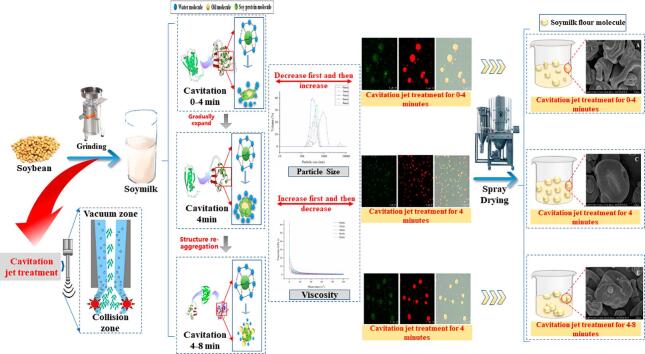
Graphical abstract
Keywords: Cavitation jet, Protein conformation, Soymilk flour, Instant solubility
Highlights
-
•
Cavitation jet technology could affect the soluble protein content and oil–water adsorption efficiency of soymilk.
-
•
Proper cavitation jet treatment could improve the instant solubility of soymilk flour by improving protein in soymilk.
-
•
Prolong-time of cavitation jet treatment made some soymilk flour particles adhere, which led to the decrease of wettability and dispersibility of soymilk flour.
Abstract
The protein conformation of soymilk is the key to affecting the instant solubility of soymilk flour. This study aimed to evaluate the effect of cavitation jet treatment time (0, 2, 4, 6, and 8 min) on the instant solubility of soymilk flour based on the conformational changes of protein in soymilk. The results showed that the cavitation jet treatment for 0–4 min significantly unfolded the protein structure of soymilk and increased the content of soluble protein, which reduced the particle size and increased the electrostatic repulsion and the viscosity of soymilk. This was beneficial for soymilk droplets fully atomized and repolymerized in the spray drying tower, forming soymilk flour particles with large size, smooth surface, and uniform distribution. When the cavitation jet treatment time was 4 min, the wettability (from 127.3 ± 2.5 s to 84.7 ± 2.1 s), dispersibility (from 70.0 ± 2.0 s to 55.7 ± 2.1 s), and solubility (from 56.54% to 78.10%) of soymilk flour were significantly improved. However, when the time of the cavitation jet treatment was extended to 8 min, the protein of soymilk aggregated and the stability of soymilk decreased, which reduced the particle size and hurt the surface characteristics of soymilk flour after spray drying. It resulted in a decrease in the instant solubility of soymilk flour. Therefore, the cavitation jet treatment with proper time increases the instant solubility of soymilk flour by improving the protein conformation of soymilk.
1. Introduction
Soymilk flour is a kind of protein beverage with high nutritional value, which can be quickly dissolved in warm water or cold water and is deeply loved by consumers [1]. However, the soymilk flour currently sold in the market has a long wetting and dispersion time, which was reflected in poor instant solubility during the actual brewing. This not only affects the quality and edibility of soymilk flour but also seriously affects consumers' interest in buying. “Instant solubility” is a general term for excellent wettability, submergence, dispersibility, and solubility. The instant solubility of soymilk flour is highly related to protein dispersion index, wetting, dispersion time, bulk density, and other factors of soymilk flour, which depend on the protein conformation of soymilk [57]. Currently, studies have shown that treatment with ultra-high pressure homogenization, ultrasound, and microfluidizer system of soymilk before spray drying can improve the instant solubility of soymilk flour (Cavender et al., 2021; [81], [40]. These methods promote the agglomerated protein of soymilk to dispersion, unfold the protein structure, and increase the protein flexibility ([83], [52]. The protein conformational changes of soymilk improve the protein dispersion index and solubility of soymilk flour, which is beneficial to the rapid dissolution of soymilk flour in water. Overall, the protein of soymilk is the key to affecting the instant solubility of soymilk flour. The processing performance for soymilk flour can be improved by processing the protein of soymilk before spray drying.
Protein conformational changes of soymilk largely depend on its processing and preparation methods [41]. It is worth mentioning that the processing technology has the greatest influence on the protein conformational of soymilk [41]. At present, novel processing technologies were used to change the protein conformation of soymilk. Zhang et al. [81] found that dual-frequency ultrasound could significantly make the molecular structure of soymilk protein loose and diminished, which decrease the particle size and improve the viscosity of soymilk. Pathania et al. [56] found that in treated soymilk with sequential microwave – ultrasound, the molecular structure of protein in soymilk was unfolded and rearranged, which enhanced the interaction between protein molecules and water molecules, and the solubility of protein significantly improved. The research results of [64] showed that high-pressure treatment of soymilk could destroy the hydrophobic effect and partial disulfide bonds of soymilk protein molecules and reduce the aggregation of protein in soymilk, reducing the viscosity of soymilk. Mu et al. [53] found that the acoustic cavitation, microstreaming, and turbulent forces caused by ultrasound might enhance the oil droplet disruption to decrease the particle size of soymilk and aggregation of protein in soymilk. These studies have shown that the protein conformation of soymilk can be modified by treating soymilk in different ways, which improves the physicochemical, and functional properties of soymilk. Therefore, whether protein in soymilk can be treated by adding effective processing technology before spray drying to improve the instant solubility of soymilk flour is a potential research field.
High-pressure homogenisation and ultrasonic processes can’t be widely used due to the large power consumption and low effort capacities of food process techniques. Therefore, it is necessary to find a technology with low power consumption and high output for the food industry. Cavitation jet technology is a new type of food processing technology, which utilizes high pressure and cavitation combined with high-speed impact, high-frequency vibration, instantaneous pressure drop, and strong shear force [42], [58]. It has the advantages of low energy consumption, rapid and large output, and can be used in the food field. The study by [28] showed that the high shear force of cavitation jet treatment reduces the particle size of oxidized SPI, the rigid part that maintains the secondary structure of protein was weakened, favoring the unfolding of the protein structure. Wu et al. (2020) showed that cavitation effect exposed hydrophobic groups buried of soy protein molecules and changed the conformation of proteins, which improved the content of soluble dietary fiber in okara. Recently, Guo et al. [25] showed the cavitation jet at a short treatment time (<6 min) broke down the disulphide bonds and protein skeleton structures, which reduced the aggregate sizes and molecular weights and increased the emulsion activities. Ren et al. [58] found that cavitation treatment unfolded the protein structure and exposed the hydrophobic residues, which significantly improved the solubility and emulsifying properties of emulsion by facilitating the formation of protein films at the interface. Collectively, cavitation jet technology could modify the conformation and improve the functional characteristics of protein. Hence, we can speculate that cavitation jet technology can modify the protein conformation of soymilk, and further affect the instant solubility of soymilk flour.
However, there are few researches on soymilk and soymilk flour of cavitation jet treatment. It is unknown regarding the influences for cavitation jet on the protein conformation of soymilk, and the instant soluble of soymilk flour. Therefore, the purpose of this study is to explore the effects of cavitation jet treatment at different time (0, 2, 4, 6, 8 min) on the instant solubility of soymilk flour. Based on different time of cavitation jet treatment on soymilk, the internal relationship between the protein conformation changes of soymilk and instant soluble of soymilk flour were further analyzed. It provides a new idea and method for soymilk flour products to process and improve the instant solubility.
2. Materials and methods
2.1. Materials
Soybean was acquired from Beidahuang Company Co., Ltd. (Harbin, China). β-Mercaptoethanol, Nile Red and Nile Blue were acquired from sigma company. Sodium hydroxide concentrated sulfuric acid, hydrochloric acid, and KBr were acquired from Beijing Chemical Reagent Factory Co., Ltd. (Beijing, China). Potassium sulfate and n-hexane were acquired from Tianjin Junbo Company Co., Ltd. (Tianjing, China). Ethanol and methanol were purchased from Nanjing Chemical Reagent Co., Ltd. (Nanjing, China). All other compounds were analytical mark, and dH2O was utilized through the research.
2.2. Methods
2.2.1. Effect of cavitation jet treatment on soymilk and soymilk protein
2.2.1.1. Preparation of soymilk
Soybean → Peeling → Screening → Cleaning → Soaking → Blanching → Grinding → Filtration → Soymilk.
Soybeans were washed three times with distilled water and then washed twice with ultrapure water. Soybeans were soaked at the ratio of 3:1 (dry soybean: water, w/w) for 12 h at 25℃. The soaked soybeans were ground with ultrapure water at a ratio of 1:7 (dry soybean: water, w/w). Then the soybeans were ground for 3 min with the high-speed blender (JP13D-800, Zhejiang Supor Co., Ltd., Zhejiang, China) to produce soymilk.
2.2.1.2. Cavitation jet (CJ) processing
A SL-2 cavitation jet homogenizer (Beijing Zhongsen Huijia Technology Development Co., Ltd., Beijing, China) was used to homogenize the soymilk. Cavitation jet treatment of the soymilk was performed using the following operation parameters: temperature, 22.5 °C; pressure, 0.01 MPa; and processing time, 2, 4, 6, and 8 min. At the same time, soymilk not treated with the cavitation jet were taken as controls.
2.2.1.3. Soluble protein content
The protein content of the supernatant of soymilk were measured using the [45] method and bovine serum albumin (BSA) served as the standard. The soymilk was centrifuged at 12,000 rpm for 10 min. After centrifugation, the supernatant was collected to determine the soluble protein content in soymilk.
2.2.1.4. Particle size and zeta potential
Particle size and zeta potential were determined according to the method described by [3] with some modification. The samples were diluted with DI water (deionized water) (pH 7.0). The particle size and zeta potential of the soymilk treated with different cavitation jet processing times were measured by using a Zetasizer Nano ZS 90 (Malvern Instruments, UK). Before measurement, samples were diluted in DI water (pH 7.0). The refractive index value was 1.460. The equilibrium time was 120 s and detection temperature was 25 ℃.
2.2.1.5. Apparent shear viscosity
The rheological properties of soymilk after different cavitation jet processing times were measured using a HAAKE MARS dynamic rheometer (Thermo Scientific, American). The experiment was used a P60 probe with a gap of 1 mm, the samples (100 mg/mL) was evenly applied to the plate, and the viscosity and shear stress was determined. The parameters were set as follows: the shear rate, temperature and time were 0 –1500 s−1, 25 ℃ and 300 s, respectively [75].
