Abstract
Background and objectives
Non-alcoholic fatty liver disease (NAFLD) develops due to impaired hepatic lipid fluxes and is a risk factor for chronic liver disease and atherosclerosis. Lipidomic studies consistently reported characteristic hepatic/VLDL “lipid signatures” in NAFLD; whole plasma traits are more debated. Surprisingly, the HDL lipid composition by mass spectrometry has not been characterised across the NAFLD spectrum, despite HDL being a possible source of hepatic lipids delivered from peripheral tissues alongside free fatty acids (FFA). This study characterises the HDL lipidomic signature in NAFLD, and its correlation with metabolic and liver disease markers.
Methods
We used liquid chromatography-mass spectrometry to determine the whole serum and HDL lipidomic profile in 89 biopsy-proven NAFLD patients and 20 sex and age-matched controls.
Results
In the whole serum of NAFLD versus controls, we report a depletion in polyunsaturated (PUFA) phospholipids (PL) and FFA; with PUFA PL being also lower in HDL, and negatively correlated with BMI, insulin resistance, triglycerides, and hepatocyte ballooning. In the HDL of the NAFLD group we also describe higher saturated ceramides, which positively correlate with insulin resistance and transaminases.
Conclusion
NAFLD features lower serum lipid species containing polyunsaturated fatty acids; the most affected lipid fractions are FFA and (HDL) phospholipids; our data suggest a possible defect in the transfer of PUFA from peripheral tissues to the liver in NAFLD. Mechanistic studies are required to explore the biological implications of our findings addressing if HDL composition can influence liver metabolism and damage, thus contributing to NAFLD pathophysiology.
Keywords: Non-alcoholic fatty liver disease (NAFLD), Obesity, Lipoprotein metabolism, Lipidomics, LC-MS
Graphical abstract
Changes in HDL and FFA composition occurring in NAFLD.
Highlights
-
•
Low serum polyunsaturated fatty acids (PUFA) in phospholipids (PL) and free fatty acids (FFA) characterise NAFLD.
-
•
PUFA-PL are specifically depleted in the HDL of NAFLD patients.
-
•
PUFA-PL and PUFA-FFA in the whole serum and HDL correlate with metabolic biochemistry andhepatocyte ballooning.
-
•
We propose that HDL composition is tightly connected to the rewiring of hepatic PUFA composition occurring in NAFLD.
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) develops due to an impairment in systemic/hepatic metabolism leading to hepatic lipid accumulation and, eventually lipotoxicity [1]. From a histological perspective, NAFLD is a continuum of presentations ranging from “simple” steatosis (NAFL; intrahepatic fat deposition in more than 5% of hepatocytes) to steatohepatitis (NASH; steatosis in the presence of inflammation and ballooning), fibrosis, and cirrhosis, which can ultimately evolve to hepatocellular carcinoma (HCC) [2].
NAFLD has reached pandemic proportions with a global prevalence of 24% in the general population, thus being a public health priority [3]. NAFLD is strongly associated with features of the Metabolic Syndrome (MetS), including obesity, insulin resistance (IR) and type 2 diabetes mellitus (T2DM), mixed dyslipidaemia [low high-density lipoprotein cholesterol (HDL-C) and increased very low-density lipoprotein triglycerides (VLDL-TG)], which partly explain the elevated cardiovascular disease (CVD) risk [4]. The latter represents the leading cause of death in these patients, and recent studies suggest that NALFD could be an independent risk factor for atherosclerosis and CVD outcomes [5]. However, the mechanisms linking both conditions are still debated [4].
Over the last decade, lipidomics has helped to understand the NAFLD pathophysiology, pointing to specific changes in lipid metabolism that can serve as candidate biomarkers [6]. Specifically, lipidomic studies of liver tissues have reported a lipid imbalance alongside the NAFLD spectrum characterised by elevated levels of saturated fatty acids (SFA) and reduced levels of phospholipids (PL) and polyunsaturated fatty acids (PUFA) [7]. Major players expected to contribute to these lipidomic changes are: 1) adipose tissue (AT) [the foremost source of fatty acids (FA) to the liver]; 2) hepatic de novo lipogenesis (DNL), enhanced by hyperinsulinaemia and increased (refined) carbohydrate intake; 3) dietary fat intake [1,8,9]. Over the years, studies have described differences in whole serum/plasma lipids, mainly focusing on TG fatty acid remodelling alongside circulating candidate biomarkers. The latter has been prompted by the risks and costs associated with liver biopsy procedures. Compared to healthy controls, NAFLD patients displayed an increased level of SFA and monounsaturated fatty acids (MUFA) and reduced PUFA within the TG fraction of the whole plasma [[10], [11], [12], [13]], partly explained by the enhanced DNL [14]. However, apart from these findings focusing on TG, whole plasma lipidomic studies have reported conflicting results regarding the abundance and composition of other lipid classes [13,[15], [16], [17]]. Several factors might contribute to the discrepancies observed in the circulating lipidome of NAFLD patients: 1) differences in inclusion criteria among studies (such as sex, dyslipidaemia, ethnicity, and dietary habits); 2) while TG composition reflects more direct changes in liver metabolism (being enriched in VLDL) and TG/VLDL metabolism is particularly stressed under the metabolic pressure of obesity/MetS/NAFLD [1], other lipids are abundant in multiple lipoprotein fractions including low-density lipoproteins (LDL) and HDL, thus rendering the biological interpretation of results generated in a complex matrix, such as whole plasma, more challenging.
The use of isolated lipoprotein fractions (lipoprotein lipidomics) can provide a higher granularity than whole plasma lipidomics, allowing a more detailed study of biological processes such as lipoprotein remodelling and organ-to-organ exchange/crosstalk.
This study aims to investigate the quantitative and qualitative lipidomic differences in patients across the NAFLD spectrum. Given the association of NAFLD with cardiovascular risk (CVR) and considering the mounting evidence associating HDL reduction with (central) obesity/IR/NAFLD [[18], [19], [20]], we posited that HDL may play a role in NAFLD acting as a possible source of hepatic lipids delivered from peripheral tissues to the liver and that, together with FFA, differences in HDL composition might be associated to NAFLD development and/or progression. With this proposition, we studied the lipidome of the whole serum and HDL (obtained through fast protein liquid chromatography) by liquid chromatography coupled with mass spectrometry in healthy and biopsy-confirmed NAFLD participants.
2. Methods
2.1. Ethics and the BioNASH study cohort
Eighty-nine patients with biopsy-proven NAFLD (patients with alternate diagnoses, aetiologies, and kidney dysfunction were excluded) and 20 healthy volunteers were involved in this study. Patients were recruited by the NASH Service at Cambridge University Hospitals NHS Foundation Trust, whereas healthy volunteers were recruited either by the NIHR Cambridge BioResource (http://www.cambridgebioresource.org.uk) or by the NHS Blood and Transplant Unit, Cambridge, UK. Participant enrolment was approved by NHS Research Ethics Committees (REC 06/Q0106/70; 12/EE/0040; 17/EE/0389). Study protocols followed the principles of the Declaration of Helsinki, and all participants gave written informed consent.
Liver biopsies were scored by an experienced liver pathologist for steatosis (0–3), ballooning (0–2), inflammation (0–2), and fibrosis (0–4) and were classified according to the Kleiner score [21] and classified into NAFL and NASH following the same algorithm proposed by Bedossa and colleagues [22]. In healthy controls, where liver biopsy was not clinically indicated, they were selected on the basis of the predicted absence of NAFLD according to the non-invasive score proposed by Kotronen et al. [based on: presence/absence of metabolic syndrome, T2DM, and levels of insulin, aspartate aminotransferase (AST) and AST/alanine aminotransferase (ALT)] [23].
