Abstract
We report the case of a 71-year-old male with essential thrombosis who presented with ground-glass lung opacity with a mosaic pattern on computed tomography, which resolved spontaneously with hospitalization. This was confused with a case of hypersensitivity pneumonitis (HP), which later turned out to be a drug-induced lung disease caused by surreptitiously administered minoxidil. This case emphasizes the importance of obtaining a correct medication history to make an accurate diagnosis, and this is the first report of minoxidil causing HP-like pulmonary illness.
Keywords: Minoxidil, Hypersensitivity pneumonitis, Drug-induced lung disease
1. Introduction
Hypersensitivity pneumonitis (HP) is challenging to diagnose [1]. To make a confident diagnosis, confirming the characteristic findings in all three domains is important: 1) exposure identification, 2) imaging pattern, and 3) bronchoalveolar lavage (BAL) lymphocytosis [1]. If any of these elements are missing, physicians should periodically reconsider the diagnosis.
Here, we describe the case of a male who presented to the pulmonary clinic with ground-glass opacity on chest computed tomography (CT), which resolved spontaneously only with hospitalization. It was confused with HP but turned out to be an adverse effect of the surreptitiously administered minoxidil. Minoxidil is known to cause pleural effusion as a pulmonary complication; however, an HP-like presentation has not been previously reported.
Although the risks of medically unsupervised medication use have been well publicized for many years, self-medication has become an increasingly common practice [2]. With the popularization of e-commerce, it has become easier than ever to purchase medications online. It is now possible to obtain medications that traditionally require prescriptions. Therefore, they can be easily overlooked unless patients report their use, which can complicate the clinical course.
Minoxidil was originally developed as a medication for hypertension. However, because it was found to cause hypertrichosis, it is now more commonly used to treat alopecia. Minoxidil is generally a prescription medicine; however, it can be easily purchased online. Due to the stigma surrounding alopecia, it is speculated that many patients hesitate to consult with a doctor about their condition and that a significant number use minoxidil without their physician's knowledge.
2. Case
A 71-year-old male with essential thrombosis (ET) was referred to the pulmonary service in March 2021 because of diffuse pulmonary ground-glass opacity (GGO) incidentally noted on a CT scan taken as part of a myelofibrosis workup. The GGO was diffusely distributed in all lobes without upper-lower or central-to-peripheral predominance (Fig. 1A). A mosaic pattern was observed.
Fig. 1.
Chest CT before and after each hospitalization. Before the first admission (A) and eight days after the admission (B). On the second admission (C) and 15 days after the second admission (D). On the third admission (E) and at the follow-up clinic visit one month after E (F).
A. Diffuse GGO with a mosaic pattern was noted.
B. There was an improvement in the GGO.
C. GGO reappeared in the lung field. Pericardial effusion was evident.
D. Both GGO and pericardial effusion had improved.
E. GGO and pericardial effusion reappeared and worsened.
F. There were improvements both in GGO and pericardial effusion.
The patient reported mild but gradually worsening dyspnea on exertion. The patient denied coughing or sputum production and reported an occasional low-grade fever. His medical history included ET, obstructive sleep apnea (OSA), chronic obstructive pulmonary disease, and cholecystectomy for cholecystitis. ET had been diagnosed nine years prior and was managed with aspirin and hydroxyurea. OSA was diagnosed three years prior and managed with continuous positive airway pressure therapy (CPAP). He was an ex-smoker with a history of smoking one pack per day for 40 years. He had quit smoking approximately ten years prior to his presentation. He resided in an air-conditioned building and did not have musty or dusty living quarters. The air conditioner filter was cleaned regularly. He used a humidifier but kept it clean. He denied direct contact with birds or bird droppings but admitted to using down quilts in winter. His medications included aspirin, hydroxyurea, esomeprazole, mecobalamin, and zolpidem. These medications had not been changed for more than a year.
On examination, his blood pressure was 131/67 mmHg, heart rate was 97 beats per minute, temperature was 36.6 °C, and oxygen saturation was 93% breathing room air. Chest auscultation revealed scant fine crackles at the base of the lungs. Otherwise, his physical examination was unremarkable, and no clubbing, heart murmur, jugular venous distention, or peripheral edema were noted.
