Abstract
Objective:
Rheumatoid arthritis (RA) is a disabling inflammatory disorder. Ginger is used for food and medicine to treat arthralgia, sprains, and muscle aches. Anti-inflammatory effects of ginger have been observed. The aim of our study was to detect the effects of ginger on experimentally induced inflammatory arthritis.
Methods:
Female Wistar albino rats (n = 21) were randomly separated into three groups (control, arthritis, and arthritis + ginger). Arthritis was generated by an appropriate method using type 2 collagen and Freund's adjuvant (collagen-induced arthritis model). The ginger group was treated starting at the first collagen injection with ginger root extract for 32 days by oral gavage (50 mg/kg/daily). Interleukin (IL)-6, IL-17, tumor necrosis factor-α (TNF-α), sclerostin, dickkopf-related protein-1 (DKK-1), and obestatin serum levels were studied by enzyme-linked immunosorbent assay method. Tissue TNF-α, IL-17, cyclooxygenase-2 (COX-2), and nuclear factor kappa B (NF-κB) levels were detected using the Western blot method.
Results:
Mean arthritis score and serum levels of TNF-α, IL-6, and IL-17 were significantly decreased in ginger group than in the arthritis group. Increased sclerostin serum level and decreased DKK-1 serum levels were detected in ginger group compared with arthritis group. The decreases of IL-17, TNF-α, COX-2, and NF-κB tissue levels were statistically significant in the ginger group compared with arthritis group. Histopathological evaluation of the ginger group showed a decrease in the inflammation score compared to arthritis group.
Conclusion:
It can be concluded that ginger has protective properties in the development of inflammatory arthritis. The antiarthritic acts of ginger are related to NF-κB activity and Wnt pathway. Thus, it may be suggested that ginger is a candidate to research in human RA treatment.
Keywords: Ginger, arthritis, inflammatory markers, cytokines, Wnt pathway
Introduction
Rheumatoid arthritis (RA), a rheumatic inflammatory disorder, causes disability, arthritis, and joint damage through chronic inflammation. 1 Many cytokines especially interleukin (IL)-1, IL-6, and tumor necrosis factor-alpha (TNF-α) have crucial pathogenic roles on RA. 2 Nuclear factor kappa B (NF-κB) manages the activity of many genes coding cytokines, acute phase reactants, adhesion molecules, monocyte chemoattractant protein-1, cyclooxygenase-2 (COX-2), and lymphotoxins. The activation of NF-κB/Rel transcriptional family through the translocation of cytoplasmic complexes to the nucleus acts a central role in the inflammation as a result of the transcription of proinflammatory genes. 3
The Wnt signaling pathway has important actions in bone homeostasis. 4 Dickkopf-related protein (DKK)-1 and sclerostin are extracellular proteins related to the Wnt signaling pathway. 5 Sclerostin, a glycoprotein, is released from mature osteocytes and inhibits osteoblastogenesis. Sclerostin levels have documented to be increased in a cohort including patients with RA. 6 In another article, DKK-1 has shown to be an independent predictor of structural damage in RA. In addition, increased DKK-1 levels have been shown to be correlated with disease activity, increased acute phase reactants, and bone resorption in RA. 7
Ginger is used in preparing vegetables and meat, and it is used as an aroma enhancer in many foods and beverage preparation. The roots of the plant have been used for more than 2,500 years in western and southern Asian medicine. 8,9 Many studies have shown the antioxidative activity of ginger. 10 The ginger components gingerols and shogaolsin have been shown to inhibit arachidonic acid metabolism and prostaglandin synthesis due to their effects on COX enzymes. 11,12 It has been shown that ginger inhibits the releases of inflammatory cytokines and chemokines from inflammatory cells, synoviocytes, and chondrocytes. 11 Ribel-Madsen et al. 13 have reported that ginger exhibits effective anti-inflammatory properties as betamethasone in the secretions of IL-6, IL-1β, and IL-8 in an in vitro study of synovial cell cultures harvested from RA and osteoarthritis patients. 13
In this study, we aimed to ascertain potential actions of ginger on the inflammatory and metabolic factors which are effective in the pathogenesis of RA, in an experimental inflammatory arthritis model (CIA: collagen-induced arthritis).
