 They provided much valuable information about the effectiveness and safety and the effect of racial, geographical, and socio-demographic differences by the treatment responses. Furthermore, such registries demonstrated the trends of biologic treatment choices and concomitant use of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs).
TURKBIO registry, established in October 2011, is the first nationwide biological database contributed by 14 different centers across Turkey (Figure 1). This study aimed to perform a general evaluation of the data collected by the registry by June 2018.
Figure 1.
Centers included in TURKBIO Registry.
Methods
The registry included adult (≥18 years) patients with rheumatoid arthritis (RA), ankylosing spondylitis (AS), nonradiographic axial spondyloarthritis (nr-AxSpA), and psoriatic arthritis (PsA). Demographic and clinical features (age, sex, diagnosis, age at disease onset and diagnosis, education level, and smoking status) were recorded at baseline. Disease activity markers and other follow-up parameters [health assessment questionnaire (HAQ), visual analog scale for pain, patient and physician global assessments, Short Form (36) Health Survey (SF-36), number of swollen and tender joints (28 counts), serum C-reactive protein (CRP) and erythrocyte sedimentation rate, disease activity score 28-CRP (DAS28-CRP), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Functional Index and Metrology Index, and Ankylosing Spondylitis Disease Activity Score], current and previous treatments [biological (b) DMARDs or targeted synthetic (ts) DMARDs, concomitant csDMARDs, and glucocorticoid therapy], adverse events, discontinuation rates and reasons, and comorbidities were registered electronically at each visit by using open-source software. Standard hand and foot X-rays for RA and PsA patients and standard pelvis, lumbosacral, thoracal, and cervical vertebra and calcaneus Xrays for axSpA were performed at baseline/diagnosis and every 2 years in the follow-up. Sacroiliac magnetic resonance imaging examinations, according to the Assessment of SpondyloArthritis International Society (ASAS) recommendations, were obtained for the diagnosis of nr-axSpA. Rheumatoid factor (RF) and anticyclic citrullinated antibody (CCP) in all RA patients and HLA-B27 in all SpA and PsA patients were studied (Table 1). Medical Dictionary for Regulatory Activities (MedDRA) was used to code adverse events (https://apps.meddra.org/selfservice/). The registration of patient-reported outcome measures was carried out electronically by the patients using touch screens. Patients with other rheumatologic diseases (systemic vasculitis, familial Mediterranean fever, Behcet’s disease, and adult Still’s disease) used biologicals, which were also recorded nonsystematically.
Table 1.
Demographic and Clinical Features of Patients
| RA | AS | nr-axSpA | PsA | |||||
|---|---|---|---|---|---|---|---|---|
| N | 2,036 | 2,459 | 129 | 428 | ||||
| Sex (female), n (%) | 1,625 (80) | 948 (39) | 75 (58) | 274 (64) | ||||
| Age (years) | 53 ± 14 | 43 ± 11 | 43 ± 13 | 46 ± 12 | ||||
| Smoking (%) | ||||||||
| Never | 62 | 37 | 45 | 60 | ||||
| Current | 21 | 43 | 38 | 24 | ||||
| Previous | 17 | 20 | 17 | 15 | ||||
