Abstract
Skeletal muscle is the most abundant tissue in the human body and has a tremendous capability to regenerate in response to muscle injuries and diseases. Induction of acute muscle injury is a common method to study muscle regeneration in vivo. Cardiotoxin (CTX) belongs to the family of snake venom toxins and is one of the most common reagents to induce muscle injury. Intramuscular injection of CTX causes overwhelming muscle contraction and lysis of myofibers. The induced acute muscle injury triggers muscle regeneration, allowing in-depth studies on muscle regeneration. This protocol describes a detailed procedure of intramuscular injection of CTX to induce acute muscle injury that could be also applied in other mammalian models.
Keywords: Cardiotoxin, Skeletal muscle injury, Intramuscular injection
Background
Skeletal muscle regeneration is a series of highly regulated processes mainly executed by muscle stem cells (MuSCs) (Hawke and Garry, 2001), including necrosis of damaged myofibers, immune cell infiltration, clearance of the damaged myofibers, MuSC activation and proliferation, myofiber formation and maturation, and muscle reconstruction. MuSCs, also named satellite cells, were first discovered by Dr. Alexander Mauro in 1961 (Mauro, 1961). In response to muscle injuries, the quiescent MuSCs are activated to enter the cell cycle, proliferate, and differentiate into new myofibers or fuse to the injured myofibers to repair muscle injury. After activation and proliferation, part of the MuSCs will return to quiescence and replenish the in vivo stem cell pool (Bischoff, 1975; Konigsberg et al., 1975; Fu et al., 2015 and 2022).
Induction of acute muscle injury is a critical step to study muscle regeneration in vivo. Several methods have been applied to induce consistent acute muscle injury, including freezing, barium chloride injection, glycerol injection, notexin injection, and cardiotoxin (CTX) injection (Mahdy et al., 2015; Hardy et al., 2016). Among the reagents to induce acute muscle injury, CTX is the most commonly used. CTX disrupts the membrane integrity of neuronal and skeletal muscle and cardiac muscle cells (Dufton and Hider 1988); it has been speculated to form pores on the cell membrane that result in membrane depolarization and influx of Ca2+, causing overwhelming muscle contraction, myofiber lysis, and acute muscle injury (Harvey et al., 1982). This protocol describes the step-by-step procedure to induce skeletal muscle injury via intramuscular injection of CTX. Following CTX injection, mice can be sacrificed at different time points for further analysis, according to variable experimental requirements.
Materials and Reagents
1 mL syringe (for intraperitoneal injection), needle 0.45 mm (Shanghai Kindly Group, 2012-3151233)
0.5 mL insulin syringe with 29 G ½ needle (for cardiotoxin injection) (EXELINT, catalog number: 26028)
0.2 µm filter membrane (Millipore, catalog number: SLGPR33RB)
Cardiotoxin (CTX) 5 mg, from Naja atra (BOYAO BioTechnology, catalog number: 9012-91-3)
Phosphate-buffered saline (PBS) (Thermo Fisher, catalog number:10010023)
2,2,2-Tribromoethanol (TBE) (Sigma, catalog number: T48402)
Tert-amyl alcohol (Sigma, catalog number: 240486)
75% ethanol in liquid or in the form of pads (if using 95% or 99% ethanol, dilute with distilled water)
Equipment
Surgical scissors
Heating pad (Optional)
Procedure
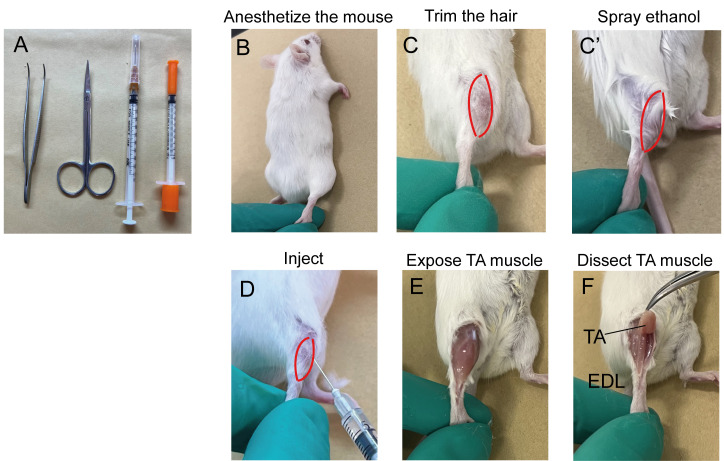
Tools used for CTX injection are shown in Figure 1A. Video 1 shows how to inject CTX in mouse’s tibialis anterior (TA) muscle.