Methods
This study was performed at the Fırat University Experimental Research Center with an approval from the Fırat University Ethical Committee for Animal Experiments. In the experiments, 21 female rats (Wistar albino: weighing 180-200 g and 8 weeks old) were used. Three study groups were defined: (1) control group, (2) arthritis group, and (3) arthritis + ginger group. The rats were fed in special cages with standard rat feed. Rats left in 12 hours light and 12 hours dark place in an environment with a ventilation system at a relative humidity of 55 ± 5% and a room temperature of 21-24 °C.
Arthritis induction
Type II collagen (Sigma–Aldrich, Saint Louis, MO, USA) was solubilized (1 mg/mL) with acetic-acid (0.1 M) to produce experimental arthritis. The prepared collagen was emulsified equally proportion with Freund's adjuvant (Difco Laboratories, Detroit, MI, USA). The prepared mixture was injected by intradermal way (total: 200 µg) to each rat in the arthritis and arthritis + ginger groups for the induction to arthritis from dorsum of tail (100 µg for each rat) and hind paws (50 µg for each paw of each rat). Booster injections of same mixture (100 µg for each rat) were applied to the tail dorsal at 7 days after the first administration. 14
Main Points
Ginger has protective properties in the development of inflammatory arthritis.
Ginger affects NF-κB and Wnt pathway in inflammatory arthritis.
Ginger is a candidate to research in rheumatoid arthritis.
After the first collagen injection, all enrolled rats were examined for the occurrence of arthritis according to the clinical arthritis scoring described previously (Table 1). 15
Table 1.
Clinically arthritis scoring.
| Findings | Score |
| No arthritis | 0 |
| Slight erythema and edema in the foot or ankle | 1 |
| Mild erythema and edema of the paw | 2 |
| Moderate erythema and edema in the paw | 3 |
| Severe edema and ankylosis, limitation of movement in the paw | 4 |
Ginger supplement
Ginger extract was obtained from Gingever ([item code: 480006025], OmniActive Health Technologies, Morristown, NJ, USA). The ginger group was treated starting at the first collagen injection with ginger root extract for 32 days by oral gavage (the dose of ginger was 50 mg/kg/day). 16
Sample collection
This study was terminated on the 33rd day, and all rats were decapitated. Harvested blood samples were centrifuged (5,000 rpm/5 min). The collected sera were kept at –20 °C until analysis. The hind paws were harvested precipitately from all rats for further Western blotting and histopathological analysis. Harvested tissue samples were divided into two parts: one part was inserted in formalin solution (10%) for later histopathological evaluations and other part was quickly frozen at –80 °C for later Western blotting analysis.
Histopathological examinations: Tissue samples fixed in solution of formalin were stored in 10% nitric acid (at least 30 days) for decalcification, and paraffin blocks were built after decalcification. Subsequently, sections from prepared paraffin blocks were stained with hematoxylin–eosin (H&E). A pathology specialist (I.H.O.) examined the sections (×20, × 40, × 100, × 200, and ×400 magnifications) to detect whether inflammatory cell infiltration of synovium, pannus formation in joints, and the destruction of bones and cartilages around the joints are present. Previously described histopathological scoring system was performed for these evaluations. 17,18
Biochemical analysis: Routine laboratory parameters (glucose, urea, creatinine, alanine aminotransferase, aspartate aminotransferase, and lipid profiles) from serum samples were researched by the standard biochemical analyzing system (Samsung Electronics, Suwon, South Korea). Enzyme-linked immunosorbent assay method was selected to measure IL-6, IL-17, TNF-α, sclerostin, DKK-1, and obestatin serum levels (Cayman Chemical, Ann Arbor, MI, USA).