| Disease duration, | 108 (64-162) | 81 (45-126) | 69 (38-99) | 90 (49-128) | ||||
| Median (Q1-Q3) (months) | ||||||||
| RF (%) | 58 | – | – | – | ||||
| Anti-CCP (%) | 60 | – | – | – | ||||
| HLA-B27 (%) | – | 65 | 39 | 16 | ||||
| Previous cDMARD use (%) | ||||||||
| MTX | 893 (44) | 267 (11) | 38 (29) | 189 (44) | ||||
| LEF | 619 (30) | 21 (1) | 8 (6) | 72 (17) | ||||
| SSZ | 430 (21) | 689 (28) | 56 (43) | 74 (17) | ||||
| HQ | 447 (22) | 21 (1) | 4 (3) | 12 (3) | ||||
| Baseline | Last | Baseline | Last | Baseline | Last | Baseline | Last | |
| DAS28-CRP | 3.96 ± 2.58 | 2.74 ± 1.33 | 2.82 ± 1.14 | 2.23 ± 0.98 | 2.54 ± 1.06 | 2.12 ± 0.72 | 3.40 ± 1.38 | 2.23 ± 1.00 |
| CRP | 17.25 ± 25.48 | 11.43 ± 20.43 | 16.04 ± 4.17 | 10.02 ± 17.61 | 15.22 ± 27.60 | 8.85 ± 14.51 | 14.99 ± 22.42 | 7.95 ± 17.19 |
| HAQ | 1.00 ± 0.73 | 0.62 ± 0.68 | 0.76 ± 0.67 | 0.40 ± 0.54 | 0.62 ± 0.61 | 0.37 ± 0.50 | 0.76 ± 0.60 | 0.38 ± 0.50 |
| BASDAI | NA | NA | 39.50 ± 22.27 | 22.89 ± 20.91 | 41.54 ± 24.53 | 26.71 ± 20.77 | 35.68 ± 20.45 | 19.56 ± 19.25 |
| Concomitant DMARD use (%) | ||||||||
| MTX | 53.0 | 12.3 | 16.7 | 6.9 | 69.8 | 7.8 | 95.8 | 14.0 |
| LEF | 52.6 | 16.2 | 2.8 | 0.8 | 15.5 | 0 | 42.1 | 4.7 |
| SSZ | 13.8 | 3.4 | 24.4 | 6.9 | 38.8 | 7.8 | 11.7 | 4.7 |
Continuous variables were presented as mean ± SD. RA, rheumatoid arthritis; AS, ankylosing spondylitis; nr-axSpA, nonradiographic axial spondyloarthritis; PsA, psoriatic arthritis; cDMARD, conventional disease-modifying antirheumatic drug; MTX, methotrexate; LEF, leflunomide; SSZ, sulphasalazine; HQ, hydroxychloroquine; DAS28-CRP, disease activity score 28-C reactive protein; HAQ, health assessment questionnaire; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; NA, nonavailable.
The Registry Project has been approved by the Drug Regulatory Authority of the Health Ministry of Turkey and the Dokuz Eylül University Ethics Committee as an observational study. It is sponsored by “The Society for Follow-up of Rheumatic Diseases (ROHIDER)” in İzmir. The coordinating center of the TURKBIO, Division of Rheumatology at Dokuz Eylül University, is also placed in this city. The Scientific Committee consisted of one responsible physician from each participating center. A nurse or a data entry staff contributed to all registration and follow-up procedures in each center. Patients signed a written informed consent form before their inclusion in the study.
Results
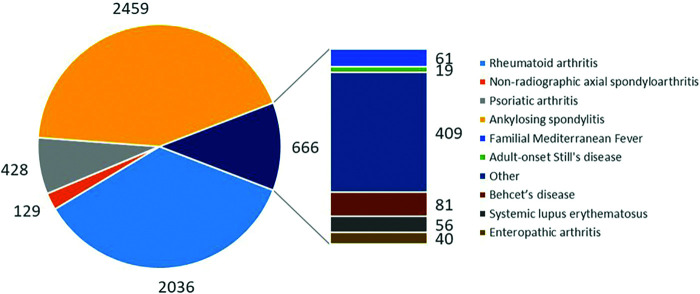
TURKBIO registry included a total of 41.145 treatment series with biologics by June 2018. There were 2.588 patients with axSpA [2.459 (43.0%) AS and 129 (2.3%) nr-axSpA], 2.036 (35.6%) patients with RA, and 428 (7.5%) patients with PsA. The total number of patients, including those with other diagnoses, was 5,718 (Figure 2).
Figure 2.
The distribution of patients by the diagnosis.
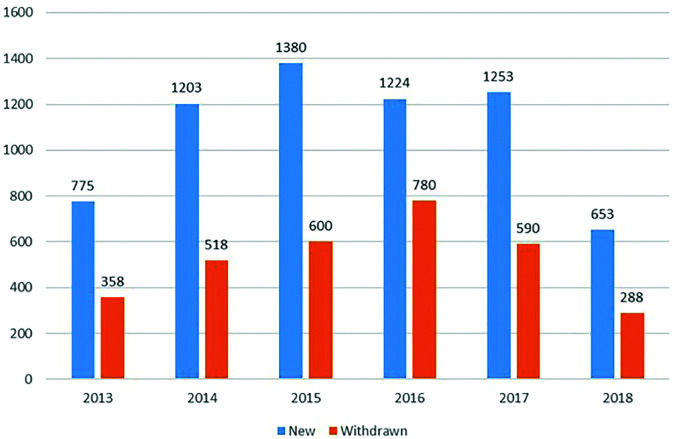
In the follow-up from 2011 to 2018, the number of patients and visits steadily increased over the years. The yearly mean number of visits per patient was found to be 2.3 (range: 2.0-2.9). It was high as 3.4 (range: 3.0-3.7) in some centers where patients were closely monitored. The number of new and withdrawn treatment series in TURKBIO was changed year by year (Figure 3).
