Abstract
Hand, foot and mouth disease (HFMD) is a major public health problem among children in the Asia-Pacific region. The optimal specimen for HFMD virological diagnosis remains unclear. Enterovirus A71 (EV-A71) neutralizing antibody titres detected in paired sera were considered the reference standard for calculating the sensitivity, specificity, positive and negative predictive value of throat swabs, rectal swabs, stool, blood samples and cerebrospinal fluid (CSF) by RT-PCR or ELISA assay. In this study, clinical samples from 276 HFMD patients were collected for analysing the sensitivity of different kind of specimens. Our results showed that stool had the highest sensitivity (88%, 95% CI: 74%–96%) and agreement with the reference standard (91%). The order of diagnostic yield for EV-A71 infection was stool sample ≥ rectal swab > throat swab > blood sample > CSF sample, and using a combination of clinical samples improved sensitivity for enterovirus detection. The sensitivity of ELISA for IgM antibody detection in sterile-site specimens was significantly higher than that of RT-PCR (serum/plasma: 62% vs. 2%, CSF: 47% vs. 0%) (P < 0.002). In conclusion, our results suggest that stool has the highest diagnostic yield for EV-A71-infected HFMD. If stool is unavailable, rectal swabs can be collected to achieve a similar diagnostic yield. Otherwise, throat swabs may be useful in detecting positive samples. Although IgM in blood or CSF is diagnostically accurate, it lacks sensitivity, missing 40%–50% of cases. The higher proportion of severe cases and shorter interval between onset and sampling contributed to the increase in congruency between clinical testing and the serological reference standard.
Keywords: Hand, foot and mouth disease (HFMD); Enterovirus A71 (EV-A71); Sensitivity; Specificity; Evaluation of diagnostic methods
Highlights
-
•
Stool samples are the single most useful specimen for the aetiological diagnosis of EV-A71 infection.
-
•
Rectal swabs achieved a similar diagnostic yield as stool samples in the virological diagnosis of HFMD.
-
•
Although ELISA was inferior to some RT-PCR methods, it nevertheless improved the diagnostic yield.
-
•
The diagnostic method, clinical severity and interval between onset and sampling can affect the diagnostic yield.
1. Introduction
Hand, foot and mouth disease (HFMD) is a common and important childhood illness affecting millions of children in the Asia-Pacific region. The case burden and mortality of HFMD in China are the highest in the world (Yu and Cowling, 2019), with 1.62–2.78 million cases reported annually between 2010 and 2019 (Wu et al., 2016). While HFMD is mainly caused by several serotypes of human enterovirus A species, HFMD caused by enterovirus A71 (EV-A71) infection is associated with a higher proportion of unfavourable outcomes, owing to the neurological complications that typically accompany it (Ooi et al., 2007). Three inactivated monovalent EV-A71 vaccines are currently available in China, but they do not confer cross-protection against other enteroviruses (Li et al., 2019), highlighting the need to improve the diagnostic workflow to isolate infected children quickly to interrupt the spread of the disease and avoid the use of unnecessary antibiotics.
As HFMD is a rash-presenting disease, its clinical manifestations are sometimes difficult to distinguish from those of chickenpox, impetigo, measles, and so on (Muppa et al., 2011, Centers for Disease and Prevention, 2012; Sinclair et al., 2014; Yan et al., 2015; Li et al., 2018; Sapia et al., 2020). Furthermore, due to symptom similarities among different serotypes, virological diagnosis of HFMD based on clinical manifestations alone is difficult (Knipe and Howley, 2013). As a result, HFMD is easily misdiagnosed, which results in delayed therapy and public health measures and subsequent epidemics (Li et al., 2018). Overall, improving diagnostic specificity can facilitate subsequent disease management.
Compared with specimens obtained from sterile sites, throat swabs and intestinal specimens collected from non-sterile sites show a higher viral detection rate, but differentiating pathogen carriage from disease causality can be challenging (Ooi et al., 2007). Moreover, the detection rate for enterovirus using different specimens from the same patient and in serial specimens from the rectum can be inconsistent (Ooi et al., 2007; Gopalkrishna et al., 2012; Yi et al., 2013; Teoh et al., 2016; Cordey et al., 2017). This is partly due to two reasons: differences between virus isolation and PCR methods and a lack of reference data for comparison.
At present, laboratory detection using molecular diagnostic tests such as RT-PCR (including qRT-PCR) have been frequently used in children with HFMD (Harvala et al., 2018). In reality, in our experience, vesicle swabs are rarely collected. Per local guidelines, cerebrospinal fluid (CSF) is collected from HFMD patients only with suspected central nervous system (CNS) involvement. Previous studies have observed low sensitivity for PCR using samples from sterile sites (Ooi et al., 2007; Gopalkrishna et al., 2012; Cordey et al., 2017). Many studies have shown that anti-EV-A71 antibody can be detected when children visit a hospital, but the correlations between pathogen detection in different clinical specimens from HFMD patients and the serological reference standard have not been reported.
To address the issues regarding the accurate virological diagnosis of HFMD described above, we conducted a study comparing serological diagnosis as the reference standard with other laboratory diagnostic tests. We used a minimum 4-fold increase in the EV-A71 neutralising antibody (NAb) titre between acute and convalescent samples or a convalescent titre of <32 to define true EV-A71 infection and to rule out EV-A71 infection, respectively. We compared the serological diagnostic results with the diagnostic results for different types of clinical specimens, and diagnostic tests (including RT-PCR and ELISA) were evaluated to provide robust evidence of the optimal specimen and diagnostic strategy for HFMD virological diagnosis and to identify associated influencing factors.
2. Materials and methods
2.1. Study design and participants
Clinically diagnosed HFMD patients (defined as rash on hands, feet, mouth or buttocks, vesicles in the mouth, with or without fever) who were hospitalized in Henan Children's Hospital (a HFMD referral centre) between February 15, 2017, and February 15, 2018, were enrolled. Among 310 patients who attended follow-up (for details, see the supplementary information), 276 with paired sera available for EV-A71 NAb detection were included in this analysis. Data were collected during hospitalization using a standardized case report form as described previously (Li et al., 2019). Severe HFMD cases were defined using four indicators, as described previously (Song et al., 2020): 1) CNS complications; 2) admission to the intensive care unit (ICU); 3) requirement for systematic corticosteroids or intravenous immunoglobulin (IVIG); 4) length of hospital stay (LOS) longer than 5 days.