Western blot: Joint tissue IL-17, TNF-α, COX-2, and NF-κB levels were analyzed using Western blotting. The joint tissue homogenates that were harvested from hind paws and rapidly frozen at –80 °C were prepared. The prepared tissue homogenates from joints were stored in ice-cold lysis buffer containing Tris–HCl (50 mM and pH, 8.0), ethylenediaminetetraacetic acid (5 mM), NaCl (150 mM), 1% triton X-100, 0.26% Na-deoxycholate, sodium fluoride (50 mM), phenyl-methyl-sulfonyl fluoride (50 µg/mL), b-glycero-phosphate (10 mM), Na-orthovanadate (0.1 mM), and leupeptin (10 µg/mL). Finally, prepared homogenates were stored on ice with 40 minutes duration. 17
The supernatant was added to a sample buffer of Na-dodecyl-sulfate-poly-acrylamide gel electrophoresis containing 2% b-mercapto-ethanol. A 20 µg protein was electrophoresed and then subsequently transferred to nitrocellulose blots (Schleicher & Schuell, Keene, NH, USA). The prepared nitrocellulose blots were washed with phosphate-buffered saline (PBS). Washed nitrocellulose was blocked with 1% bovine serum albumin (diluted in PBS) with the duration of 1 hour until administration of the primary antibodies. Primary antibodies against IL-17, TNF-α, COX-2, and NF-κB (Abcam, Cambridge, England) were diluted (1: 1,000) in 0.05% tween-20. Subsequently, the nitrocellulose blots were incubated with primary antibody at 4 °C overnight. The blots were applied by horseradish peroxidase conjugated with goat antimouse immunoglobulin G (Abcam, Cambridge, England). Specific binding was analyzed using hydrogen-peroxide and di-amino-benzidine. The check for protein loading was performed using the monoclonal β-actin mouse antibody (Sigma-Aldrich, Saint Louis, MO, USA). Measurements were performed three times for the confirmation of the results, and their densitometric analysis performed on an image analysis system (Image J, Bethesda, MD, USA).
Statistical analysis
The sample size of the present study was seven per group, and it was calculated based on a power of 85% and a P value of .05. All analyses were performed using the general linear model procedure. Differences among groups were analyzed by Kruskal–Wallis variance analysis and post hoc Mann–Whitney U test. Statistical analysis was performed using the IBM-SPSS software, version 21.0 (IBM Corp., Armonk, NY, USA). Values of P < .05 were considered as statistically significant.
Results
Arthritis developed in the rats at 13-15 days after the first collagen injections. The mean values of arthritis score on the 15th day and 33rd day, respectively, were 1.86 ± 0.34 and 3.71 ± 0.18 in the arthritis group and 0.43 ± 0.20 and 0.86 ± 0.26 in the ginger group (Table 2). Mean arthritis scores of 15th and 33rd days were significantly lower in the ginger group compared with arthritis group (P < .01 and P < .001, respectively).
Table 2.
The effect of ginger extracts on arthritis scores and biochemical parameters in rats with collagen-induced arthritis.
| Items | Control | Arthritis | Ginger |
| Fifteenth day arthritis score | – | 1.86 ± 0.34 | 0.43 ± 0.20a |
| Thirty-third day arthritis score | – | 3.71 ± 0.18 | 0.86 ± 0.26b |
| GLU, mg/dL | 103.14 ± 3.71 | 104.29 ± 2.50 | 102.00 ± 3.71 |
| ALT, U/L | 62.57 ± 6.34 | 65.14 ± 5.87 | 61.57 ± 7.18 |
| AST, U/L | 356.86 ± 25.00 | 350.00 ± 26.68 | 361.14 ± 29.87 |
| CREA, mg/dL | 2.03 ± 0.15 | 1.97 ± 0.15 | 1.98 ± 0.16 |
| CHOL, mg/dL | 101.71 ± 3.33 | 99.71 ± 2.56 | 98.29 ± 4.65 |
| TRIG, mg/dL | 123.71 ± 6.29 | 121.86 ± 3.80 | 120.57 ± 10.18 |
| HDL, mg/dL | 22.86 ± 1.49 | 21.71 ± 1.39 | 23.00 ± 1.60 |
| LDL, mg/dL | 48.00 ± 2.48 | 47.14 ± 3.00 | 46.43 ± 4.50 |
GLU, glucose; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CREA: creatinine; CHOL, cholesterol; TRIG, triglycerides; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
The data presented as mean ± standard error.
Mean values within same row are statistically different for aP < .01, bP < .001 as compared with the arthritis group.
Serum IL-6 levels were higher in the arthritic group compared with control group (P < .001). In the ginger group, the serum IL-6 level was lower than the arthritis group (P < .001) (Table 3). Similarly, serum TNF-α and IL-17 levels were statistically significantly higher in the arthritis group when compared with the control group (P < .001 for both). Conversely, serum TNF-α and IL-17 levels were statistically significantly lower in the ginger group than in the arthritis group (P < .001 for both).
>Table 3.