Figure 3.
The number of new and withdrawn treatment series in TURKBIO by years (first 6 months for 2018).
The demographic and clinical features of patients with RA, AS, nr-axSpA, and PsA at baseline were shown in Table 1. Female predominance was observed among RA, PsA, and nr-axSpA patients, whereas AS patients were predominantly male. The mean age of RA patients was higher compared to others. Among RA patients, 58% had RF, and 60% had anti-CCP antibodies. The frequency of HLA-B27 was found to be 65%, 39%, and 16% in patients with AS, nr-axSpA, and PsA, respectively. The most frequently used csDMARD was methotrexate (MTX) in patients with RA and PsA and sulphasalazine (SSZ) in patients with AS and nr-axSpA starting of biologic therapy.
In all the patient groups, statistically significant improvements in disease activity markers, including DAS28 (RA), BASDAI (AxSpA), and serum CRP levels, and health assessment parameters, were observed following the biological treatments (Table 1).
Biologics were often given in combination with a csDMARD (mostly MTX and LEF) in patients with RA. The frequency of concomitant csDMARD use was also significantly decreased at the last visits than baseline visits (Table 1).
A total of 187 serious adverse events were reported during the 41,145 treatment courses. Infections were the most commonly seen serious adverse events (74 patients, including 12 with tuberculosis), followed by allergic reactions (34 patients). Malignancy was observed in 18 patients (12 with RA and six SpA) during the study period. Among six patients, two each had lung, skin (basal cell), and breast cancers. Other malignancies reported in the remaining 12 patients were lymphoma, multiple myeloma, cranial plasmacytoma, acute lymphocytic leukemia, brain and thyroid tumors, renal and bladder cancers, gastrointestinal stromal tumor, colon and stomach cancers, and germ cell tumor, respectively. Seventeen patients were using TNFi, including adalimumab (ADA), etanercept (ETA), infliximab (INF) and golimumab (GOL), and one abatacept when they developed malignancy. Treatment-related mortality was reported in 31 patients. There were seven paradoxical events (six anterior uveitis and one psoriasis).
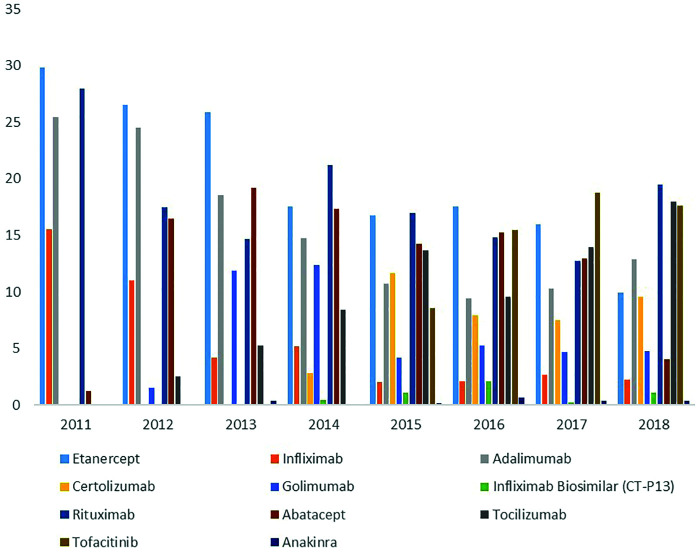
In the follow-up duration of 7 years, the number of biologics approved and reimbursed gradually increased, and therefore, their using percentages were changed in the whole group of RA patients (Figure 4). Mean biological switch numbers were similar in patients with RA (2.85 ± 1.20), AS (2.55 ± 0.95), nr-axSpA (2.72 ± 1.14), and PsA (2.69 ± 1.00). The drug retention rate in patients with RA who used their first biologics (TNFi, abatacept, tocilizumab, and rituximab) or tofacitinib was found to be 63.6%. 21.6% of RA patients required switching to second and 9.1% switching to third agents. Among axSpA patients, the first biologic survival rate (69%) was found to be higher than that in RA patients. The rates of the first and the second biological switches were 20.6% and 6.5%, respectively. Patients with PsA had a first biological survival rate (TNFi) of 58.6%. The rates of the first and the second biological switches were 33.7% and 11.7%, respectively.
Figure 4.