2.2. Specimen collection
Throat and rectal swabs, and stool, blood, vesicle, and CSF samples were collected from enrolled patients. Briefly, throat and rectal swabs were simultaneously collected within 48 h of enrolment, vesicle and stool samples were collected if possible, and CSF was collected from children with suspected CNS involvement. Sera were collected during hospitalization and at follow-up visits after discharge (Qiu et al., 2021). All samples were stored at −80 °C until testing.
2.3. Laboratory assays
An overall flow chart of the laboratory assays conducted is shown in Supplementary Figs. S1–A. Paired sera were tested by an EV-A71 neutralization (NT) assay at the laboratory of Fudan University. All available samples except sera were tested by RT-PCR. For auxiliary diagnosis, acute serum and CSF samples were tested using a commercial EV-A71 IgM-ELISA kit in the hospital laboratory, as previously described (Zhang et al., 2020).
2.3.1. Test 1: EV-A71 NT assay
Serum samples were serially diluted 2-fold (1:8 to 1:4096) in duplicate after inactivation, mixed with an equal volume of 100 TCID50 EV-A71 virus strain FY1708 (C4a) and incubated for 2 h. The serum-virus mixture was seeded with a human rhabdomyosarcoma cell suspension and incubated for 7 days. Control wells comprising cells alone, cells with virus (no serum), positive serum control and test serum alone (for serum toxicity) were included on each plate. NAb titres were defined as the highest dilution that inhibited cytopathic effects in 50% of the wells as calculated by the method of Karber (WHO, 2004). If the titre was higher than 4096, the serum sample was retested within a 1:8 to 1:16384 dilution.
2.3.2. Test 2: commercial EV-A71 IgM-ELISA
The assay was conducted according to the manufacturer's instructions (Beier, Beijing, China). Briefly, the test sample was added to wells containing anti-human IgM monoclonal antibody. Bound antibodies were detected by the addition of horseradish peroxidase-labelled recombinant EV-A71 antigen, followed by substrate. Stop solution was added, and the optical density at 450 nm was measured.
2.3.3. Test 3: RT-PCR
Real-time RT-PCR targeting pan-EV and EV-A71 primers and probes (Supplementary Table S1) was performed to rapidly screen for EV-positive samples, and several nested RT-PCRs were conducted for EV genotype identification (Supplementary Figs. S1–B) as previously described (Li et al., 2019; Zhou et al., 2021).
2.4. EV-A71 infection definition
NAb criteria were used as the reference standard to identify EV-A71-infected HFMD patients. EV-A71 infection was defined as a convalescent EV-A71 NAb titre >512 (reasons listed in the supplementary materials) and a minimum 4-fold increase over acute serum NAb titre within three months. Non-EV-A71 infection was defined as an EV-A71 NAb in paired sera <32. The following results were considered possible EV-A71 infection: ① titres of paired sera within 32–512; ② titres changing within 2-fold, with that of second serum titre >512 within three months; ③ NAb titre decreasing more than 2-fold; ④ second serum sample collected at more than three months with a titre >512.
2.5. Statistical analysis
All analyses were performed with R software version 3.4.4 (http://www.R-project.org). Categorical variables were compared by the χ2 test or Fisher's exact test. Medians of continuous variables were compared using the Kruskal-Wallis H test.
The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for each sample type were calculated using NAb criteria as the reference standard. Sensitivity analyses were conducted by parameter sensitivity analysis: all HFMD patients considered to have possible EV-A71 infection were categorized as having proven EV-A71 infection or non-EV-A71 infection for analysis. The 95% confidence intervals (CIs) for parameters were calculated according to the efficient-score method (corrected for continuity) described by Newcombe (1998).
To analyse the effects of clinical variables on testing with different laboratory diagnostic methods (such as ELISA) and the reference standard, Student's t-test was employed to analyse the effects of different specimen types and the interval between onset and sample collection. To account for the nonrandomized nature of severity and diagnosis according to the reference standard, we used dependent propensity scores to match pairs of individuals, and Pearson's chi-square test with Yates' continuity correction was performed to determine the influence of clinical severity.
Using the specimen giving the greatest agreement with the reference standard as a reference, logistic regression was applied to analyse agreement between the reference standard and the different types of clinical specimens considering the diagnostic method, clinical severity and the interval between onset and sampling.
The logistic model was as follows: where represents the congruence between the results of the common diagnostic methods and those of the reference standard, indicates the interval between onset and sampling of different specimens, and indicates the estimated coefficient of the reference method.
All statistical tests were two-tailed, and the significance level was set as α=0.05.
3. Results
3.1. Overview
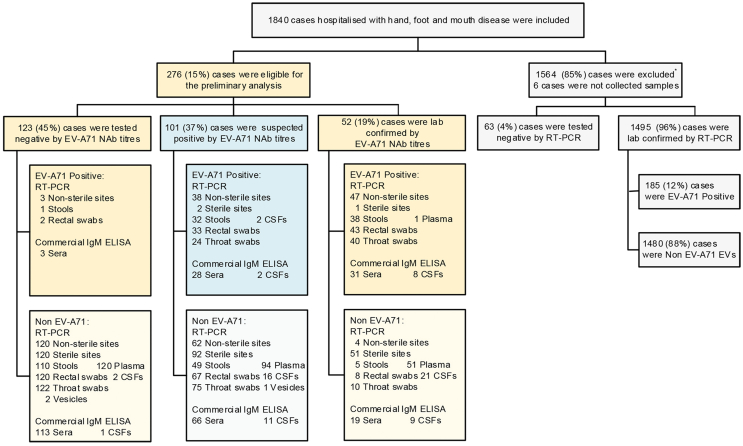
Overall, 1357 (74%) out of 1840 enrolled, clinically diagnosed HFMD patients (Li et al., 2019) were invited to participate in follow-up, and 23% (310/1357) of them attended the follow-up visits. After excluding 34 patients for whom paired sera were not available, 89% (276/310) of the clinically diagnosed HFMD patients were eligible for this study (Fig. 1), 179 (65%) of whom were male. The median age was 2 years [interquartile range (IQR): 1–3 years]. The median duration from onset to admission was 2 days (IQR: 1–3 days). Sex ratio and median age were similar between the 276 (15%) patients who participated in the study and the 1564 patients who were excluded. Among participants, the median interval between onset and admission (P = 0.0237) and the median LOS (P < 0.0001) were longer and the proportion of severe HFMD was higher than among nonparticipants (P < 0.0001, Table 1).
Fig. 1.
Flowchart of patient enrolment. “∗” indicates that the patient met one of the following criteria: previous paediatric intensive care unit (PICU) admission/ventilation (123, 54%); premature birth (before 37 weeks) (91, 40%); any prior chronic respiratory, cardiac, or other illness (i.e., congenital hypothyroidism, congenital epilepsy, asthma) (37, 16%); prior delayed development or neurodevelopment (34, 15%); or prior learning disability or neurological regression (8, 4%). In addition, some patients met more than 1 criterion.