Sample collection and processing for serum and lipoprotein lipidomics have been previously described [20] and are here reported in supplementary materials.
2.2. Statistical analyses
Data are shown as mean ± standard deviation unless otherwise specified. Normality was visually assessed from plots of the data (skewness/kurtosis) obtained with the lm function in R, and logarithmic transformations were applied to non-normally distributed data. Comparisons of clinical data between healthy and NAFLD patients were assessed using three-way and two-way ANOVA controlling for sex and the presence of T2DM, followed by the Tukey HSD post hoc test to estimate the statistical significance among groups. Regarding categorical variables, a chi-square test was adopted. Whole serum lipidomic data were analysed using three-way ANOVA controlling for sex, presence of T2DM and interaction between sex, T2DM and disease state, followed by the Tukey HSD post hoc test to estimate the statistical significance among groups. A p-value <0.05 was considered significant. However, when lipids were investigated as independent hits, multiple testing correction [Benjamini-Hochberg procedure to control the False Discovery Rate (FDR)] was applied as specified in the legend to tables. Lipoprotein lipidomics data, where participants were only males, were analysed using two-way ANOVA controlling for the presence of T2DM and the interaction between disease state and T2DM, followed by the Tukey HSD post hoc test to estimate the statistical significance among groups. A p-value <0.05 was considered significant. As with whole serum, when lipids were considered as an independent unit, FDR was reported along with the raw p-value. To assess the power of this study, whole serum and lipoprotein lipidomics, we performed a Post Hoc Power Analysis with G∗power software. Variables with an effect size (f) below 0.3 (whole serum) and 0.5 (lipoproteins) fell below an acceptable power level of 0.7, therefore being potentially exposed to type 2 error. All the significant variables had an optimal power (>0.8). Univariate correlations were carried out using the Pearson Correlation Coefficient. Statistical analysis and graphs were performed with R version 4.2.1.
2.3. Other experimental procedures
Detailed experimental procedures are described in the supplementary files.
3. Results
3.1. Clinical characteristics and whole serum lipidomic profile of healthy and NAFLD patients
This study involved 109 participants, including 20 healthy volunteers (age and sex-matched), 36 NAFL, and 53 NASH (Supplementary Table 1). The patients in the NAFLD spectrum displayed significantly higher BMI, insulin resistance (as assessed by the Homeostasis Model Assessment 2 of Insulin Resistance HOMA2-IR), and mixed dyslipidaemia (higher TG and lower HDL-C), along with increased liver enzymes (ALT, AST), while LDL-C and total cholesterol were not significantly different across the groups (Supplementary Table 1). NASH patients displayed a worse metabolic profile (significantly higher glucose, insulin, HOMA2-IR, AST) compared to NAFL, despite similar BMI (Supplementary Table 1).
Lipids were analysed as total lipid class (sum of each lipid measured), and according to their acyl chain saturation levels (saturated, monounsaturated and polyunsaturated), as fatty acids saturation level imbalances have been described in the livers of NAFLD patients and are involved in the pathophysiology of NAFLD.
The whole serum lipidomic analysis revealed a quantitative and qualitative depletion of several phospholipid classes [phosphatidylcholines (PC), phosphatidylethanolamines (PE), phosphatidylglycerols (PG), and sphingomyelins (SM)] in the NAFLD (both NAFL and NASH) groups as compared to the controls (Figure 1 A-G). The lower levels of total PC and PE were mainly driven by a depletion in their PUFA component, while PG were markedly lower irrespectively of their acyl chain saturation levels (Figure 1B,D,F). Lower SM levels in NAFLD groups were attributable to a reduction in MUFA and PUFA components (Figure 1H). Furthermore, we observed a significant sex effect on most of the PL measured (Figure 1), in line with known differences in the lipoprotein metabolism among sexes [24,25]. While between NAFLD and controls total free fatty acids (FFA) were not significantly different (Figure 1I), NAFLD patients showed markedly higher saturated FFA, coupled with a depletion in polyunsaturated FFA (Figure 1J). Similar results were found in the triglyceride fraction (Supplementary Fig. 1a,b), likely reflecting the known activation of DNL programs previously described in NAFLD [11,14,26,27]. Within the lysophosphatidylcholines (LPC), the only significant difference observed was in the saturated fraction, which was higher in NAFLD than controls (Supplementary Fig. 1c,d). In contrast, within the lysophosphatidylethanolamines (LPE), only the MUFA content was significantly lower in NAFL compared to controls and NASH (Supplementary Fig. 1e,f). Ceramides (Cer) did not show significant differences between NAFLD and controls (Supplementary Fig. 1g,h) but were characterised by a significant sex effect, as reported in the literature [[28], [29], [30]].
Figure 1.
Whole serum levels of major lipid classes in healthy volunteers and NAFLD patients. (A,B) PC, (C,D) PE, (E,F) PG, (G,H) SM were lower across the NAFLD spectrum compared to controls. (I,J) FFA were higher in SFA and lower in PUFA in NAFLD as compared to controls. All lipid species were analysed by LC-MS. Statistical significance was assessed using three-way ANOVA controlling for sex, presence of type 2 diabetes mellitus (T2DM) and interaction between sex, T2DM and disease state, with a p-value <0.05 considered significant. Tukey HSD post hoc test was used to estimate the statistical significance among groups. Lowercase red letters indicate post hoc analysis significance: “a” means different from controls “CTRL”, and “b” means different from NAFL. Data are represented as mean ± standard deviation; expression data of participants are represented as dot plots. In Supplementary Table 2 are reported all the specific lipid species analysed. Abbreviations: PC, phosphatidylcholines; PE, phosphatidylethanolamines; PG, phosphatidylglycerols; SM, sphingomyelins; FFA, free fatty acids; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids.
Lastly, within the NASH group, we clustered the patients against fibrosis (Supplementary Table 3). Apart from a mild increase of LPE MUFA, and a trend in lower SFA/MUFA-TG and FFA in NASH F3-4 vs. NASH F0-2, no major changes were observed in the lipid classes when clustering the patients against fibrosis (Supplementary Table 4). This trend to deflection in SFA/MUFA-TG (including TG enriched in DNL products) in end-stage NASH F3-4, was previously described by others and explained by liver dysfunction [7]; in our cohort, it also reflects similar trends in BMI, Insulin/HOMA-IR and TG (Supplementary Table 3) that could be associated to a deflection in sterol regulatory element-binding protein 1 (SREBP-1) activation, as we previously described (by next generation sequencing in a partially overlapping cohort) [26].
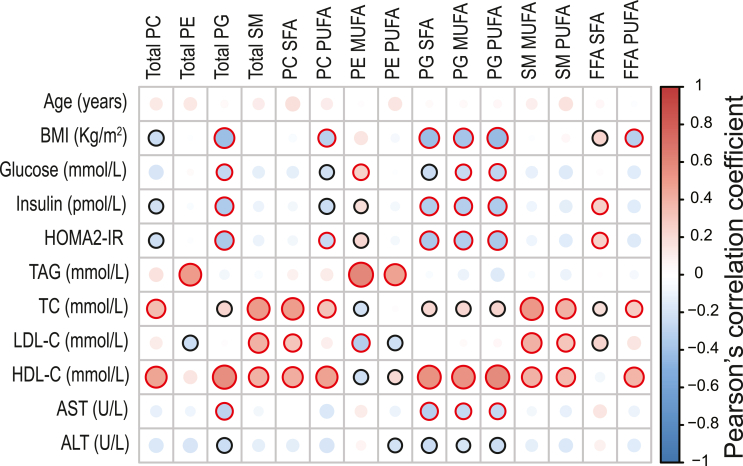
To better understand the extent to which whole serum lipidomic data related to metabolic impairment, we correlated the significantly different lipids from Figure 1 with critical clinical data (Figure 2). Pearson correlation analysis showed that PG (total, SFA, MUFA, PUFA) had the strongest inverse correlation with obesity and insulin resistance, whereas SFA from FFA were positively correlated with HOMA2-IR (Figure 2). Moreover, most of the lipids significantly reduced in the NAFLD groups were also positively correlated with HDL-C (Figure 2). No significant correlation between whole serum lipids and liver histology were found (data not shown), aligning with previous studies showing small/no differences across the NAFLD spectrum [17,31].