The patient's laboratory data are presented in Table 1. The blood count was significant for elevated myelocyte lineage and mildly suppressed erythrocytes, reflecting a history of ET. The C-reactive protein (CRP) level was not elevated at 0.12 mg/dL. The interstitial pneumonia markers were moderately elevated, with Krebs von den Lungen-6 (KL-6) at 942 U/mL and surfactant protein D (SP-D) at 117 ng/mL. The serological panel results for connective tissue disease were unremarkable. Serum angiotensin-converting enzyme levels were within normal limits. Serum IgG4 levels were also within normal limits. The brain natriuretic peptide (BNP) level was moderately elevated to 94.6 pg/mL. The electrocardiogram was unremarkable only with incomplete right-bundle-branch block.
Table 1.
Laboratory data.
| Item | Value | Reference range |
|---|---|---|
| WBC | 14,600/μL | 3300–8600/μL |
| Differential count | ||
| Neutrophil | 11,500/μL, 78.6% | 1700–630/μL, 40.6–76.4% |
| Lymphocyte | 1000/μL, 6.6% | 1000–3100/μL, 16.5–49.5% |
| Monocyte | 900/μL, 6.3% | 100-600/μL, 2.0–10% |
| Eosinophil | 800/μL, 5.6% | <500/μL, <8.5% |
| Basophil | 400/μL, 2.9% | <200/μL, <2.5% |
| Hb | 11.0 g/dL | 13.7–16.8 g/dL |
| Plt | 327 × 103/μL | 158–348 × 103/μL |
| CRP | 0.12 mg/dL | <0.14 mg/dL |
| KL-6 | 942 U/mL | <500 U/mL |
| SP-D | 117 ng/mL | <110 ng/mL |
| ACE | 7.2 U/mL | <22 U/mL |
| IgG4 | 63.9 mg/dL | 11–121 mg/dL |
| Serologic panel for CTD | ||
| ANA | 40 times, speckled | <40 times |
| Anti-ds-DNA | <10 IU/mL | <10 IU/mL |
| Anti-CCP | <0.6 U/mL | <0.6 U/mL |
| RF | <5.0 IU/mL | <5.0 IU/mL |
| Anti-ScL-70 | <1.0 U/mL | <1.0 U/mL |
| Anti-centromere | <2.0 U/mL | <2.0 U/mL |
| Anti-RNP | 2.0 U/mL | <10 U/mL |
| Anti-SSA | <1.0 U/mL | <1.0 U/mL |
| Anti-ARS | 7.9 | <25 |
| Anti-MDA5 | <5 | <32 |
| PR3-ANCA | <1.0 | <3.5 |
| MPO3-ANCA | <1.0 | <3.5 |
| BNP | 94.6 pg/mL | <18.5 pg/mL |
| D-dimer | 0.5 μg/mL | <1.0μg/mL |
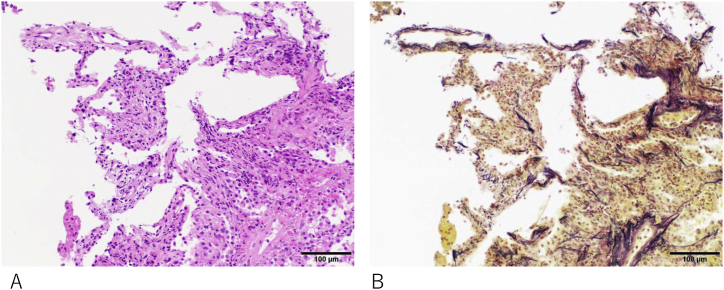
HP was suspected based on the CT findings, elevated KL-6 levels, and otherwise generally unrevealing laboratory investigations. Bronchoscopy was performed, and the patient was admitted to the hospital to avoid possible inciting antigens for both diagnostic and therapeutic purposes. Surprisingly, the analysis of BAL fluid (BALF) showed a near-normal cell count: total cell count 2.33 × 105 cells/mL, macrophages 95%, lymphocytes 1%, and neutrophils 4%. The CD4/CD8 ratio was unremarkable (0.93). Cytology and cultures were also unremarkable. Transbronchial lung biopsy revealed only a mildly inflamed interstitium with mild inflammatory cell infiltration (Fig. 2).