2.2.1.6. Confocal laser scanning microscopy (CLSM)
The distribution of the soymilk under different cavitation jet treatment time was evaluated according to the method of Geremias-Andrade et al.[22]. First, Nile red was dissolved in isopropanol to obtain a 0.1% dyeing solution. Second, Nile blue was dissolved in isopropanol to obtain 1% dyeing solution. Subsequently, 20 μL Nile Red and 25 μL Nile Blue was added to 0.5 mL of soymilk and stained for 30 min. The stained soymilk was placed on concave microscope slides and covered with glycerol-coated coverslips to obtain confocal images.
2.2.2. Effect of cavitation jet treating on the soymilk flour quality
2.2.2.1. Soymilk flour preparation
Soybean → Peeling → Screening → Cleaning → Soaking → Blanching → Grinding → Filtration → Cavitation Jet Treatment → Concentration → Spray Drying → Soymilk flour.
Take 1000 mL of soymilk with different cavitation jet treatment time. The soymilk was concentrated in vacuum to make the solids content in soymilk reach about 15%. Spray drying the obtained concentrate. Spray drying processing of the concentrate was performed using the following operation parameters: inlet temperature, 185 °C; outlet temperature, 185 °C. The obtained solid powder is soymilk flour.
2.2.2.2. Particle size
The particle size of the soymilk flour was determined using a Malvern S3500 (Malvern Instruments, Malvern, UK) image analysis-based particle characterization system. The measurement was made using refractive indices of 1.54 for the particle and the general-purpose model for irregular particles was used to analyse the data [7].
2.2.2.3. Bulk density
With a few minor adjustments, bulk density was carried out in accordance with the procedure outlined earlier by [72]. In a graduated glass cylinder with a 10 mL capacity, one gramme of each soymilk flour was added. Glass cylinder was shaken for 5 min at 25 °C with agitation set at 300 rpm on a shaker (Unimax1010, Heidolph, Germany). The quantities of soymilk flour in a glass cylinder were measured after a five-min exposure to vibration. Bulk density, which is measured in milligrams of soymilk flour per milliliter (mg/mL), was computed as the ratio of soymilk flour mass to measured soymilk flour volume.
2.2.2.4. Dispersibility
With minor adjustments, the approach of Felix da Silva, Ahrné et al.[4] was used to calculate the dispersibility. Distilled water (30 mL) was put into a heated magnetic stirrer to keep the temperature at 40℃. Slowly add 1 g of soymilk flour into the beaker. The amount of time needed to completely moisten and disperse the soymilk flour agglomeration from the beginning of the agitation was then noted. To assess the dispersibility of the soymilk flour, the test was conducted three times, with the average value used as the dispersion time.
2.2.2.5. Wettability
To determine the wettability a glass funnel held on a stand was set over the beaker. An amount of distilled water (200 mL) at 25 ± 1 ℃ was poured into a 250 mL beaker than 1 g of the soymilk flour was added. Finally, the time was recorded by using stopwatch when the soymilk flour became completely wetted (visually assessed as when all the soymilk flour particles penetrated the surface of the water) [33].
2.2.2.6. Solubility
The solubility of the soymilk flour was determined as previously reported by Cano-Chauca et al.[11]. Exactly 1 g sample was dissolved in 30 mL DW at 27.5 ± 2.5 ℃and put in a centrifuge tube. The centrifuge tubes were placed in 30 ℃ water for 5 min and then taken them out. This sample was centrifuged at 3000 rpm for 10 min (SIGMA Co., Ltd, Germany). The precipitate was again centrifuged once by repeating the above operation. The precipitate was dried at 105℃ for 8 h. The solubility of the sample was calculated according to the formula:
where m, m1, and m2represent the bulk of the soymilk flour before dehydrating, the bulk of the crucible and soymilk flour after dehydrating, and the bulk of the crucible before dehydrating, orderly.
2.2.2.7. Protein dispersion index
Soymilk flour were added to 150 mL of deionised water in 400 mL beakers to attain 1% (w/v) protein suspensions. The Protein dispersion index of the soymilk flour was determined according to the method of Anema et al.[6]with slight modification. Aliquots of stirred soymilk flour solutions were withdrawn and the supernatants were obtained by centrifugation at 2000 g for 10 min at 25 ℃. Then, the content of protein in the supernatant was determined as the protein dispersion index of soymilk flour.
2.2.2.8. Scanning electron microscope (SEM)
The microstructure of the soymilk flour was measured by a Tungsten-filament SEM (JSM-6390LV, JEOL, Japan) according to a previously described method with some modifications [62]. Before scanning electron microscopy, gold was plated with an ion sputter coater. The samples were plated with approximately 20 nm thick gold–palladium in a high vacuum evaporator in an argon atmosphere. The sputtering time was about 30 s, and the acceleration voltage was 15 kV.
2.2.3. Statistical analysis
All tests were conducted in triplicate, and the results are presented as means ± standard deviations. Analysis of variance (ANOVA) was used to determine significant differences among the means at p < 0.05, using Duncan’s multiple range test with SPSS20 software (SPSS Inc., Chicago, IL, USA).
3. Results and discussion
3.1. Effect of cavitation jet treatment on soymilk
3.1.1. Soluble protein content
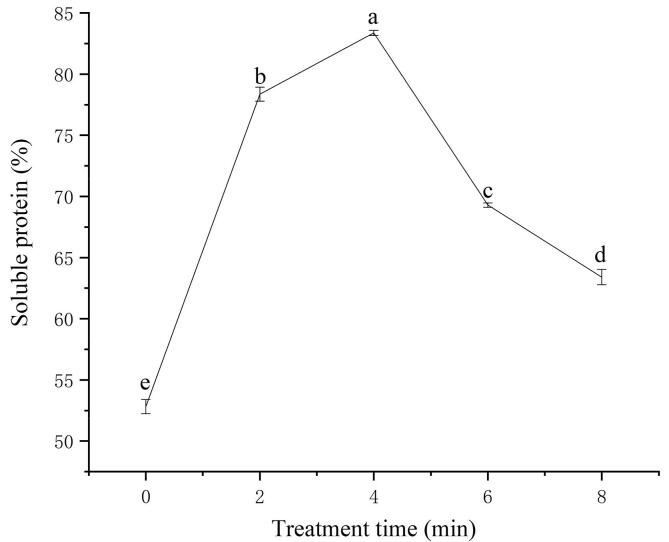
The content of soluble protein in soymilk affects the quality of soymilk and further affects the solubility and dispersibility of soymilk flour [63]. Higher content of soluble protein in soymilk usually produces smooth surfaced and spherical primary particles of soymilk flour. This could be mainly attributed to the formation of a dense film on the surface of soymilk droplets, which reduces the exposure of oil droplets. This effectively prevents the lipid inside the soymilk flour particles from migrating to the surface, forming particles with smooth surface. Lower content of soluble protein can’t wrap the oil droplets completely of soymilk flour particles. This leads to the exposure of oil droplets and affects the apparent morphology of soymilk flour particles. The presence of lipid at soymilk flour surfaces may play a critical role in large cohesive strength experienced in the soymilk flour and ultimately act to limit their solubility [60]. Fig. 1 shows that the content of soluble protein in soymilk increases first and then decreases with the expansion of cavitation jet treating time. When the cavitation jet treatment time at 4 min, the contents of soluble protein in soymilk reaches the maximum. It is speculated that the rise contents of soluble proteins in soymilk may be owing to the extra-speed shear and cavitation effects formed by cavitation treatment, which destroys the spatial structure and intermolecular force of insoluble protein molecules namely the H-bonds and hydrophobic forces between proteins and different subunits [31]. This is conducive to cracking the insoluble proteins of soymilk transformation into soluble proteins through high-speed shear and cavitation effects, [27] increasing the content of soluble proteins. Hu et al. [27] pointed out in their research on the effect of ultrasonic waves on soybean protein isolate that ultrasonic and cavitation destroy some noncovalent interactions in protein, which increase the content of soluble protein. According to the results of [21], cavitation jet treatment unfolds the protein structure and changes the conformational of insoluble proteins, and insoluble proteins were transformed into soluble proteins by increasing the area where water molecules and protein molecules. These results were in line with a related study by Deng et al.[16], in which a change of protein conformation caused by severe physical force enhanced the protein-water interaction, increasing the solubility of soy protein isolates. Moreover, cavitation jet treatment might make small insoluble proteins in homogenized dispersions oscillate violently, and remarkably slow down the association of these insoluble protein aggregates [70]. Cavitation jet treatment might accelerate the process of new balanced interactions between insoluble protein and other protein components present in the dispersion [30]. These two processes promote the formation of soluble protein components and increase the soluble protein content of soymilk. However, excessive cavitation jet treatment reduces soluble protein content of soymilk. Excessive cavitation jet treatment causes part protein molecules of soymilk to form insoluble macromolecular aggregates through hydrophobic interaction [25]. This leads to a decrease in the soluble protein content of soymilk. Therefore, cavitation jet treatment with appropriate time promotes the transformation of insoluble proteins into soluble proteins, increasing the content of soluble proteins in soymilk. This may improve the quality of the soymilk flour and the functional properties such as the solubility of the soymilk flour.
Fig. 1.
Effect of cavitation jet treatment time on soluble protein content of soymilk.