Figure 2.
Correlations between significant whole-serum lipid species and clinical data. Heatmap representing a correlation matrix among significantly different whole serum lipid species and clinical data in healthy volunteers and NAFLD patients: colour represents the Pearson correlation coefficient (red: positive; blue: negative), and the size of the circle represents significance (black bold borders highlight correlations with p < 0.05; red bold borders highlight correlations with p < 0.01). Abbreviations: PC, phosphatidylcholines; PE, phosphatidylethanolamines; PG, phosphatidylglycerols; SM, sphingomyelins; FFA, free fatty acids; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; BMI, body mass index; HOMA2-IR, Homeostasis Model Assessment 2 of Insulin Resistance; TAG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; AST, aspartate aminotransaminase; ALT, alanine aminotransaminase.
Taken together, these data show that in NAFLD, the whole serum lipidome is depleted of specific PL, and their PUFA content drove these changes, suggesting a close relationship between the circulating lipidome, IR and HDL metabolism.
3.2. HDL lipidomics of NAFLD patients suggest a reduced reverse PUFA transport from peripheral tissues to the liver
Based on the strong correlations between PL/PUFA-containing lipids and HDL-C, we posited an implication of HDL particles in the observed whole serum differences; specifically, we investigated if the lipidomic differences we observed in the whole serum were due to the sole reduction of HDL concentration or also driven by an impairment of HDL composition.
To address this question, we isolated HDL by fast protein liquid chromatography (FPLC) and studied its lipidome in a sub-cohort of 40 age-matched male subjects (9 healthy, 11 NAFL, and 20 NASH; Supplementary Table 5). We focused on male participants to reduce the variability attributable to sex differences in HDL metabolism.
Compared to controls, and in agreement with whole serum lipidomics, the HDL lipidomic profile of NAFLD patients was characterised by lower PC levels, mainly driven by their PUFA content, with NASH being most significantly affected (Figure 3A,B). This observation confirmed our hypothesis that PC differences in whole serum lipidomics tightly reflect HDL abundance and composition. Indeed, reduced PC (and especially their PUFA content) have been reported in peripheral tissues (including AT and macrophages) of obese patients with IR [[32], [33], [34], [35], [36]]; these tissues are involved in HDL metabolism.
Figure 3.
HDL levels of major lipid classes in healthy volunteers and NAFLD patients, and their correlation with clinical and liver histological data. (A,B) PC were lower in NAFLD as compared to controls. (C–-E) SM and PG were lower across the NAFLD spectrum as compared to controls. (F,G) Total and SFA Cer were higher in NASH compared to controls. Statistical significance was assessed using two-way ANOVA controlling for presence of type 2 diabetes mellitus (T2DM) and interaction between T2DM and disease state, with a p-value <0.05 considered significant. Tukey HSD post hoc test was used to estimate the statistical significance among groups. Lowercase red letters indicate post hoc analysis significance: “a” means different from controls “CTRL”, and “b” means different from NAFL. Data are represented as mean ± standard deviation; expression data of participants are represented as dot plots. In Supplementary Table 6 are reported all the specific lipid species analysed within the HDL fraction. (H) Heatmap representing a correlation matrix among significantly different HDL lipid species and clinical data in healthy volunteers and NAFLD patients: colour represents the Pearson correlation coefficient (red: positive; blue: negative), and the size of the circle represents significance (black bold borders highlight correlations with p < 0.05; red bold borders highlight correlations with p < 0.01). (I) Heatmap representing a correlation matrix among significantly different HDL lipid species and liver histological data in NAFLD patients (n = 31): colour represents the Pearson correlation coefficient (red: positive; blue: negative), and the size of the circle represents significance (black bold borders highlight correlations with p < 0.05; red bold borders highlight correlations with p < 0.01). All lipid species were analysed by LC-MS and normalised to its internal standard (IS) as with whole serum (list of IS used reported in the method section), in addition to the ApoA-I concentration (Supplementary Fig. 2). Abbreviations: PC, phosphatidylcholines; SM, sphingomyelins; PG, phosphatidylglycerols; Cer, ceramides; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids.
Furthermore, compared to controls, the NAFLD group showed lower absolute levels of total SM (mostly MUFA and PUFA) and total PG (Figure 3C-E). Low HDL SM concentration has been associated with a decreased HDL cholesterol efflux capacity [37]; this has also been suggested for PG, even though this lipid class represents a minor component of the HDL lipidome [38] (in our data, with few exceptions, most PG were undetectable). We also observed higher HDL total Cer in NASH patients, mainly driven by their SFA component (Figure 3F,G). No significant differences were observed in LPC, TG and PE among the groups (Supplementary Fig. 3). When clustering NASH patients against fibrosis (Supplementary Table 7), no major differences were observed in the HDL lipid classes, with the exception of a significant decline in HDL TG SFA concentration (Supplementary Table 8).
To further assess the extent to which the HDL lipidome related to metabolic parameters and liver histology, we correlated the significantly different lipids with critical clinical and histological data (Figure 3H,I). Pearson correlation analysis showed that the lipid species significantly reduced in the NAFLD group were negatively correlated with insulin resistance and, within these species, (Total/MUFA/PUFA) SM had the strongest correlation. On the other hand, we found the positive correlation of total and SFA Cer with insulin resistance and liver enzymes particularly intriguing, with AST showing the strongest correlation (Figure 3H). We also observed a significant negative correlation between hepatocyte ballooning and total PG, Total/MUFA/PUFA SM (Figure 3I). As already described for the whole serum lipidome, none of HDL lipid classes correlated with fibrosis.
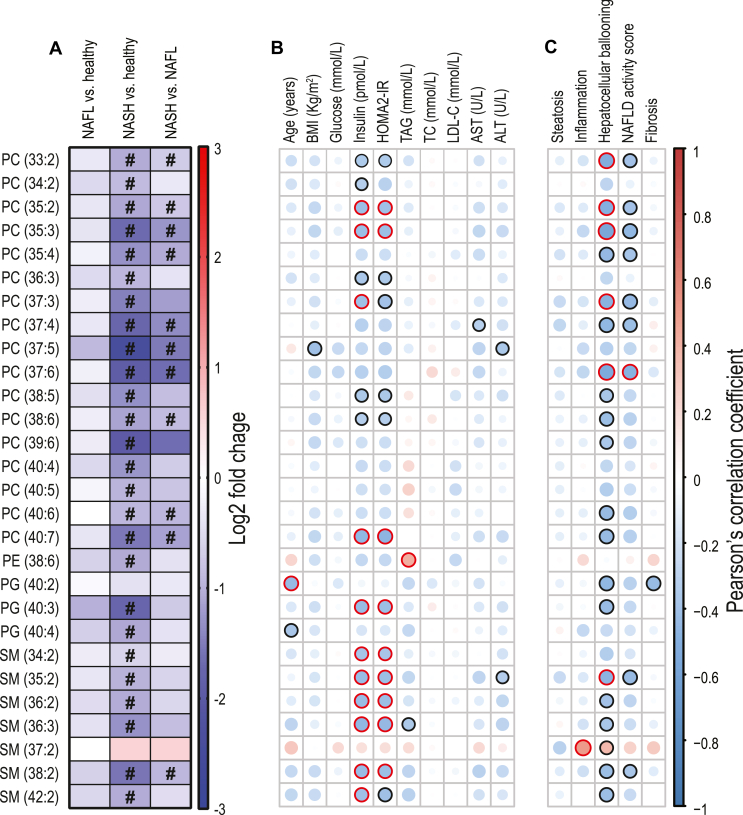
We then focussed on the specific HDL PUFA-containing species where we found a generalised depletion in NASH of PUFA-PL (Figure 4A) including PC containing odd-chain fatty acids (such as PC 37:5, PC 37:6, 35:2, 35:3). Moreover, most of these PUFA-containing lipid species negatively correlated with insulinemia, HOMA2-IR (Figure 4B) and hepatocyte ballooning (Figure 4C).
