Abstract
Microplastics represent an emerging environmental contaminant, with large gaps in our understanding of human health impacts. Furthermore, environmental factors may modify the plastic chemistry, further altering the toxic potency. Ultraviolet (UV) light is one such unavoidable factor for airborne microplastic particulates and a known modifier of polystyrene surface chemistry. As an experimental model, we aged commercially available polystyrene microspheres for 5 weeks with UV radiation, then compared the cellular responses in A549 lung cells with both pristine and irradiated particulates. Photoaging altered the surface morphology of irradiated microspheres and increased the intensities of polar groups on the near-surface region of the particles as indicated by scanning electron microscopy and by fitting of high-resolution X-ray photoelectron spectroscopy C 1s spectra, respectively. Even at low concentrations (1–30 µg/ml), photoaged microspheres at 1 and 5 µm in diameter exerted more pronounced biological responses in the A549 cells than was caused by pristine microspheres. High-content imaging analysis revealed S and G2 cell cycle accumulation and morphological changes, which were also more pronounced in A549 cells treated with photoaged microspheres, and further influenced by the size, dose, and time of exposures. Polystyrene microspheres reduced monolayer barrier integrity and slowed regrowth in a wound healing assay in a manner dependent on dose, photoaging, and size of the microsphere. UV-photoaging generally enhanced the toxicity of polystyrene microspheres in A549 cells. Understanding the influence of weathering and environmental aging, along with size, shape, and chemistry, on microplastics biocompatibility may be an essential consideration for incorporation of different plastics in products.
Keywords: plastics, photoaging, cytotoxicity, high-content imaging, particles, airway, lung
Global accumulation of plastic waste has led to the ubiquitous presence of degraded microparticles that have incompletely understood impacts on ecological and human health (Larue et al., 2021). It is currently estimated that one metric ton of plastic waste exists for every human on earth (Geyer et al., 2017). Microplastics vary dramatically in chemical composition, size, and shape; all of these factors can affect exposure, local or systemic uptake, and cellular and molecular interactions with the plastics (Brachner et al., 2020; Shupe et al., 2021; Wang et al., 2021a; Yong et al., 2020). Toxicological studies have only been conducted in recent years, and the factors that influence adverse health outcomes from microplastics exposures remain poorly characterized (Yong et al., 2020). Most research to date has been conducted on freshly manufactured materials, and limited shapes and types of plastics have been examined in this context. Plasticizers, such as bisphenols and phthalates (Sorensen et al., 2021), may also leach from microplastics to impact organisms (Liu et al., 2021). Furthermore, plastics in the environment are exposed to varying conditions of temperature, humidity, microbes, and sunlight (Brachner et al., 2020; Guo and Wang, 2019). Environmental weathering (ie, photooxidation and mechanical stress) was shown to induce physical degradation and chemical changes in commodity plastics, releasing smaller particles called secondary microplastics (Akanyange et al., 2021; Andrady, 2017; Roblin et al., 2020; Song et al., 2017). How such environmental factors modify the biological interactions with—and health risks of—microplastics remains unclear.
Microplastics can be emitted into the air or resuspended by wind, posing a specific risk as an inhaled pollutant. Inhaled carbonaceous particulates, broadly speaking, are clearly linked with respiratory, cardiovascular, neurological, and gestational adverse outcomes (Garcia et al., 2021; Pope et al., 2004; Volk et al., 2013). Definitive evidence of plastics contamination in the ambient airshed exists (Cox et al., 2019); however, accurate quantitation is hampered by technical challenges of detection amidst particles made of elemental and organic carbon and other major crustal elements (Ca, Si, Fe), especially for sizes below 1 µm (Kelly and Fussell, 2020; Wang et al., 2021b). Regardless, studies have documented the presence of airborne microplastics and, given the concerns for the unabated growth of plastics waste, they clearly represent an emerging human exposure (Cox et al., 2019; Dris et al., 2017; Liu et al., 2021). Potential negative effects of plastic particles exposure have been reported in limited toxicity studies showing different patterns of cellular uptake, reactive oxygen species production and pro-inflammatory responses with respect to particles type/size, and human cell lines (Dong et al., 2020; Schirinzi et al., 2017; Stock et al., 2019; Yong et al., 2020). Understanding how the physicochemical properties of plastic particles under environmental weathering (eg, photooxidation) affect the mechanisms of cellular toxicity and biocompatibility is important to identify the potential health risks.
Airborne microplastics released in the environment are routinely exposed to the ultraviolet (UV) radiation from sunlight prior to inhalation, potentially impacting the toxicity. We investigated how this inevitable environmental factor could alter the surface chemistry and toxicity of polystyrene microspheres, as a consistent experimental model. Polystyrene represents one of the most manufactured polymers and is commonly reported in airborne microplastics composition (Dong et al., 2020; Liao et al., 2021; Sangkham et al., 2022). We used different size distributions (1 µm, 5 µm, and a mixture of 1 and 5 µm) and dose exposures (1–30 µg/l) that are commonly reported and examined in airborne microplastics and other environmental exposure studies (Liao et al., 2021; Sangkham et al., 2022). While environmental microplastics represent a wide mixture of materials with varied chemical composition, size, shape, and condition, the investigation of specific impacts conferred by photoaging required the use of a consistent model system in a well-established human airway cell line, A549 cells.
Materials and methods
Polystyrene microspheres
In total, 1 and 5 µm polystyrene microspheres were purchased (Degradex by Phosphorex, Hopkinton, MA) and either stored in the original packaging/solution (0.1% tween in water) or pipetted onto a glass microscope slide and placed in a biosafety cabinet with a UV lamp (UVC with 40 µW/cm2 light intensity) turned on continuously for 5 weeks. The duration of UV exposure was based on previous physicochemical studies that showed a prominent impact on polystyrene particles from an exposure time of 800 h (Meides et al., 2021). Prior to use, the UV-aged microspheres were resuspended in the storage vehicle and stored at 4°C under dark conditions. “Fresh” or “pristine” microspheres were purchased at the same time, but maintained in dark, cold (4°C) conditions until use. The polystyrene microspheres were washed by successive centrifugation steps and diluted in cell media to be prepared for cells exposure experiments. The stability and the size of polystyrene microspheres in suspension were tested in F-12K cell culture media by measuring the hydrodynamic diameter by dynamic light scattering (DLS) at 37°C for 72 h. In total, 30 μg/ml of particles suspension was tested as it corresponds to the highest concentration used in our experiments. DLS measurements were conducted to the 1 μm microspheres (fresh and aged), as this measurement is mainly considered accurate for particles with a hydrodynamic diameter ≤1 μm. The surface charge of microspheres (fresh and aged) was determined by zeta potential analysis. Hydrodynamic size and zeta potential data were acquired on a Malvern Zetasizer Nano-ZS equipped with a He-Ne laser (633 nm) and non-invasive backscatter optics.
Solid analyses
The surface physicochemical changes between the fresh and UV-aged polystyrene were studied by SEM, X-ray photoelectron spectroscopy (XPS), and Raman spectroscopy. SEM images were obtained using TESCAN Vega3 using an accelerating voltage of 10–15 kV and beam currents of 1–4 nA. XPS measurements were performed on a Kratos Ultra DLD spectrometer using a monochromatic Al Kα source operating at 150 W (1486.6 eV). The operating pressure was 5 × 10−9 Torr. Charge compensation was accomplished using low-energy electrons. All spectra were charged referenced by adjusting the C 1s region to 285 eV. Survey spectra and high-resolution C 1s spectra were acquired at a pass energies of 160 and 20 eV, respectively. XPS data were processed using Casa XPS software. Raman measurements were made using a WITec Alpha 300R Confocal Raman microscope with a 532 nm laser.
High-content imaging
A549 cells (ATCC, cat#CCL-185), chosen for their wide utilization as model airway epithelial cells in the recent toxicity studies of microplastics and particulates (El Hayek et al., 2021; Goodman et al., 2021; Shi et al., 2021; Zhu et al., 2020), grown in F-12K media (Kaighn’s Modification of Ham’s F-12 medium) with 10% fetal bovine serum and Penn/Strep, cultured at 37°C and 5% CO2, were harvested and resuspended in media containing 10% serum at 1.6 × 105 cells/ml at 300 µl/well in three 96-well plates (Costar 3599) and placed in the incubator (37°C, 5% CO2) overnight. Cells were treated with fresh or UV-aged 1 µm and 5 µm polystyrene spheres at 0, 1, 3, 10, and 30 µg/ml (n = 5 wells/condition on each plate). An additional 8 wells/plate were treated with Cadmium (CdCl2, anhydrous, ACS, 99.0% min, Thermo Scientific) at 2.5 µg/ml as positive control. Plates were incubated at 37°C for 24, 48, or 72 h. At each timepoint, the appropriate plate was removed from the incubator, wells were washed with phosphate-buffered saline (PBS), and then fixed with 4% formaldehyde for 20 min at 22°C.