3.1.2. Particle size
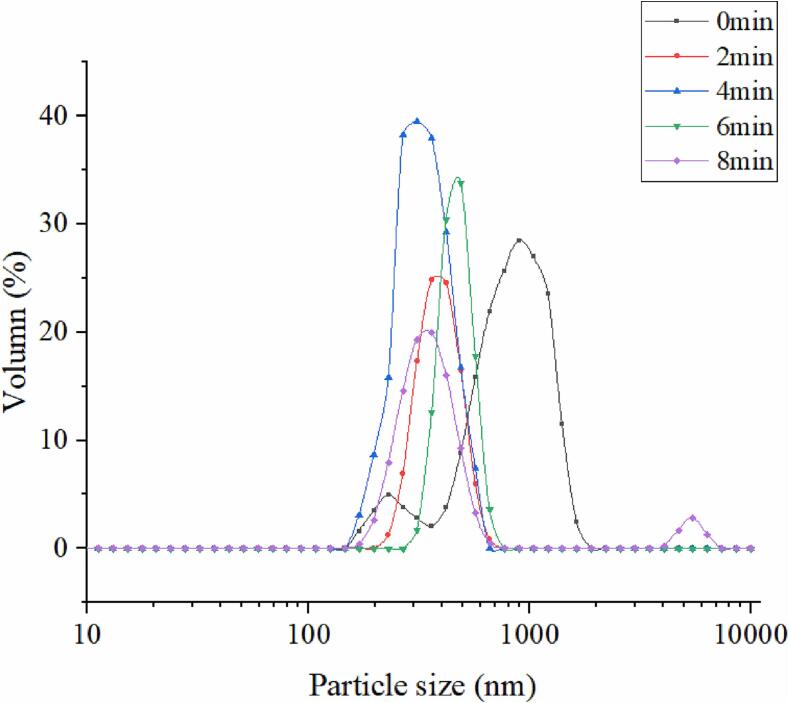
The particle size of soymilk not only affects the physical and chemical characteristics of soymilk, but also affects the particle size, wettability, and solubility of soymilk flour [36]. Broadly, the particle size distribution of the resultant powder is related to the initial spray in terms of initial drop size. Soymilk droplets with small size and uniform distribution could produce large soymilk flour particles by spray drying [54]. This is beneficial to improve the bulk density of soymilk flour. Smaller droplets of soymilk are difficult to form large particles of soymilk flour. As the soymilk flour with smaller particle size increased occluded air content, facilitating greater soymilk flour particle compaction, yielding increased interparticle interactions (i.e., cohesion) and lower bulk density [60]. The particle size distribution of soymilk at several cavitation jet treatment times were shown in Fig. 2. Particle size in untreated cavitation jet soymilk showed a bimodal distribution. The particle size of soymilk shifts to a smaller peak as the cavitation jet duration grows from 0 to 4 min, changing the distribution from bimodal to unimodal. Cavitation jet technology has been proved to be able to improve particle size. First, physical forces like turbulence and shear produced by cavitation jet during the treatment break up fat globules and reduce the size of oil droplets in soymilk [3]). Secondly, cavitating jet treatment facilitated the movement of more soluble proteins in soymilk to the oil–water interface, enhancing the adsorption and directional reordering of soluble protein molecules at the oil–water interface. It declines the interfacial tension of the oil–water interface and adsorbs more oil droplets that caused a reduction of soymilk droplets particle size [77]. This suggests that cavitation jet could decline the soymilk particle size by enhancing the contents of soluble protein and oil/water adsorption efficiency and reducing the size of oil drops of soymilk [73]. The decrease of soymilk particle size not only improves the viscosity and stability of soymilk, but also improve the wetting, dispersion, and solubility of soymilk flour [70]. Soymilk belongs to an unstable colloidal system [53]. The cavitation, collision, and shear forces produced by cavitation jet destroy the hydrophobic interaction between the protein molecules of soymilk. The large protein aggregates, oil droplets, and other aggregates in soymilk are fine-grained, which reduces the particle size of soymilk. Fat globules of soymilk are more tightly wrapped in protein and evenly distributed in soymilk. The uniformity and stability of the soymilk are improved. As the cavitation jet treating time increased from 4 to 6 min, the particle size of soymilk gradually increased. When the treatment time was extended to 8 min, the particle size of soymilk demonstrated bimodal distribution, and compared with the sample treated for 6 min, it showed a left shift, but a second small peak appeared at 2000 nm. Excessive cavitation jet treatment reduces the soluble protein content and protein molecular flexibility of soymilk [77]. It is not conducive to the protein of soymilk adsorption and rearrangement at the oil–water interface, resulting in increasing the particle size of the soymilk droplet. Moreover, excessive cavitation jet treatment could cause the demulsification phenomenon of droplets in soymilk, which promotes the aggregation of oil droplets in soymilk. Thus, the particle size of soymilk increases. This may hurt the wetting, dispersion, and solubility of soymilk flour. These results indicate that cavitation jet treatment with proper time can improve droplet size distribution in soymilk.
Fig. 2.
Effect of cavitation jet treatment time on particle size distribution of soymilk.
3.1.3. Zeta potential
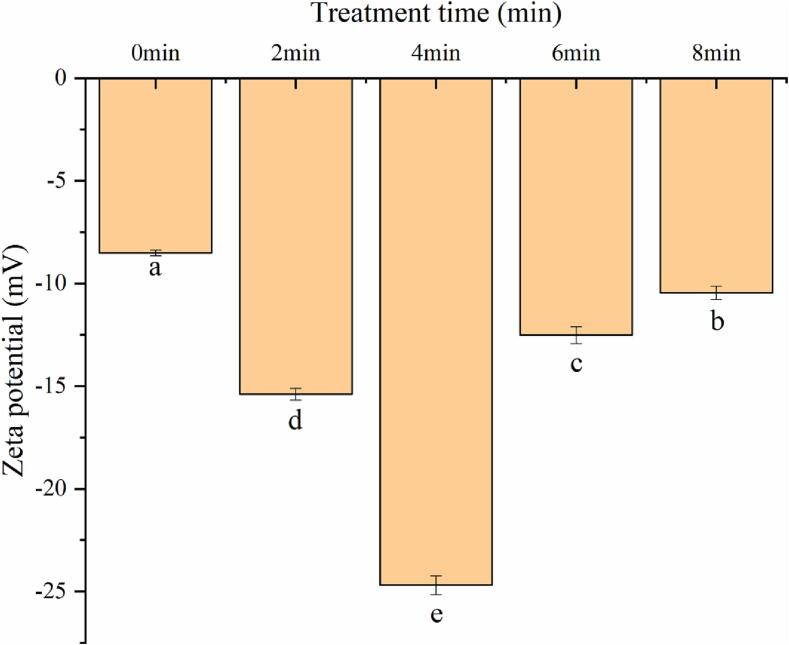
Electrostatic repulsion between soymilk droplets can influence the stability of soymilk which could be estimated based on their zeta potential [47]. High electrostatic repulsion between soymilk droplets is beneficial for stabilizing soymilk [9]. The increase of electrostatic repulsion of soymilk prevents the aggregation between droplets, thus producing soymilk flour particles with uniform distribution. The decrease of electrostatic repulsion leads to the attraction of soymilk droplets through electrostatic interaction. It is difficult for mutually aggregated soymilk droplets to form smaller atomized droplets with a large specific surface area. The water evaporation rate and solubility of soymilk flour particles are seriously affected [26]. The zeta potential of the soymilk after cavitation jet treatment is portrayed in Fig. 3. The lowest absolute value of zeta potential was observed in soymilk before cavitation jet treatment. In comparison, soymilk with cavitation jet treatment exhibited a higher absolute value of zeta potential. With the expansion of cavitation jet time, the absolute value of zeta potential of soymilk increased first and then declined, and reached the maximum value at 4 min. The absolute value of zeta potential increased by cavitation jet treatment for 0–4 min, which might be explained by the fact that shear force and turbulence by cavitation jet treatment promoted the exposure of the charged groups of soymilk [5], [46]. Moreover, the cavitation jet treatment decreased the particle size of soymilk globules and increased the specific surface of soymilk droplets, resulting in the increasing of electrostatic repulsion among soymilk droplets. It is beneficial for enhancing the steadiness of soymilk in the production process [3]. However, treatment of cavitating jet for a too long time can reduce the absolute value of the zeta potential of soymilk. This might be owing to the partial protein aggregation of soymilk caused by extreme cavitation jet treatment, which covered part of charged groups. This would increase the particle size and decrease the interfacial film strength of soymilk, and negatively affect the zeta potential of the soymilk droplets [3], [74]. Overall, cavitation jet treatment with proper time is contributed to improving the electrostatic repulsion and stability of soymilk, which is related to the treating time of the cavitation jet.
Fig. 3.
Effect of cavitation jet treatment time on zeta potential of soymilk.
3.1.4. CLSM analysis
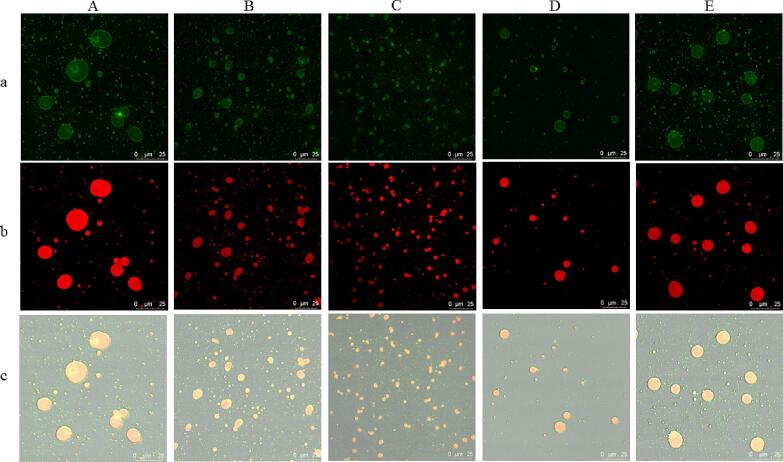
Evenly distributed soymilk droplets are beneficial to produce evenly distributed soymilk flour particles after spray drying. Evenly distributed soymilk flour particles show improved functionalities including better flow properties, higher storage stability, as well as higher mixing capacity, wetting, dispersion, solubility, and controlled release properties [15]. The CLSM micrograph of soymilk is shown in Fig. 4. The soymilk droplets without cavitation jet treatment had large particle size and uneven distribution, and droplet flocculation (aggregation of two or more emulsion droplets to form a floc) and coalescence (merging of droplets) occurred. With the prolongation of cavitation jet treatment time, the soymilk droplets are distributed evenly, and the droplet size decreases gradually. After 4 min of cavitation jet treatment, the size of soymilk droplets is the lowest and the distribution is the most uniform. This is consistent with the results of particle size distribution in 3.1.2. Earlier studies have indicated that cavitation jet treating can improve the flexibility of protein and reduce the interfacial tension by exposing hydrophobic clusters to the exterior of protein molecules [78]. It is beneficial to promote the movement of protein in soymilk to the oil–water interface and increase the content of oil–water interface protein, to form a more uniform and stable soymilk system. Li et al. [39] also showed that cavitation jet treatment can enhance the stability and dispersion characteristics of the emulsion, which may be related to the surface hydrophobicity/hydrophilicity and its aggregation state (or particle size) balance of SPI. According to Stoke law, the creaming rate of oil droplets is proportional to the radius of oil droplets. Smaller droplets contribute to the physical stabilization of emulsion [74]. Hence, cavitation jet treatment can improve emulsion stability by reducing the size of oil droplets. However, when the cavitation jet treatment time was extended, the soymilk droplet size rapidly increased, the dispersion became unstable, and local aggregation started to happen at 8 min. The soymilk had obvious demulsification after long-term cavitation jet treatment, which causes oil droplets to aggregate in the soymilk. This is not conducive to the adsorption and rearrangement of protein in soymilk at the oil–water interface and the rapid formation of viscoelastic protein film, resulting in the inability to form a evenly soymilk system [17], [77]. Therefore, cavitation jet treatment with proper time can uniform distribution of soymilk droplets.