Figure 4.
Significantly lower PUFA-containing phospholipids within HDL, and their correlations with clinical and liver histological data. (A) Log2 fold change among the significantly different HDL PUFA in healthy volunteers (CTRL) versus NAFLD patients, with “#” indicating a Tukey HSD post hoc significant difference p < 0.05 (details in Supplementary Table 6). (B) Heatmap representing a correlation matrix among significantly different HDL PUFA species and clinical data in healthy volunteers and NAFLD patients: colour represents the Pearson correlation coefficient (red: positive; blue: negative), and the size of the circle represents significance (black bold borders highlight correlations with p < 0.05; red bold borders highlight correlations with p < 0.01). (C) Heatmap representing a correlation matrix among significantly different HDL PUFA species and liver histological data in NAFLD patients (n = 31): colour represents the Pearson correlation coefficient (red: positive; blue: negative), and the size of the circle represents significance (black bold borders highlight correlations with p < 0.05; red bold borders highlight correlations with p < 0.01). Abbreviations: PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; SM, sphingomyelin; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; BMI, body mass index; HOMA2-IR, Homeostasis Model Assessment 2 of Insulin Resistance; TAG, triglycerides; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; AST, aspartate aminotransaminase; ALT, alanine aminotransaminase.
In summary, these data show that, independently from the lower HDL-C concentration, HDL is characterised by PUFA PL depletion and enrichment of SFA Cer levels in NAFLD that might be intimately linked to IR, and associated to liver damage. The fact that the liver reuses HDL-derived PL, either incorporating them into membranes or converting them into different lipid classes, has been previously suggested with tracer experiments in preclinical models [39]. Our results, despite being observational, show for the first time that the HDL lipidome of NAFLD patients carries lipidomic signatures similar to those previously described in both peripheral tissues and liver of patients with MetS/NAFLD [7,[32], [33], [34], [35], [36]], therefore potentially implicating HDL as a contributor to NAFLD development and progression in IR states.
4. Discussion
NAFLD is characterised by an imbalance in hepatic lipid fluxes [1], with lipoproteins (alongside FFA) being deeply involved in these processes. Apart from the characteristic elevated hepatic fat content (mainly TG), it is increasingly recognised that other lipid species, such as phospholipids and sphingolipids, are involved in the onset and progression of this condition [40]. Indeed, some lipid species are referred to as “lipotoxic species” because of their capability to induce cell toxicity.
While reports regarding characteristic hepatic lipidomic signatures of NAFLD are coherent (including higher SFA across different lipid species, oxidised lipids, ceramides or lower PUFA/PL), whole serum lipidomic studies have produced conflicting results, also sometimes discordant with hepatic findings [41]. Discordances have also been reported among major plasma lipidomic classes: for example, PL have been described higher (PC/SM [16]; PC/PE/PG [42]), and/or lower (PC/LPC/SM [13]; LPC [43]) in NAFLD compared to controls. This might be partly due to the recruiting criteria (e.g., differences in clinical parameters such as HDL-C, TG, and BMI between diseased and controls across studies) and diet, potentially affecting the circulating lipidome. Moreover, the lipidomic profiling of the whole serum/plasma provides averaged information on lipoproteins concentration and composition varying according to disease state, dietary habits, sex and many other factors [44,45]. Investigating the specific lipoprotein lipidomic profile provides a more accessible matrix to compare among studies (e.g., lipoproteins are normalised to their protein/apolipoprotein content) and give a more biologically relevant interpretation (e.g., VLDL as a more direct proxy of liver output). Understandably, mass-spectrometry-based lipoprotein lipidomics of NAFLD has mainly focused on VLDL [[46], [47], [48]], with limited information regarding the other lipoprotein fractions.
In this study, we first started describing NAFLD's whole serum lipidomic signature compared to healthy controls. We showed that NAFLD is characterised by a depletion in PL (PC, PE, PG, SM) specifically driven by their PUFA fraction. Moreover, NAFLD patients had higher saturated and lower polyunsaturated FFA. Circulating FFA are released by the AT, and elevated levels of FFA have been attributed to enhanced lipolysis due to AT-IR [1,9]. Elevated FFA play a major role in the onset and development of NAFLD [49,50]. The higher saturated FFA in NAFLD vs. controls that we reported is aligned with previous whole serum studies [[51], [52], [53]] as well as with the acyl chain saturation profile of the whole hepatic lipidome [17,54,55].
We also observed lower levels of PUFA in FFA and PL in whole serum of NAFLD patients (compared to controls); these results find confirmation in previous reports [40] (although polyunsaturated FFA depletion is a debated finding [[51], [52], [53]]). Preclinical studies have provided mechanistic insights as to how PUFA (especially essential fatty acids, EFA) deficiency promotes hepatic steatosis. EFA can negatively modulate the DNL machinery toward the negative modulation of the Liver X Receptor (LXR), of SREBP-1 and/or of the carbohydrate response element binding protein (ChREBP) [[56], [57], [58]]. Also, PUFA can activate the peroxisome proliferator–activated receptor-α (PPARα) promoting fatty acid oxidation [59]. Despite the solid evidence of the role of PUFA in modulating hepatic lipid metabolism, it should be said that omega-3 supplementation has yielded unsatisfactory results in NAFLD trials [60]. Our data do not clearly point to EFA deficiency but rather to a global PUFA depletion: FFA composition, a robust predictor of adipose tissue fatty acid composition (that, in turn is considered the gold standard for the representation of long term dietary fatty acids storage due to the slow turnover time [[61], [62], [63]]) showed a depletion in linoleic acid (FFA 18:2) that is not confirmed by other EFA and their derivatives. Reduced PUFA PL in NAFLD could therefore be also attributable to enhanced utilisation (catabolism or conversion to second messengers such as eicosanoids) [64,65].