High-content assays (HCAs), including nuclear morphology, cell cycle, DNA damage responses (phosphorylated H2A histone family member X, γ-H2AX), and perturbation of the cytoskeleton barrier, were conducted as previously reported (Liang et al., 2017; Yin et al., 2020). Briefly, after washing twice with PBS-Tween 20, the cells were permeabilized in PBS supplemented with 0.1% Triton X-100 and blocked with bovine serum albumin (BSA) blocking buffer. The cells were then incubated with primary mouse anti-phospho-histone H2AX (Ser139) (γ-H2AX; Developmental Studies Hybridoma Bank, Iowa, IA), mouse anti-alpha tubulin, and rabbit anti-focal adhesion kinase (FAK) (Proteintech, Rosemont, IL) in PBS/BSA/0.5% Tween-20 overnight at 4°C. After washing twice with PBS-Tween 20, the cells were incubated with matching species of secondary antibodies, Dylight conjugated 488, 650, and 550 (Thermo Scientific, Waltham, Massachusetts). The cell nuclei were stained with Hoechst 33342 (Thermo Scientific) and F-actin filaments were stained with Alexa Fluor 488 Phalloidin (Cell Signaling, Danvers, Massachusetts).
Multi-channel images were then automatically acquired using a Laser-based confocal CX7 LZR High-Content Screening (HCS) Platform with the HCS Studio 4.0 software (Thermo Scientific). Forty-nine fields (images) per well, per channel were acquired at ×20 magnification. Multiple parameters of nuclei were quantified for each cell, including nuclei number, nuclear area, shape, and total intensity. The total and average intensities of γ-H2AX, and F-actin, FAK, and alpha-tubulin of the individual cells were quantified. With 49 images per well, at least 8000 cells were analyzed, and single cell-based data were extracted for further nuclear morphology, cell cycle, DNA damage response, and cytoskeletal analysis. The data obtained from the HCS Studio 4.0 Target Activation BioApplication Analyst were exported and further analyzed using the JMP statistical analysis package (SAS Institute, North Carolina). To account for intra-plate normalization, data were normalized to the overall scaling factors, which were calculated by obtaining the geometric mean of the vehicle controls in each plate. The data extracted from the single-cell-based image represented the phenotype of a single cell and were geometrically averaged for each field for a traditional field-based statistical analysis. We used the heavy metal cadmium (Cd) at 2.5 µM as a positive control toxicant.
HCA-based cell cycle analysis was conducted as previously reported (Liang et al., 2017; Yin et al., 2020). The distributions of total DNA intensity of all individual nuclei were calculated and plotted for each experimental condition in a custom script written in Python 3.5.2 (Python Software Foundation, Oregon). Cell cycle profiles with discrete sub-G1 (apoptotic cells), G0/1, S, or G2/M populations in the controls and treatments were determined by selecting appropriate thresholds of these gates (Liang et al., 2017).
Metabolic assessment
Changes in basal glycolytic rate were measured in A549 cells (N = 6 wells/dose) treated overnight with 0, 1, 10, or 20 µg/ml of fresh and UV-aged 1 µm polystyrene microplastics, using a Seahorse platform as previously described (Merkley et al., 2022).
Monolayer barrier integrity and wound healing assay
A549 cells, grown as above, were harvested and resuspended in media containing 10% serum at 2.0 × 105 cell/ml at 300 µl/well in a 96-well electric cell-substrate impedance system (ECIS) plate (Applied Biophysics, Cat# 96W1E+). The plate was placed in the ECIS system recorder for 48 h until a stable resistance baseline plateau was achieved. The cells were then treated with 1 µm, 5 µm, or with a mixture of 1 µm and 5 µm polystyrene microspheres, either fresh or UV-aged, at 1 µg/ml or 10 µg/ml (n = 6–7 wells/condition). Following 24 h incubation with polystyrene microspheres or vehicle, the wounding sequence was initiated without removing or changing the supernatant. The cells were then allowed to regrow for 24 h while recording the wound recovery.
Statistics
For barrier integrity and wound healing assays, each condition included 6–7 separate replicates and a 2-way analysis of variance (ANOVA) considering treatment and time was used with Tukey’s post hoc testing for individual timepoints. Three-way ANOVA testing was conducted to assess statistical significance for high-content imaging data, considering dose, microsphere size, and UV-aging across each timepoint. A p value of less than .05 confirmed significant difference compared between groups.
Results
Surface chemistry alterations from UV radiation
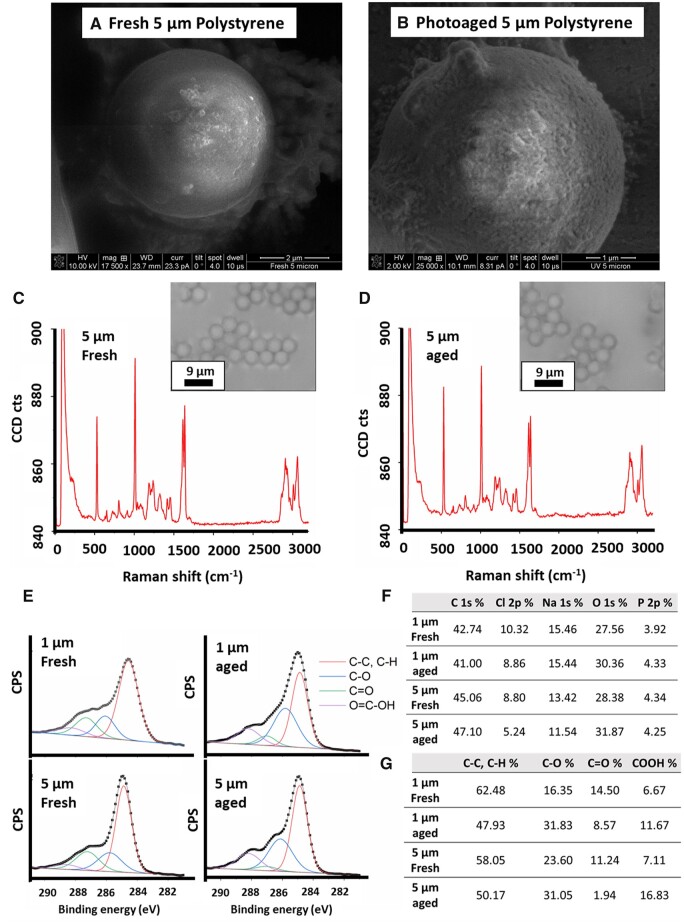
Compared with the pristine polystyrene microspheres (Fig. 1A), UV irradiation for 5 weeks caused a visible alteration to the surface of the polystyrene microspheres, as observed by scanning electron microscopy (SEM) (Fig. 1B); notably, the microspheres appeared otherwise intact. The primarily smooth and rounded surfaces of particles transformed under UV light into rough and deformed surfaces with no detectable changes in the size of particles (Figs. 1A and 1B). Raman spectroscopy revealed a consistent polystyrene spectrum with no detectable changes between aged and fresh microspheres. (Figs. 1C and 1D). DLS confirmed the size and the relative stability of the aqueous dispersion of fresh and aged micropsheres (1 μm) by showing an average hydrodynamic diameter of 905 ± 32 nm and 956 ± 99 nm for a duration of 72 h (Supplementary Fig. 1). The zeta potential of suspended microspheres (1 μm) in cell media was negative for both fresh (−13 ± 0.2 mV) and aged particles (−38 ± 1.1 mV), confirming the aqueous dispersion of particles during the cells exposure.
Figure 1.
UV-photoaging alters the surface chemistry of polystyrene microspheres. SEM of fresh (A) and photoaged (B) polystyrene microspheres. Raman spectroscopy confirmed that the surface chemistry of the particles retained the fingerprint for polystyrene in both pristine (C) and photoaged (D) microspheres. E, XPS spectroscopy, however, demonstrated alterations to the surface chemistry due to photoaging, with an approximately 10% increase in the surface oxygen content (F) for photoaged microspheres of both 1 and 5 µm sizes. Single (C–O) and carboxylic acid (COOH) group levels were increased due to the UV light exposure (G).