The effect of ginger extracts on serum IL-6, TNF-α, obestatin, sclerostin, and DKK-1 levels in rats with collagen-induced arthritis.
| Items | Control | Arthritis | Ginger |
| IL-6, pg/mL | 11.14 ± 1.16 | 49.43 ± 3.97c | 23.29 ± 2.50a,f |
| IL-17, pg/mL | 23.57 ± 1.72 | 58.14 ± 4.44c | 34.00 ± 1.05a,f |
| TNF-α, pg/mL | 26.00 ± 1.91 | 60.43 ± 2.66c | 37.85 ± 1.79b,f |
| Obestatin, pg/mL | 265.57 ± 11.42 | 123.00 ± 3.71c | 140.71 ± 3.55c |
| Sclerostin, ng/mL | 0.51 ± 0.03 | 0.22 ± 0.02c | 0.33 ± 0.02c,d |
| DKK-1, pg/mL | 587.43 ± 27.95 | 1707.14 ± 31.22c | 1298.57 ± 61.26c,e |
IL-6, interleukin; TNF-α, tumor necrosis factor alpha; DKK-1, dickkopf-related protein 1.
Data are means ± standard error.
aP < .05, bP < .01, cP < .001 as compared with control group; dP < .05, eP < .01, fP < .001 as compared with arthritis group.
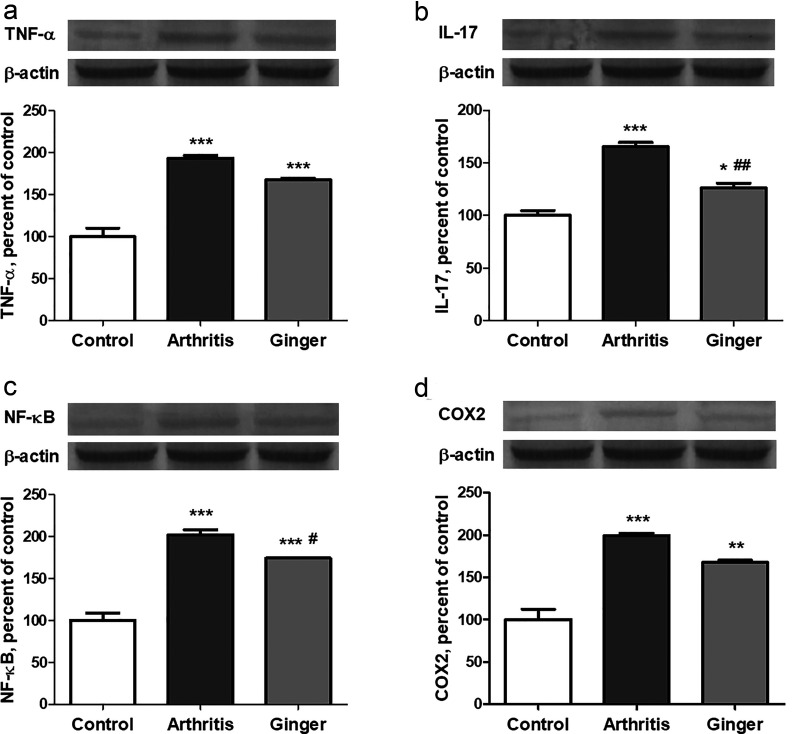
Tissue TNF-α and IL-17 levels were statistically significantly higher in the arthritis group compared with the control group (P < .001 for both). Tissue TNF-α level was relatively decreased (P > .05), while tissue IL-17 level was statistically significantly decreased (P < .01), in the ginger group compared with the arthritis group (Figure 1).
Figure 1.
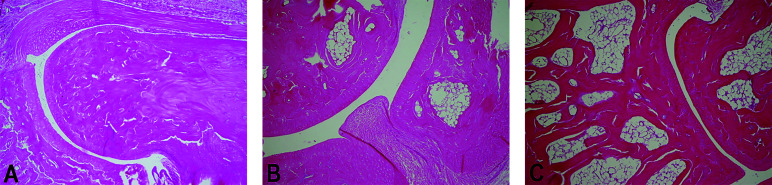
The effect of ginger extracts on histopathological changes (H&E × 200) in rats with collagen-induced arthritis. (a) Normal perisynovial tissue and cartilage-bone appearance in the control group. (b) Obvious perisynovial inflammation and destruction of cartilage-bone in the arthritis group. (c) Decreased perisynovial inflammation and synovial hyperplasia in the ginger group.