-
Prepare 10 µM CTX by reconstituting CTX powder in PBS.
CTX PREPARATION: Calculate the volume of PBS that is needed for the reconstitution of CTX powder. CTX used for this protocol is from Naja atra and has a molecular weight of approximately 7,100 Da. Hence, the volume of PBS is 5 mg/(10 µM × 7,100 g/mol) = 70.4 mL. Filter the solution through a membrane with 0.2 μm or smaller pore size. Aliquot and store at -20 °C or lower temperature. Do not freeze and thaw frequently.
-
Anesthetize the mice by intraperitoneal injection of TBE (Avertin) or other equivalent anesthesia (Figure 1B). 200–250 mg of TBE per kilogram of mice body weight is recommended. For example, for a mouse weighing 30 g, 200–250 mg/kg × 30 g = 6–7.5 mg of TBE is needed. If using 2.5% working solution, injection volume will be 6–7.5 mg/2.5% (2.5% equals 2.5 g/100 mL) = 240–300 µL.
TBE PREPARATION: To prepare 100% TBE solution, mix 10 g of TBE with 10 mL tert-amyl alcohol. Make sure it is fully dissolved. To prepare working solution, dilute 100% stock to 1.25%–2.5%, v/v, in water or PBS. Stir vigorously until it is thoroughly dissolved. Filter the solution through a 0.2 μm filter membrane. Both the 100% stock and working solutions should be stored in the dark at 4 °C to prevent decomposition. The working solution is preferred to be prepared freshly from stock aliquots and is stable for at least a month.
To get a clear visualization of the TA muscle, either trim the hair or spray the hind limbs with 70% ethanol (Figure 1C and C’).
-
Determine the injection volume of CTX and aspirate a proper volume of the solution into the insulin syringe by pulling the plunger back slowly. To remove the air bubbles, hold the syringe with the needle upward and further gently pull the plunger down. Tap the side of the syringe to drive the air bubbles to top. Press the plunger until a few drops of liquid come out of the needle.
A different volume of CTX solution needs to be applied based on different purposes. For a muscular regeneration experiment, a total of 50–100 µL of CTX with 5–10 injection sites is recommended for each TA muscle. For induction of muscular damage in muscle stem cell transplantation experiments, a total of 10–20 µL of CTX with 1–2 injection spots is recommended.
Locate the center of the TA muscle, insert the needle at a 10°–30° angle, 2–3 mm deep, and perform the injection (Figure 1D). Approximately 10 µL of CTX solution is injected at each site. Leave the needle in the muscle for 2–3 s to prevent leakage of CTX. Slowly pull out the needle and continue the next injection until desired volume of injection is reached (Video 1).
After the procedure, place the cage on a heated pad until the mice are awake.
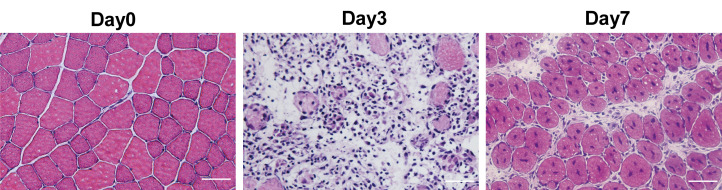
Perform muscle histology characterization using hematoxylin & eosin (H&E) staining. A detailed protocol of muscle dissection (Figure 1E and 1F) and H&E staining was published previously (Wang et al., 2017). H&E staining allows for the evaluation of the morphology of the regeneration process post CTX injury during skeletal muscle regeneration (Figure 2). Before injury, H&E–stained transverse sections show highly organized muscle structure with eosin-stained cytoplasm and hematoxylin-stained nuclei placed at the periphery of the fibers. At day 3 post CTX injury, the muscle structure is completely destroyed, and both necrotic myofibers and numerous mononucleated cells are observed. Along with the regeneration process, myofibers are newly regenerated by fusion and differentiation of myoblasts. Newly formed myofibers display centrally located nuclei and smaller size in the cross-sectional area compared to that of uninjured state (Day7 in Figure 2).