Additional analyses were conducted using XPS to identify any weathering effect on the organic functional groups by measuring the signal of C 1s on the near-surface region of the fresh and aged microspheres (Fig. 1E). Curve fitting of high-resolution C 1s spectra reveals the photooxidation of UV-irradiated polystyrene microspheres at the top near-surface region by showing changes in peak intensity of polar functional groups compared with fresh microspheres. Overall oxygen content, measured by XPS, was increased roughly 10% on both sizes of polystyrene microspheres exposed to UV light (Fig. 1F). Increased single and double carbon-oxygen bonds were also detected in the 5 µm microspheres after UV-aging with an increase in the relative percent of C–O groups of 95% and 32% in 1 and 5 µm microspheres, respectively (Fig. 1G). Similarly, carboxyl (COOH) groups increased by 75% and 137% in 1 and 5 µm microspheres, respectively. A different pattern was observed for the non-polar bonds C-C, C-H % by showing a decrease of the corresponding peaks intensity in high-resolution C 1s spectra after UV-aging. It should be noted that there was also a decrease in the relative percent of C=O functional groups after UV-aging for both 1 µm and 5 µm microspheres. Meides et al. (2021) reported an increase in ketone groups only after a longer exposure of PS particles to UV radiation (>2000 h). The non-detectable changes in functional chemistry by Raman spectroscopy (depth resolution about 1 µm) confirm that the increase in polarity is limited to the top 5–10 nm of the near-surface region of the particles, which could only be detected by XPS analysis. Our microscopic and spectroscopic findings confirm that photoaging of 5 weeks causes physical alteration of the surface of the microspheres and increases the polar groups of polystyrene chains on the near-surface region that may affect the interactions of particles with mammalian cells. The detected increase in polar groups and oxygen content on the surface of microspheres was supported with an increase in the negative charge as measured by zeta potential (Supplementary Fig. 1). Given that the surface chemistry is altered after UV-aging, we set out to understand if this change would impact the lung epithelium.
Polystyrene microspheres affect cellular morphology and metabolism
To understand how both pristine or photoaged microplastics impacted epithelial homeostasis, we applied high-content analysis to examine effects of fresh or UV-aging, dose (1, 3, 10, and 30 µg/ml), and size (1 and 5 µm microspheres) at multiple timepoints (24, 48, and 72 h) on A549 cells. It should be noted that our selected doses of particles are still in the lowest end of the range of concentrations used in other studies (Prata et al., 2020).
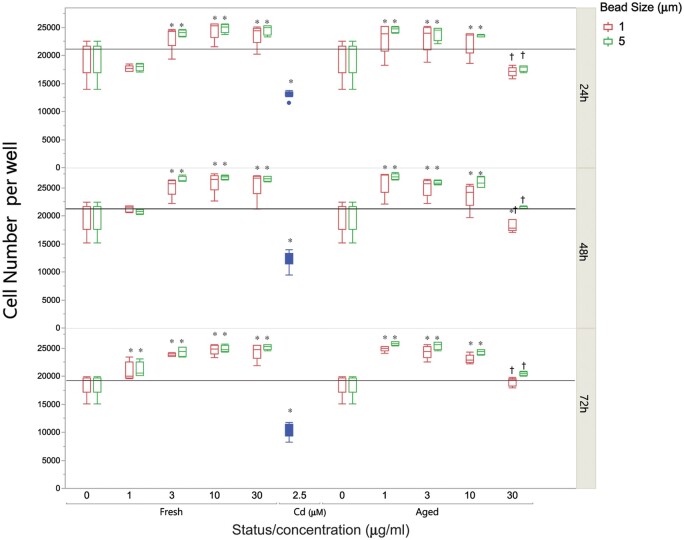
Overall DNA intensity measures were unchanged by microsphere treatment (Supplementary Fig. 2) and there was no significant alteration of γ-H2AX intensity, suggesting no double-strand DNA breaks (Supplementary Fig. 3). Cell numbers per well were significantly altered by microplastics treatment. Pristine polystyrene microspheres, at both 1 and 5 µm sizes, caused a dose-dependent increase in cell numbers per well compared with untreated cells (Fig. 2). On the other hand, aged microsphere exposure led to increased cell numbers at the lowest dose (1 µg/ml), but cell numbers per well decreased at higher doses. These dose-related patterns, for both pristine and UV-aged microplastics, were evident and consistent at 24, 48, and 72 h. It is possible that the microplastics exhibit a biphasic effect on cell growth that culminates in loss of cells due to cytotoxicity, and that the aged microplastics exhibit a dose-response relationship that has shifted due to more potent toxicity.
Figure 2.
Polystyrene microspheres alter growth patterns in A549 cells. Cell number changes after polystyrene microsphere treatment across dose (0–30 µg/ml), UV-photoaging, size (1 or 5 µm), and time (24, 48, and 72 h). Relative to untreated controls, greater cell numbers were observed at all doses, sizes, and aging conditions (*P < .0001), except for the high dose (30 µg/ml) of the aged polystyrene microspheres (†P < .01 compared with 10 µg/ml). Fresh polystyrene microspheres displayed an increasing trend even at 30 µg/ml, while photoaged microspheres reduced cell numbers to control (untreated) levels.
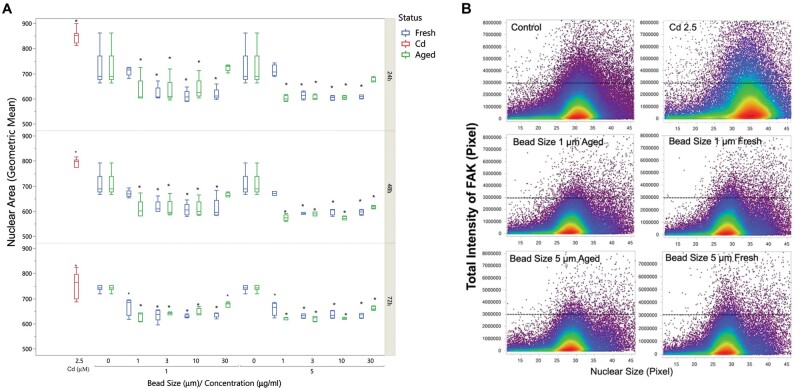
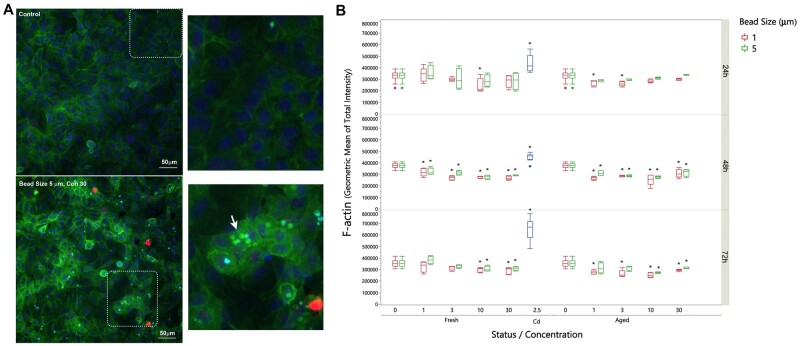
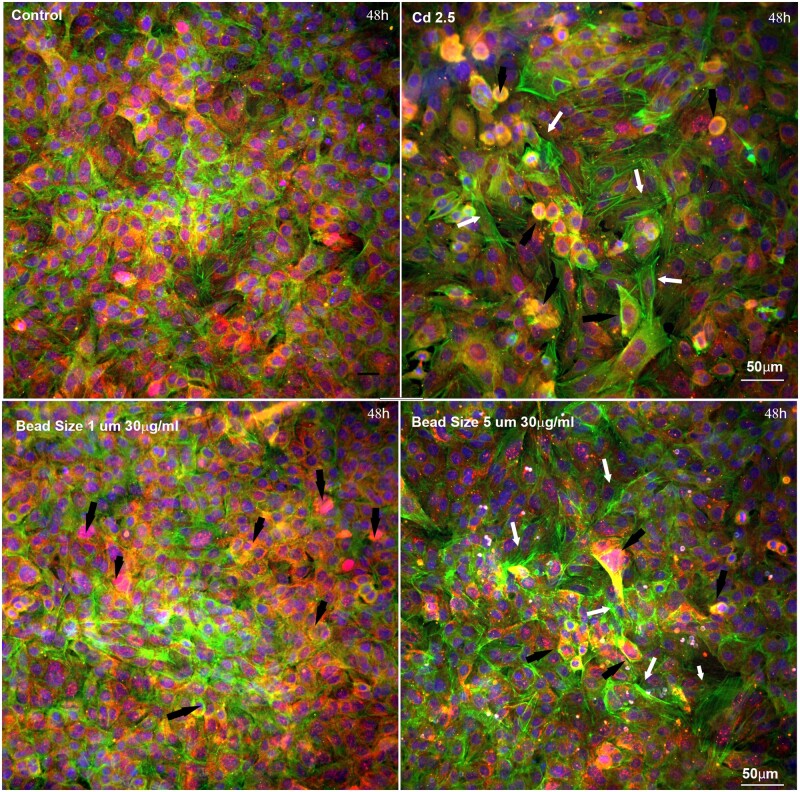
Nuclear size was significantly reduced in A549 cells treated with either size of polystyrene microspheres across all doses (Fig. 3). Changes in nuclear shape can occur through alterations in nuclear lamina or the cytoplasm and cellular morphology (Webster et al., 2009). Since F-actin is involved in maintaining cell structure and rigidity, we sought to determine if microplastics caused morphological changes in A549 cells by examining cytoskeleton F-actin via high-content imaging analysis. F-actin was evenly distributed across the whole well in control (untreated) cells; however, treatment with UV-aged microplastics disrupted F-actin structure (Fig. 4). The distribution of the F-actin was different when treated with UV-aged microplastics. We observed increased intensity of F-actin around these plastic particles, but the quantification of total F-actin and the average of F-actin per cell dose-dependently decreased at 48 and 72 h with the treatment. Green microparticles indicated that these particles were enwrapped inside of the F-actin and internalized into A549 (white arrow); while the blue particles indicated that these particles were outside of the cell body (black arrow).