Tissue NF-κB and COX-2 levels were significantly higher in the arthritis group than in control group (P < .001 for both). In the ginger group, tissue NF-κB level significantly decreased (P < .05) and tissue COX-2 level relatively (P > .05) decreased when compared with arthritis group (Figure 1).
Serum sclerostin level was significantly decreased in arthritic group when compared with control group (P < .001). The serum sclerostin level in the ginger group was higher when compared with arthritis group (P < .05). Unlike the sclerostin level, serum DKK-1 level was elevated in the arthritic group compared with the control group (P < .001) and lower in ginger group compared with arthritis group (P < .01) (Table 3). Serum obestatin level was decreased in arthritis group when compared to control group (P < .001). The increase in serum obestatin level in ginger group compared with arthritis group was not statistically significant (P > .05) (Table 3).
In the arthritis group, obvious inflammatory cell infiltrations in synovium and destructions of cartilages and bones were observed by histopathological examination (Figure 2). On the other hand, ginger supplement decreased perisynovial inflammation, synovial hyperplasia, and the destruction of cartilage-bone. Mean histopathological inflammation score was higher compared with the control group (P < .001). Conversely, it was lower in the ginger group compared with the arthritis group (P < .01).
Figure 2.
The effect of ginger extracts on protein levels of tumor necrosis factor alpha (TNF-α; a), interleukin 17 (IL-17; b), nuclear factor-kappa B (NF-κB; c), and cyclooxygenase-2 (COX-2; d) in rats with collagen-induced arthritis. The intensity of the bands was quantified by densitometric analysis, and β-actin was included to ensure equal protein loading. Data are expressed as percent of control value. Each bar represents the mean ± standard error. Blots were repeated at least three times. *P < .05, **P < .01, ***P < .001 as compared with control group; # P < .05, ## P < .01 as compared with arthritis group.
Discussion
RA which is a chronic and inflammatory disorder often results in disability and morbidity. T cells, B cells, macrophages, and synovial fibroblasts infiltrate synovial membrane and cause joint destruction in RA. 1,2 Cytokines (TNFα, IL-1, and IL-6) deriving from fibroblast-like synoviocytes and macrophages have been proposed as key mediators of RA. 2,19 Macrophages and fibroblast-like synoviocytes are sources of these proinflammatory cytokines, which serve as autologous stimuli of other cytokines. After the discovery of IL-17 producing CD4 T cells in RA synovium, it has been understood that Th17 cells are the ancestors of CD4+ T cells different from classical Th1 and Th2 cells, which play a role in various autoimmune and inflammatory disorders. 20,21 The potent arthritogenic effect of Th17 cells is mainly based on the pleiotropic effect of IL-17A acting on different cells forming the synovial tissue. 22,23 The IL-17 synergistically encompasses the production of several cytokines, including TNFα, IL-1, and IL-6, by acting on macrophages and synovial fibroblasts. 24 IL-17 also causes osteoclast differentiation leading to erosions on bones and destructions on cartilages. 25
In our study, serum TNF-α, IL-6, and IL-17 levels and tissue TNF-α and IL-17 levels were increased in the CIA model. In the ginger-treated group, it was observed that the clinical and histopathological findings of arthritis were regressed; moreover, serum TNF-α, IL-6, and IL-17 levels and tissue TNF-α and IL-17 levels were decreased. It can be argued that ginger reduces the expression of IL-17 by an inhibitory effect on Th17 cells. Thus, it can be achieved that synovial macrophages decrease the production of several cytokines such as TNF-α, IL-1, and IL-6.
