Abstract
Viruses are important components of the human body. Growing evidence suggests that they are engaged in the physiology and disease status of the host. Even though the vaginal microbiome is involved in human papillomavirus (HPV) infection and cervical cancer (CC) progression, little is known about the role of the vaginal virome. In this pilot exploratory study, using unbiased viral metagenomics, we aim to investigate the vaginal eukaryotic virome in women with different levels of cervical lesions, and examine their associations with different cervical disease status. An altered eukaryotic virome was observed in women with different levels of lesions and Lactobacillus profiles. Anelloviruses and papillomaviruses are the most commonly detected eukaryotic viruses of the vaginal virome. Higher abundance and richness of anelloviruses and papillomaviruses were associated with low-grade squamous intraepithelial lesion (LSIL) and CC. Besides, higher anellovirus abundance was also associated with lactobacillus-depleted microbiome profiles and bacterial community state (CST) type IV. Furthermore, increased correlations between Anelloviridae and Papillomaviridae occurred in the women with increased cervical disease severity level from LSIL to CC. These data suggest underlying interactions between different microbes as well as the host physiology. Higher abundance and diversity of both anelloviruses and papillomaviruses shared by LSIL and CC suggest that anellovirus may be used as a potential adjunct biomarker to predict the risk of HPV persistent infection and/or CC. Future studies need to focus on the clinical relevance of anellovirus abundance with cervical disease status, and the evaluation of their potential as a new adjunct biomarker for the prediction and prognoses of CC.
Keywords: Vaginal virome, Cervical cancer, Anellovirus, Papillomavirus
Highlights
-
•
Higher eukaryotic viral abundance and diversity were associated with cervical lesion severity and cervical cancer (CC).
-
•
Anellovirus was highly associated with CC and low-grade squamous intraepithelial lesion (LSIL).
-
•
More correlations between different viral families were observed in higher levels of cervical lesion (HSIL and CC).
-
•
Specific viruses are attractive targets for studying their role in host physiology and developing alternative biomarkers.
1. Introduction
More than 600,000 new cases and 342,000 deaths worldwide were related to cervical cancer (CC) in 2020, making it the fourth most prevalent cancer and the fourth leading cause of cancer death in women (Sung et al., 2021). Persistent infection with human papillomavirus (HPV), especially high-oncogenic-risk HPV (hrHPV), is a major risk factor for cervical lesion and CC development (Curty et al., 2019; Castanheira et al., 2021). However, hrHPV infection alone is a necessary but not sufficient factor for CC development since most hrHPV infections are transient and regress spontaneously (Castellsagué, 2008). Many additional factors are also involved in HPV persistence and cervical disease progression or regression, such as host-related factors including immunity status, smoking, parity and sexual behavior, mechanical factors like vaginal douching, and other biological factors like sexually transmitted infections (STIs) (Castellsagué, 2008; Audirac-Chalifour et al., 2016).
The microbiome, in most cases the bacterial microbiome, has long been determined to play an essential role in maintaining homeostasis and physiological functions (Gilbert et al., 2018). The vaginal bacterial microbiome (VMB) has been shown to be associated with cervical disease progression (Mitra et al., 2016; Kyrgiou et al., 2017; Champer et al., 2018; Łaniewski et al., 2020). The bacterial microbiome in the healthy vaginal tract is mainly dominated by Lactobacillus and is less diverse than other anatomical sites such as the gut. According to relative abundance of the bacterial profiles, five distinct bacterial community state types (CSTs I–V) are established (Ravel et al., 2011). The VMB composition is dynamic, and its alterations may influence the local immune response and the vaginal microenvironment, thereby involving in HPV infection control and cervical oncogenesis (Chase et al., 2015; Castanheira et al., 2021). For example, the decreasing abundance of certain Lactobacillus species and increasing microbiome diversity (especially the enrichment of different anaerobes) are associated with increasing lesion severity and CC progression (Mitra et al., 2015; Audirac-Chalifour et al., 2016; Usyk et al., 2020; Gardella et al., 2022; Zhang et al., 2022). However, the change of vaginal bacterial microbiome could also be a consequence of altered local immune response and vaginal environment.