Figure 3.
HCA reveals nuclear area and FAK expression reductions following polystyrene microsphere treatment. A, Nuclear area derived from HCA revealed consistent reductions in the size of the nucleus in A549 cells treated with polystyrene microspheres. This effect was relatively consistent across dose, time, size, and photo-aging, although the lowest dose of fresh microspheres did not induce this outcome. Horizontal line provides a visual based on the mean of the control cells (N = 5 wells per condition, >8,000 cells per well). B, FAK intensity relative to the size of the nucleus for the 10 µg/ml dose at 48 h is shown for select groups. In general, polystyrene treated cells displayed smaller nuclear areas and reduced FAK intensity. Horizontal bar set arbitrarily at 300,000 to highlight the threshold reduction in 1 and 5 µm microspheres.
Figure 4.
Representative images of F-actin and microspheres in A549 cells. A, F-actin, stained with phalloidin (green), was evenly distributed across the whole well in control (untreated) cells; γ-H2AX was evident (red) and nuclei were stained with Hoechst 33342 (blue). At 72 h, treatment with 5 µm UV-aged microspheres (30 µg/ml) resulted in disruption of F-actin structure. Green puncta indicate plastic microparticles enwrapped inside of the F-actin (white arrow), while the blue puncta indicated that these particles were outside of the cell body (black arrow). B, Quantitation of overall F-actin staining reveals reductions in a dose- and time-dependent manner at 48 and 72 h (*P < .01 compared with untreated control).
We further examined the effects of MPs on the formation of alpha-tubulin (green) and the expression of FAK (red, Fig. 5). Using Cd as a positive control, the quantitative analysis of the geometric mean of total intensity FAK, or alpha-tubulin, revealed that Cd treatment significantly increased both FAK and alpha-tubulin. Higher levels of FAK expression were observed in these affected cells in comparison to the control along with the increase of stress fibers, which is in good agreement with the literature (Katoh, 2017; Margadant et al., 2007). Stress fibers are contractile actin bundles found in non-muscle cell to form a highly regulated actomyosin structure within non-muscle cells (Sackmann, 2015). An alteration in stress fibers can affect cellular contractility, cell adhesion and tight junction. In the treatment with 30 µg/ml of UV-aged microplastics (size of 5 µm), we observed a few cells with up-regulated FAK, but much less than those observed in Cd treatment (Fig. 5). The increase of stress fiber formation of alpha-tubulin was significantly observed with microplastics treatment at 1 µg/ml and inconsistently elevated in higher concentrations (Supplementary Fig. 4).
Figure 5.
Representative images of alpha-tubulin and FAK in polystyrene microsphere-treated A549 cells. Effects of microplastics on formation of alpha-tubulin (green) and the expression of FAK (red); nuclei are stained with Hoechst 33342 (blue). Using Cd as a positive control to show cellular disruption, we observed disrupted FAK expression, which increased stress fiber formation. Treatment with 30 µg/ml of UV-aged microplastics also disrupted the FAK (black arrow) and increased the stress fiber formation of alpha-tubulin (white arrow), similar to what was observed for Cd treatment.
Stress can cause changes in cytoskeletal architecture, resulting in changes in cellular metabolism (Willson, 2020). Therefore, we utilized an assay to measure the proton efflux, which is derived from both glycolysis and mitochondrial/tricarboxylic acid cycle activity to determine the rate of glycolysis in A549 cells after overnight exposure to pristine or photoaged microplastics at various concentrations (0, 1, 10, or 20 µg/ml). Briefly, the inhibition of mitochondrial function via rotenone and antimycin A enables the calculation of mitochondrial-associated acidification (mitochondrial oxygen consumption rate, mitoOCR). Subtraction of mitoOCR from the total proton efflux rate (PER) enables the calculation of PER derived from glycolysis, denoted as the glycolytic PER. All doses and status of microplastics increased basal glycolysis in A549 cells compared with unstimulated A549 cells (Fig. 6). Taken together, the change in cellular metabolism along with the alterations in the cytoskeleton architecture suggests that microplastics are damaging the cell membrane.
Figure 6.
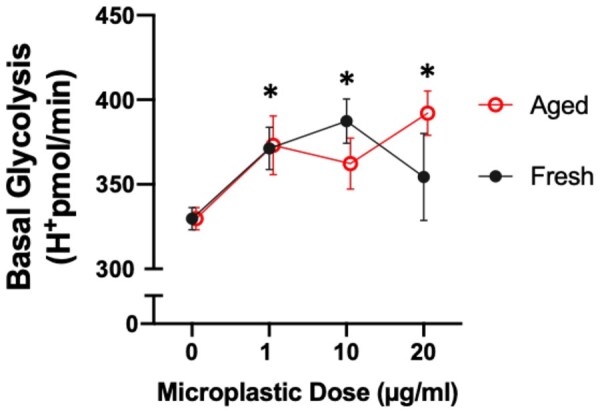
Polystyrene microspheres induce basal glycolysis in A549 cells. Basal glycolysis changes in A549 cells treated overnight with 0, 1, 10, or 20 µg/ml of fresh and UV-aged 1 µm polystyrene microplastics, using a Seahorse platform as previously described (Merkley et al., 2022). Asterisks indicate significant difference from untreated cells by 2-way ANOVA (considering dose and UV-aging as factors). All doses increased basal glycolysis, but UV-aging had no significant influence on this outcome.
Polystyrene microspheres-induced cellular stress causes G2 arrest
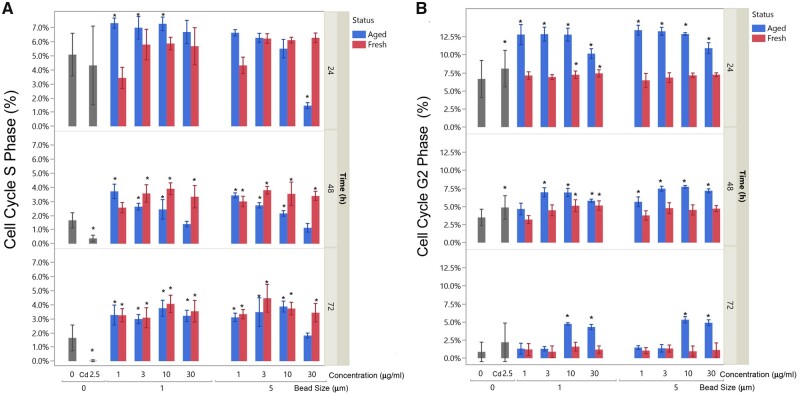
As shown in Figure 7, cell population in S and G2 phases significantly decreased at 48 h and 72 h compared with 24 h, since cells reached 100% confluence with minimum DNA synthesis and cell proliferation. Treatment with Cd significantly decreased the S populations at 48 and 72 h. We found statistical significance (p < .001) of a 4-way factorial model analysis of cells in S population versus concentration (1–30 µg/ml), time (24, 48, and 72 h), status (fresh and UV-aged), and bead size (1 and 5 µm). Two-way ANOVA comparison at each timepoint showed that there are significant differences in cell population in S phase between the fresh and UV-aged microplastics at all 3 timepoints. Significant increase of cells in S phase at the dose of 1, 3, and 10 µg/ml of UV-aged microplastics of bead size of 1 µm at 24 h and decrease at the dose of 30 µg/ml of UV-aged microplastics of bead size of 5 µm. At 48 h, regardless of bead size, all doses of fresh or UV-aged microplastics except 30 µg/ml of UV-aged microplastics increased the cells in S phase. At 72 h, regardless of bead size, all doses of fresh or UV-aged microplastics except 30 µg/ml of UV-aged microplastics with a bead size of 5 µm increased the cells in S phase. Increases of cells in G2 phase after treatment with Cd were observed in 24 and 48 h. Treatment of UV-aged microplastics at both 1 and 5 µm increased the numbers of cells in G2 phase at 24 and 48 h at all doses (except 1 dose at 1 µg/ml of 1 µm bead size at 48 h). Significant increases of cells in G2 phases at 72 h were only observed at UV-aged microplastics at 10 and 30 µg/ml for both 1 and 5 µm bead size. Changes in G2 phase in the fresh microplastics were only observed in 10 and 30 µg/ml in the bead size of 1 µm.