Wnt/β-catenin signal pathway regulates various cell homeostasis processes such as differentiation, proliferation, migration, and adhesion. 4 Human and animal studies have shown that the classical Wnt signaling pathway controls the formation, repairing, and remodeling of bone by regulating osteoblast and osteoclast differentiation. 5 DKK-1 has shown to be an important regulator of joint remodeling in inflammatory arthritis in RA and to be associated with bone erosion. 26 In a study conducted, DKK-1 levels have been found to be high in active RA patients when compared with healthy controls. 27 Garnero et al. 28 have shown that the changes in serum DKK-1 levels could be a potential biomarker for disease activity and radiological progression. In addition, reduction in DKK-1 levels has been shown in patients treated with an inhibitor of TNF-α or IL-1. 29 In this study, the serum DKK-1 level was higher in arthritis group and lower in ginger-treated group. This finding indicates the possible effect of the ginger on the Wnt/ β-catenin pathway. In a previous study, ginger was shown to inhibit apoptosis in human meningioma cells via depletion of the Wnt/ β-catenin pathway, and treatment could be added to recurrent meningiomas. 30 In another study, ginger was seen as a potential therapeutic tool in inducing apoptosis through the inhibition of mTOR and Wnt/β catenin signaling pathway in colorectal cancer. 31
Sclerostin, a Wnt antagonist, has a role in local and systemic bone loss in RA. Restrictive repairing of bone erosion in RA appears to be associated with the induction of signals which can block bone formations. The sclerostin expression is induced by inflammation, and sclerostin suppresses bone formation, and thus inhibits the repairing of bone erosion. 6 In a study conducted by Lim et al., 32 serum C-terminal telopeptide of type 1 collagen and sclerostin levels after 12 weeks of treatment with etanercept have been shown to be statistically significantly increased compared to baseline. Increased sclerostin levels have been found to be positively correlated with CTX-1 and bone-specific alkaline phosphatase levels. 32 In our study, serum sclerostin level was decreased in the arthritis group and increased in the ginger group.
In a meta-analysis conducted by Shi et al. 33 to identify serum sclerostin level association in RA patients, serum sclerostin levels are not statistically different when compared with healthy controls. Mehaney et al. 34 have documented that serum sclerostin levels in RA have been similar in healthy controls and that sclerostin serum levels are not related to disease activity, bone mineral density, and radiological injury grade. Although some studies on sclerostin suggest that increased sclerostin levels are related to restructuring bone metabolism, we think that detailed studies should be done because of conflicting results. However, we conclude that DKK-1 and sclerostin levels, which are altered by ginger administration, may have protective actions on joint destruction and bone remodeling by acting on the Wnt/β-catenin pathway rather than the effects of ginger on RA pathogenesis.
In our study, tissue NF-κB level was increased in rats with inflammatory arthritis and decreased in ginger supplied rats. Previous scientific investigations have shown that NF-κB activation increases much earlier than the development of clinical arthritis. 35 In our study, decreased NF-κB activity in ginger-supplied rats compared with inflammatory arthritis rats could be thought to prevent or delay the development of arthritis by decreasing proinflammatory cytokine-chemokine levels. It can also be used to explain the positive effects of ginger on inflammatory arthritis pathogenesis.
Ginger, a plant originated in Southeast Asia, has been used for food and medicine since antiquity. 8 There has recently been increased attention to the use of ginger as an herbal dietary supplement, especially in the treatment of chronic inflammatory conditions. 36 Ginger's anti-inflammatory properties have been documented in many studies. 37,38 In several studies, it has been shown that ginger inhibits chemokine and cytokine expressions by cells in in-vitro settings. 37,38 Tzeng et al. 39 have investigated the protective role of subtropical ginger, a so-called zerumbone, on hyperglycemia-induced phytochemically induced retinal damage in experimentally induced diabetic rats. Zerumbone has been shown to improve diabetes-induced TNF-α, IL-1, and IL-6 up-regulations. Moreover, zerumbone has been shown to inhibit vascular endothelial growth factor and intracellular adhesion molecule-1 overexpressions, to suppress NF-κB expression, and to prevent apoptosis in retinal cells. 39 Zehsaz et al. 40 have investigated the effects of ginger on the levels of cytokines on well-trained male endurance runners. In this study, plasma levels of TNF-α, IL-1β, and IL-6 collected at postexercise period have been found to be lower in the ginger-administered runners than in controls. 40 Hoseinzadeh et al. 41 have shown that healthy women volunteers using the ginger extract 1 hour before exercise have reduced muscle pain and IL-6 levels compared with the placebo group.
As a result, our study suggests that ginger has protective properties in the development of inflammatory arthritis. In addition, the ginger affects the cytokine levels, tissue NF-κB, COX-2 levels, and Wnt pathway in the CIA model. Promoting consumption of ginger as a dietary supplement, which has been used since ancient times, can be considered to have a positive effect on inflammatory arthritis specimens such as RA. Although it is a very ambitious opinion, community-based broad-based work is needed to support this hypothesis.
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Animal Care of Fırat University (Approval number: 2016/15-147).