Table 1.
Comparison of demographics between participants in the study and excluded patients in Zhengzhou city, China, from Feb. 15, 2017 to Feb. 15, 2018.
| Characteristics | Included patients N= 276, n (%) |
Excluded patients N= 1564, n (%) |
P value |
|---|---|---|---|
| Male sex | 179 (65) | 983 (63) | 0.5247 |
| Age, median [IQR, yrs] | 2 (1–3) | 2 (1–3) | 0.0501 |
| Age group | |||
| 0∼ yrs | 1 (0.4) | 21 (1) | 0.2369 |
| 1∼ yrs | 102 (37) | 662 (42) | |
| 2∼ yrs | 82 (30) | 424 (27) | |
| 3∼ yrs | 49 (18) | 257 (16) | |
| 4∼ yrs | 29 (11) | 118 (8) | |
| 5∼ yrs | 7 (3) | 56 (4) | |
| 6∼ yrs | 6 (2) | 26 (2) | |
| Severe | 127 (46) | 381 (24) | <0.0001 |
| Median LOS [IQR, days] | 5 (4–6) | 4 (4–5) | <0.0001 |
Note: LOS= length of hospital stay.
Data are no. (%) of patients or median value. IQR=interquartile range.
P values were estimated by the Kruskal‒Wallis H test for median age [interquartile range (IQR), years], median interval time between illness onset and admission [IQR, days], and median LOS [IQR, days] and by χ2 tests or Fisher's exact test for all other characteristics.
3.2. Specimens
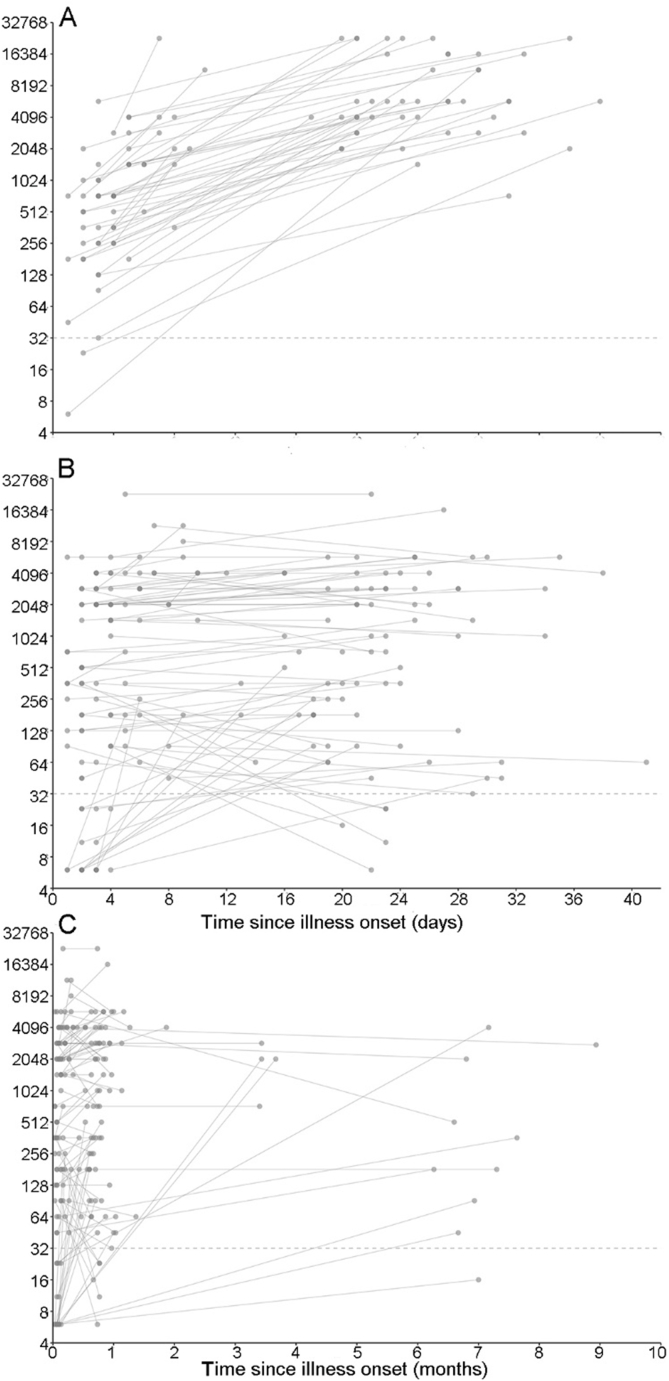
We collected 1644 specimens from the 276 included patients. Of these, 1641 specimens (552 sera, 271 throat swabs, 273 rectal swabs, 235 stool samples, 266 plasma samples, 3 vesicle samples, and 41 CSF samples) were tested at the laboratory of Fudan University, while 291 specimens (260 sera and 31 CSF) were tested at the hospital laboratory. A total of 260 acute-phase sera and 28 CSF samples were analysed in both laboratories (Supplementary Table S2). Ninety-six percent of plasma samples, 95% of throat and rectal swabs, 91% of stool samples, 88% of CSF samples (RT-PCR detection), 77% of CSF samples (ELISA detection) and 95% of acute-phase sera were collected within 7 days after onset (Fig. 2). For patients with paired sera, the median time between onset and the first serum/plasma collection was 3 days (IQR: 2–4 days), and that between onset and the second serum collection was 22 days (IQR: 19–28 days). The median intervals between onset and sampling of throat and rectal swabs, stool samples, and CSF samples were 4 days (IQR: 3–5 days), 4 days (IQR: 3–6 days), and 6 days (IQR: 4–7 days), respectively.
Fig. 2.
Interval between onset and sampling (days) by sample type. A The distribution of intervals between onset and sampling (days) among the different sample types. B The cumulative probability of interval time between onset and sampling (day) among the different sample types. Acute-phase serum samples (NT) were tested by the NT assay. CSF (IgM) and acute-phase serum samples (IgM) were tested by ELISA. Other five samples were tested by RT-PCR.