Since 1) NAFLD liver biopsies show depletion in PC and PUFA in multiple lipid classes [40]; 2) HDL are the primary carriers of circulating PC; 3) total and PUFA PC were strongly correlated with HDL-C; we sought to better understand the lipid composition of isolated HDL to investigate whether these results were due to HDL concentration or composition. The HDL lipidome confirmed the reduction of PC in NAFLD, being once again driven by their PUFA component and strongly negatively correlated with IR. The effects of changes in HDL PL content has been studied in the context of HDL physical properties and reverse cholesterol transport: in preclinical experiments, lower PC levels in reconstituted HDL and HDL-mimicking micelles have been associated with reduced cholesterol efflux capacity (CEC), thus rendering these particles potentially less athero-protective [66,67]. This might go along with some reports suggesting a lower CEC in NAFLD compared to healthy controls (although this matter is also debated) [[68], [69], [70]]: the lower PC we observed in HDL of NAFLD patients could justify, at least in part, the lower CEC described in NAFLD. Intriguingly, HDL PUFA PL were mainly depleted in NASH and negatively correlated with hepatocyte ballooning: despite this finding will require future mechanistic studies to explain the association, we are tempted to speculate that differences in HDL PL composition might contribute to hepatocyte damage. It is worth considering that HDL (together with FFA, diet, DNL [1]) might be contributing as input to the hepatic fat pool, promoting a “reverse (phospho)lipid transport” since: 1) PL constitute nearly 50% of total lipids within HDL [45]; 2) tracer studies in mice suggest that about 50% of hepatic PC is derived from the circulation (being HDL the main carriers) [39]; 3) HDL PL can be used to build up membranes and/or disassembled to use or esterify FA (into TG) [39]. The fact that our HDL lipidomics results are aligned with hepatic lipidomic signatures described in NAFLD [41] might support the hypothesis that HDL PL composition might have a direct impact on hepatocyte (dys)-function. However, the understanding of HDL fate in hepatocytes is currently at its infancy. While mechanisms are established with regards to HDL-C uptake (with the scavenger receptor class B type I (SR-BI) being a key player in RCT) [71], less studied is the uptake of HDL lipids: it has been suggested that SR-BI might also contribute to HDL-PC uptake but other mechanisms (e.g., particle endocytosis, hydrolysis of PC by phospholipases, and other unknown pathways) seem to be also at play [39,72].
Here we also report strong correlations between HDL PUFA composition and IR that merits further investigation in light of the tight association between adipose tissue dysfunction, IR, and HDL metabolism and function [20,73,74]. However, we cannot rule out the hypothesis that depletion of PUFA in the liver would influence AT via VLDL, and then fire back to the liver (via FFA and HDL-PL) in a vicious cycle [9]. Moreover, the central role of the liver in the HDL biogenesis (including its participation into HDL lipidation), renders even more difficult to disentangle in this setting the contribution of periphery vs. liver in the final HDL lipid composition.
We also reported a depletion of odd-chain PC-FA within the HDL: these fatty acids have been previously associated with a reduced incidence of T2DM [75,76]. Not surprisingly, the lower HDL odd-chain fatty acids observed in NAFLD finds confirmation in an independent cohort of patients with metabolic syndrome that we previously reported [20]. Whether HDL odd chain FA exert any metabolic effect is to be established and so is their derivation as dietary sources [77], gut microbiota [78], alpha-oxidation [79], and mitochondrial catabolism of BCAA [80] can potentially contribute to the pool of these lipids.
Lastly, our data show that HDL Cer were higher in NASH compared to controls and that their abundance in HDL correlated with hepatic necro-inflammatory markers. Some studies described increased hepatic Cer in NAFLD livers, and its association with lipotoxic damage [17,81]. The liver is considered a major Cer synthesis site through different pathways (de novo, salvage and sphingomyelin hydrolysis) and of its export to circulation [82]; however Cer can be produced in other organs and in circulation (by sphingomyelin hydrolysis) [83]. Our findings can either reflect hepatic Cer concentrations (since HDL can receive Cer from VLDL/LDL due to the activity of lipoprotein lipid transport enzymes [84]) or, vice-versa, suggest that HDL influence hepatic Cer pool. These findings, although intriguing, need further mechanistic exploration.
This study is subject to different limitations. First, the study's cross-sectional nature provides a correlation between lipids and disease states. Thus, causation cannot be drawn. Second, the contribution of HDL lipids to its functionality alongside the liver lipid pool is only speculative and requires adequate investigation. Third, dietary habits were not recorded thus the impact of diet on our findings cannot be inferred. Fourth, HDL lipidomics was performed only on males. Last, patients were not profiled for the major genetic risk alleles for NAFLD, but the marked IR state observed favours more the metabolic rather than genetic nature of NAFLD.
In conclusion, by using mass-spectrometry-based lipidomics weshow that NAFLD is characterised by substantial changes in HDL PL composition and impaired reverse PUFA transport (via FFA and HDL-PL) and that HDL PUFA-PL correlate with hepatocyte ballooning. How HDL lipidome can influence the hepatic lipid pool and function requires further investigation.
Author contributions
G.M., M.A., A.V·P., M.V. and J.L.G. conceived and designed the study. G.M., M.V. and J.L.G. wrote the manuscript. M.A., V.A., G.M., M.F., recruited the participants. G.M., B.J., Z.H., A.M., A.K., performed lipidomic analyses. G.M. and M.V. performed statistical analyses and prepared figures. G.M. performed HDL lipoprotein isolation with support from L.V·H.-M. All the authors provided useful criticism during the study, critically reviewed the manuscript and agreed to the published version of the manuscript.
Funding
The cohort was initially generated with the support of the Evelyn Trust (to M.A.). G.M., Z.H., M.V., and J.L.G. were funded by the Medical Research Council (MRC) (Lipid Profiling and Signalling, MC UP A90 1006 & Lipid Dynamics and Regulation, MC PC 13030 to J.L.G.; MR/W019132/1 to Z.H.). MV is supported by the University of Bari (Horizon Europe Seed cod. id. S06-miRNASH), the Foundation for Liver Research (Intramural Funding), Associazione Italiana Ricerca sul Cancro (IG2022 Grant n. 27521) and Ministry of University and Research on Next Generation EU Funds (Cod PE00000003, CUP: H93C22000630001, DD MUR 1550, Title: “ON Foods - Research and innovation network on food and nutrition Sustainability, Safety and Security – Working ON Foods”; Cod: CN00000041, CUP: H93C22000430007, Title PNRR “National Center for Gene Therapy and Drugs based on RNA Technology”, M4C2-Investimento 1.4; Code: CN00000013, CUP: H93C22000450007, Title PNRR: “National Centre for HPC, Big Data and Quantum Computing”). G.M. was also partly funded by NIHR Cambridge Biomedical Research Centre and the Foundation for Liver Research. A.K. and B.J. are supported by the National Institute for Health Research Cambridge Biomedical Research Centre (IS-BRC-1215-20014). M.F. is funded by the British Heart Foundation (FS/18/53/33863) and is supported by the National Institute for Health and Care Research Exeter Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the funders (e.g. NIHR or the Department of Health and Social Care). V.A. was funded by the University of Cambridge, the Mason Medical Research Trust, the Academy of Medical Sciences and the NIHR Cambridge Biomedical Research Centre.
Acknowledgments
The authors are indebted to the following colleagues and institutions: (1) Katherine Stott, Matthew Watson and Sonia Liggi (Department of Biochemistry, University of Cambridge) and Damon Parkington and his team (Amanda McKillion and Tabasum Tabasum; NIHR Cambridge BRC Nutritional Biomarker Laboratory) for training, useful conversations, and access to instrumentation; (2) the MRC Metabolic Diseases Unit (MC_UU_12012/5), Richard Kay, Fiona Gribble and Frank Reimann (IMS-MRL Peptidomics and Proteomics Core) for the apolipoprotein A-I measurement, and the Biochemistry Assay Lab (Keith Burling and collaborators) for serum biochemistry.
Handling Editor: Dr Harrison & Dr Krones-Herzig
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2023.101728.
Contributor Information
Albert Koulman, Email: ak675@medschl.cam.ac.uk.
Julian L. Griffin, Email: jules.griffin@abdn.ac.uk.