Fig. 2.
Serial sections of a transbronchial lung biopsy specimen stained with Hematoxylin and Eosin (A) and Elastica van Gieson (B). The specimen appears crushed, but alveolar elastic fibers are visible in B. A mild increase in macrophages in the alveolar space and septa suggests mild inflammation. Gross inflammation with lymphocyte or neutrophil infiltration is not observed. Vascular congestion is difficult to assess with this specimen. No hemosiderin-laden macrophages, which are indicative of chronic pulmonary congestion, are found.
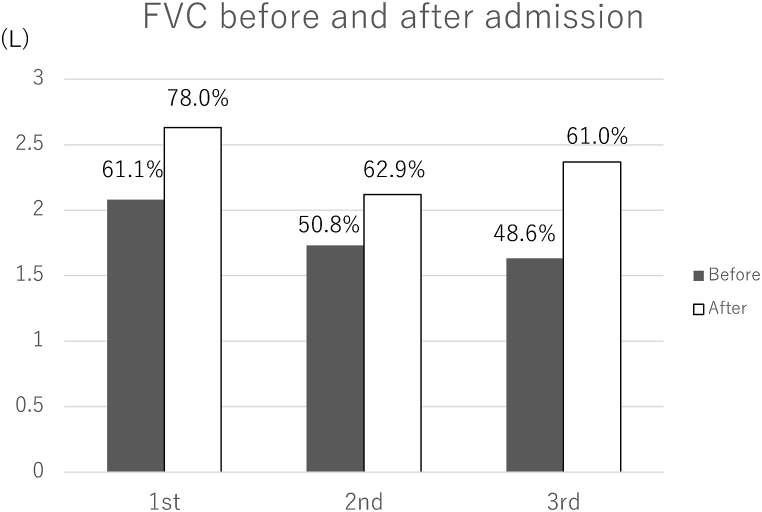
The patient was observed for approximately two weeks without significant changes in his treatment. After one week of hospital stay, the patient reported improvement in dyspnea on exertion. The CT (Fig. 1B) and spirometry (Fig. 3) findings also improved. Antibody tests against bird-related antigens showed high titers (Table 2). Given this clinical course and the high titer of the antibody against bird-related antigens, we made a diagnosis of hypersensitivity pneumonitis, possibly due to avian antigen exposure, even though the BALF findings were not entirely consistent with HP. The patient was instructed to stop using and remove the down quilt, avoid any contact with birds, and clean his house. He was discharged home.
Fig. 3.
Spirometry before or on admission and after hospitalization. There was a significant improvement in forced vital capacity at each admission.
Table 2.
Antibodies to bird-related antigens.
| Suggested cutoff valuea | ||
|---|---|---|
| Budgerigar IgG | 13.31 mgA/L | 8.7 mgA/L |
| Pigeon IgG | 32.88 | 24.6 mgA/L |
| Parrot IgG | 28.42 | 14.0 mgA/L |
Allergol Int 2021; 70:208–214.
Approximately ten months after the initial admission, in late January, he returned to the pulmonary clinic complaining of dyspnea recurrence. CT demonstrated the reappearance of diffuse GGO with a mosaic pattern. A significant increase in pericardial effusion was noted. The patient was afebrile, and his CRP level was only slightly elevated at 0.81 mg/dL. KL-6 and SP-D levels were again elevated, but only mildly, at 618 U/mL and 131 ng/mL, respectively. The BNP increased to 163.1 pg/mL. Echocardiography revealed normal systolic function with an ejection fraction of 64.3%, diastolic dysfunction, a moderate amount of pericardial effusion without signs of tamponade, moderately elevated pulmonary artery pressure, and an estimated pulmonary artery systolic pressure (PASP) of 54 mmHg. The patient was admitted to the hospital again for probable recurrence of HP, even though he denied restarting the down quilt or any exposure to birds. The etiology of the new cardiac findings was unclear, but diuresis was initiated as symptomatic treatment.
His condition improved again after two weeks of hospitalization without significant medical intervention other than diuretics. CT and spirometry showed improvement (Figs. 1D and 3). Pericardial effusion also decreased with diuretics (Fig. 1D). As he was using an air humidifier and CPAP, fungi were suspected to be the culprit. He was discharged with instructions to stop using the humidifier, change the CPAP tubing, and clean the machine diligently.