3.3. Laboratory test results
The overall results are shown in Fig. 3. For the 276 patients with paired sera, the EV-A71 NT assay confirmed EV-A71 infection in 52 (19%) patients; 123 (45%) patients were considered negative for EV-A71 infection, and 101 (37%) patients were considered to have possible EV-A71 infection. Among the 52 patients with confirmed infection, the median titre of the first serum sample was 724 (IQR: 256–1448), and that of the second serum sample was 4944.5 (IQR: 2896–11585). For the 101 patients with possible EV-A71 infection, the median titre of the first serum sample was 512 (IQR: 64–2896), and that of the second serum sample was 724 (IQR: 181–2896) (Supplementary Fig. S2, for raw data, see the supplemental dataset). The samples from 63 (24%) of 260 patients were subjected to EV-A71 IgM-ELISA and tested positive. Eighty-eight (32%) of 276 patients with available samples for RT-PCR had sequence-confirmed EV-A71 infection. By combining the two diagnostic methods, 96 (35%) of the 276 patients were considered positive for EV-A71 infection (Supplementary Table S3).
Fig. 3.
Overview of patient enrolment and detection results. “∗” indicates the 1564 excluded patients, as shown in Fig. 1. Non-sterile samples included throat swabs, rectal swabs and stool samples. Sterile samples included CSF, plasma and vesicle fluid. Sterile sites (266 cases) and non-sterile sites (274 cases) were both classified based on the following: EV-A71 positivity was defined as EV-A71 detection in at least one sample, and EV-A71 negativity was defined as no EV-A71 detection in all samples for which EVs were or were not detected.
3.4. Diagnostic accuracy
We used the data from 175 patients with definitively positive or negative EV-A71 NT assay results as the reference standard to assess the performance of different samples (Table 2).
Table 2.
Neutralization tests for assessing the diagnostic performance of different samples and methods.
| Methods | Specimens | No. of patients# | SEN, % (95% CI) | SPE, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|---|---|
| RT-PCR | Sterile sites (n=266) | 172 | 2 (0.1–12) | 100 (96–100) | 100 (6–100) | 70 (63–77) |
| CSF (n=41) | 23 | 0 (0–19) | 100 (20–100) | – | 9 (2–30) | |
| Plasma (n=266) | 172 | 2 (0.1–12) | 100 (96–100) | 100 (6–100) | 70 (63–77) | |
| Non-sterile sites (n=274) | 174 | 92 (80–98) | 98 (93–99) | 94 (83–98) | 97 (91–99) | |
| Throat swab (n=271) | 172 | 80 (66–90) | 100 (96–100) | 100 (90–100) | 92 (86–96) | |
| Rectal swab (n=273) | 173 | 84 (71–93) | 98 (94–100) | 96 (84–99) | 94 (88–97) | |
| Stool sample (n=235) | 154 | 88 (74–96) | 99 (94–100) | 97 (85–100) | 96 (90–98) | |
| Commercial EV-A71 IgM-ELISA | Sera (n=260) | 166 | 62 (47–75) | 97 (92–99) | 91 (75–98) | 86 (78–91) |
| CSF (n=31) | 18 | 47 (24–72) | 100 (6–100) | 100 (60–100) | 10 (0.5–46) |
Note: SEN= sensitivity; SPE= specificity; PPV= positive predictive value; NPV= negative predictive value. Sterile sites are CSF, plasma and vesicle swabs; non-sterile sites are throat swabs, rectal swabs and stool samples. “#” indicates that HFMD patients with unclear detection results were excluded.
For RT-PCR, the most sensitive single sample type was stool (sensitivity: 88%, 38/43), with no significant difference between stool samples and rectal swabs (sensitivity: 84%, 43/51) or throat swabs (sensitivity: 80%, 40/50). The proportion of patients correctly diagnosed with EV-A71 infection (the diagnostic yield) increased to 92% (47/51) when the results of all non-sterile site samples were combined. The sensitivity of samples from sterile sites was very low (<2%). All samples had satisfactory specificity (>97%) and PPV (>94%). The CSF had the lowest NPV (9%, 2/23), followed by plasma (70%, 120/171); all the others achieved an NPV higher than 92%.
The sensitivity of IgM antibody detection in serum and CSF in the diagnosis of EV-A71 infection was 62% (31/50) and 47% (8/17), respectively, and their specificity and PPV were higher than 91%. The NPVs for serum and CSF were 86% (113/132) and 10% (1/10), respectively, and were similar to those for RT-PCR detection. Compared with RT-PCR, the sensitivity of the commercial EV-A71 IgM-ELISA was much higher in CSF (P =0.0017) and serum/plasma (P < 0.0001). The specificity, PPV and NPV were not significantly different between the testing methods (Supplementary Table S4).
A total of 101 HFMD patients with possible EV-A71 infection were classified as having proven EV-A71 infection or non-EV-A71 infection for sensitivity analysis (for details, see the Supplementary information). After the sensitivity analysis, HFMD due to EV-A71 infection was redefined as an increasing trend in the EV-A71 NAb titre, a within 2-fold change from the acute to convalescent samples within three months, and a convalescent serum titre >512; HFMD caused by non-EV-A71 infection was defined as EV-A71 NAb titres <512 or a more than 2-fold decrease in the NAb titres of paired sera within 3 months. On the basis of these definitions, 97 (35%) patients were classified as having a proven EV-A71 infection, and 179 (65%) were considered to be non-EV-A71 infection (Supplementary Fig. S3); only the sensitivity and NPV of some of the sensitivity analysis results were significantly different before and after the sensitivity analysis (Supplementary Table S5). In addition, there was no difference in the overall conclusions for 175 patients with conclusive NT assay results compared with that for the 276 patients after sensitivity analysis (P > 0.05, Supplementary Table S3).
3.5. Analysis of influencing factors
The influence of clinical factors on the diagnostic performance of the different laboratory methods is shown in Supplementary Table S5. In samples whose diagnoses did not agree with the reference standard serological diagnosis (i.e., false negatives), there was, on average, a longer interval between onset and sampling than for samples whose diagnosis did agree with that of the reference standard, implying a limited window for virus detection after onset. Based on the RT-PCR results, plasma showing concordant results with NAb detection (“true positives”) was collected on average 3 days after symptom onset, whereas negative plasma in cases with a definitive diagnosis by NAb detection (“false negatives”) was collected 0.9 days later (P = 0.0002). Overall, stool sample, throat swab, rectal swab, serum and CSF sample types showed no relationship between the time of collection and agreement with the reference standard (Supplementary Table S6). Agreement between RT-PCR results (from stool samples and rectal swabs) and serological reference standard was higher in severe cases (P < 0.05, Supplementary Table S7).
According to our results, the diagnoses from the stool samples exhibited the greatest agreement with the serological reference standard diagnoses (91%, 213/235). The logistic regression analysis results (Table 3) showed no significant difference between the results of the rectal swabs and stool samples, and the results of the other samples were less useful than those of the stool samples. Similar to the results of the univariate analyses, increasing clinical severity and a shorter interval between onset and sampling led to higher agreement with the reference standard-based diagnosis (P < 0.0001).