Fig. 4.
Effect of cavitation jet treatment time on confocal laser scanning microscope (CLSM) of soymilk. The image was obtained at 25℃. The bar represents 30 μm. a) Protein Network; b) Fat sphere; c) soymilk particle. A)0min; B) 2 min; C) 4 min; D) 6 min; E) 8 min.
3.1.5. Apparent viscosity
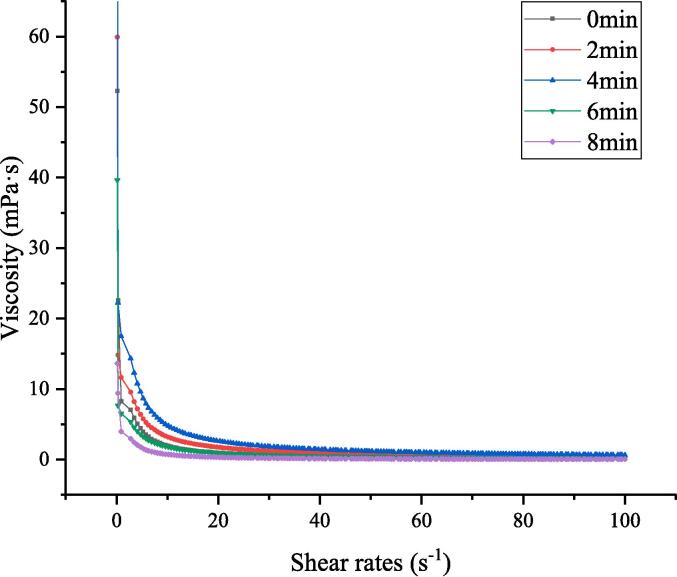
The apparent viscosity of soymilk depends on the composition and the interaction between soymilk droplets. It can affect the solubility of soymilk flour by affecting the spray drying process of soy flour. The too-low viscosity of soymilk will affect the feeding and atomization of soy flour during spray drying, resulting in uneven dispersion and low solubility of soymilk flour particles. Appropriate viscosity is beneficial to feed during spray drying, forming uniform and dispersed soy flour particles, and improving the solubility of soymilk flour [26]). The impact of cavitation treating time on the viscosity of soymilk is shown in Fig. 5. All soymilk were shown to behave non-Newtonian fluids, that shear rate increased, the shear viscosity decreased and the shear thinning pseudoplastic fluid appeared [10]. According to Fig. 5, the viscosity of soymilk increased initially, reduced as the cavitation jet treatment duration grew, and reached its peak at 2 min. According to [79] reported, the ordered protein network structure was conducive to increasing the apparent viscosity of emulsion. Rod-like molecules in proteins tend to overlap and entangle to form the ordered protein network structure, then higher viscosity may be obtained via the steric hindrance and internal friction within the protein chain. The cavitation effect and shear force produced by cavitation jet treatment break the oil droplets of soymilk and expose the flexible interval of the protein in the soymilk. It is beneficial for the combination of protein and fat globules of soymilk to produce a more stable network, which in turn improves the viscosity of soymilk. It has been suggested that potential and viscosity have a specific relationship, which was called the electro-viscous effect [12]. Generally speaking, the more surface charges on particles, the greater the electro-viscosity effect and the higher the viscosity. The cavitation jet treatment for 0–4 min resulted in the improvement of the surface charge of soymilk protein, which affected the protein–protein and protein-solvent interactions and resulted in the increase of viscosity. In addition, Vélez-Erazo et al.[71]showed that the decrease in emulsions particle size was partly responsible for the increase in apparent viscosity. The decrease in soymilk droplet size may lead to an increase in viscosity. After cavitation treatment for 0–4 min, the surface potential of soymilk droplets was boosted, and particle size declined. In these circumstances, the aggregation and flocculation of soymilk droplets are inhibited. Therefore, the greater secondary electroviscous effects, which can arise due to increased overlapping of the electrical double layer and raised the interparticle potential of smaller soymilk particles, may have imparted greater viscosity[18], [38]. In addition, the strong sheer force of the cavitation jet reduces the particle size of oil droplets in soymilk and increases the specific surface area of soymilk droplets, causing the increase of oil–water interface proteins[69]. This is beneficial to the interface protein interacting with water through hydrogen bonds and stabilizing the balance between the aqueous and oil phases of soymilk, promoting the increase of the viscosity of soymilk. These behaviors were consistent with the changes in particle size and zeta potential, confirming that the increase in viscosity is related to the decrease of particle size and the increase in zeta potential. However, long-term cavitation jet treatment causes the apparent viscosity of soymilk to decrease. The decline in viscosity might be associated with the disruption of intermolecular binding among soymilks stimulated by cavitation effect (Ren & Li, 2019). Under the long-term treatment of cavitation jet, the ordered soymilk droplet network is transformed into a disordered structure, which leads to lower viscosity. Moreover, excessive cavitation jet treatment could cause the demulsification phenomenon of droplets in soymilk, which promotes the aggregation of oil droplets in soymilk, resulting in decreased viscosity of soymilk [66]. Therefore, the high shear stress of proper time for cavitation jet treatment may increase the viscosity of soymilk and be beneficial to the spray drying process of soymilk flour.
Fig. 5.
Effect of cavitation jet treatment time on viscosity of soymilk.
3.2. Impact of cavitation jet treating on soymilk flour
3.2.1. Sem
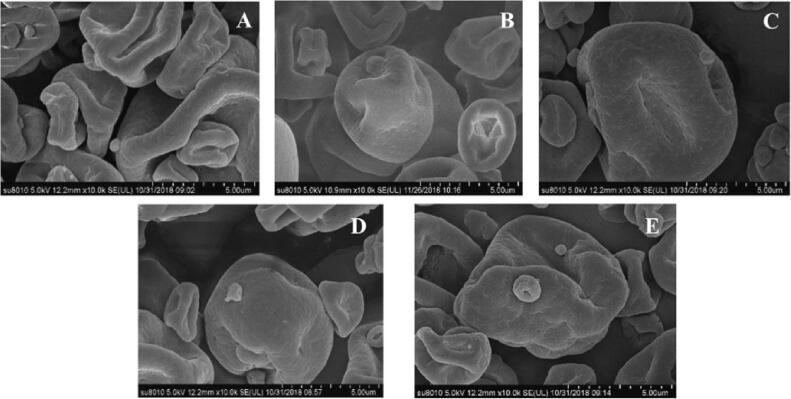
Changes in particle size and shape largely affect the bulk density of a powder [26]. For soymilk flour, due to its shape with small size and wrinkles, the surface properties of these powders allow for extensive van der Waals and electrostatic interactions to occur, leading to such powders having high cohesive strength. This would seriously affect the bulk density and hydration of soymilk flour. Soymilk flour particles with large size and smooth surface are beneficial to improve the bulk density and rehydration of soymilk flour [26]. The microstructure of soymilk flour treated by cavitation jet at various times is displayed in Fig. 6. The microstructure of soymilk flour changed significantly under different cavitation jet treatment time. The soymilk flour without cavitation jet tended to be oblate, with serious surface collapse and wrinkle but no obvious cracks or cracks, and there was obvious aggregation among soymilk flour particles. With the treating time of the cavitation jet prolonged to 4 min, the soymilk flour particles gradually tend to spherical structure, the agglomeration phenomenon among soymilk flour particles gradually decreased, and the surface collapse and wrinkling were weakened. In the case of spray drying, the soymilk droplets encounter hot and dehydrated air and the soymilk droplets shrink due to the high flux leaving the droplets [68], [81]. The complex interaction among the changes of water diffusion rate of soymilk droplets, surface tension of the air–liquid interface, and interaction force of interface film will affect the shape and surface morphology of soymilk flour [23]. Proper cavitation jet treatment reduces the particle size of soymilk and forms a dense protein film by increasing the content of soluble protein and the oil/water adsorption efficiency [24]. By increasing the electrostatic repulsion between soymilk droplets, the thickness of protein film at the interface of soymilk can be increased, and soymilk droplets with uniform distribution and higher stability can be formed, which is beneficial to improve the diffusion coefficient of water in soymilk. The interfacial protein film has an extraordinarily strong concentration-dependent water diffusivity [81]. When the protein content and the thickness of the interfacial protein film increase, it is valuable to increase the amplitude of water diffusivity and hasten the evaporation of water. This is conducive to promoting the centrifugal force of spray drying chamber to overcome the surface tension of soymilk droplets, forming small atomized droplets with large specific surface areas and uniform distribution. The small atomized droplets extended the residence time in the spray dehydration chamber and accelerated the heat transfer between the droplets and the drying medium [8]. It accelerates the diffusion of water inside the atomized droplet and the evaporation of water on the droplet’s surface. The increase in viscosity of soymilk is conducive to slow entry into the dehydration chamber, and the number of atomized droplets produced by the atomizer tends to be constant per unit of time. The centrifugal force and heat generated by the spray dryer meet the heat required for the evaporation of soymilk droplets. Soymilk droplets can be fully atomized and formed to disperse into uniform small atomized droplets. Therefore, the surface layer of soymilk flour particles can quickly form a hard protein film. It prevents the protein particles from continuously shrinking, forming a smooth surface and uniform distribution of soymilk flour particles. Nevertheless, when the treating time of cavitation jet continues to be prolonged to 8 min, the surface of soymilk flour particles collapses seriously and the folds deepen again. The number of small soymilk flour particles increases, and the particle size distribution of soymilk is uneven. This phenomenon was due to the “over-treatment effect” caused by too long-time cavitation jet on soymilk [74]. Excessive cavitation jet treatment caused the rise of soymilk particle size and uneven distribution, and the decline of electrostatic repulsion force and interfacial film strength. The decrease of the interfacial protein film strength of soymilk droplets decreases the diffusion rate of protein-water in soymilk and exposes oil droplets, which leads to adhesion between soymilk droplets. This is not conducive to the formation of uniformly distributed small atomized droplets and the outward diffusion of water inside the atomized droplets during the atomization process. In addition, excessive cavitation jet treatment has a negative impact on the viscosity of soymilk and the surface tension of soymilk droplets. The decrease in viscosity and the rise of surface tension led to the increasing of soymilk flow into the dehydrating chamber, and the number of atomized droplets produced by the atomizer per unit of time increased [14]. As a result, the soymilk droplets are not atomized enough and it is difficult to disperse into small atomized droplets. Confused atomized droplets may lead to incomplete evaporation of water in large atomized droplets, while small atomized droplets are excessively dry [70]. Excessively dry small soymilk flour particles are easy to adhere to the surface of large particles, resulting in uneven distribution of particles. In addition, the decrease in the water diffusion rate of atomized droplets will lead to the inconsistency of the water evaporation rate inside and outside droplets [82]. The water on the surface of soymilk droplets rapidly evaporates and the surface wrinkles deepen. Therefore, proper time for cavitation jet treatment of soymilk is helpful to improve the appearance of soymilk flour particles.