Figure 1. Illustration of cardiotoxin (CTX) injection in mouse’s TA muscle.
A. Tools used to inject CTX; B–D. Key steps for CTX injection; E and F. Key steps for muscle dissection. TA: Tibialis anterior; EDL: Extensor digitorum longus.
Video 1. Demonstration of cardiotoxin (CTX) injection in mouse’s tibialis anterior muscle.
Figure 2. H&E staining of uninjured and injured tibialis anterior (TA) muscle induced by cardiotoxin (CTX).
Scale bar, 50 µm.
Acknowledgments
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Science (XDA16020400 to PH), National Natural Science Foundation of China (32170804 to PH). This protocol is derived from the original research paper (Zhou et al., 2020).
Competing interests
All authors declare that there are no competing interests.
References
- 1.Bischoff R.(1975). Regeneration of single skeletal muscle fibers in vitro. Anat Rec 182(2): 215-235. [DOI] [PubMed] [Google Scholar]
- 2.Dufton M. J. and Hider R. C.(1988). Structure and pharmacology of elapid cytotoxins. Pharmacol Ther 36(1): 1-40. [DOI] [PubMed] [Google Scholar]
- 3.Fu X., Wang H. and Hu P.(2015). Stem cell activation in skeletal muscle regeneration. Cell Mol Life Sci 72(9): 1663-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu X., Zhuang C. L. and Hu P.(2022). Regulation of muscle stem cell fate. Cell Regen 11(1): 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy D., Besnard A., Latil M., Jouvion G., Briand D., Thepenier C., Pascal Q., Guguin A., Gayraud-Morel B., Cavaillon J. M., et al.(2016). Comparative Study of Injury Models for Studying Muscle Regeneration in Mice. PLoS One 11(1): e0147198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvey A. L., Marshall R. J. and Karlsson E.(1982). Effects of purified cardiotoxins from the Thailand cobra(Naja naja siamensis) on isolated skeletal and cardiac muscle preparations. Toxicon 20(2): 379-396. [DOI] [PubMed] [Google Scholar]
- 7.Hawke T. J. and Garry D. J.(2001). Myogenic satellite cells: physiology to molecular biology. J Appl Physiol(1985) 91(2): 534-551. [DOI] [PubMed] [Google Scholar]
- 8.Konigsberg U. R., Lipton B. H. and Konigsberg I. R.(1975). The regenerative response of single mature muscle fibers isolated in vitro. Dev Biol 45(2): 260-275. [DOI] [PubMed] [Google Scholar]
- 9.Mahdy M. A., Lei H. Y., Wakamatsu J., Hosaka Y. Z. and Nishimura T.(2015). Comparative study of muscle regeneration following cardiotoxin and glycerol injury. Ann Anat 202: 18-27. [DOI] [PubMed] [Google Scholar]
- 10.Mauro A.(1961). Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9(2): 493-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C., Yue F. and Kuang S.(2017). Muscle Histology Characterization Using H&E Staining and Muscle Fiber Type Classification Using Immunofluorescence Staining. Bio Protoc 7(10): e2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou S., Zhang W., Cai G., Ding Y., Wei C., Li S., Yang Y., Qin J., Liu D., Zhang H., et al.(2020). Myofiber necroptosis promotes muscle stem cell proliferation via releasing Tenascin-C during regeneration. Cell Res 30(12): 1063-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]