Distribution of biologics as a new treatment across a year of prescription in patients with rheumatoid arthritis.
When baseline disease activity parameters were evaluated, a trend to start using biologics in RA, AS, and nr-axSpA patients with less disease activity over the years was seen (mean DAS28-CRP: 5.2 in 2011 vs 4.2 in 2017, P = .002 for RA; mean BASDAI: 46.8 in 2011 vs 35.9 in 2017, P = .0001 for AS; and mean BASDAI: 53.4 in 2011 vs 32.1 in 2017, P = .0001 for nr-axSpA). There was no such trend in PsA patients (mean DAS28-CRP: 3.3 in 2012 vs 3.7 in 2017, P = .334).
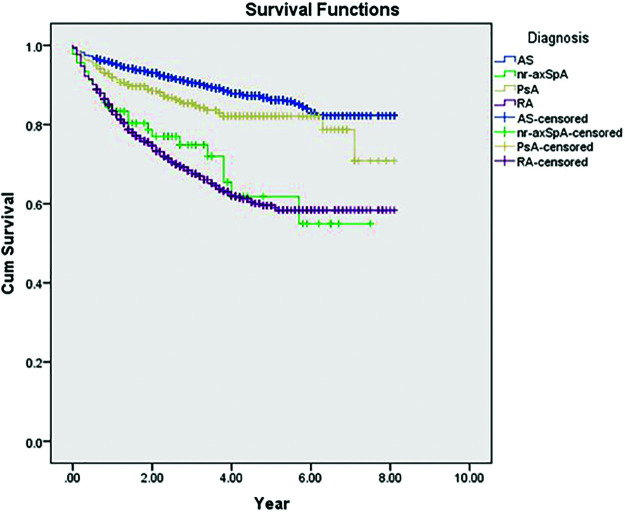
Comparing the first-line TNFi survival rate revealed a significant difference between the patients with RA, AS, nr-axSpA, and PsA (Figure 5).
Figure 5.
The comparison of first-line TNFi survival in patients with RA, AS, nr-axSpA, and PsA (P < .001). RA: rheumatoid arthritis; AS: ankylosing spondylitis; nr-axSpA: nonradiographic axial spondyloarthritis; PsA: psoriatic arthritis.
Discussion
The TURKBIO registry created several opportunities to evaluate the real-life experience with biological treatments in rheumatologic diseases in Turkey. The registry data demonstrated the increasing number of biological treatment series and the expanding diversity in the routine rheumatology practice over the years. The majority of inflammatory rheumatic diseases, including RA, AS, nr-axSpA, and PsA, were included in TURKBIO; therefore, it allowed determining and comparing various biologicals’ efficacy in different disease groups.
In Turkey, the prevalence of RA3 and AS4 was estimated to be 0.5% for each. When the whole group of SpA was evaluated, the prevalence increased to 1.0-1.8%.4,5 According to the ASAS classification criteria, the estimated prevalence for axSpA was 1.3% (0.5% for radiographic axSpA and 0.8% for nr-axSpA).4 When considering the high prevalence of inflammatory rheumatic diseases and the need for effective treatments in patients unresponsive to conventional treatments, it was no surprise to have the increased use of biologics in daily rheumatology practice.
TURKBIO database has very similar properties to DANBIO because it was initially developed as its Turkish version. TURKBIO allows us to investigate the efficacy of different biologics and adverse events related to treatments in all patient groups and provide essential data on the differences between age/sex groups and subtypes of diseases such as spondyloarthritis. Furthermore, drug survival, switch ratios, and the treatment response in “switchers” could have been investigated using longitudinal data obtained by the registry. The online registration helped to follow-up patients on biologics more strictly. Therefore, treatment responses were evaluated at regular intervals in the individual patient, and necessary treatment changes have been done rapidly. Such tight control of the treatment is associated with better outcome measures.6,7 The use of MedDRA for the coding of adverse events provided more detailed and standardized reporting. These reports also contributed to the Health Ministry Registration Systems.
It was seen that as the clinical experience with biologics increased over the years, they were begun to be prescribed in patients with less severe disease activity compared to earlier years. In the follow-up of patients, no unexpected adverse events were observed. The frequency of adverse events was similar to previous observations.