Table 3.
Logistic regression analysis results.
| Variable | Coefficient | SD | Z value | P value |
|---|---|---|---|---|
| Stool sample RT-PCR (reference) (n=143) | 2.29454 | 0.28672 | 8.003 | <0.0001 |
| Sera ELISA (n=163) | −0.95277 | 0.29895 | −3.187 | 0.0014 |
| CSF ELISA (n=26) | −1.70205 | 0.46746 | −3.641 | 0.0003 |
| Rectal swab RT-PCR (n=169) | −0.49809 | 0.31035 | −1.605 | 0.1085 |
| Throat swab RT-PCR (n=168) | −0.66284 | 0.30567 | −2.169 | 0.0301 |
| CSF RT-PCR (n=34) | −2.87922 | 0.44676 | −6.445 | <0.0001 |
| Plasma RT-PCR (n=165) | −2.08158 | 0.29242 | −7.118 | <0.0001 |
| Interval between onset and sampling | −0.16706 | 0.02938 | −5.687 | <0.0001 |
| Severity | 0.50829 | 0.126 | 4.025 | <0.0001 |
Notes: Coefficient indicates the regression coefficient which is the change in log odds of having the outcome per unit change in the predictor, SD means standard deviation, Z value is the Wald statistic (estimatio/SD).
4. Discussion
This was a prospective, hospital-based cohort study on HFMD patients in China. RT-PCR testing revealed that the order of diagnostic yield for EV-A71 infection was stool ≥ rectal swab > throat swab > plasma/serum > CSF and that using a combination of clinical samples for EV detection can improve sensitivity. Although ELISA was generally inferior to RT-PCR, performing the anti-EV-A71 IgM-ELISA with serum samples improved the diagnostic yield, and thus this test may be useful in cases for which EV-specific RT-PCR of throat swabs or intestinal specimens is negative and a diagnosis is elusive, for example, when sampling occurs late in the course of illness.
The aetiological diagnosis of EV infection mainly includes virus isolation, serological detection and PCR-based molecular diagnosis methods. Virus isolation and paired sera testing with the NT assay is time and laboratory resource intensive, and some serotypes of EVs are uncultivable (Knipe and Howley, 2013; Harvala et al., 2018). Commercial IgM-ELISA has been widely used in routine clinical practice (Harvala et al., 2018), but it is prone to serological cross-reactivity and exhibits limited sensitivity. In contrast, the efficiency, specificity and sensitivity of molecular diagnostic methods (Oberste et al., 1999) are suitable for different kinds of samples (Kupila et al., 2005; Harvala et al., 2018), simplifying EV diagnosis.
One review found that researchers preferred to test respiratory samples (31%) rather than gastrointestinal samples (25%) for enterovirus diagnosis (Brouwer et al., 2021); however, the sample type used for diagnosis is generally determined according to experience, without much evidence as support. Unlike sterile-site specimens, which require invasive collection procedures, throat swabs, rectal swabs, and stool samples can be collected noninvasively, making sampling more convenient. The cooperation of children for throat swab collection is relatively poorer than that for stool samples. Our study found that the sensitivity of stool samples (88%) was higher than that of throat swabs (80%). This is the seventh study to report this result (Kupila et al., 2005; Gopalkrishna et al., 2012; Yi et al., 2013; Teoh et al., 2016; Cordey et al., 2017; Zhou et al., 2017), supporting the notion that the virus is cleared more quickly from the throat, reducing the sensitivity of throat swab testing for diagnosis (Zhu et al., 2013).
Interestingly, the sensitivity, specificity, PPV, NPV and agreement with the reference standard were the same in stool samples and rectal swabs tested by RT-PCR. This is consistent with the results of a previous study which indicated that the virus detection rates in both stool samples and rectal swabs were approximately 95%, with no significant difference between them (Teoh et al., 2016). In addition, using a combination of samples for detection can improve the sensitivity. A study by Ooi et al. (2007) showed that testing multiple samples can improve the positive EV detection rate (throat swabs: 49%, vesicles: 48%, rectal swabs: 28%, ulcer swabs: 26%, all four specimens: 78%), increasing the validity of our findings.
In our study, the sensitivity of RT-PCR testing using samples obtained from sterile sites was very low, consistent with previous studies (Gopalkrishna et al., 2012; Cordey et al., 2017). Two possible explanations for this are as follows: first, the lithium heparin anticoagulant used in our study may have interfered with the assays (Lam et al., 2004), and second, the EV viral load in plasma may be low and difficult to detect (Zhao et al., 2017). However, the sensitivity of IgM antibody detection in blood and CSF is much higher than that of RT-PCR detection (blood samples 62% vs. 2%; CSF 47% vs. 0%), meaning that serum samples have substantial diagnostic utility. When patients with HFMD have symptoms suggesting CNS involvement, enterovirus IgM antibody detection in collected CSF samples can help in identifying encephalopathy or viral encephalitis. A previous study found a similar pattern in Japanese encephalitis virus (JEV) detection results in samples obtained from patients with suspected CNS infection. This study showed that eleven (27%, 11/41) CSF and serum samples were anti-JEV MAC-ELISA positive (IgM), but JEV RNA was not detected by RT-qPCR in the same samples (Bharucha et al., 2019).
Previous studies have reported that serological cross-reactivity occurs when enterovirus IgM is detected by ELISA. However, Zhang et al. in a study using RT-PCR as the reference method, obtained overall ELISA specificities similar to ours (Zhang et al., 2020) and comparatively higher than those of other studies, in which the specificity ranged from 72.1% to 90.0% (Xu et al., 2010; Lin et al., 2011; Yu et al., 2012; Aw-Yong et al., 2016; Zhang et al., 2016). This may be because Zhang T. et al. and our group used the same ELISA kit, while that used in the other studies had a number of deficiencies other than the cross-reactivity in IgM detection.
Only the study conducted by Ooi et al. used virus isolation from vesicles as a reference to evaluate the diagnostic values of other clinical specimens (Ooi et al., 2007). The positive detection rates of all samples from all sites in their study were less than 50%, leaving a significant number of patients without virological confirmation. Compared with the sensitivity in their study, the sensitivity of throat and rectal swabs in our study was significantly higher (throat swabs: 67% vs. 80%; rectal swabs: 31% vs. 84%). The sensitivity of oral ulcer swabs in their study was 28%, which was statistically significantly lower than that of all non-sterile site specimens (stools, rectal swabs and throat swabs) in our study. Furthermore, the specificities, PPVs, NPVs and agreement with the reference standards of throat swabs, rectal swabs and oral ulcer swabs in their study were all lower than those in our study. It is likely that the limit of detection for viral isolation was higher than that for the EV-A71 NT assay and RT‒PCR used in our study.