Although he strictly followed the instructions, dyspnea recurred once he returned home. One month later, the patient was readmitted to the hospital. CT confirmed the reappearance of GGO and pericardial effusion (Fig. 1E). On admission, he brought in two medicines he purchased through an online store: minoxidil (10 mg/day orally) and finasteride. He took these medications for hair loss. He did not remember exactly when he started these medicines, but probably a few years before the first admission. He reported that he temporarily stopped taking these medications while he was in the hospital at the previous admissions, but he restarted taking them when he returned home. We suspected that these medications, especially minoxidil, were the cause of his lung GGO and pericardial effusion and asked him to stop taking these medications entirely. His condition improved again with diuretics and hospitalization (Figs. 1F and 3). The drug lymphocyte stimulation test was negative for minoxidil and finasteride. He was discharged and remained well at the latest clinic visit eleven months later. Echocardiography was repeated approximately two months after discharge. It demonstrated normal systolic function, unchanged diastolic dysfunction, decreased pericardial effusion, and pulmonary pressure (PASP = 43 mmHg).
3. Discussion
HP is challenging to diagnose [1]. The joint guideline published in 2020 by American Thoracic Society, Japan Respiratory Society, and Asociación Latinoamericana del Tórax (the 2020 ATS/JRS/ALAT guideline) proposed that diagnoses should be categorized as definite (≧90% confidence), high-confidence (80–89%), moderate-confidence (70–79%), and low-confidence (51–69%) diagnoses rather than conventional met-or-unmet dichotomous diagnostic criteria [1]. To determine confidence, the importance of three domains is emphasized: 1) exposure identification, 2) imaging pattern, and 3) BAL lymphocytosis [1]. The presence of an inciting antigen is often inferred by confirming clinical improvement after two to four weeks of hospitalization (antigen avoidance test) [3]. In our case, HP was suspected because the CT findings were consistent with nonfibrotic HP [1] and the patient's condition improved only with hospital admission (i.e., a positive antigen avoidance test). The antigen avoidance test is practical and widely utilized. However, physicians should be cautious about interpreting the results because it only confirms that something in the environment outside the hospital contributes to the disease process, and it does not specifically identify the causative agent. BALF analysis in our case was not typical of HP, which lowered our confidence in the diagnosis. KL-6 and SP-D are useful interstitial pneumonia biomarkers that correlate with the clinical course of HP [4]. The elevation of KL-6 in our case was only mild and did not correlate with this patient's symptoms, which also lowered our confidence. As the 2020 ATS/JRS/ALAT guideline cautions, physicians should maintain a high index of suspicion for another possibility and reconsider the diagnosis at subsequent visits if the confidence is not high, as in our case.
Obtaining an accurate complete medication list, including over-the-counter medications, dietary supplements, and herbal medications, can be challenging. One study revealed that as many as 25% of the prescription drugs in use were not recorded in the hospital medical records [5]. It is natural to expect that the number is even higher in the case of drugs that carry a stigma, such as those for baldness treatment, like minoxidil in our case. The patient hesitated to report the use of minoxidil and finasteride to the medical staff, and it was only when he was found to carry the medications with him on admission that we learned that he was taking these medications. It is probably less embarrassing for patients to hand in such medications along with their other medications than to inform medical staff directly. This may be a useful method to use to encourage patients to inform the medical staff about their use of certain medications while avoiding embarrassment.