Fig. 6.
Effect of cavitation jet treatment time on scanning electron microscope (SEM) of soymilk flour. A) 0 min; B) 2 min.; C) 4 min.; D) 6 min.; E) 8 min.
3.2.2. Particle size
The particle size distribution formed soymilk flour during spray drying and the relationship between the particle size affect the wettability, dispersibility, and solubility characteristics of soymilk flour [26]. The most traditional approach to increase a powder's solubility is to increase the size of the particles that make up a powders bulk, allowing the forces encouraging movement (such as gravity or pressurised air) to outweigh the forces of cohesion, thereby, allowing the powder particles to soluble. Larger particle structures contain increased volumes of interstitial air in the form of capillaries, increasing the powder's bulk density and allowing for easier penetration and movement of water into and through the particle structures through capillary action on rehydration [26]. The particle size of soymilk flour achieved by spray drying is tabularized in Table 1, including the average particle diameter (D50), the equivalent diameter (D10), the equivalent diameter (D90), and the volume average particle size. After cavitation jet treatment, the average particle size of soymilk flour rose at first and then declined and reached the maximum at 4 min. On the contrary, the particle size distribution of soymilk flour decreased at first and then expanded, achieving the minimum level at 4 min. The strong shear force and cavitation effect of the cavitation jet form a denser protein film on the surface of soymilk droplets, improving the electrostatic repulsion among droplets. To avoid soymilk droplets from sticking together, soymilk is supplied into the atomizer from the center and then flows radially outward and towards the atomizer's edge as a thin film of liquid that is dispersed into tiny individual atomizing droplets at the wheel's edge [43]. During the spray-drying process, these atomizing droplets turn into individual powder particles as a result of the water in the primary drier chamber evaporating [51]. The term repolymerization (mainly used within the food industry) is used to describe the processes of increasing the particle size of a powder by combining numerous individual primary powder particles into large cluster-like structures where the individual particle may still be distinguishable. These clusters of particles are of much greater size than that of the original particle [26]. The process of repolymerization of soymilk flour particles occurs in the primary drier chamber. Repolymerization process promotes the particle size of soymilk flour to increase, which is caused by repolymerization between small atomizing droplets or repolymerization between small atomizing droplets and completely dried particles [33]. The size of atomized droplets is particularly important for the repolymerization process of soymilk flour particles. The size of atomized droplets produced by atomization strongly depends on the inherent properties of soymilk [61]. After cavitation jet treatment for 0–4 min, the particle size of soymilk drops decreased and the distribution was uniform. This is conducive to the creation of tinier and dispersed atomized droplets in the atomization chamber. Small atomized droplets accelerate the evaporation of water on the surface of soymilk droplets. These changes are beneficial to increase the collision probability and repolymerize between atomized liquid droplets, forming soymilk flour particles with large particle size and uniform distribution. However, further prolonging the treatment time of the cavitation jet lead to a decrease of soymilk viscosity, the increase in soymilk droplet size and uneven distribution [32]. As a result, soymilk droplets are fed too fast in the spray drying process, and it is difficult to form smaller and more uniform atomized droplets. The internal and external water evaporation of larger atomized droplets is inconsistent, which is not conducive to the repolymerize between particles and leads to the particle size reduction of soymilk flour [32]. Uneven distribution of soymilk droplets forms atomized droplets with different sizes during spray drying. The large atomize droplets and the small atomize droplets have different rates of moisture drying [60]. This results in different degrees of drying between the droplets and the formation of different sized soymilk flour particles. [13] research shows that small and uneven distribution of powder particles may have a negative impact on the edible quality of powder. Cavitation jet treatment with proper time to soymilk changes the edible quality by varying the particle size characteristics of soymilk flour.
Table 1.
Effect of cavitation jet treatment on the particle size of soymilk flour.
| Treatment time of cavitation jet(min) | Particle size-D10(μm) | Particle size-D50(μm) | Particle size-D90(μm) | Volume average particle size(μm) | Particle size distribution |
|---|---|---|---|---|---|
| 0 | 0.87 ± 0.02d | 2.67 ± 0.03d | 9.76 ± 0.11e | 4.40 ± 0.05d | 3.33 ± 0.02a |
| 2 | 1.34 ± 0.04a | 6.33 ± 0.05a | 17.99 ± 0.03a | 6.43 ± 0.08c | 2.63 ± 0.03b |
| 4 | 1.19 ± 0.05b | 5.70 ± 0.16b | 12.87 ± 0.11c | 7.95 ± 0.07a | 2.05 ± 0.08e |
| 6 | 0.97 ± 0.06c | 5.11 ± 0.09c | 12.26 ± 0.23d | 6.95 ± 0.08b | 2.21 ± 0.02d |
| 8 | 0.94 ± 0.06cd | 5.05 ± 0.08c | 13.77 ± 0.17b | 6.59 ± 0.16c | 2.54 ± 0.01c |
Note: Comparisons were carried out between values of the same column; values with a different letter(s) indicate a significant difference at p ≤ 0.05.
3.2.3. Bulk density
The bulk density is a significant parameter for soymilk flour quality as it modifies the fluidity and solubility of soymilk flour. The low bulk density leads to the agglomeration and oxidation of soymilk flour particles, and it is difficult to flow. Appropriately increasing the bulk density of soymilk flour can prevent agglomeration and oxidation among soymilk flour particles, which improves the solubility and fluidity of soymilk flour [35]. The bulk density of soymilk flour after spray drying of soymilk treated with different cavitation jet time is shown in Table 2. The bulk density of soymilk flour is affected by many factors, comprising particle size, particle size distribution, shape, and friction between particles. With the rise of the treatment time of the cavitation jet, the bulk density of the soymilk flour firstly increases and then decreases and reaches the maximum at 4 min. Tan et al.' s (2015) research shows that large particles with approximate spherical shape, smooth surface, and uniform distribution contributed to increased bulk density. Such favorable morphological features would be beneficial to the orderly accumulation of particles, reducing the gap between particles and improving the bulk density. After cavitation jet treatment for 0–4 min, soymilk flour particles tend to be spherical, with smooth surface, increased particle size, and uniform distribution. We speculate that one of the reasons for the high bulk density of soymilk flour might be favorable morphological features. Additionally, [50] claimed that the particle size distribution is also one of the significant factors affecting the bulk density of particle. Particle size distribution with high proportion of large particles and low proportion of small particles contributes to improving bulk density. The small particles can be tightly filled between the large ones and the gap between particles is reduced, which improves the bulk density. Combined with results in 3.2.2, it shows that cavitation jet treating has changed the particle size distribution of soymilk flour and increased the proportion of large soymilk flour particles. This might also be one of the key reasons for increasing the bulk density of soymilk flour. Therefore, proper cavitation jet treatment improves the bulk density by changing the particle size, and particle size distribution morphology of soymilk flour. However, long-term cavitation jet treatment will cause the bulk density of soymilk flour particles to decrease. With the time of cavitation jet treatment prolonged to 8 min, the particle size and sphericity of soymilk flour decreased, and the surface of the particle was wrinkled again. The decrease in the particle size of soymilk flour leads to the increase in the number of small particles in soymilk flour. Small particles tend to stick to one another leading to not being conducive to filling [2]. The sticked soymilk flour particles lead to the separation of the filled soymilk flour particles, which makes the filling structure looser and reduces the bulk density. The wrinkles on the surface of soymilk flour particles increase the friction between particles and hinder the orderly arrangement of particles, which is not conducive to the close filling of soymilk flour particles.
Table 2.
Effect of cavitation jet treatment on the particle size of soymilk flour.
| Treatment time of cavitation jet(min) | Bulk density(g/cm3) | Dispersibility(s) | Wettability(s) |
|---|---|---|---|
| 0 | 0.2067 ± 0.0010d | 70.0 ± 2.0a | 127.3 ± 2.5a |
| 2 | 0.2181 ± 0.0016c | 65.3 ± 2.5b | 112.7 ± 4.7b |
| 4 | 0.2529 ± 0.0052a | 55.7 ± 2.1c | 84.7 ± 2.1c |
| 6 | 0.2320 ± 0.0022b | 53.3 ± 1.5c | 87.7 ± 4.0c |
| 8 | 0.2350 ± 0.0037b | 68.6 ± 0.5ab | 117.7 ± 2.5b |
Note: Comparisons were carried out between values of the same column; values with a different letter(s) indicate a significant difference at p ≤ 0.05.