Most patients (72%, 1329/1840) hospitalized with HFMD in our study experienced mild illness. The high proportion of mild illness might be explained by the desire of local physicians to avoid missing the rapidly deteriorating cases. Additionally, many parents may be nervous about HFMD due to extensive health education messaging about the disease by the government and the health departments. Furthermore, inpatients receive larger reimbursement amounts because of the healthcare insurance system requirements in the mainland of China (Gao et al., 2018). To account for the nonrandomized nature of severity and diagnosis by NAb titre, we used dependent propensity scores to match pairs of individuals. We confirmed that greater severity and a shorter interval between onset and sampling were associated with agreement between the common diagnostic methods and the reference standard. This is possibly because clinical severity is positively correlated with the viral genomic load (Song et al., 2020), and the sampling time and the dynamic changes in virus excretion can affect the positive detection rate and hence the sensitivity of the diagnostic method. The limited window for virus detection in plasma after onset is consistent with low plasma viraemia in patients with enterovirus infection (Zhao et al., 2017).
Our study has several limitations. First, NAb can be detected in children at the time of presentation to the hospital, which may lead to an underestimation of the number of EV-A71 infections when using the chosen reference standard for diagnosis. Nevertheless, we applied strict criteria in our analysis to increase specificity. Our study also showed that the reference standard had a higher sensitivity than virus isolation. Second, the interval between onset and sampling was short, typically less than 7 days, and more than half of the samples were collected within 3–5 days after onset. This interval had an impact on agreement with the diagnostic results, but given the lack of variation, our results cannot be used to guide the actual sampling time, which may be compounded by the use of various clinical specimens in this study. Third, most of the mildly affected patients declined to participate in follow-up. However, we used dependent propensity score matching to establish matched pairs of individuals to account for the nonrandomized nature of severity and diagnosis by NAb titre. Fourth, the number of EV-A71-infected patients/samples was small. Only 1% of cases collected vesicle swabs. Nonetheless, our conclusions remained unchanged after sensitivity analysis, suggesting that our methods were reliable despite these weaknesses.
5. Conclusions
In conclusion, we used serological reference standard diagnosis to compare the diagnostic accuracy of RT-PCR and IgM-ELISA and further compared stool samples, rectal swabs, throat swabs, plasma/serum and CSF samples to identify the optimal specimen and strategy for HFMD virological diagnosis in this study. We found that stool samples had the highest diagnostic yield, with the added advantage of convenient collection. If stool samples are unavailable, rectal swabs can achieve a similar diagnostic yield. The diagnosis was more accurate in those with severe disease and a shorter interval between disease onset and sample collection than in their counterparts. As different enterovirus serotypes have similar structures, physical properties, pathological mechanisms, transmission routes, and general epidemiological and laboratory characteristics (Knipe and Howley, 2013), our results can be extrapolated to the diagnosis of HFMD caused by non-EV-A71 enteroviruses.
Data availability
Sequences obtained in this study were submitted to the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov) under accession numbers MN938504–MN938830 and MT710348–MT710695.
Ethics statement
The study protocol was reviewed by the Institutional Review Boards of the Chinese Centre for Disease Prevention and Control, the Public Health School of Fudan University, and Henan Children's Hospital. Informed consent was obtained from all subjects involved in the study or their parents/guardians.
Author contributions
Yonghong Zhou: Conceptualization, Methodology, Investigation, Software, Formal analysis, Resources, Data curation, Writing – original draft preparation, Visualization. Chongchen Zhou: Investigation. Kai Wang: Investigation. Qi Qiu: Investigation. Yibing Cheng: Investigation. Yu Li: Investigation. Peng Cui: Investigation. Lu Liang: Investigation. Peng Li: Investigation. Xiaowei Deng: Formal analysis. Lili Wang: Investigation. Wen Zheng: Investigation. Hui Gong: Investigation. Fang Wang: Investigation. Meng Xu: Validation. Justin Jang Hann Chu: writing-review, editing. Lance Turtle: writing-review, editing, Funding acquisition. Hongjie Yu: Conceptualization, Methodology, Resources, writing-review, Project administration, Funding acquisition.
Conflict of interest
Hongjie Yu received investigator-initiated research funding from Sanofi Pasteur, GlaxoSmithKline, and Yichang HEC Changjiang Pharmaceutical Company and Shanghai Roche Pharmaceutical Company, none of which is related to HFMD and enteroviruses. All other authors report no competing interests.
Acknowledgements
This work was supported by the National Natural Science Fund for Distinguished Young Scholars of China (No. 81525023) and, in whole or in part, by a Wellcome Trust fellowship awarded to LT [205228/Z/16/Z]. For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author-Accepted Manuscript version arising from this submission. LT is also supported by the National Institute for Health Research Health Protection Research Unit in Emerging and Zoonotic Infections (grant no. NIHR200907) at University of Liverpool in partnership with Public Health England (PHE), in collaboration with Liverpool School of Tropical Medicine and the University of Oxford. LT is based at the University of Liverpool. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health or Public Health England.
We thank the staff members of Henan Children's Hospital for helping with field investigation, administration and data collection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virs.2022.11.004.
Appendix ASupplementary data
The following are the Supplementary data to this article:
Suppl Fig S1.
Suppl Fig S.
2
Suppl Fig S3.