Figure 7.
Polystyrene microspheres cause S and G2 phase arrest in A549 cells. Cell cycle changes resulting from fresh and UV-aged polystyrene microspheres. Data for S phase (A) and G2 phase (B) are shown, highlighting complex changes influenced by microsphere dose, microsphere size, UV-aging (blue versus red), and time. Data are compared with untreated controls (labeled as 0) and a positive control of Cd at 2.5 µg/ml (Cd 2.5), both in gray. Asterisks denote significant difference from untreated controls by a 3-way ANOVA (considering dose, UV-aging status, and size, at each timepoint).
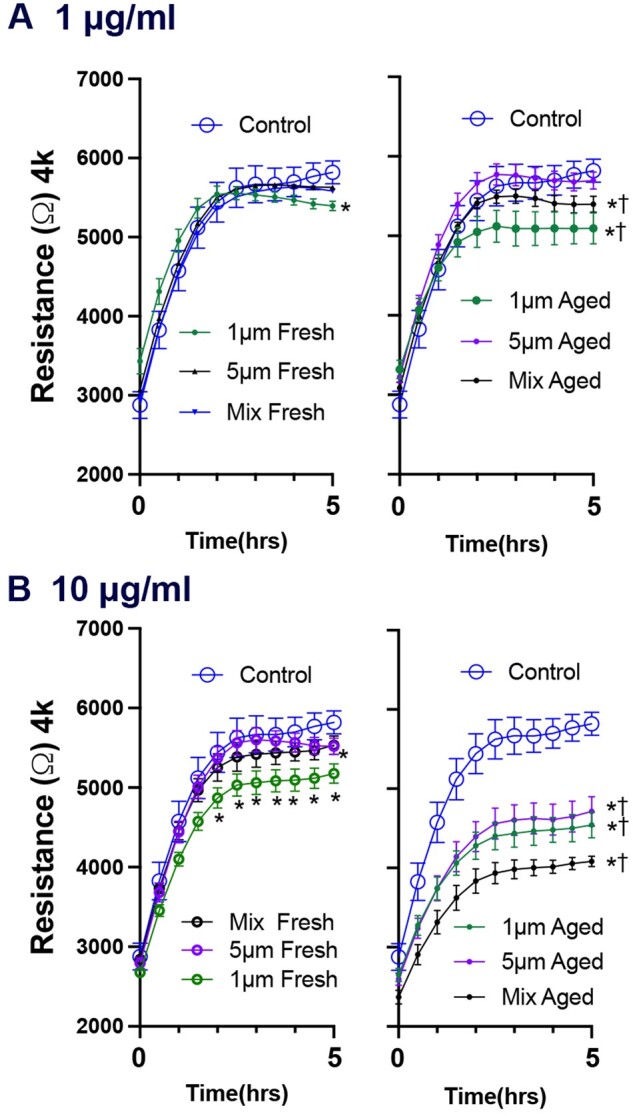
UV-aged polystyrene microspheres impact cell monolayer integrity and wound healing
Confluent A549 cells were treated with fresh and photoaged microspheres (1 µm, 5 µm, or a mix of both sizes) at 1 or 10 µg/ml. Electrical resistance was measured across the A549 monolayer as an index of barrier integrity. After the initial dosing, which included a change of media and a brief increase in resistance, all cells treated with fresh polystyrene microspheres at 1 or 10 µg/ml trended toward a lower resistance level compared with untreated controls (Fig. 8). The smaller particles induced a greater loss of resistance. A greater loss of monolayer resistance was observed in cells treated with photoaged microspheres than in cells treated with fresh polystyrene microspheres. This suggests that surface chemistry changes could affect biocompatibility.
Figure 8.
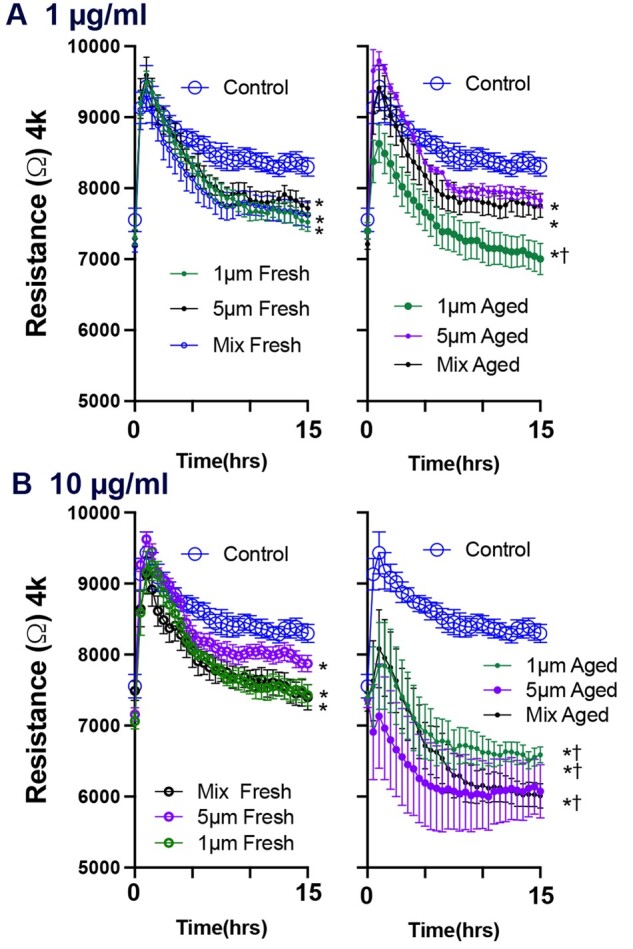
UV-aging enhances loss of barrier integrity in A549 cell treated with polystyrene microspheres. After achieving a stable resistance baseline plateau, cells were treated with the different concentrations/sizes of microspheres. Concentrations of 1 (A) and 10 μg/ml (B) were used. The barrier function responses in confluent A549 cells treated with polystyrene microspheres are showed in each figure for the first 15 h following cells treatment. Each figure compares 1 µm, 5 µm, and a mix of both sizes. Photoaged microspheres, the figures to the right of the fresh particles, were invariably more potent for both barrier and wound healing responses. N = 7 per group. Three-way ANOVAs were conducted to consider the effects of photoaging, size, and time. Asterisks (*) indicate a significant effect of microspheres compared with control and cross (†) indicated significant effect between photoaged and pristine microspheres.
Another integral part of barrier integrity is the ability to heal after an insult/injury. Therefore, stably confluent cells were treated with a brief electric current (Mostovenko et al., 2019) to eliminate cells covering the electrodes on the microwells, following 24 h incubation with polystyrene microspheres or vehicle. The electric current led to a substantial drop in resistance in all wells, which recovered over the next 5 h as cells regrew over the detection electrode (Fig. 9). Fresh 1 µm polystyrene microspheres at the low dose (1 µg/ml) only exhibited a modest reduction in wound healing, with 5 µm microspheres having no effect; the higher dose (10 µg/ml) potentiated this effect. UV-aging of the plastics exacerbated the wound recovery deficits at both doses. The high-dose mixture of 1 µm and 5 µm photoaged plastics reduced regrowth by 65%. Taken together, our data suggest the exposure of the lung epithelial cells to pristine or photoaged microplastics could prove detrimental to airway function and injury recovery.
Figure 9.
UV-aging potentiates polystyrene microsphere-induced impairment of wound recovery in A549 cells. Wound healing responses in A549 cells treated with polystyrene microspheres. Concentrations of 1 µg/ml (A) and 10 µg/ml (B) were used. The wounding sequence was initiated after 24 h of cells treatment with the different exposure conditions. The wound recovery was achieved over the first 5 h as shown in the corresponding figures. Each figure compares 1 µm, 5 µm, and a mix of both sizes. Photoaged microspheres, the figures to the right of the fresh particles, were invariably more potent for both barrier and wound healing responses. N = 7 per group. Three-way ANOVAs were conducted to consider the effects of photoaging, size, and time. Asterisks (*) indicate a significant effect of microspheres compared with control and cross (†) indicated significant effect between photoaged and pristine microspheres.