3.2.4. Wettability and dispersibility
The wettability and dispersibility characteristics of powdered foods are important during the hydration process. The solubility of soymilk flour depends greatly on its wettability and dispersibility[20]. The wettability and dispersibility of soymilk flour treated with different cavitation jet time after spray drying are demonstrated in Table 2. The shorter the wetting and dispersion time per unit mass for soymilk flour, the better wettability and dispersibility of soymilk flour. With the treatment time of cavitation jet lasting to 8 min, the wettability and dispersity of soymilk flour particles first decreased and then increased. This change is probably the result of a combination of cavitation jet and spray drying process. Cavitation effect and high shear force cause the conformational changes of protein in soymilk, such as loose structure, dissociation, and unfolding, increasing the flexibility of protein in soymilk with great affinity to water. This could favor the formation of protein shells with higher affinity to water on the surface of soymilk flour particles. In addition, the turbulent force of cavitation jet promoted the repolymerize process of soymilk droplets during the spray drying process to form large soymilk flour particles. The adhesion between large soymilk flour particles is smaller, which increases the fluidity of the particles [61]. This is beneficial to the diffusion of water molecules to the surface of soymilk flour particles and moistens them, and enhances the dispersibility of soymilk flour particles in water. Mcsweeney et al. (2021b) showed that large particle size of powder improves the wettability and dispersibility. Cavitation jet treatment for 0–4 min is favored to form smooth spherical and evenly distributed soymilk flour particle. The improvement of the morphology of soymilk flour particles is beneficial to reduce the friction between soymilk flour particles, which increases the spreading speed of water molecules on the surface of soymilk flour and the wetting rate inside soymilk flour [67]. Therefore, the wettability and dispersibility of soymilk flour were improved. On the contrary, when the treatment time of cavitation jet extended to 8 min, the droplet size of soymilk increased and droplet aggregation occurred. The soymilk flour particles have smaller size, wrinkled surface, and uneven distribution after spray drying. It is reported that uneven distribution of particles and wrinkles on the surface of particles is considered to have a negative impact on wettability [2]. A large proportion of small particles and a small proportion of large particles lead to the adhesion of soymilk flour particles. High adhesion of soymilk flour has a lower affinity for and interaction with water, which is not conducive to the spreading and wetting of water molecules on the surface of soymilk flour particles. Additionally, the wrinkles on the surface of soymilk flour particles and the smaller particles have a larger specific surface area. It has stronger adsorption on gas and forms gas film on the surface of particles [65]. This finally hinders the contact of water molecules and soymilk flour particles, resulting in lower wettability and dispersibility of soymilk flour particles.
3.2.5. Solubility
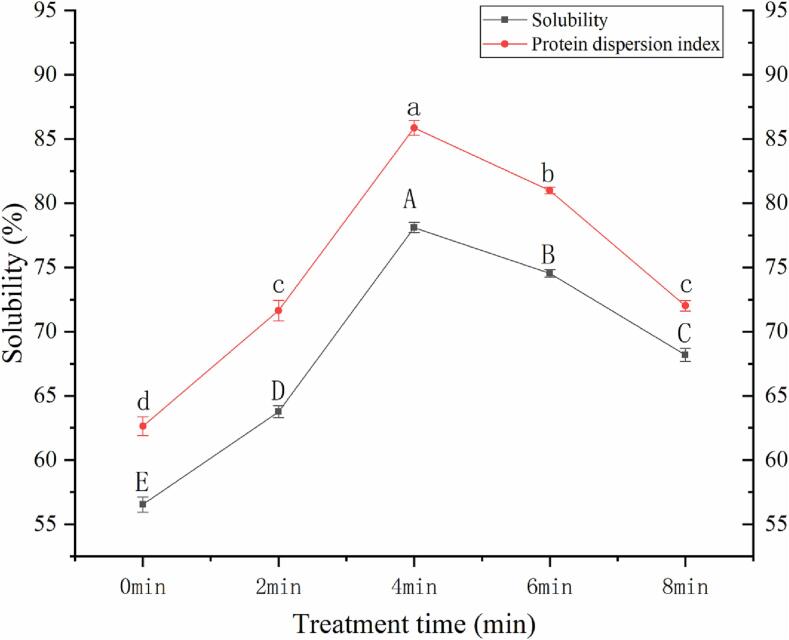
Solubility is an important index of the quality of soymilk flour, and it is the basis for evaluating the instant soluble of soymilk flour [19]. Protein Dispersion Index (PDI) refers to the percentage of protein that can be dispersed in water over the total proteins of soymilk flour, which is used to characterize the solubility of the protein in soymilk flour [2]. Generally, the PDI and solubility of soymilk flour particles not only depend on the particle size and particle morphology of soymilk flour particles, but also depend on the interaction of protein on the surface of soymilk flour with water [61]. The effect of cavitation jet treatment time on the solubility and protein dispersion index of soymilk flour is shown in Fig. 7. The solubility and PDI of soymilk flour without cavitation jet treatment showed the lowest value. With the prolongation of cavitation jet treatment up to 4 min, the PDI and solubility of soymilk flour increased obviously. It is worth mentioning that the solubility of soymilk flour is highly related to the protein dispersion index [2]. Cavitation jet treatment for 0–4 min raises the particle size of soymilk flour and makes the particle distribution of soymilk flour more uniform. The protein dispersion index of soymilk flour exhibited the same trend. The large soymilk flour particles treated by cavitation jet for 4 min shows the highest protein dispersion index, which increases the affinity between the protein shell of soymilk flour and water. This could be one of the reasons for improving the solubility of soymilk flour particles. Secondly, cavitation jet treatment for 0–4 min prevents the exposure of insoluble substances such as oil droplets to the surface of soymilk flour particles after spray drying, and forms soymilk flour with a complete surface. The wettability will be improved by the decrease of lipid content and the improvement of shape of soymilk flour. It is easy for water molecules to diffuse into the interior of the soymilk flour particles, which improves the solubility of soymilk flour particles [59]. However, the dispersion index and solubility of protein decreased when the cavitation treatment time was further extended to 8 min. The “over-treatment effect” produced by long-time cavitation jet treatment leads to the particle size reduction of soymilk flour after spray drying and the surface wrinkling of soymilk flour. Adhesion becomes dominant over gravity for small soymilk flour particles, which leads to agglomeration by gravity. It disfavors water molecules transfer to the interiors of soymilk flour particles, and prolongs the wetting and dispersion time of soymilk flour in water. In addition, the soymilk flour particles with smaller particle size have a lower protein dispersion index [55]. Small soymilk flour particles with low protein dispersion index may form insoluble hydration film when contacting with water, which is not conducive to the interaction between the protein shell of soymilk flour particles and water [49], [48]. The apparent morphology of soymilk flour particles will also affect its solubility. Soymilk flour particles full of wrinkles increase the friction and cohesion between powder particles through the interlocking effect of particles, resulting in the decrease of solubility of soymilk flour particles ([35]; Cavender et al., 2021). Therefore, proper jet treatment improves the protein dispersion index and solubility by enhancing the affinity between soymilk flour particles and water molecules.
Fig. 7.
Effect of cavitation jet treatment time on protein dispersion index and solubility of soymilk flour.
4. Conclusion
The present study demonstrates that cavitation jet treatment can change the protein conformation of soymilk for improving the instant solubility of soymilk flour. Depending on the increasing treatment time, higher soluble protein content, smaller particle size, more uniform distribution, and higher viscosity were determined in the cavitation jet treated soymilk. Soymilk flour produced with cavitation jet-treated soymilk at 4 min has larger particle size, higher bulk density, wettability, dispersibility, solubility, and better apparent morphology than those produced with non-cavitation jet treated. Therefore, the instant solubility of soymilk flour was improved. In conclusion, the cavitation jet treatment can be an effective method for significantly improving the instant solubility of soymilk flour.
Funding
The authors would like to thank the National Major Research and Development Plan (2022YFF1100603), Heilongjiang Province Key R&D Plan (GY2021ZB0204), Heilongjiang Province Million Project (2021ZX12B02), Shandong Province Major Research and Development Plan (2022CXGC010603), National Major Research and Development Plan (2021YFD2100401), National Natural Science Fund (3220228), Heilongjiang Province Major Research and Development Plan (GA21B001), Heilongjiang Province Support Major Scientific and Technological Achievements Transformation Project (CG19A002), Harbin Manufacturing Science and Technology Innovation Talents Project (2022HBRCCGO05), and China postdoctoral Project (2022M721995) for the support.
CRediT authorship contribution statement
Qi Gong: Conceptualization, Software, Writing – original draft. Caihua Liu: Data curation. Yachao Tian: Methodology. Yuxuan Zheng: Investigation. Libin Wei: Visualization. Tianfu Cheng: Investigation. Zhongjiang Wang: Investigation. Zengwang Guo: Supervision. Linyi Zhou: Funding acquisition, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Zengwang Guo, Email: gzwname@163.comdor.
Linyi Zhou, Email: isneau@126.com.