References
- Aw-Yong K.L., Tan C.W., Koh M.T., Sam I.C., Chan Y.F. Diagnosis of human enterovirus A71 infection in Malaysia using a commercial IgM-capture enzyme-linked immunosorbent assay and an IgM-colloidal gold immunochromatographic assay. Trop. Biomed. 2016;33:238–245. [PubMed] [Google Scholar]
- Bharucha T., Sengvilaipaseuth O., Seephonelee M., Vongsouvath M., Vongsouvath M., Rattanavong S., Piorkowski G., Lecuit M., Gorman C., Pommier J.D., Garson J.A., Newton P.N., De Lamballerie X., Dubot-Pérès A. Viral RNA degradation makes urine a challenging specimen for detection of Japanese encephalitis virus in patients with suspected CNS infection. Open Forum Infect. Dis. 2019;6:ofz048. doi: 10.1093/ofid/ofz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer L., Moreni G., Wolthers K.C., Pajkrt D. World-wide prevalence and genotype distribution of enteroviruses. Viruses. 2021;13:434. doi: 10.3390/v13030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease, C.,Prevention Notes from the field: severe hand, foot, and mouth disease associated with coxsackievirus A6 - Alabama, Connecticut, California, and Nevada, November 2011-February 2012. Morb. Mortal. Wkly. Rep. 2012;61:213–214. [PubMed] [Google Scholar]
- Cordey S., Schibler M., L'huillier A.G., Wagner N., Goncalves A.R., Ambrosioni J., Asner S., Turin L., Posfay-Barbe K.M., Kaiser L. Comparative analysis of viral shedding in pediatric and adult subjects with central nervous system-associated enterovirus infections from 2013 to 2015 in Switzerland. J. Clin. Virol. 2017;89:22–29. doi: 10.1016/j.jcv.2017.01.008. [DOI] [PubMed] [Google Scholar]
- Gao L., Zou G., Liao Q., Zhou Y., Liu F., Dai B., Liu J., Chen Z., Xing W., Yang L., Liang H., Zhang Y., Chen Z., Luo L., Li Q., Luo K., Wu P., Mo X., Wang L., Lan K., Horby P.W., Cowling B.J., Simmonds P., Altmeyer R., Van Doorn H.R., Yu H. Spectrum of enterovirus serotypes causing uncomplicated hand, foot, and mouth disease and enteroviral diagnostic yield of different clinical samples. Clin. Infect. Dis. 2018;67:1729–1735. doi: 10.1093/cid/ciy341. [DOI] [PubMed] [Google Scholar]
- Gopalkrishna V., Patil P.R., Patil G.P., Chitambar S.D. Circulation of multiple enterovirus serotypes causing hand, foot and mouth disease in India. J. Med. Microbiol. 2012;61:420–425. doi: 10.1099/jmm.0.036400-0. [DOI] [PubMed] [Google Scholar]
- Harvala H., Broberg E., Benschop K., Berginc N., Ladhani S., Susi P., Christiansen C., Mckenna J., Allen D., Makiello P., Mcallister G., Carmen M., Zakikhany K., Dyrdak R., Nielsen X., Madsen T., Paul J., Moore C., Von Eije K., Piralla A., Carlier M., Vanoverschelde L., Poelman R., Anton A., Lopez-Labrador F.X., Pellegrinelli L., Keeren K., Maier M., Cassidy H., Derdas S., Savolainen-Kopra C., Diedrich S., Nordbo S., Buesa J., Bailly J.L., Baldanti F., Macadam A., Mirand A., Dudman S., Schuffenecker I., Kadambari S., Neyts J., Griffiths M.J., Richter J., Margaretto C., Govind S., Morley U., Adams O., Krokstad S., Dean J., Pons-Salort M., Prochazka B., Cabrerizo M., Majumdar M., Nebbia G., Wiewel M., Cottrell S., Coyle P., Martin J., Moore C., Midgley S., Horby P., Wolthers K., Simmonds P., Niesters H., Fischer T.K. Recommendations for enterovirus diagnostics and characterisation within and beyond Europe. J. Clin. Virol. 2018;101:11–17. doi: 10.1016/j.jcv.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Knipe D.M., Howley P.M. sixth ed. Lippincott Williams & Wilkins; 2013. Fields Virology. [Google Scholar]
- Kupila L., Vuorinen T., Vainionpaa R., Marttila R.J., Kotilainen P. Diagnosis of enteroviral meningitis by use of polymerase chain reaction of cerebrospinal fluid, stool, and serum specimens. Clin. Infect. Dis. 2005;40:982–987. doi: 10.1086/428581. [DOI] [PubMed] [Google Scholar]
- Lam N.Y., Rainer T.H., Chiu R.W., Lo Y.M. EDTA is a better anticoagulant than heparin or citrate for delayed blood processing for plasma DNA analysis. Clin. Chem. 2004;50:256–257. doi: 10.1373/clinchem.2003.026013. [DOI] [PubMed] [Google Scholar]
- Li X.W., Ni X., Qian S.Y., Wang Q., Jiang R.M., Xu W.B., Zhang Y.C., Yu G.J., Chen Q., Shang Y.X., Zhao C.S., Yu H., Zhang T., Liu G., Deng H.L., Gao J., Ran X.G., Yang Q.Z., Xu B.L., Huang X.Y., Wu X.D., Bao Y.X., Chen Y.P., Chen Z.H., Liu Q.Q., Lu G.P., Liu C.F., Wang R.B., Zhang G.L., Gu F., Xu H.M., Li Y., Yang T. Chinese guidelines for the diagnosis and treatment of hand, foot and mouth disease (2018 edition) World J. Pediatr. 2018;14:437–447. doi: 10.1007/s12519-018-0189-8. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhou Y., Cheng Y., Wu P., Zhou C., Cui P., Song C., Liang L., Wang F., Qiu Q., Guo C., Zeng M., Long L., Cowling B.J., Yu H. Effectiveness of EV-A71 vaccination in prevention of paediatric hand, foot, and mouth disease associated with EV-A71 virus infection requiring hospitalisation in Henan, China, 2017-18: a test-negative case-control study. Lancet Child Adolesc. Health. 2019;3:697–704. doi: 10.1016/S2352-4642(19)30185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Wen K., Pan Y., Wang Y., Che X., Wang B. Cross-reactivity of anti-EV71 IgM and neutralizing antibody in series sera of patients infected with Enterovirus 71 and Coxsackievirus A 16. J. Immunoassay Immunochem. 2011;32:233–243. doi: 10.1080/15321819.2011.559297. [DOI] [PubMed] [Google Scholar]
- Muppa R., Bhupatiraju P., Duddu M., Dandempally A. Hand, foot and mouth disease. J. Indian Soc. Pedod. Prev. Dent. 2011;29:165–167. doi: 10.4103/0970-4388.84692. [DOI] [PubMed] [Google Scholar]