Informed Consent: Informed consent was not obtained due to the nature of this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.S.K.; Design - K.Ş.; Materials - V.J.; Data Collection and/or Processing - C.O.; Analysis and/or Interpretation - M.T., N.Ş., İ.H.Ö., P.D.Ö.; Literature Search - S.S.K.; Writing - B.Ö., S.S.K.; Critical Review - S.S.K.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature . 2003;423(6937):356–61.. [DOI] [PubMed] [Google Scholar]
- Feldmann M,, Brennan FM, Maini R. Cytokines in autoimmune disorders. Int Rev Immunol . 1998;17(1-4):217–28.. [DOI] [PubMed] [Google Scholar]
- Baldwin Jr AS. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol . 1996;14:649–83.. [DOI] [PubMed] [Google Scholar]
- Pinzone JJ, Hall BM, Thudi NK, et al. The role of dickkopf-1 in bone development, homeostasis, and disease. Blood . 2009; 113(3): 517–25.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli C, Piemontese M, Lumetti S, et al. The importance of WNT pathways for bone metabolism and their regulation by implant topography. Eur Cell Mater . 2012;24:46–59.. [DOI] [PubMed] [Google Scholar]
- Matzelle MM, Gallant MA, Condon KW, et al. Resolution of inflammation induces osteoblast function and regulates the Wnt signaling pathway. Arthritis Rheum . 2012;64(5):1540–50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seror R, Boudaoud S, Pavy S, et al. Increased dickkopf-1 in recent-onset rheumatoid arthritis is a new biomarker of structural severity. Sci Rep . 2016;6:18421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal M, Al-Hadidi D, Menon M, et al. Ginger: an ethnomedical, chemical and pharmacological review. Drug Metabol Drug Interact . 2001; 18(3-4): 159–90. [DOI] [PubMed] [Google Scholar]
- Grant KL, Lutz RB. Ginger. Am J Health Syst Pharm . 2000;57(10):945–7.. [DOI] [PubMed] [Google Scholar]
- Promthep K, Eungpinichpong W, Sripani-dkulchai B, Chatchawan U. Effect of kaempferia parviflora extract on physical fitness of soccer players: a randomized double-blind placebo-controlled trial. Med Sci Monit Basic Res . 2015;21:100–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale roscoe): a review of recent research. Food Chem Toxicol . 2008;46(2): 409–20.. [DOI] [PubMed] [Google Scholar]
- Black CD, Herring MP, Hurley DJ, O’Connor PJ. Ginger (Zingiber officinale) reduces muscle pain caused by eccentric exercise. J Pain . 2010; 11(9):894–903.. [DOI] [PubMed] [Google Scholar]
- Ribel-Madsen S, Bartels EM, Stockmarr A, et al. A synoviocyte model for osteoarthritis and rheumatoid arthritis: response to ibuprofen, betamethasone, and ginger extract—a cross-sectional in vitro study. Arthritis . 2012;2012:1–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med . 1977;146(3):857–68.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson P, Kleinau S, Holmdahl R, Klareskog L. Homologous type II-collagen-induced arthritis in rats. Characterization of the disease and demonstration of clinically distinct forms of arthritis in two strains of rats after immunization with the same collagen preparation. Arthritis Rheum . 1990;33(5):693–701.. [DOI] [PubMed] [Google Scholar]
- Shrivastava S. The influence of gingerol treatment on aluminum toxicity in rats. J Environ Pathol Toxicol Oncol . 2015;34(1):11–21.. [DOI] [PubMed] [Google Scholar]
- Choi J, Yoon BJ, Han YN, et al. Antirheumatoid arthritis effect of Rhus verniciflua and of the active component, sulfuretin. Planta Med . 2003;69(10):899–904.. [DOI] [PubMed] [Google Scholar]
- Barsante MM, Roffê E, Yokoro CM, et al. Anti-inflammatory and analgesic effects of atorvastatin in a rat model of adjuvant-induced arthritis. Eur J Pharmacol . 2005;516(3):282–9.. [DOI] [PubMed] [Google Scholar]
- Udalova IA, Mantovani A, Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol . 2016;12(8):472–85.. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4 + effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol . 2005;6(11):1123–32.. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol . 2005;6(11):1133–41.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P. Interleukin-17 in rheumatoid arthritis: if T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum . 2003;48(3):594–601.. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity . 2004;21(4):467–76.. [DOI] [PubMed] [Google Scholar]