Discussion
Surface chemistry and morphology of polystyrene microspheres are modified by long-term exposure to UV light, and these effects augment cellular toxicity. Many factors likely influence the biological interactions with micro- and nanoplastics in the environment. Size, composition, and shape are obvious factors, but plastics are exposed to light, heat, moisture, and microbes in the natural environment, all of which likely influence their toxicity. Our ability to predict the safety of microplastics is limited when we do not also investigate this range of natural decomposition mechanisms. Innovative biodegradable plastics, for instance, may offer benefits in terms of waste disposal, but may actually develop enhanced toxicity during their abbreviated lifespan; testing the biocompatibility of new plastics products across the lifespan of those materials should be considered in the decision matrix for product implementation. Our findings showed that UV-aging mainly affected the surface chemistry of the particles by increasing the oxygen content (approximately 10%), single carbon-oxygen bonds, and carboxyl groups on the near-surface region for both microsphere sizes with moderate physical modifications. Particle degradation or size reduction were not observed under our experimental conditions. The surface properties of particles (eg, functional groups and carbon/oxygen content) are determining factors of their cellular and molecular interactions and subsequently their biocompatibility (Yang et al., 2013). Xu et al. (2016) showed differential cellular toxicity and biocompatibility of graphene oxide particles depending on particles surface functionalization (Xu et al., 2016). The integrity of cell membrane is a recognized parameter in determining particles biocompatibility (Wu et al., 2015). Our findings showed that polystyrene microspheres reduced monolayer barrier integrity and slowed regrowth in a wound healing assay in a manner dependent on dose and size of particles but most importantly, on photoaging. This reduction in membrane integrity was accompanied with the most pronounced toxic effect of microspheres on the cells. For instance, under our experimental conditions, the photooxidation of microplastics was found to reduce the nuclear size, alter the cytoskeleton and cellular metabolism, and disrupt the FAK expression. These mechanisms suggest that the increase of polar groups on the surface of polystyrene microplastics enhanced the cellular interactions with the particles resulting in an increased toxicity and decreased biocompatibility.
Limited research on micro- and nanoplastics toxicity to lungs or airway cells has been conducted to date. Fresh polystyrene microspheres have been shown to reduce A549 cell proliferation and metabolism, an effect that was consistent across a wider range of concentrations (0.05–100 µg/ml) than were employed in the present study (1–30 µg/ml) (Goodman et al., 2021). Shi et al. (2021) similarly found only modest biological responses to fresh polystyrene nanospheres (100 nm diameter) at even greater concentrations (20 µg/ml) on A549 cells (Shi et al., 2021). At much higher concentrations (200 µg/ml), they observed inflammatory responses and reduced cell viability. They further demonstrated that toxicity to certain phthalates was reduced in the presence of nanoplastics, most likely due to the absorption of phthalates into the polystyrene. A remaining question from our study is how the UV aging protocol alters the content and bioavailability of plasticizers like phthalates or bisphenol.
Photoaging of phenol-formaldehyde resin microparticles led to chemical changes of the surfaces that included redox-active species, increased carbonyl formation, and enhanced oxidative potential (Zhu et al., 2020). Photoaging also enhanced cytotoxicity of these particulates, in terms of cell viability and lactate dehydrogenase activity (Zhu et al., 2020). The microparticle chemical changes and resultant enhancement of cytotoxicity are consistent with the present study, making a clear statement regarding the role of environmental modifications to plastics that likely impact health risks from exposures. Our findings are in good agreement with the recent study by Volkl et al. (2022) where polystyrene (2 µm) cytotoxicity in murine macrophages was amplified following the augmentation of surface oxidation and physical fragmentation by UV weathering (Volkl et al., 2022). Plastic particle size is another essential factor to consider, as Goodman et al. (2021) noted cellular uptake and nuclear accumulation of 1 µm polystyrene particles but only modest cellular responses. In comparison, Xu et al. (2019) noted more profound inflammatory responses to 25 and 70 nm polystyrene particles. Microplastics size in the present study was not as crucial as photoaging in terms of cytotoxicity enhancement, but the 1 and 5 µm particles did cause modestly different responses, qualitatively-speaking. Notably, the mixture of 1 and 5 µm aged plastics caused the greatest decrement in epithelial cell wound healing. These findings in wound healing assays have underlined the potential effect that can have the heterogeneity in size distribution on particles toxicity. Such increase in cellular toxicity was also observed with the heterogeneous size distributions of carbon-rich metal-bearing particles in comparison to homogeneous distribution of particles (El Hayek et al., 2021).
The cell cycle consists of a series of stages in which DNA is duplicated and cellular components such as organelles are divided to produce 2 daughter cells. The stages of the cell cycle include Gap 0 (G0), Gap 1 (G1), DNA Synthesis (S), Gap 2 (G2), and mitosis (M). Prior to moving to the next stage, the cell must undergo internal verification to detect any potential defects. These detection points are the G1/S and the G2/M checkpoints and they ensure the cell is ready to proceed to the next stage. The aged polystyrene microspheres exhibited a differential effect on cell cycle progression, primarily apparent at the 48 h timepoint, with aged polystyrene microspheres causing a dose-dependent reduction in the numbers of cells in S-phase, while fresh polystyrene microplastics caused a dose-dependent increase. A proportion of cells exposed to microplastics arrested in S phase. With the aged microplastics, these data are consistent with previous findings and suggest alteration in cyclin levels that prevent cell cycle progression. Another proportion of cells were arrested in G2 with a large population arresting during the first 24 h of exposure. While this accumulation could suggest DNA damage, damage to the repair machinery, or increased activation of the checkpoint; the absence of significant increase in γ-H2AX suggests the inhibition of cyclin-dependent kinases that is specific to checkpoint activation. FAK has a role in integrin-mediated cell migration and cell proliferation. Reduced FAK activation/signaling inhibits cell proliferation (Lim et al., 2008), which along with the absence of significant γ-H2AX signal in particle-exposed cells helps illuminate mechanistic underpinnings of the S and G2 arrest seen after microplastics treatment (Xu et al., 2019). One possible mechanism is an increase in cyclin-dependent kinase inhibitors, which would cause arrests in the G1/S and G2/M phases of the cell cycle. FAK, in particular, has been shown to increase during cancer metastasis and inhibit CKIs in order to increase proliferation (Jones et al., 2019; Zhang et al., 2019).
Photoaging clearly enhanced the toxicity of polystyrene microspheres in the present study, but limitations of the study design must be noted in terms of the global health problems emerging from plastics pollution. For one, polystyrene reflects an important but a fraction of the types of plastics in the environment (Dong et al., 2020; Liao et al., 2021; Sangkham et al., 2022), and whether photoaging similarly enhances toxicity of other plastics remains unknown. Our data confirm that the size of particle influences cellular response. Given the enhanced public health concerns around nanomaterials for pulmonary health (Landsiedel et al., 2012; Miller et al., 2017), assessing the airway epithelial response to smaller plastics particles will be needed. The spherical nature of polystyrene microplastics may not actually be representative of the smaller fraction of plastics shapes in the environment, with studies documenting fibers as being a predominant (>90%) airborne contaminant (Kelly and Fussell, 2020; Wright et al., 2020). Lastly, while studies of the photoaging influence of toxicity could have been conducted on environmentally derived, “real-world” microplastics, it would be difficult to isolate sufficient quantity and adequately remove endotoxins and pyrogens that may independently drive inflammatory responses. Delineating the contribution of specific factors (shape, size, surface chemistry, etc.) is essential to understanding the interactions of plastics with mammalian cells, but mixtures of such exposures may potentiate outcomes, as we observed with 2 sizes of microspheres in wound healing assays.
Human exposure to degrading plastics is ongoing and inevitable. Inhaled plastics will lead to biological responses simply to eliminate such particulates from the airway. Although inhaled particles can be cleared from the lungs by mucociliary clearance, many nano- or micro-sized particles may penetrate deeper into the respiratory tract where removal is dependent on less expedient macrophage activity. Different inhalation studies showed that polystyrene nanoparticles are retained in the lung tissues of female mice without apparent excretion (Zhao et al., 2022) and cause inflammation in rats lungs (Lim et al., 2021). Recently reported detection of microplastics in human tissues highlights the urgent need for standard procedures for plastic particles analysis and quantification (Jenner et al., 2022; Ragusa et al., 2021). Physicochemical modifications to plastic microparticle surfaces that augment inflammatory or immunological responses in the lung may exacerbate disease states; further study on susceptible models is needed. Our experimental photoaging process allowed the induction of the surface oxidation on polystyrene microspheres and highlighted the significant effects of photooxidation on the cellular response in A549. More studies are needed to further investigate the effects of real-world exposure conditions of photoaging (eg, UV light A/B) as an essential factor defining plastic particles toxicity in the environment. Further mechanistic and translational studies should investigate the association between the physicochemistry of inhaled plastic particles surfaces and the alteration of particular biomarkers in human diseases. With new forms of plastics being developed (even ecologically minded biodegradable subtypes)—and with engineering solutions to recycling and reuse lagging—our findings highlight the need to consider human health outcomes across the lifespan of the material in the decision-making process for product incorporation.