To date, we performed several studies using TURKBIO data and presented them in prestigious Rheumatology meetings. First, we investigated the poor prognostic factors8 and the effect of different concomitant csDMARDs9 and glucocorticoid use on the response to biologics among patients with RA. The effectiveness of tofacitinib was compared between RA patients with and without previous biologicals.10 Furthermore, we evaluated the effect of body mass index on the response to rituximab11 and tofacitinib12 in RA patients. The other three studies investigated the impact of cigarette smoking on the treatment response in patients with axSpA13 and PsA14 using TNFi and in patients with RA using tofacitinib,15 respectively. Data from the registry also allowed to determine the long-term survival of TNFi in axSpA subgroups16 and compare the effectiveness and survival of original TNFi and biosimilar treatments.17
In the last of 2017, TURKBIO participated in EuroSpA Research Collaboration Network in Spondyloarthritis, consisting of 15 European registries. In the first pooled data from EuroSpA Research Collaboration Network, drug retention and response rates of TNFi treatment in 21,470 patients with axSpA18 and 13,170 patients with PsA,19 respectively, were investigated. The third paper from EuroSpA investigated drug retention, inactive disease, and response rates in 1,860 patients with ax-SpA initiating secukinumab treatment.20
In conclusion, the TURKBIO registry provided valuable information about biologics’ efficacy and safety in real-life patients with various inflammatory rheumatic diseases in Turkey. It also allowed us to investigate these treatments’ survival and influencing factors on drug efficacy and survival. The collaboration with other biological registries in Europe provided to investigate the effects of racial, geographical, and socio-demographic differences by the treatment responses.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Dokuz Eylül University (Date:13.04.2017; Number: 304-SBKAEK-2017/05-06).
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - F.O., S.A., S.S.K., N.A.; Design - F.O., S.A., A.T., N.A.; Supervision - F.O., S.S.K., N.S.K.; Resources - E.D., A.T., N.S.K.; Materials - G.C., E.D., S.S.K., A.T., C.B.; Data Collection and/or Processing - F.O., G.C., S.C., Y.P., S.S., S.A., S.S.K., A.T., A.Y., S.Y., N.I., I.S., M.B., D.S., A.C., M.A.O., S.Y., N.S.K., N.Y., S.E., C.B., O.S.G., B.G., S.H., S.Y., G.Y.C., F.Y., H.D., N.A.; Analysis and/or Interpretation - S.C., E.D., Y.P., S.S., S.A., A.Y., I.S., M.B., D.S., A.C., S.Y., S.E., C.B., O.S.G., S.H., S.Y., F.Y., H.D., N.A.; Literature Search - G.C., S.S., S.A., S.S.K., M.A.O., S.Y., N.Y., S.E., B.G., S.H., G.Y.C.; Writing Manuscript - F.O., N.A.; Critical Review - F.O., S.A., S.Y., N.I., I.S., M.B., N.A.
Acknowledgments: Thanks to all the Turkish departments of rheumatology for reporting to the TURKBIO registry.
Declaration of Interests: The authors have no conflicts of interest to declare.
Funding: The study was sponsored by “The Society for Follow-up of Rheumatic Diseases (ROHIDER),” who did not influence the study design, analyses, interpretation, or the decision to publish the results.
References
- 1. Hetland ML. DANBIO: A nationwide registry of biological therapies in Denmark. Clin Exp Rheumatol . 2005;23:(5 Suppl. 39):S205–S207.. [PubMed] [Google Scholar]
- 2. Kvien TK, Heiberg LE, Kaufmann C.et al. A Norwegian DMARD register: Prescriptions of DMARDs and biological agents to patients with inflammatory rheumatic diseases. Clin Exp Rheumatol . 2005;23:(5 Suppl. 39):S188–S194.. [PubMed] [Google Scholar]
- 3. Akar S, Birlik M, Gurler O.et al. The prevalence of rheumatoid arthritis in an urban population of Izmir-Turkey. Clin Exp Rheumatol . 2004;22:(4):416–420.. [PubMed] [Google Scholar]
- 4. Onen F, Solmaz D, Cetin P.et al. Prevalence of inflammatory back pain and axial spondyloarthritis among university employees in Izmir, Turkey. J Rheumatol . 2015;42:(9):1647–1651.. 10.3899/jrheum.141600 [DOI] [PubMed] [Google Scholar]
- 5. Onen F, Akar S, Birlik M.et al. Prevalence of ankylosing spondylitis and related spondyloarthritides in an urban area of Izmir, Turkey. J Rheumatol . 2008;35:(2):305–309.. [PubMed] [Google Scholar]