- Ogura H, Murakami M, Okuyama Y, et al. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity . 2008;29(4):628–36.. [DOI] [PubMed] [Google Scholar]
- Takayanagi H. New developments in osteoimmunology. Nat Rev Rheumatol . 2012;8(11):684–9.. [DOI] [PubMed] [Google Scholar]
- Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med . 2007;13(2):156–63.. [DOI] [PubMed] [Google Scholar]
- Wang SY, Liu YY, Ye H, et al. Circulating dickkopf-1 is correlated with bone erosion and inflammation in rheumatoid arthritis. J Rheumatol . 2011;38(5):821–7.. [DOI] [PubMed] [Google Scholar]
- Garnero P, Tabassi NC, Voorzanger-Rousselot N. Circulating Dickkopf-1 and radiological progression in patients with early rheumatoid arthritis treated with etanercept. J Rheumatol . 2008;35(12):2313–5.. [DOI] [PubMed] [Google Scholar]
- Adami G, Orsolini G, Adami S, et al. Effects of TNF inhibitors on parathyroid hormone and Wnt signaling antagonists in rheumatoid arthritis. Calcif Tissue Int . 2016;99(4):360–4.. [DOI] [PubMed] [Google Scholar]
- Das A, Miller R, Lee P, et al. A novel component from citrus, ginger, and mushroom family exhibits antitumor activity on human meningioma cells through suppressing the Wnt/β-catenin signaling pathway. Tumor Biol . 2015; 36(9):7027–34.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee LH, Morad NA, Aan GJ, et al. Mechanism of chemoprevention against colon cancer cells using combined Gelam honey and ginger extract via mTOR and wnt/β-catenin pathways. Asian Pac J Cancer Prev . 2015;16(15):6549–56.. [DOI] [PubMed] [Google Scholar]
- Lim MJ, Kwon SR, Joo K, et al. Early effects of tumor necrosis factor inhibition on bone homeostasis after soluble tumor necrosis factor receptor use. Korean J Intern Med . 2014; 29(6):807–13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Ying H, Du J, Shen B. Serum sclerostin levels in patients with ankylosing spondylitis and rheumatoid arthritis: a systematic review and meta-analysis. Biomed Res Int . 2017;2017: 9295313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehaney DA, Eissa M, Anwar S, Fakhr El-Din S. Serum sclerostin level among Egyptian rheumatoid arthritis patients: relation to disease activity, bone mineral density and radiological grading. Acta Rheumatol Port . 2015;40(3): 268–74.. [PubMed] [Google Scholar]
- Han Z, Boyle DL, Manning AM, Firestein GS. AP-1 and NF-kappaB regulation in rheumatoid arthritis and murine collagen-induced arthritis. Autoimmunity . 1998;28(4):197–208.. [DOI] [PubMed] [Google Scholar]
- Srivastava KC. Aqueous extracts of onion, garlic and ginger inhibit platelet aggregation and alter arachidonic acid metabolism. Biomed Biochim Acta . 1984;43(8-9):335–46.. [PubMed] [Google Scholar]
- Ojewole JA. Analgesic, antiinflammatory and hypoglycaemic effects of ethanol extract of Zingiber officinale (Roscoe) rhizomes (Zingiberaceae) in mice and rats. Phytother Res . 2006; 20(9):764–72.. [DOI] [PubMed] [Google Scholar]
- Kim HW, Murakami A, Abe M, et al. Suppressive effects of mioga ginger and ginger constituents on reactive oxygen and nitrogen species generation, and the expression of inducible pro-inflammatory genes in macrophages. Antioxid Redox Signal . 2005; 7(11-12): 1621–9.. [DOI] [PubMed] [Google Scholar]
- Tzeng TF, Liou SS, Tzeng YC, Liu IM. Zerumbone, a phytochemical of subtropical ginger, protects against hyperglycemia-induced retinal damage in experimental diabetic rats. Nutrients . 2016;8(8):E449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehsaz F, Farhangi N, Mirheidari L. The effect of Zingiber officinale R. rhizomes (ginger) on plasma pro-inflammatory cytokine levels in well-trained male endurance runners. Cent Eur J Immunol . 2014;39(2):174–80.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoseinzadeh K, Daryanoosh F, Baghdasar PJ, Alizadeh H. Acute effects of ginger extract on biochemical and functional symptoms of delayed onset muscle soreness. Med J Islam Repub Iran . 2015;29:261. [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a