References
- 1.Adrian R. Modification of the soluble protein content of heat-processed soybean flour. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2010;38:2. [Google Scholar]
- 2.Adjei-Fremah S., Worku M., De Erive M.O., He F., Wang T., Chen G. Effect of microfluidization on microstructure, protein profile and physicochemical properties of whole cowpea flours. Innov. Food Sci. Emerg. Technol. 2019;57:102207. [Google Scholar]
- 3.Ahmed T., Hu T., Zhang Z., Bakry A., Khalifa I., Pan P., Hu H. Effect of different oils and ultrasound emulsification conditions on the physicochemical properties of emulsions stabilized by soy protein isolate. Ultrason. Sonochem. 2018;49:283–293. doi: 10.1016/j.ultsonch.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Ahrné L., Larsen F., Flemming L., Anni H., Richard I. Physical and functional properties of cheese powders affected by sweet whey powder addition before or after spray drying. Powder Technol. Internat. J. Sci. Technol. Wet Dry Part. Syst. 2018;323:139–148. [Google Scholar]
- 5.Amiri A., Sharifian P., Soltanizadeh N., Parisa S., Nafiseh. Application of ultrasound treatment for improving the physicochemical, functional and rheological properties of myofibrillar proteins. Internat. J. Biol. Macromol. Struct. Funct. Interact. 2018;111:139–147. doi: 10.1016/j.ijbiomac.2017.12.167. [DOI] [PubMed] [Google Scholar]
- 6.Anema S.G., Pinder D.N., Hunter R.J., Hemar Y. Effects of storage temperature on the solubility of milk protein concentrate (MPC85) Food Hydrocoll. 2006;20(2):386–393. [Google Scholar]
- 7.Augustin M.A., Sanguansri P., Williams W., Andrews H. High shear treatment of concentrates and drying conditions influence the solubility of milk protein concentrate powders. J. Dairy Res. 2012;79(4):459–468. doi: 10.1017/S0022029912000489. [DOI] [PubMed] [Google Scholar]
- 8.Brishti F.H., Chay S.Y., Muhammad K., Ismail-Fitry M.R., Zarei M., Karthikeyan S., Saari N. Effects of drying techniques on the physicochemical, functional, thermal, structural and rheological properties of mung bean (Vigna radiata) protein isolate powder. Food Res. Int. 2020;138:109783. doi: 10.1016/j.foodres.2020.109783. [DOI] [PubMed] [Google Scholar]
- 9.Cavender G., Jiang N., Singh R.K., Chen J., Solval K.M. Improving the survival of lactobacillus plantarum nrrl b-1927 during microencapsulation with ultra-high-pressure-homogenized soymilk as a wall material. Food Res. Int. 2020;139(2) doi: 10.1016/j.foodres.2020.109831. [DOI] [PubMed] [Google Scholar]
- 10.Cai Z.X., Zhang F., Jin Q., Li X., Yadav M.P., Zhang H.B. A comparison of corn fiber gum, hydrophobically modified starch, gum arabic and soybean soluble polysaccharide: Interfacial dynamics, viscoelastic response at oil/water interfaces and emulsion stabilization mechanisms. Food Hydrocoll. 2017;70:329–344. [Google Scholar]
- 11.Cano-Chauca M., Stringheta P.C., Ramos A.M., Cal-Vidal J. Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov. Food Sci. Emerg. Technol. 2005;6(4):420–428. [Google Scholar]
- 12.Ciron C.I.E., Gee V.L., Kelly A.L., Auty M.A.E. Comparison of the effects of high-pressure microfluidization and conventional homogenization of milk on particle size, water retention and texture of non-fat and low-fat yoghurts. nternational Dairy Journal. 2010;20(5):314–320. [Google Scholar]
- 13.Cruz-Tirado J.P., Martins J.P., Olmos B.D.F., Condotta R., Kurozawa L.E. Impact of glass transition on chemical properties, caking and flowability of soymilk powder during storage. Powder Technol. 2021;386:20–29. [Google Scholar]
- 14.Cui R., Zhu F. Effect of ultrasound on structural and physicochemical properties of sweetpotato and wheat flours. Ultrason. Sonochem. 2020;66:105118. doi: 10.1016/j.ultsonch.2020.105118. [DOI] [PubMed] [Google Scholar]
- 15.Cuq B., Mandato S., Jeantet R., Saleh K., Ruiz T. Agglomeration/ granulation in food powder production. Handbook Food Powders. 2013:150–177. [Google Scholar]
- 16.Deng X., Ma Y., Lei Y., Zhu X., Zhang L., Hu L., Lu S., Guo X., Zhang J. Ultrasonic structural modification of myofibrillar proteins from Coregonus peled improves emulsification properties. Ultrason. Sonochem. 2021;76:105659. doi: 10.1016/j.ultsonch.2021.105659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickinson E. Biopolymer-based particles as stabilizing agents for emulsions and foams. Food Hydrocoll. 2016;68:219–231. [Google Scholar]
- 18.Dissanayake M., Liyanaarachchi S., Vasiljevic T. Functional properties of whey proteins microparticulated at low pH. J. Dairy Sci. 2012;95(4):1667–1679. doi: 10.3168/jds.2011-4823. [DOI] [PubMed] [Google Scholar]
- 19.Fernandes R.V.B., Borges S.V., Silva E.K., Silva Y.F., Souza H.J.B., Carmo E.L., Oliveira C.R., Yoshida M.I., Botrel D.A. Study of ultrasound-assisted emulsions on microencapsulation of ginger essential oil by spray drying. Ind. Crop. Prod. 2016;94:413–423. [Google Scholar]
- 20.Gagneten M., Corfield R., Mattson M.G., Sozzi A., Leiva G., Salvatori D., Schebor C. Spray-dried powders from berries extracts obtained upon several processing steps to improve the bioactive components content. Powder Technol. 2019;342:1008–1015. [Google Scholar]
- 21.Gallego-Juárez J.A., Elvira-Segura L., Rodríguez-Corral G. A power ultrasonic technology for deliquoring. Ultrasonics. 2003;41(4):255–259. doi: 10.1016/s0041-624x(02)00449-3. [DOI] [PubMed] [Google Scholar]
- 22.Geremias-Andrade I.M., Souki N.P.D.B.G., Moraes C.F., Pinho S.C. Rheological and mechanical characterization of curcumin-loaded emulsion-filled gels produced with whey protein isolate and xanthan gum. LWT. 2017;86:166–173. [Google Scholar]
- 23.Gong K., Shi A.M., Liu H.Z., Liu L., Hu H., Adhikari B., Wang Q. Emulsifying properties and structure changes of spray and freeze-dried peanut protein isolate. J. Food Eng. 2016;170:33–40. [Google Scholar]
- 24.Goyal A., Sharma V., Sihag M.K., Tomar S.K., Arora S., Sabikhi L., Singh A.K. Development and physico-chemical characterization of microencapsulated flaxseed oil powder: A functional ingredient for omega-3 fortification. Powder Technol. 2015;286:527–537. [Google Scholar]
- 25.Guo Y., Li B., Cheng T., Hu Z., Liu S., Liu J., Sun F., Guo Z., Wang Z. Effect of cavitation jet on the structural, emulsifying properties and rheological properties of soybean protein-oxidised aggregates. Int. J. Food Sci. Technol. 2022;58:343–354. [Google Scholar]
- 26.Hazlett R., Schmidmeier C., O’Mahong J.A. Approaches for improving the flowability of high-protein dairy powders post spray drying – A review. Powder Technol. 2021;388:26–40. [Google Scholar]
- 27.Hu H., Wu J., Li-Chan E.C.Y., Zhu L., Zhang F., Xu X., Fan G., Wang L., Huang X., Pan S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013;30(2):647–655. [Google Scholar]
- 28.He M., Wu C., Li L., Zheng L.i., Tian T., Jiang L., Li Y., Teng F. Effects of cavitation jet treatment on the structure and emulsification properties of oxidized soy protein isolate. Foods. 2020;10(1):2. doi: 10.3390/foods10010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jafari S.M., Assadpoor E., He Y., Bhandari B. Re-coalescence of emulsion droplets during high-energy emulsification. Food Hydrocoll. 2008;22(7):1191–1202. [Google Scholar]
- 31.Jiang S., Ding J., Andrade J., Rababah T.M., Almajwal A., Abulmeaty M.M., Feng H. Modifying the physicochemical properties of pea protein by pH-shifting and ultrasound combined treatments. Ultrason. Sonochem. 2017;38:835–842. doi: 10.1016/j.ultsonch.2017.03.046. [DOI] [PubMed] [Google Scholar]
- 32.Jin H., Sun S., Sun Z., Wang Q.i., Jin Y., Sheng L. Ultrasonic-assisted spray drying as a tool for improving the instant properties of egg white powder. Food Struct. 2022;33:100289. [Google Scholar]
- 33.Jinapong N., Suphantharika M., Jamnong P. Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J. Food Eng. 2008;84(2):194–205. [Google Scholar]
- 35.Jovanovic,M., Jovanović , M., Ćujić-Nikolić ,N., Drinić ,Z., Janković,T., Marković ,S., Petrović ,P., & Šavikin a,K. (2021). Spray drying of Gentiana asclepiadea L. root extract: Successful encapsulation into powders with preserved stability of bioactive compounds. Industrial Crops and Products, 172-172.
- 36.Juliane F., Desrumaux A., Lardières J. Effect of high-pressure homogenization on droplet size distributions and rheological properties of model oil-in-water emulsions. Innov. Food Sci. Emerg. Technol. 2000;1(2):127–134. [Google Scholar]
- 38.Kzab C., Mao Z., Huang Y., Yun Y., Huang C., Guo Y., Ren X., Liu C. Ultrasonic assisted water-in-oil emulsions encapsulating macro-molecular polysaccharide chitosan: Influence of molecular properties, emulsion viscosity and their stability. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2020.105018. [DOI] [PubMed] [Google Scholar]
- 39.Li Q., Zhang J., Ge G., Zhao M., Sun W. Impact of heating treatments on physical stability and lipid-protein co-oxidation in oil-in-water emulsion prepared with soy protein isolates. Food Hydrocoll. 2019;100 [Google Scholar]
- 40.Li Y.-T., Chen M.-S., Deng L.-Z., Liang Y.-Z., Liu Y.-K., Liu W., Chen J., Liu C.-M. Whole soybean milk produced by a novel industry-scale micofluidizer system without soaking and filtering. J. Food Eng. 2021;291:110228. [Google Scholar]
- 41.Li Y., Wan Y., Mamu Y., Liu X., Guo S. Protein aggregation and Ca∼(2+)-induced gelation of soymilk after heat treatment under slightly alkaline conditions. Food Hydrocoll. 2022;10274:1–11. [Google Scholar]
- 42.Li L., Zhou Y., Teng F., Guo Z., Tian T., Wang Z. Effects of jet cavitation on maillard reaction and conjugate structure and emulsifying properties of soy protein isolate. Trans. Chin. Soc. Agricul. Machinery. 2019;50(8):372–378. [Google Scholar]
- 43.Lima D., Busanello M., Prudencio S.H., Garcia S. Soymilk with okara flour fermented by Lactobacillus acidophilus : Simplex-centroid mixture design applied in the elaboration of probiotic creamy sauce and storage stability. LWT- Food Sci. Technol. 2018;93:339–345. [Google Scholar]
- 45.Lowry OliverH., Rosebrough NiraJ., Farr A.L., Randall RoseJ. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 46.Matsumiya K., Takahashi W., Inoue T., Matsumura Y. Effects of bacteriostatic emulsifiers on stability of milk-based emulsions. J. Food Eng. 2010;96(2):185–191. [Google Scholar]