- 6. Grigor C, Capell H, Stirling A.et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): A single-blind randomised controlled trial. Lancet . 2004;364:(9430):263–269.. 10.1016/S0140-6736(04)16676-2 [DOI] [PubMed] [Google Scholar]
- 7. Coates LC, Moverley AR, McParland L.et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): A UK multicentre, open-label, randomised controlled trial. Lancet . 2015;386:(10012):2489–2498.. 10.1016/S0140-6736(15)00347-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inanc N, Ertürk Z, Ozen G.et al. AB0278 investigation of poor prognostic factors among rheumatoid arthritis patients in TURKBIO registry. Ann Rheum Dis . 2018;77:(Suppl. 2):1319. [Google Scholar]
- 9. Inanc N, Ozen G, Yalcinkaya Y.et al. FRI0134. Is there any difference in Ra patients for methotrexate use vs. leflunomide use as a concomitant treatment with biological and targeted synthetic DMARDS in TURKBIO registry?. Ann Rheum Dis . 2018;77:(Suppl. 2):611.2–612.. [Google Scholar]
- 10. Zengin B, Inanç N, Akar S.et al. AB0482. Similar efficacy of tofacitinib on disease activity in rheumatoid arthritis patients with and without previous biologicals; results from the TURKBIO registry. Ann Rheum Dis . 2018;77:(Suppl. 2):1401.3–1402.. [Google Scholar]
- 11. Koca SS, Karatas A, Oz B.et al. Index does not affect response to rituximab in patients with rheumatoid arthritis: Results from Turkbio registry [abstract]. In ACR/ARHP Annual Meeting, . American College of Rheumatology, 2017: 69. [Google Scholar]
- 12. Babaoglu H, Goker B, İnanç N.et al. Impact of obesity on drug survival of tofacitinib in patients with rheumatoid arthritis: Analysis from the Turkbio registry [abstract]. In ACR/ARHP Annual Meeting, . American College of Rheumatology, 2018: 1712–1714.. [Google Scholar]
- 13. Yarkan TH, Can G, Çapar S.et al. SAT0184 The effect of smoking on response to tumor necrosis factor-alpha inhibitor treatment in ankylosing spondylitis patients: Results from the turkbio registry. Ann Rheum Dis . 2018;77:(Suppl. 2):953.1-953. [Google Scholar]
- 14. Yarkan H, Kenar G, Capar S.et al. The effect of smoking on response to tumour necrosis factor-alpha inhibitor treatment in psoriatic arthritis patients: Results from the turkbio registry [abstract]. In ACR/ARHP Annual Meeting . American College of Rheumatology, 2018. [Google Scholar]
- 15. Karatas A, Oz B, Dalkiliç E.et al. Cigarette Smoking does not affect treatment response to tofacitinib in rheumatoid arthritis [abstract]. In ACR/ARHP Annual Meeting, . American College of Rheumatology, 2018: 70. [Google Scholar]
- 16. Can G, Dalkilic E, Pehlivan Y.et al. AB0835 Comparison of long term anti-tnf survival in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis; data from turkbio registry. Ann Rheum Dis . 2018;77:(Suppl. 2):1545.2–1546.. [Google Scholar]
- 17. Uslu S, Can G, Senel S.et al. THU0218 The efficacy and drug survival of the biosimilar infliximab (CT-P13) compared to the original reference infliximab in inflammatory rheumatic diseases; results from the turkbio registry. Ann Rheum Dis . 2018;77:(Suppl. 2):329. [Google Scholar]
- 18. Ørnbjerg LM, Brahe CH, Askling J.et al. Treatment response and drug retention rates in 24 195 biologic-naïve patients with axial spondyloarthritis initiating TNFi treatment: Routine care data from 12 registries in the EuroSpA collaboration. Ann Rheum Dis . 2019;78:(11):1536–1544.. 10.1136/annrheumdis-2019-215427 [DOI] [PubMed] [Google Scholar]
- 19. Brahe CH, Ørnbjerg LM, Jacobsson L.et al. Retention and response rates in 14 261 PsA patients starting TNF inhibitor treatment—Results from 12 countries in EuroSpA. Rheumatology . 2020;59:(7):1640–1650.. 10.1093/rheumatology/kez427 [DOI] [PubMed] [Google Scholar]
- 20. Michelsen B, Lindström U, Codreanu C.et al. Drug retention, inactive disease and response rates in 1860 patients with axial spondyloarthritis initiating secukinumab treatment: Routine care data from 13 registries in the EuroSpA collaboration. RMD Open . 2020;6:(3):e001280. 10.1136/rmdopen-2020-001280 [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Content of this journal is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.