Michele Vacca, Email: michele.vacca@uniba.it.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Azzu V., Vacca M., Virtue S., Allison M., Vidal-Puig A. Adipose tissue-liver cross talk in the control of whole-body metabolism: implications in nonalcoholic fatty liver disease. Gastroenterology. 2020;158(7):1899–1912. doi: 10.1053/j.gastro.2019.12.054. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 3.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4.Targher G., Byrne C.D., Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69(9):1691–1705. doi: 10.1136/gutjnl-2020-320622. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A., Csermely A., Petracca G., Beatrice G., Corey K.E., Simon T.G., et al. Non-alcoholic fatty liver disease and risk of fatal and non-fatal cardiovascular events: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(11):903–913. doi: 10.1016/S2468-1253(21)00308-3. [DOI] [PubMed] [Google Scholar]
- 6.Masoodi M., Gastaldelli A., Hyotylainen T., Arretxe E., Alonso C., Gaggini M., et al. Metabolomics and lipidomics in NAFLD: biomarkers and non-invasive diagnostic tests. Nat Rev Gastroenterol Hepatol. 2021;18(12):835–856. doi: 10.1038/s41575-021-00502-9. [DOI] [PubMed] [Google Scholar]
- 7.Guerra S., Mocciaro G., Gastaldelli A. Adipose tissue insulin resistance and lipidome alterations as the characterizing factors of non-alcoholic steatohepatitis. Eur J Clin Invest. 2022;52(3) doi: 10.1111/eci.13695. [DOI] [PubMed] [Google Scholar]
- 8.Morigny P., Boucher J., Arner P., Langin D. Lipid and glucose metabolism in white adipocytes: pathways, dysfunction and therapeutics. Nat Rev Endocrinol. 2021;17(5):276–295. doi: 10.1038/s41574-021-00471-8. [DOI] [PubMed] [Google Scholar]
- 9.Mocciaro G., Gastaldelli A. Obesity-related insulin resistance: the central role of adipose tissue dysfunction. Handb Exp Pharmacol. 2022;274:145–164. doi: 10.1007/164_2021_573. [DOI] [PubMed] [Google Scholar]
- 10.Mayo R., Crespo J., Martinez-Arranz I., Banales J.M., Arias M., Minchole I., et al. Metabolomic-based noninvasive serum test to diagnose nonalcoholic steatohepatitis: results from discovery and validation cohorts. Hepatol Commun. 2018;2(7):807–820. doi: 10.1002/hep4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders F.W.B., Acharjee A., Walker C., Marney L., Roberts L.D., Imamura F., et al. Hepatic steatosis risk is partly driven by increased de novo lipogenesis following carbohydrate consumption. Genome Biol. 2018;19(1):79. doi: 10.1186/s13059-018-1439-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang R.X., Hu C.X., Sun W.L., Pan Q., Shen F., Yang Z., et al. Serum monounsaturated triacylglycerol predicts steatohepatitis in patients with non-alcoholic fatty liver disease and chronic hepatitis B. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-11278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y., Oresic M., Leivonen M., Gopalacharyulu P., Hyysalo J., Arola J., et al. Noninvasive detection of nonalcoholic steatohepatitis using clinical markers and circulating levels of lipids and metabolites. Clin Gastroenterol Hepatol. 2016;14(10):1463–1472 e1466. doi: 10.1016/j.cgh.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 14.Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capel F., Bongard V., Malpuech-Brugere C., Karoly E., Michelotti G.A., Rigaudiere J.P., et al. Metabolomics reveals plausible interactive effects between dairy product consumption and metabolic syndrome in humans. Clin Nutr. 2020;39(5):1497–1509. doi: 10.1016/j.clnu.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Tiwari-Heckler S., Gan-Schreier H., Stremmel W., Chamulitrat W., Pathil A. Circulating phospholipid patterns in NAFLD patients associated with a combination of metabolic risk factors. Nutrients. 2018;10(5) doi: 10.3390/nu10050649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ooi G.J., Meikle P.J., Huynh K., Earnest A., Roberts S.K., Kemp W., et al. Hepatic lipidomic remodeling in severe obesity manifests with steatosis and does not evolve with non-alcoholic steatohepatitis. J Hepatol. 2021;75(3):524–535. doi: 10.1016/j.jhep.2021.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Stadler J.T., Marsche G. Obesity-related changes in high-density lipoprotein metabolism and function. Int J Mol Sci. 2020;21(23) doi: 10.3390/ijms21238985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seyres D., Cabassi A., Lambourne J.J., Burden F., Farrow S., McKinney H., et al. Transcriptional, epigenetic and metabolic signatures in cardiometabolic syndrome defined by extreme phenotypes. Clin Epigenetics. 2022;14(1):39. doi: 10.1186/s13148-022-01257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mocciaro G., D'Amore S., Jenkins B., Kay R., Murgia A., Herrera-Marcos L.V., et al. Lipidomic approaches to study HDL metabolism in patients with central obesity diagnosed with metabolic syndrome. Int J Mol Sci. 2022;23(12) doi: 10.3390/ijms23126786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 22.Bedossa P., Poitou C., Veyrie N., Bouillot J.L., Basdevant A., Paradis V., et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56(5):1751–1759. doi: 10.1002/hep.25889. [DOI] [PubMed] [Google Scholar]
- 23.Kotronen A., Peltonen M., Hakkarainen A., Sevastianova K., Bergholm R., Johansson L.M., et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137(3):865–872. doi: 10.1053/j.gastro.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Meikle P.J., Summers S.A. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat Rev Endocrinol. 2017;13(2):79–91. doi: 10.1038/nrendo.2016.169. [DOI] [PubMed] [Google Scholar]
- 25.Beyene H.B., Olshansky G., Aa T.S., Giles C., Huynh K., Cinel M., et al. Correction: high-coverage plasma lipidomics reveals novel sex-specific lipidomic fingerprints of age and BMI: evidence from two large population cohort studies. PLoS Biol. 2020;18(12) doi: 10.1371/journal.pbio.3001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azzu V., Vacca M., Kamzolas I., Hall Z., Leslie J., Carobbio S., et al. Suppression of insulin-induced gene 1 (INSIG1) function promotes hepatic lipid remodelling and restrains NASH progression. Mol Metab. 2021;48 doi: 10.1016/j.molmet.2021.101210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith G.I., Shankaran M., Yoshino M., Schweitzer G.G., Chondronikola M., Beals J.W., et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J Clin Invest. 2020;130(3):1453–1460. doi: 10.1172/JCI134165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bui H.H., Leohr J.K., Kuo M.S. Analysis of sphingolipids in extracted human plasma using liquid chromatography electrospray ionization tandem mass spectrometry. Anal Biochem. 2012;423(2):187–194. doi: 10.1016/j.ab.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 29.Hammad S.M., Pierce J.S., Soodavar F., Smith K.J., Al Gadban M.M., Rembiesa B., et al. Blood sphingolipidomics in healthy humans: impact of sample collection methodology. J Lipid Res. 2010;51(10):3074–3087. doi: 10.1194/jlr.D008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mielke M.M., Bandaru V.V., Han D., An Y., Resnick S.M., Ferrucci L., et al. Demographic and clinical variables affecting mid- to late-life trajectories of plasma ceramide and dihydroceramide species. Aging Cell. 2015;14(6):1014–1023. doi: 10.1111/acel.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vvedenskaya O., Rose T.D., Knittelfelder O., Palladini A., Wodke J.A.H., Schuhmann K., et al. Nonalcoholic fatty liver disease stratification by liver lipidomics. J Lipid Res. 2021;62 doi: 10.1016/j.jlr.2021.100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrara P.J., Rong X., Maschek J.A., Verkerke A.R., Siripoksup P., Song H., et al. Lysophospholipid acylation modulates plasma membrane lipid organization and insulin sensitivity in skeletal muscle. J Clin Invest. 2021;131(8) doi: 10.1172/JCI135963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pietilainen K.H., Rog T., Seppanen-Laakso T., Virtue S., Gopalacharyulu P., Tang J., et al. Association of lipidome remodeling in the adipocyte membrane with acquired obesity in humans. PLoS Biol. 2011;9(6) doi: 10.1371/journal.pbio.1000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borkman M., Storlien L.H., Pan D.A., Jenkins A.B., Chisholm D.J., Campbell L.V. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med. 1993;328(4):238–244. doi: 10.1056/NEJM199301283280404. [DOI] [PubMed] [Google Scholar]