In our case, both minoxidil and finasteride were candidates for the causative agents of the lung infiltrate and pericardial effusion. However, multiple reports have documented pericardial effusion as an adverse effect of minoxidil [[6], [7], [8], [9], [10], [11], [12], [13]], whereas no such reports were found for finasteride. Hence, we believe that minoxidil was the cause. Pleural effusion was reported as an adverse pulmonary effect of minoxidil, but pulmonary parenchymal infiltrate dominated in our case. To our knowledge, this is the first case of minoxidil-induced lung disease presenting as HP-like lung infiltrate. CT demonstrated GGO with a mosaic pattern that was compatible with HP [1]. However, BALF analysis revealed near-normal cell counts and differentials. TBLB showed only mild inflammatory changes. These results are not consistent with those of HP. The underlying mechanism remains unclear. However, considering that previous reports on adverse events from minoxidil included protein-rich pericardial effusion, pleural effusion, and peripheral edema [[6], [7], [8], [9], [10], [11], [12], [13]], minoxidil may increase vascular permeability in addition to vasodilation [14,15], which may induce permeability pulmonary edema that appears as GGO on CT. Mosaic patterns can be observed not only in small airway diseases but also in the aberrant pulmonary circulation [16,17]. The uneven effect of minoxidil on the pulmonary vasculature bed may have produced a mosaic pattern. Congestion may also have played a role in our case, based on the moderately elevated BNP and diastolic dysfunction noted on echocardiography. However, congestion could not fully explain the CT findings because the diastolic dysfunction was unchanged in the repeated echocardiogram, even after the improvement of his symptoms and CT images. Mechanistically, minoxidil is known to cause vasodilation by opening ATP-sensitive K channels [15], and opening the ATP-sensitive K channel was reported to cause an increase in pleural exudative effusions and neutrophil extravasation in a pleuritis rat model [14]. Another report showed that minoxidil increased the permeability of the blood-brain barrier through the ROS/RhoA/PI3K/PKB signaling pathway [15].
The limitations of this study include a considerable time gap between the onset of minoxidil administration and the development of symptoms, as well as the fact that finasteride was also taken and discontinued along with minoxidil. These factors weakens the possibility of minoxidil as a cause of pulmonary disease. However, we still believe that minoxidil as the cause, because the improvement and deterioration of the pulmonary disease coincided with the discontinuation and resumption of minoxidil, and the pulmonary disease did not recur after the permanent discontinuation of minoxidil. We think finasteride is much less likely the primary cause, because of the reasons discussed above.
In conclusion, we have presented the first case of minoxidil-induced HP-like lung disease. Surreptitious use of minoxidil only when the patient was at home confused the treating physician because it resulted in a positive “antigen avoidance test.” The importance of accurate medication history cannot be overemphasized. Asking patients to show their actual medications may improve the accuracy of their medication history. In certain cases, minoxidil-induced HP-like lung infiltrates are difficult to distinguish from real HP in terms of imaging and exposure history. The lack of lymphocytic leukocytosis in the BALF and the associated pleuropericardial effusions may indicate minoxidil as a cause.
-
-
Minoxidil can present with pulmonary infiltrate similar to HP.
-
-
Minoxidil-induced HP-like lung lesion lacks typical features of HP such as lymphocytosis in the BALF and high serum KL-6 and SP-D.
-
-
Physicians should maintain high index of suspicion for medically unsupervised use of medications.
Funding
This study received no funding.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We would like to express our appreciation to Dr. Nei Fukasawa from the Department of Pathology for his invaluable assistance with the pathological findings reported in this study.
References