Supplementary Material
Acknowledgments
Not applicable.
Contributor Information
Eliane El Hayek, Department of Pharmaceutical Sciences, College of Pharmacy, The University of New Mexico, Albuquerque, New Mexico, USA.
Eliseo Castillo, Division of Gastroenterology, Department of Internal Medicine, School of Medicine, The University of New Mexico, Albuquerque, New Mexico, USA; Clinical and Translational Science Center, The University of New Mexico, Albuquerque, New Mexico, USA.
Julie G In, Division of Gastroenterology, Department of Internal Medicine, School of Medicine, The University of New Mexico, Albuquerque, New Mexico, USA.
Marcus Garcia, Department of Pharmaceutical Sciences, College of Pharmacy, The University of New Mexico, Albuquerque, New Mexico, USA.
Jose Cerrato, Department of Civil Engineering, College of Engineering, The University of New Mexico, Albuquerque, New Mexico, USA.
Adrian Brearley, Department of Earth and Planetary Sciences, College of Arts and Sciences, The University of New Mexico, Albuquerque, New Mexico, USA.
Jorge Gonzalez-Estrella, College of Engineering, Oklahoma State University, Stillwater, Oklahoma, USA.
Guy Herbert, Department of Pharmaceutical Sciences, College of Pharmacy, The University of New Mexico, Albuquerque, New Mexico, USA.
Barry Bleske, Department of Pharmacy Practice and Administrative Sciences, College of Pharmacy, The University of New Mexico, Albuquerque, New Mexico, USA.
Angelica Benavidez, Center for Micro-Engineered Materials, The University of New Mexico, Albuquerque, New Mexico, USA.
Hsuan Hsiao, ReproTox Biotech, Albuquerque, New Mexico, USA.
Lei Yin, ReproTox Biotech, Albuquerque, New Mexico, USA.
Matthew J Campen, Department of Pharmaceutical Sciences, College of Pharmacy, The University of New Mexico, Albuquerque, New Mexico, USA; Clinical and Translational Science Center, The University of New Mexico, Albuquerque, New Mexico, USA.
Xiaozhong Yu, College of Nursing, The University of New Mexico, Albuquerque, New Mexico, USA.
Supplementary data
Supplementary data are available at Toxicological Sciences online.
Declarations
Ethics approval and consent to participate
No animals or humans were involved in these studies.
Availability of data and materials
All data related to this study are publicly available upon reasonable request to the corresponding authors.
Declaration of conflicting interests
The authors declare no conflicts of interest with the content of this manuscript.
Funding
This project was funded through the National Institutes of Health (R01 ES014639, P42 ES025589, KL2 TR001448, P20 GM130422, T32 HL007736, K12 GM088021, and P50 MD1570606).
Author contributions
E.E.H., E.C., J.G.I., M.G., J.C., A.Br., J.G.-E., B.B., M.J.C., and X.Y.: manuscript writing. G.H., M.J.C., E.C., H.H., L.Y., and X.Y.: exposures. E.E.H., E.C., G.H., A.Br., A.Be., M.J.C., X.Y., L.Y., H.H., and J.G.I.: outcome measurements. E.E.H., E.C., A.Br., A.Be., J.G.-E., B.B., M.J.C., and X.Y.: study design. E.E.H., J.C., E.C., M.G., A.Br., and M.J.C.: funding. All authors have read and approved the submitted manuscript.
References
- Akanyange S. N., Lyu X. Y., Zhao X. H., Li X., Zhang Y., Crittenden J. C., Anning C., Chen T. P., Jiang T. L., Zhao H. Q. (2021). Does microplastic really represent a threat? A review of the atmospheric contamination sources and potential impacts. Sci. Total Environ. 777, 146020. [DOI] [PubMed] [Google Scholar]
- Andrady A. L. (2017). The plastic in microplastics: A review. Mar. Pollut. Bull. 119, 12–22. [DOI] [PubMed] [Google Scholar]
- Brachner A., Fragouli D., Duarte I. F., Farias P. M. A., Dembski S., Ghosh M., Barisic I., Zdzieblo D., Vanoirbeek J., Schwabl P., et al. (2020). Assessment of human health risks posed by nano- and microplastics is currently not feasible. Int. J. Environ. Res. Public Health 17, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K. D., Covernton G. A., Davies H. L., Dower J. F., Juanes F., Dudas S. E. (2019). Human consumption of microplastics. Environ. Sci. Technol. 53, 7068–7074. [DOI] [PubMed] [Google Scholar]
- Dong C. D., Chen C. W., Chen Y. C., Chen H. H., Lee J. S., Lin C. H. (2020). Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J. Hazard. Mater. 385, 121575. [DOI] [PubMed] [Google Scholar]
- Dris R., Gasperi J., Mirande C., Mandin C., Guerrouache M., Langlois V., Tassin B. (2017). A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 221, 453–458. [DOI] [PubMed] [Google Scholar]
- El Hayek E., Medina S., Guo J., Noureddine A., Zychowski K. E., Hunter R., Velasco C. A., Wiesse M., Maestas-Olguin A., Brinker C. J., et al. (2021). Uptake and toxicity of respirable carbon-rich uranium-bearing particles: insights into the role of particulates in uranium toxicity. Environ. Sci. Technol. 55, 9949–9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M., Salazar R., Wilson T., Lucas S., Herbert G., Young T., Begay J., Denson J. L., Zychowski K., Ashley R., et al. (2021). Early gestational exposure to inhaled ozone impairs maternal uterine artery and cardiac function. Toxicol. Sci. 179, 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer R., Jambeck J. R., Law K. L. (2017). Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman K. E., Hare J. T., Khamis Z. I., Hua T., Sang Q. A. (2021). Exposure of human lung cells to polystyrene microplastics significantly retards cell proliferation and triggers morphological changes. Chem. Res. Toxicol. 34, 1069–1081. [DOI] [PubMed] [Google Scholar]
- Guo X., Wang J. L. (2019). The chemical behaviors of microplastics in marine environment: A review. Mar. Pollut. Bull. 142, 1–14. [DOI] [PubMed] [Google Scholar]
- Jenner L. C., Rotchell J. M., Bennett R. T., Cowen M., Tentzeris V., Sadofsky L. R. (2022). Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci. Total Environ. 831, 154907. [DOI] [PubMed] [Google Scholar]
- Jones M. C., Zha J., Humphries M. J. (2019). Connections between the cell cycle, cell adhesion and the cytoskeleton. Philos. Trans. R Soc. Lond. B Biol. Sci. 374, 20180227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K. (2017). Activation of rho-kinase and focal adhesion kinase regulates the organization of stress fibers and focal adhesions in the central part of fibroblasts. PeerJ 5, e4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly F. J., Fussell J. C. (2020). Toxicity of airborne particles-established evidence, knowledge gaps and emerging areas of importance. Philos. Trans. A Math. Phys. Eng. Sci. 378, 20190322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsiedel R., Ma-Hock L., Haussmann H. J., van Ravenzwaay B., Kayser M., Wiench K. (2012). Inhalation studies for the safety assessment of nanomaterials: Status quo and the way forward. WIREs Nanomed. Nanobiotechnol. 4, 399–413. [DOI] [PubMed] [Google Scholar]
- Larue C., Sarret G., Castillo-Michel H., Pradas Del Real A. E. (2021). A critical review on the impacts of nanoplastics and microplastics on aquatic and terrestrial photosynthetic organisms. Small 17, e2005834. [DOI] [PubMed] [Google Scholar]
- Liang S., Yin L., Shengyang Yu K., Hofmann M. C., Yu X. (2017). High-content analysis provides mechanistic insights into the testicular toxicity of bisphenol A and selected analogues in mouse spermatogonial cells. Toxicol. Sci. 155, 43–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z., Ji X., Ma Y., Lv B., Huang W., Zhu X., Fang M., Wang Q., Wang X., Dahlgren R., et al. (2021). Airborne microplastics in indoor and outdoor environments of a coastal city in eastern China. J. Hazard. Mater. 417, 126007. [DOI] [PubMed] [Google Scholar]
- Lim D., Jeong J., Song K. S., Sung J. H., Oh S. M., Choi J. (2021). Inhalation toxicity of polystyrene micro(nano)plastics using modified OECD TG 412. Chemosphere 262, 128330. [DOI] [PubMed] [Google Scholar]
- Lim S. T., Chen X. L., Lim Y., Hanson D. A., Vo T. T., Howerton K., Larocque N., Fisher S. J., Schlaepfer D. D., Ilic D. (2008). Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol. Cell 29, 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zeng X., Dong G., Venier M., Xie Q., Yang M., Wu Q., Zhao F., Chen D. (2021). Plastic additives in ambient fine particulate matter in the pearl river delta, China: high-throughput characterization and health implications. Environ. Sci. Technol. 55, 4474–4482. [DOI] [PubMed] [Google Scholar]