- 35.Singaraja R.R., Van Eck M., Bissada N., Zimetti F., Collins H.L., Hildebrand R.B., et al. Both hepatic and extrahepatic ABCA1 have discrete and essential functions in the maintenance of plasma high-density lipoprotein cholesterol levels in vivo. Circulation. 2006;114(12):1301–1309. doi: 10.1161/CIRCULATIONAHA.106.621433. [DOI] [PubMed] [Google Scholar]
- 36.Prieur X., Mok C.Y., Velagapudi V.R., Nunez V., Fuentes L., Montaner D., et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60(3):797–809. doi: 10.2337/db10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sano O., Kobayashi A., Nagao K., Kumagai K., Kioka N., Hanada K., et al. Sphingomyelin-dependence of cholesterol efflux mediated by ABCG1. J Lipid Res. 2007;48(11):2377–2384. doi: 10.1194/jlr.M700139-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Camont L., Lhomme M., Rached F., Le Goff W., Negre-Salvayre A., Salvayre R., et al. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arterioscler Thromb Vasc Biol. 2013;33(12):2715–2723. doi: 10.1161/ATVBAHA.113.301468. [DOI] [PubMed] [Google Scholar]
- 39.van der Veen J.N., Lingrell S., Vance D.E. The membrane lipid phosphatidylcholine is an unexpected source of triacylglycerol in the liver. J Biol Chem. 2012;287(28):23418–23426. doi: 10.1074/jbc.M112.381723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musso G., Cassader M., Paschetta E., Gambino R. Bioactive lipid species and metabolic pathways in progression and resolution of nonalcoholic steatohepatitis. Gastroenterology. 2018;155(2):282–302 e288. doi: 10.1053/j.gastro.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 41.Kartsoli S., Kostara C.E., Tsimihodimos V., Bairaktari E.T., Christodoulou D.K. Lipidomics in non-alcoholic fatty liver disease. World J Hepatol. 2020;12(8):436–450. doi: 10.4254/wjh.v12.i8.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anjani K., Lhomme M., Sokolovska N., Poitou C., Aron-Wisnewsky J., Bouillot J.L., et al. Circulating phospholipid profiling identifies portal contribution to NASH signature in obesity. J Hepatol. 2015;62(4):905–912. doi: 10.1016/j.jhep.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Oresic M., Hyotylainen T., Kotronen A., Gopalacharyulu P., Nygren H., Arola J., et al. Prediction of non-alcoholic fatty-liver disease and liver fat content by serum molecular lipids. Diabetologia. 2013;56(10):2266–2274. doi: 10.1007/s00125-013-2981-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding M., Rexrode K.M. A review of lipidomics of cardiovascular disease highlights the importance of isolating lipoproteins. Metabolites. 2020;10(4) doi: 10.3390/metabo10040163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kontush A., Lhomme M., Chapman M.J. Unraveling the complexities of the HDL lipidome. J Lipid Res. 2013;54(11):2950–2963. doi: 10.1194/jlr.R036095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boon J., Hoy A.J., Stark R., Brown R.D., Meex R.C., Henstridge D.C., et al. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes. 2013;62(2):401–410. doi: 10.2337/db12-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carlier A., Phan F., Szpigel A., Hajduch E., Salem J.E., Gautheron J., et al. Dihydroceramides in triglyceride-enriched VLDL are associated with nonalcoholic fatty liver disease severity in type 2 diabetes. Cell Rep Med. 2020;1(9) doi: 10.1016/j.xcrm.2020.100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kotronen A., Velagapudi V.R., Yetukuri L., Westerbacka J., Bergholm R., Ekroos K., et al. Serum saturated fatty acids containing triacylglycerols are better markers of insulin resistance than total serum triacylglycerol concentrations. Diabetologia. 2009;52(4):684–690. doi: 10.1007/s00125-009-1282-2. [DOI] [PubMed] [Google Scholar]
- 49.Bevilacqua S., Bonadonna R., Buzzigoli G., Boni C., Ciociaro D., Maccari F., et al. Acute elevation of free fatty acid levels leads to hepatic insulin resistance in obese subjects. Metabolism. 1987;36(5):502–506. doi: 10.1016/0026-0495(87)90051-5. [DOI] [PubMed] [Google Scholar]
- 50.Lomonaco R., Ortiz-Lopez C., Orsak B., Webb A., Hardies J., Darland C., et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology. 2012;55(5):1389–1397. doi: 10.1002/hep.25539. [DOI] [PubMed] [Google Scholar]
- 51.de Almeida I.T., Cortez-Pinto H., Fidalgo G., Rodrigues D., Camilo M.E. Plasma total and free fatty acids composition in human non-alcoholic steatohepatitis. Clin Nutr. 2002;21(3):219–223. doi: 10.1054/clnu.2001.0529. [DOI] [PubMed] [Google Scholar]
- 52.Feng R., Luo C., Li C., Du S., Okekunle A.P., Li Y., et al. Free fatty acids profile among lean, overweight and obese non-alcoholic fatty liver disease patients: a case - control study. Lipids Health Dis. 2017;16(1):165. doi: 10.1186/s12944-017-0551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gambino R., Bugianesi E., Rosso C., Mezzabotta L., Pinach S., Alemanno N., et al. Different serum free fatty acid profiles in NAFLD subjects and healthy controls after oral fat load. Int J Mol Sci. 2016;17(4):479. doi: 10.3390/ijms17040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiappini F., Coilly A., Kadar H., Gual P., Tran A., Desterke C., et al. Metabolism dysregulation induces a specific lipid signature of nonalcoholic steatohepatitis in patients. Sci Rep. 2017;7 doi: 10.1038/srep46658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puri P., Baillie R.A., Wiest M.M., Mirshahi F., Choudhury J., Cheung O., et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46(4):1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 56.Ducheix S., Montagner A., Polizzi A., Lasserre F., Marmugi A., Bertrand-Michel J., et al. Essential fatty acids deficiency promotes lipogenic gene expression and hepatic steatosis through the liver X receptor. J Hepatol. 2013;58(5):984–992. doi: 10.1016/j.jhep.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 57.Sekiya M., Yahagi N., Matsuzaka T., Najima Y., Nakakuki M., Nagai R., et al. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology. 2003;38(6):1529–1539. doi: 10.1016/j.hep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 58.Dentin R., Benhamed F., Pegorier J.P., Foufelle F., Viollet B., Vaulont S., et al. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J Clin Invest. 2005;115(10):2843–2854. doi: 10.1172/JCI25256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neschen S., Morino K., Dong J., Wang-Fischer Y., Cline G.W., Romanelli A.J., et al. n-3 Fatty acids preserve insulin sensitivity in vivo in a peroxisome proliferator-activated receptor-alpha-dependent manner. Diabetes. 2007;56(4):1034–1041. doi: 10.2337/db06-1206. [DOI] [PubMed] [Google Scholar]