- 1.Raghu G., Remy-Jardin M., Ryerson C.J., Myers J.L., Kreuter M., Vasakova M., Bargagli E., Chung J.H., Collins B.F., Bendstrup E., Chami H.A., Chua A.T., Corte T.J., Dalphin J.C., Danoff S.K., Diaz-Mendoza J., Duggal A., Egashira R., Ewing T., Gulati M., Inoue Y., Jenkins A.R., Johannson K.A., Johkoh T., Tamae-Kakazu M., Kitaichi M., Knight S.L., Koschel D., Lederer D.J., Mageto Y., Maier L.A., Matiz C., Morell F., Nicholson A.G., Patolia S., Pereira C.A., Renzoni E.A., Salisbury M.L., Selman M., Walsh S.L.F., Wuyts W.A., Wilson K.C. Diagnosis of hypersensitivity pneumonitis in adults. An official ATS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2020;202(3):e36–e69. doi: 10.1164/rccm.202005-2032ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennadi D. Self-medication: a current challenge. J. Basic Clin. Pharm. 2013;5(1):19–23. doi: 10.4103/0976-0105.128253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsutsui T., Miyazaki Y., Okamoto T., Tateishi T., Furusawa H., Tsuchiya K., Fujie T., Tamaoka M., Sakashita H., Sumi Y., Inase N. Antigen avoidance tests for diagnosis of chronic hypersensitivity pneumonitis. Respir Investig. 2015;53(5):217–224. doi: 10.1016/j.resinv.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto T., Fujii M., Furusawa H., Tsuchiya K., Miyazaki Y., Inase N. The usefulness of KL-6 and SP-D for the diagnosis and management of chronic hypersensitivity pneumonitis. Respir. Med. 2015;109(12):1576–1581. doi: 10.1016/j.rmed.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Lau H.S., Florax C., Porsius A.J., De Boer A. The completeness of medication histories in hospital medical records of patients admitted to general internal medicine wards. Br. J. Clin. Pharmacol. 2000;49(6):597–603. doi: 10.1046/j.1365-2125.2000.00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin W.B., Spodick D.H., Zins G.R. Pericardial disorders occurring during open-label study of 1,869 severely hypertensive patients treated with minoxidil. J. Cardiovasc. Pharmacol. 1980;2(Suppl 2):S217–S227. doi: 10.1097/00005344-198000022-00016. [DOI] [PubMed] [Google Scholar]
- 7.Houston M.C., McChesney J.A., Chatterjee K. Pericardial effusion associated with minoxidil therapy. Arch. Intern. Med. 1981;141(1):69–71. [PubMed] [Google Scholar]
- 8.Webb D.B., Whale R.J. Pleuropericardial effusion associated with minoxidil administration. Postgrad. Med. 1982;58(679):319–320. doi: 10.1136/pgmj.58.679.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shirwany A., D'Cruz I.A., Munir A. Very large pericardial effusion attributable to minoxidil: resolution without drainage of fluid. Echocardiography. 2002;19(6):513–516. doi: 10.1046/j.1540-8175.2002.00513.x. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqui A., Ansari M., Shakil J., Chemitiganti R. Minoxidil-associated exudative pleural effusion. South. Med. J. 2010;103(5):458–460. doi: 10.1097/SMJ.0b013e3181be3415. [DOI] [PubMed] [Google Scholar]
- 11.Nautiyal A., Wong T., Kumar S., Mukherjee J.T., Schick E.C. Very large incidental pericardial effusion attributable to minoxidil: resolution without drainage. J. Cardiovasc. Med. 2011;12(3):186–188. doi: 10.2459/jcm.0b013e328334fb07. [DOI] [PubMed] [Google Scholar]
- 12.Cilingiroglu M., Akkus N., Sethi S., Modi K.A. Large pericardial effusion induced by minoxidil. Turk Kardiyol. Dernegi Arsivi. 2012;40(3):255–258. doi: 10.5543/tkda.2012.63904. [DOI] [PubMed] [Google Scholar]
- 13.Gbadamosi W.A., Melvin J., Lopez M. Atypical case of minoxidil-induced generalized anasarca and pleuropericardial effusion. Cureus. 2021;13(6) doi: 10.7759/cureus.15424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Da Silva-Santos J.E., Santos-Silva M.C., Cunha Fde Q., Assreuy J. The role of ATP-sensitive potassium channels in neutrophil migration and plasma exudation. J. Pharmacol. Exp. Therapeut. 2002;300(3):946–951. doi: 10.1124/jpet.300.3.946. [DOI] [PubMed] [Google Scholar]
- 15.Gu Y.T., Xue Y.X., Wang Y.F., Wang J.H., Chen X., ShangGuan Q.R., Lian Y., Zhong L., Meng Y.N. Minoxidil sulfate induced the increase in blood-brain tumor barrier permeability through ROS/RhoA/PI3K/PKB signaling pathway. Neuropharmacology. 2013;75:407–415. doi: 10.1016/j.neuropharm.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Stern E.J., Swensen S.J., Hartman T.E., Frank M.S. CT mosaic pattern of lung attenuation: distinguishing different causes. AJR Am. J. Roentgenol. 1995;165(4):813–816. doi: 10.2214/ajr.165.4.7676972. [DOI] [PubMed] [Google Scholar]
- 17.Kligerman S.J., Henry T., Lin C.T., Franks T.J., Galvin J.R. Mosaic attenuation: etiology, methods of differentiation, and pitfalls. Radiographics. 2015;35(5):1360–1380. doi: 10.1148/rg.2015140308. [DOI] [PubMed] [Google Scholar]