- Margadant C., van Opstal A., Boonstra J. (2007). Focal adhesion signaling and actin stress fibers are dispensable for progression through the ongoing cell cycle. J. Cell Sci. 120, 66–76. [DOI] [PubMed] [Google Scholar]
- Meides N., Menzel T., Poetzschner B., Loder M. G. J., Mansfeld U., Strohriegl P., Altstaedt V., Senker J. (2021). Reconstructing the environmental degradation of polystyrene by accelerated weathering. Environ. Sci. Technol. 55, 7930–7938. [DOI] [PubMed] [Google Scholar]
- Merkley S. D., Moss H. C., Goodfellow S. M., Ling C. L., Meyer-Hagen J. L., Weaver J., Campen M. J., Castillo E. F. (2022). Polystyrene microplastics induce an immunometabolic active state in macrophages. Cell Biol. Toxicol. 38, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. R., Raftis J. B., Langrish J. P., McLean S. G., Samutrtai P., Connell S. P., Wilson S., Vesey A. T., Fokkens P. H. B., Boere A. J. F., et al. (2017). Inhaled nanoparticles accumulate at sites of vascular disease. ACS Nano 11, 4542–4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostovenko E., Young T., Muldoon P. P., Bishop L., Canal C. G., Vucetic A., Zeidler-Erdely P. C., Erdely A., Campen M. J., Ottens A. K. (2019). Nanoparticle exposure driven circulating bioactive peptidome causes systemic inflammation and vascular dysfunction. Part. Fibre Toxicol. 16, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C. A. 3rd, Burnett R. T., Thurston G. D., Thun M. J., Calle E. E., Krewski D., Godleski J. J. (2004). Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation 109, 71–77. [DOI] [PubMed] [Google Scholar]
- Prata J. C., da Costa J. P., Lopes I., Duarte A. C., Rocha-Santos T. (2020). Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 702, 134455. [DOI] [PubMed] [Google Scholar]
- Ragusa A., Svelato A., Santacroce C., Catalano P., Notarstefano V., Carnevali O., Papa F., Rongioletti M. C. A., Baiocco F., Draghi S., et al. (2021). Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 146, 106274. [DOI] [PubMed] [Google Scholar]
- Roblin B., Ryan M., Vreugdenhil A., Aherne J. (2020). Ambient atmospheric deposition of anthropogenic microfibers and microplastics on the western periphery of Europe (Ireland). Environ. Sci. Technol. 54, 11100–11108. [DOI] [PubMed] [Google Scholar]
- Sackmann E. (2015). How actin/myosin crosstalks guide the adhesion, locomotion and polarization of cells. Biochim. Biophys. Acta. 1853, 3132–3142. [DOI] [PubMed] [Google Scholar]
- Sangkham S., Faikhaw O., Munkong N., Sakunkoo P., Arunlertaree C., Chavali M., Mousazadeh M., Tiwari A. (2022). A review on microplastics and nanoplastics in the environment: their occurrence, exposure routes, toxic studies, and potential effects on human health. Mar. Pollut. Bull. 181, 113832. [DOI] [PubMed] [Google Scholar]
- Schirinzi G. F., Perez-Pomeda I., Sanchis J., Rossini C., Farre M., Barcelo D. (2017). Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 159, 579–587. [DOI] [PubMed] [Google Scholar]
- Shi Q., Tang J., Wang L., Liu R., Giesy J. P. (2021). Combined cytotoxicity of polystyrene nanoplastics and phthalate esters on human lung epithelial A549 cells and its mechanism. Ecotoxicol. Environ. Saf. 213, 112041. [DOI] [PubMed] [Google Scholar]
- Shupe H. J., Boenisch K. M., Harper B. J., Brander S. M., Harper S. L. (2021). Effect of nanoplastic type and surface chemistry on particle agglomeration over a salinity gradient. Environ. Toxicol. Chem. 40, 1820–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y. K., Hong S. H., Jang M., Han G. M., Jung S. W., Shim W. J. (2017). Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ. Sci. Technol. 51, 4368–4376. [DOI] [PubMed] [Google Scholar]
- Sorensen L., Groven A. S., Hovsbakken I. A., Puerto O., Krause D. F., Sarno A., Booth A. M. (2021). UV degradation of natural and synthetic microfibers causes fragmentation and release of polymer degradation products and chemical additives. Sci. Total Environ. 755, 143170. [DOI] [PubMed] [Google Scholar]
- Stock V., Böhmert L., Lisicki E., Block R., Cara-Carmona J., Pack L. K., Selb R., Lichtenstein D., Voss L., Henderson C. J., et al. (2019). Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch. Toxicol. 93, 1817–1833. [DOI] [PubMed] [Google Scholar]
- Volk H. E., Lurmann F., Penfold B., Hertz-Picciotto I., McConnell R. (2013). Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry 70, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkl M., Jerome V., Weig A., Jasinski J., Meides N., Strohriegl P., Scheibel T., Freitag R. (2022). Pristine and artificially-aged polystyrene microplastic particles differ in regard to cellular response. J. Hazard. Mater. 435, 128955. [DOI] [PubMed] [Google Scholar]
- Wang C. H., Zhao J., Xing B. S. (2021a). Environmental source, fate, and toxicity of microplastics. J. Hazard. Mater. 407, 1–17. [DOI] [PubMed] [Google Scholar]
- Wang Y., Huang J., Zhu F., Zhou S. (2021b). Airborne microplastics: A review on the occurrence, migration and risks to humans. Bull. Environ. Contam. Toxicol. 107, 657–664. [DOI] [PubMed] [Google Scholar]
- Webster M., Witkin K. L., Cohen-Fix O. (2009). Sizing up the nucleus: Nuclear shape, size and nuclear-envelope assembly. J. Cell Sci. 122, 1477–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson J. (2020). Stress fibres fuel glycolysis. Nat. Rev. Mol. Cell Biol. 21, 180. [DOI] [PubMed] [Google Scholar]
- Wright S. L., Ulke J., Font A., Chan K. L. A., Kelly F. J. (2020). Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 136, 105411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Wang C., Zheng J., Luo C., Li Y., Guo S., Zhang J. (2015). Vacuolization in cytoplasm and cell membrane permeability enhancement triggered by micrometer-sized graphene oxide. ACS Nano 9, 7913–7924. [DOI] [PubMed] [Google Scholar]
- Xu M., Halimu G., Zhang Q., Song Y., Fu X., Li Y., Li Y., Zhang H. (2019). Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total Environ. 694, 133794. [DOI] [PubMed] [Google Scholar]
- Xu M., Zhu J., Wang F., Xiong Y., Wu Y., Wang Q., Weng J., Zhang Z., Chen W., Liu S. (2016). Improved in vitro and in vivo biocompatibility of graphene oxide through surface modification: Poly(acrylic acid)-functionalization is superior to PEGylation. ACS Nano 10, 3267–3281. [DOI] [PubMed] [Google Scholar]
- Yang K., Feng L., Shi X., Liu Z. (2013). Nano-graphene in biomedicine: Theranostic applications. Chem. Soc. Rev. 42, 530–547. [DOI] [PubMed] [Google Scholar]
- Yin L., Siracusa J. S., Measel E., Guan X., Edenfield C., Liang S., Yu X. (2020). High-content image-based single-cell phenotypic analysis for the testicular toxicity prediction induced by bisphenol A and its analogs bisphenol S, bisphenol AF, and tetrabromobisphenol A in a three-dimensional testicular cell co-culture model. Toxicol. Sci. 173, 313–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong C. Q. Y., Valiyaveetill S., Tang B. L. (2020). Toxicity of microplastics and nanoplastics in mammalian systems. Int. J. Environ. Res. Public Health 17, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Stockwell S. R., Elbanna M., Ketteler R., Freeman J., Al-Lazikani B., Eccles S., De Haven Brandon A., Raynaud F., Hayes A., et al. (2019). Signalling involving MET and FAK supports cell division independent of the activity of the cell cycle-regulating CDK4/6 kinases. Oncogene 38, 5905–5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Wang Y., Ji Y., Mei R., Chen Y., Zhang Z., Wang X., Chen L. (2022). Polystyrene nanoplastics demonstrate high structural stability in vivo: A comparative study with silica nanoparticles via SERS tag labeling. Chemosphere 300, 134567. [DOI] [PubMed] [Google Scholar]
- Zhu K., Jia H., Sun Y., Dai Y., Zhang C., Guo X., Wang T., Zhu L. (2020). Enhanced cytotoxicity of photoaged phenol-formaldehyde resins microplastics: Combined effects of environmentally persistent free radicals, reactive oxygen species, and conjugated carbonyls. Environ. Int. 145, 106137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data related to this study are publicly available upon reasonable request to the corresponding authors.