- 60.Scorletti E., Byrne C.D. Omega-3 fatty acids and non-alcoholic fatty liver disease: evidence of efficacy and mechanism of action. Mol Aspects Med. 2018;64:135–146. doi: 10.1016/j.mam.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Hodson L., Skeaff C.M., Fielding B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47(5):348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 62.Leaf D.A., Connor W.E., Barstad L., Sexton G. Incorporation of dietary n-3 fatty acids into the fatty acids of human adipose tissue and plasma lipid classes. Am J Clin Nutr. 1995;62(1):68–73. doi: 10.1093/ajcn/62.1.68. [DOI] [PubMed] [Google Scholar]
- 63.Jacobsen B.K., Trygg K., Hjermann I., Thomassen M.S., Real C., Norum K.R. Acyl pattern of adipose tissue triglycerides, plasma free fatty acids, and diet of a group of men participating in a primary coronary prevention program (the Oslo Study) Am J Clin Nutr. 1983;38(6):906–913. doi: 10.1093/ajcn/38.6.906. [DOI] [PubMed] [Google Scholar]
- 64.Parry S.A., Rosqvist F., Cornfield T., Barrett A., Hodson L. Oxidation of dietary linoleate occurs to a greater extent than dietary palmitate in vivo in humans. Clin Nutr. 2021;40(3):1108–1114. doi: 10.1016/j.clnu.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 65.Burke J.E., Dennis E.A. Phospholipase A2 biochemistry. Cardiovasc Drugs Ther. 2009;23(1):49–59. doi: 10.1007/s10557-008-6132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwendeman A., Sviridov D.O., Yuan W., Guo Y., Morin E.E., Yuan Y., et al. The effect of phospholipid composition of reconstituted HDL on its cholesterol efflux and anti-inflammatory properties. J Lipid Res. 2015;56(9):1727–1737. doi: 10.1194/jlr.M060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hong K., Yu M., Crowther J., Mei L., Olsen K., Luo Y., et al. Effect of lipid composition on the atheroprotective properties of HDL-mimicking micelles. Pharmaceutics. 2022;14(8) doi: 10.3390/pharmaceutics14081570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCullough A., Previs S.F., Dasarathy J., Lee K., Osme A., Kim C., et al. HDL flux is higher in patients with nonalcoholic fatty liver disease. Am J Physiol Endocrinol Metab. 2019;317(5):E852–E862. doi: 10.1152/ajpendo.00193.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Costanzo A., Ronca A., D'Erasmo L., Manfredini M., Baratta F., Pastori D., et al. HDL-mediated cholesterol efflux and plasma loading capacities are altered in subjects with metabolically- but not genetically driven non-alcoholic fatty liver disease (NAFLD) Biomedicines. 2020;8(12) doi: 10.3390/biomedicines8120625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fadaei R., Poustchi H., Meshkani R., Moradi N., Golmohammadi T., Merat S. Impaired HDL cholesterol efflux capacity in patients with non-alcoholic fatty liver disease is associated with subclinical atherosclerosis. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-29639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zannis V.I., Fotakis P., Koukos G., Kardassis D., Ehnholm C., Jauhiainen M., et al. HDL biogenesis, remodeling, and catabolism. Handb Exp Pharmacol. 2015;224:53–111. doi: 10.1007/978-3-319-09665-0_2. [DOI] [PubMed] [Google Scholar]
- 72.Robichaud J.C., van der Veen J.N., Yao Z., Trigatti B., Vance D.E. Hepatic uptake and metabolism of phosphatidylcholine associated with high density lipoproteins. Biochim Biophys Acta. 2009;1790(6):538–551. doi: 10.1016/j.bbagen.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 73.Borggreve S.E., De Vries R., Dullaart R.P. Alterations in high-density lipoprotein metabolism and reverse cholesterol transport in insulin resistance and type 2 diabetes mellitus: role of lipolytic enzymes, lecithin:cholesterol acyltransferase and lipid transfer proteins. Eur J Clin Invest. 2003;33(12):1051–1069. doi: 10.1111/j.1365-2362.2003.01263.x. [DOI] [PubMed] [Google Scholar]
- 74.D'Amore S., Hardfeldt J., Cariello M., Graziano G., Copetti M., Di Tullio G., et al. Identification of miR-9-5p as direct regulator of ABCA1 and HDL-driven reverse cholesterol transport in circulating CD14+ cells of patients with metabolic syndrome. Cardiovasc Res. 2018;114(8):1154–1164. doi: 10.1093/cvr/cvy077. [DOI] [PubMed] [Google Scholar]
- 75.Prada M., Wittenbecher C., Eichelmann F., Wernitz A., Drouin-Chartier J.P., Schulze M.B. Association of the odd-chain fatty acid content in lipid groups with type 2 diabetes risk: a targeted analysis of lipidomics data in the EPIC-Potsdam cohort. Clin Nutr. 2021;40(8):4988–4999. doi: 10.1016/j.clnu.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 76.Imamura F., Sharp S.J., Koulman A., Schulze M.B., Kroger J., Griffin J.L., et al. A combination of plasma phospholipid fatty acids and its association with incidence of type 2 diabetes: the EPIC-InterAct case-cohort study. PLoS Med. 2017;14(10) doi: 10.1371/journal.pmed.1002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abdullah M.M., Cyr A., Lepine M.C., Labonte M.E., Couture P., Jones P.J., et al. Recommended dairy product intake modulates circulating fatty acid profile in healthy adults: a multi-centre cross-over study. Br J Nutr. 2015;113(3):435–444. doi: 10.1017/S0007114514003894. [DOI] [PubMed] [Google Scholar]
- 78.Weitkunat K., Schumann S., Nickel D., Hornemann S., Petzke K.J., Schulze M.B., et al. Odd-chain fatty acids as a biomarker for dietary fiber intake: a novel pathway for endogenous production from propionate. Am J Clin Nutr. 2017;105(6):1544–1551. doi: 10.3945/ajcn.117.152702. [DOI] [PubMed] [Google Scholar]
- 79.Jenkins B.J., Seyssel K., Chiu S., Pan P.H., Lin S.Y., Stanley E., et al. Odd chain fatty acids; new insights of the relationship between the gut microbiota, dietary intake, biosynthesis and glucose intolerance. Sci Rep. 2017;7 doi: 10.1038/srep44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wallace M., Green C.R., Roberts L.S., Lee Y.M., McCarville J.L., Sanchez-Gurmaches J., et al. Enzyme promiscuity drives branched-chain fatty acid synthesis in adipose tissues. Nat Chem Biol. 2018;14(11):1021–1031. doi: 10.1038/s41589-018-0132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luukkonen P.K., Zhou Y., Sadevirta S., Leivonen M., Arola J., Oresic M., et al. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J Hepatol. 2016;64(5):1167–1175. doi: 10.1016/j.jhep.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 82.Iqbal J., Walsh M.T., Hammad S.M., Cuchel M., Tarugi P., Hegele R.A., et al. Microsomal triglyceride transfer protein transfers and determines plasma concentrations of ceramide and sphingomyelin but not glycosylceramide. J Biol Chem. 2015;290(43):25863–25875. doi: 10.1074/jbc.M115.659110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chaurasia B., Summers S.A. Ceramides in metabolism: key lipotoxic players. Annu Rev Physiol. 2021;83:303–330. doi: 10.1146/annurev-physiol-031620-093815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patanapirunhakit P., Karlsson H., Mulder M., Ljunggren S., Graham D., Freeman D. Sphingolipids in HDL - potential markers for adaptation to pregnancy? Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866(8) doi: 10.1016/j.bbalip.2021.158955. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.