- Newcombe R.G. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat. Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Oberste M.S., Maher K., Kilpatrick D.R., Pallansch M.A. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J. Virol. 1999;73:1941–1948. doi: 10.1128/jvi.73.3.1941-1948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi M.H., Solomon T., Podin Y., Mohan A., Akin W., Yusuf M.A., Del Sel S., Kontol K.M., Lai B.F., Clear D., Chieng C.H., Blake E., Perera D., Wong S.C., Cardosa J. Evaluation of different clinical sample types in diagnosis of human enterovirus 71-associated hand-foot-and-mouth disease. J. Clin. Microbiol. 2007;45:1858–1866. doi: 10.1128/JCM.01394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Q., Zhou J., Cheng Y., Zhou Y., Liang L., Cui P., Xue Y., Wang L., Wang K., Wang H., Li P., Chen J., Li Y., Turtle L., Yu H. Kinetics of the neutralising antibody response in patients with hand, foot, and mouth disease caused by EV-A71: a longitudinal cohort study in Zhengzhou during 2017-2019. EBioMedicine. 2021;68 doi: 10.1016/j.ebiom.2021.103398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapia E.Y., Maroni C., Groisman C., Kromer H., Lihue Rojo G., Dastugue M., Valinotto L. Atypical hand-foot-mouth disease virus genotyping in a pediatric hospital in Buenos Aires city, Argentina (in Chinese) Arch. Argent. Pediatr. 2020;118:e199–e203. doi: 10.5546/aap.2020.e199. [DOI] [PubMed] [Google Scholar]
- Sinclair C., Gaunt E., Simmonds P., Broomfield D., Nwafor N., Wellington L., Templeton K., Willocks L., Schofield O., Harvala H. Atypical hand, foot, and mouth disease associated with coxsackievirus A6 infection, Edinburgh, United Kingdom, January to February 2014. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.12.20745. [DOI] [PubMed] [Google Scholar]
- Song C., Li Y., Zhou Y., Liang L., Turtle L., Wang F., Wu P., Qiu Q., Yang J., Wang K., Cui P., Cheng Y., Zhang T., Guo C., Zeng M., Long L., Peiris M., Zhou C., Cowling B.J., Yu H. Enterovirus genomic load and disease severity among children hospitalised with hand, foot and mouth disease. EBioMedicine. 2020;62 doi: 10.1016/j.ebiom.2020.103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh H.L., Mohammad S.S., Britton P.N., Kandula T., Lorentzos M.S., Booy R., Jones C.A., Rawlinson W., Ramachandran V., Rodriguez M.L., Andrews P.I., Dale R.C., Farrar M.A., Sampaio H. Clinical characteristics and functional motor outcomes of enterovirus 71 neurological disease in children. JAMA Neurol. 2016;73:300–307. doi: 10.1001/jamaneurol.2015.4388. [DOI] [PubMed] [Google Scholar]
- WHO . fourth ed. WHO; 2004. Polio Laboratory Manual.https://apps.who.int/iris/handle/10665/68762 [Google Scholar]
- Wu J.T., Jit M., Zheng Y., Leung K., Xing W., Yang J., Liao Q., Cowling B.J., Yang B., Lau E.H., Takahashi S., Farrar J.J., Grenfell B.T., Leung G.M., Yu H. Routine pediatric enterovirus 71 vaccination in China: a cost-effectiveness analysis. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F., Yan Q., Wang H., Niu J., Li L., Zhu F., He S., Zhang S., Weng Z., Cheng T., Cai Y., He D., Chen Y., Ge S., Yeo A.E., Zhang J., Ng M.H., Xia N. Performance of detecting IgM antibodies against enterovirus 71 for early diagnosis. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Zhang Z.Z., Yang Z.H., Zhu C.M., Hu Y.G., Liu Q.B. Clinical and etiological characteristics of atypical hand-foot-and-mouth disease in children from chongqing, China: a retrospective study. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/802046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L., Liwen J., Lufang J., Lei S., Wenliang Z., Qi S., Mingyi C., Wei M., Qingwu J. Comparison of enterovirus detection rates in paired specimens of throat swabs and anus swabs from patients in different phases of hand-foot-mouth disease. Chin. J. Infect. Dis. 2013;31:170–172. (in Chinese) [Google Scholar]
- Yu H., Cowling B.J. Remaining challenges for prevention and control of hand, foot, and mouth disease. Lancet Child Adolesc. Health. 2019;3:373–374. doi: 10.1016/S2352-4642(19)30065-3. [DOI] [PubMed] [Google Scholar]
- Yu N., Guo M., He S.J., Pan Y.X., Chen X.X., Ding X.X., Hao W., Wang Y.D., Ge S.X., Xia N.S., Che X.Y. Evaluation of human enterovirus 71 and coxsackievirus A16 specific immunoglobulin M antibodies for diagnosis of hand-foot-and-mouth disease. Virol. J. 2012;9:12. doi: 10.1186/1743-422X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Weng Z.X., Du H.L., Xu F.H., He S.Z., He D.L., Cheng T., Zhang J., Ge S.X., Xia N.S. Development and evaluation of rapid point-of-care tests for detection of Enterovirus 71 and Coxsackievirus A16 specific immunoglublin M antibodies. J. Virol. Methods. 2016;231:44–47. doi: 10.1016/j.jviromet.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Zhang T., Cheng Y., Li Y., Yang J., Liang L., Yang J., Cui P., Song C., Zhou Y., Kang D., Qiu Q., Cui N., Guo C., Jing Y., Zeng M., Liu Q., Long L., Zhou C., Yu H. Evaluation of the diagnostic performance and its associated factors of a commercial anti-EV-A71 IgM-capture ELISA kit in hospitalized children with clinical diagnostic HFMD. J. Clin. Virol. 2020;130 doi: 10.1016/j.jcv.2020.104582. [DOI] [PubMed] [Google Scholar]
- Zhao T., Zhang Z., Zhang Y., Feng M., Fan S., Wang L., Liu L., Wang X., Wang Q., Zhang X., Wang J., Liao Y., He Z., Lu S., Yang H., Li Q. Dynamic interaction of enterovirus 71 and dendritic cells in infected neonatal rhesus macaques. Front. Cell. Infect. Microbiol. 2017;7:171. doi: 10.3389/fcimb.2017.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.F., Chen Z.Y., Yang S.M., Chen J.Z., Zhou L.Y., Wang Y.F., Wang G., Yu X.J., Zhang W.H. Clinical features and peripheral blood T lymphocyte subsets in hand, foot, and mouth disease according to different pathogens. Indian J. Pediatr. 2017;84:124–127. doi: 10.1007/s12098-016-2198-8. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Van Tan L., Luo K., Liao Q., Wang L., Qiu Q., Zou G., Liu P., Anh N.T., Hong N.T.T., He M., Wei X., Yu S., Lam T.T., Cui J., Van Doorn H.R., Yu H. Genetic variation of multiple serotypes of enteroviruses associated with hand, foot and mouth disease in southern China. Virol. Sin. 2021;36:61–74. doi: 10.1007/s12250-020-00266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D., Zhao X.Y., Yao Y., Dai F.F., He H., Li R.Q., Jin R.H., Liang L.C., Li N. A new factor influencing pathogen detection by molecular assay in children with both mild and severe hand, foot, and mouth disease. Diagn. Microbiol. Infect. Dis. 2013;76:162–167. doi: 10.1016/j.diagmicrobio.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequences obtained in this study were submitted to the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov) under accession numbers MN938504–MN938830 and MT710348–MT710695.