- 47.McClements D.J., Rao J. Food-grade nanoemulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011;51(4):285–330. doi: 10.1080/10408398.2011.559558. [DOI] [PubMed] [Google Scholar]
- 48.McSweeney D.J., Maidannyk V., O'Mahony J.A., McCarthy N.A. Influence of nitrogen gas injection and agglomeration during spray drying on the physical and bulk handling properties of milk protein concentrate powders. J. Food Eng. 2021;293:110399. [Google Scholar]
- 49.McSweeney D.J., Maidannyk V., O'Mahony J.A., McCarthy N.A. Rehydration properties of regular and agglomerated milk protein concentrate powders produced using nitrogen gas injection prior to spray drying. J. Food Eng. 2021;305:110597. [Google Scholar]
- 50.Mcsweeney D.J., O'Mahony J.A., Mccarthy N.A. Strategies to enhance the rehydration performance of micellar casein-dominant dairy powders. Int. Dairy J. 2021;122:122. [Google Scholar]
- 51.Moghaddam A.D., Pero M., Askari G.R.J. Optimizing spray drying conditions of sour cherry juice based on physicochemical properties, using response surface methodology (RSM) J. Food Sic. Technol. 2017;54(1):174–184. doi: 10.1007/s13197-016-2449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morales-de la Peña M., Martín-Belloso O., Welti-Chanes J. High-power ultrasound as pre-treatment in different stages of soymilk manufacturing process to increase the isoflavone content. Ultrason. Sonochem. 2018;49:154–160. doi: 10.1016/j.ultsonch.2018.07.044. [DOI] [PubMed] [Google Scholar]
- 53.Mu Q., Su H., Zhou Q.i., Xiao S., Zhu L., Xu X., Pan S., Hu H. Effect of ultrasound on functional properties, flavor characteristics, and storage stability of soybean milk. Food Chem. 2022;381:132158. doi: 10.1016/j.foodchem.2022.132158. [DOI] [PubMed] [Google Scholar]
- 54.O'Sullivan J.J., Norwood E.-A., O'Mahony J.A., Kelly A.L. Atomisation technologies used in spray drying in the dairy industry: A review. J. Food Eng. 2019;243:57–69. [Google Scholar]
- 55.Park C.W., Drake M.A. The effect of homogenization pressure on the flavor and flavor stability of whole milk powder. J. Dairy Sci. 2017;100(7):5195–5205. doi: 10.3168/jds.2017-12544. [DOI] [PubMed] [Google Scholar]
- 56.Pathania S., Ho Q.T., Hogan S.A., Mccarthy N., Tobin J.T. Applications of hydrodynamic cavitation for instant rehydration of high protein milk powders. J. Food Eng. 2018;225:18–25. [Google Scholar]
- 57.Radha C., Prakash V. Structural and functional properties of heat-processed soybean flour: effect of proteolytic modification. Food Sci. Technol. Int. 2009;15(5):453–463. [Google Scholar]
- 58.Ren X., Li C., Yang F., Huang Y., Huang C., Zhang K., Yan L. Comparison of hydrodynamic and ultrasonic cavitation effects on soy protein isolate functionality. J. Food Eng. 2019;265:109697. [Google Scholar]
- 59.Sert D., Mercan E. Production and characterisation of goat milk powder made from sonicated whole milk concentrates. Int. Dairy J. 2022;129:129. [Google Scholar]
- 60.Singh P., Bilyeu L., Krishnaswamy Spray drying process optimization: Drought resistant variety (W82) soymilk powder using response surface methodology (RSM) LWT. 2022;166 [Google Scholar]
- 61.Song B.o., Yao P., Zhang Y., Pang X., Zhang S., Lv J. Ultrasound pretreatment prior to spray drying improve the flowability and water sorption properties of micellar casein concentrate. Ultrason. Sonochem. 2022;87:106049. doi: 10.1016/j.ultsonch.2022.106049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmidmeier C., Wen Y., Drapala K.P., Dennehy T., McGuirke A., Cronin K., O'Mahony J.A. The effect of agglomerate integrity and blending formulation on the mechanical properties of whey protein concentrate powder tablets. J. Food Eng. 2019;247:160–167. [Google Scholar]
- 63.Sun J., Mu Y., Jing H., Obadi M., Xu B. Effects of single- and dual-frequency ultrasound on the functionality of egg white protein. J. Food Eng. 2020;277 [Google Scholar]
- 64.Yanjun S., Jianhang C., Shuwen Z., Hongjuan L.i., Jing L.u., Lu L., Uluko H., Yanling S.u., Wenming C., Wupeng G.e., Jiaping L.v. Effect of power ultrasound pre-treatment on the physical and functional properties of reconstituted milk protein concentrate. J. Food Eng. 2014;124:11–18. [Google Scholar]
- 65.Syll O., Khalloufi S., Schuck P. Dispersibility and morphology of spray-dried soy powders depending on the spraying system. Dairy Sci. Technol. 2013;93(4-5):431–442. [Google Scholar]
- 66.Taboada M.L., Müller E., Fiedler N., Karbstein H.P., Gaukel V. Oil droplet breakup during pressure swirl atomization of emulsions: Influence of emulsion viscosity and viscosity ratio. J. Food Eng. 2022;321:110941. [Google Scholar]
- 67.Tan S.P., Kha T.C., Parks S.E., Stathopoulos C.E., Roach P.D. Effects of the spray-drying temperatures on the physiochemical properties of an encapsulated bitter melon aqueous extract powder. Powder Technol. 2015;28:165–175. [Google Scholar]
- 68.Tang L., Liu H., Huang G., Yuan Z., Fu M., Bu Z., Wen J., Xu Y. The structural characterization, physicochemical properties, and stability of gardenia yellow pigment microcapsules. LWT. 2022;162:113507. [Google Scholar]
- 69.Tong X., Cao J., Tian T., Bo L., Jiang L. Changes in structure, rheological properties and antioxidant activity of soy protein isolate fibrils by ultrasound pretreatment and EGCG. Food Hydrocoll. 2021;7 [Google Scholar]
- 70.Vela A.J., Villanueva M., Solaesa N.G., Ronda F. Impact of high-intensity ultrasound waves on structural, functional, thermal and rheological properties of rice flour and its biopolymers structural features. Food Hydrocoll. 2020;113106480 [Google Scholar]
- 71.Vélez-Erazo E.M., Bosqui K., Rabelo R.S., Kurozawa L.E., Hubinger M.D. High Internal Phase Emulsions (Hipe) Using Pea Protein And Different Polysaccharides As Stabilizers. Food Hydrocoll. 2020;105:105775. [Google Scholar]
- 72.Vidovi S.S., Vladi J.Z., VaTag G., Zekovi Z.P., Popovi L.M. Maltodextrin as a carrier of health benefit compounds in Satureja montana dry powder extract obtained by spray drying technique. Powder Technol. 2014;258:209–215. [Google Scholar]
- 73.Sui X., Bi S., Qi B., Wang Z., Zhang M., Li Y., Jiang L. Impact of ultrasonic treatment on an emulsion system stabilized with soybean protein isolate and lecithin: Its emulsifying property and emulsion stability. Food Hydrocoll. 2017;63:727–734. [Google Scholar]
- 74.Wang N., Zhou X., Wang W., Wang L., Jiang L., Liu T., Yu D. Effect of high intensity ultrasound on the structure and solubility of soy protein isolate-pectin complex. Ultrason. Sonochem. 2021;80:105808. doi: 10.1016/j.ultsonch.2021.105808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang P., Wang W., Chen C., Fu X., Liu R. Effect of Fructus Mori. bioactive polysaccharide conjugation on improving functional and antioxidant activity of whey protein. Int. J. Biol. Macromol. 2020;148:761–767. doi: 10.1016/j.ijbiomac.2020.01.195. [DOI] [PubMed] [Google Scholar]
- 77.Wang T., Wang N., Li N.a., Ji X., Zhang H., Yu D., Wang L. Effect of high-intensity ultrasound on the physicochemical properties, microstructure, and stability of soy protein isolate-pectin emulsion. Ultrason. Sonochem. 2022;82:105871. doi: 10.1016/j.ultsonch.2021.105871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu C., Teng F., McClements D.J., Zhang S., Li Y., Wang Z. Effect of cavitation jet processing on the physicochemical properties and structural characteristics of okara dietary fiber. Food Res. Int. 2020;134:109251. doi: 10.1016/j.foodres.2020.109251. [DOI] [PubMed] [Google Scholar]
- 79.Yan X., Zhao J., Zeng Z., Ma M., Xia J., Tian W., Zhang G., Gong X., Gong D., Yu P. Effects of preheat treatment and polyphenol grafting on the structural, emulsifying and rheological properties of protein isolate from Cinnamomum camphora seed kernel. Food Chem. 2022;377:132044. doi: 10.1016/j.foodchem.2022.132044. [DOI] [PubMed] [Google Scholar]
- 81.Zhang L., Wang X., Hu Y., Abiola Fakayode O., Ma H., Zhou C., Hu Z., Xia A., Li Q. Dual-frequency multi-angle ultrasonic processing technology and its real-time monitoring on physicochemical properties of raw soymilk and soybean protein. Ultrason. Sonochem. 2021;80:105803. doi: 10.1016/j.ultsonch.2021.105803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu J., Li X., Liu L.u., Li Y., Qi B., Jiang L. Preparation of spray-dried soybean oil body microcapsules using maltodextrin: Effects of dextrose equivalence. LWT. 2022;154:112874. [Google Scholar]
- 83.Cui S., Xu L., Wang Y., Zhang H. Influence of Ultrasonic Treatment on Sulfhydryl Content, Surface Hydrophobicity and Particle Size of Low-Purine Defatted Soybean Powder[J] Food Science. 2015;21:50–55. [Google Scholar]
Further reading
- 29.Insang S., Kijpatanasilp I., Jafari S., Assatarakul K. Ultrasound-assisted extraction of functional compound from mulberry (Morus alba L.) leaf using response surface methodology and effect of microencapsulation by spray drying on quality of optimized extract. Ultrason. Sonochem. 2022;82 doi: 10.1016/j.ultsonch.2021.105806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jing W., Li M., Zhang J., Lv Y., Li X., Guo S. Effects of high-temperature pressure cooking. on cold-grind and blanched soymilk: physico-chemical properties, in vitro digestibility and sensory quality. Food Res. Int. 2021;149 doi: 10.1016/j.foodres.2021.110669. [DOI] [PubMed] [Google Scholar]
- 37.Kurozawa L.E., Morassi A.G., Vanzo A.A., Park K.J., Hubinger M.D. Influence of spray drying conditions on physicochemical properties of chicken meat powder. Drying Technol. 2009;27(11):1248–1257. [Google Scholar]
- 44.Liu H.H., Kuo M.I. Ultra high pressure homogenization effect on the proteins in soy flour. Food Hydrocoll. 2016;52:741–748. [Google Scholar]
- 76.Wang T., Wang N., Li N., Ji X., Zhang H., Yu D. Effect of ultrasound on the preparation of soy protein isolate-maltodextrin embedded hemp seed oil microcapsules and the establishment of oxidation kinetics models. Ultrason. Sonochem. 2022;77(7) doi: 10.1016/j.ultsonch.2021.105700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang J., Luo D., Xiang J., Xu W., Xu B., Li P., Huang J. Structural Variations of Wheat Proteins Under Ultrasound Treatment. J. Cereal Sci. 2021;99:103219. [Google Scholar]