The collection and complex network of commensal and pathogenic eukaryotic viruses and bacteriophages termed the virome (Virgin, 2014; Carroll et al., 2018; Ren et al., 2021). Many studies that focused on limited disease types and body compartments suggested an important role of the virome in human physiology and disease (Virgin, 2014; Norman et al., 2015; Li et al., 2019; Liang and Bushman, 2021; Cao et al., 2022). However, whether it is the cause of disease status and/or adverse conditions is not clear. As it was discussed in a recent review (Madere and Monaco, 2022), the virome could also be involved in different gynecological health and disease. For example, higher vaginal eukaryotic viral diversity was found to be associated with preterm birth, exposure to antibiotics and reproduction failure (Wylie et al., 2018; Eskew et al., 2020). Furthermore, the bacterial compositions may play an important role in the bacterial vaginosis (BV) status. A recent study investigated the association of cervicovaginal DNA virome with local microenvironment factors that influence HPV persistence and progression to CC development, and revealed that an altered vaginal virome featured by the blooms of Anelloviridae was highly associated with genital inflammation and Lactobacillus frequency (Kaelin et al., 2022). However, the vaginal virome and its association with cervical cancer is understudied.
These preliminary evidences highlight an important yet unappreciated role of the vaginal virome in women health, as either a result or a cause of the disease status. Besides HPV, however, current knowledge is sparse about whether other vaginal eukaryotic virome is linked with cervical lesion severity and CC development. In this exploratory study, we enrolled women with different cervical disease status, and investigated their vaginal eukaryotic virome profiles using an unbiased viral metagenomic sequencing method. We aim to determine the total viral components that present in women's vaginal tract and examine possible associations between the eukaryotic virome trait and certain lesion stages.
2. Materials and Methods
2.1. Study design and cohort
To investigate the vaginal virome and its association with different cervical disease status, women who visited the cervical clinic during summer of 2021 at the Obstetrics and Gynecology Hospital of Fudan University were enrolled in this cohort. The exclusion criteria included ongoing pregnancy, immunosuppression by drugs, the use of antibiotics during the last one month, and previous history of cervical treatment or surgery. Women were classified into different cervical disease groups according to hrHPV test, cytology, colposcopy inspections and pathological diagnosis. In total, 46 women with low-grade squamous intraepithelial lesion (LSIL), 36 women with high-grade squamous intraepithelial lesion (HSIL) and 26 women with cervical cancer (CC) were included. Women without any signs of cervical tissue lesions (normal tissue, NT) were selected based on the 2019 American Society of Colposcopy and Cervical Pathology (ASCCP) guideline (Schiffman et al., 2020): HPV negative and normal cytology result; or HPV positive and normal colposcopy inspections. Fifty-three women with NT were included. Demographics, such as age, douching habits and contraception methods, as well as basic clinical features were recorded upon their visits (Table 1). The sample collection was approved by the Ethics Committees of Obstetrics and Gynecology Hospital of Fudan University. Written informed consents were obtained before sample collection.
Table 1.
Patient characteristic of 161 patients included in study cohort.
| NT (n = 53) | LSIL (n = 46) | HSIL (n = 36) | CC (n = 26) | Total (n = 161) | P-valuea | |
|---|---|---|---|---|---|---|
| Age, years | 39.4 | 41.4 | 38.4 | 48.3 | 41.2 | 0.005 |
| Mean (SD, range) | (11.1,23–74) | (9.8, 26–69) | (10.8, 23–69) | (13.5, 20–75) | ||
| Ethnicity (Han), n (%) | 53 (100) | 46 (100) | 36 (100) | 26 (100) | 161 (100) | − |
| Vaginal douching, n (%) | 0.555 | |||||
| Yes | 8 (15.1) | 3 (6.5) | 5 (13.9) | 3 (11.5) | 19 (11.8) | |
| No | 45 (84.9) | 43 (93.3) | 31 (86.1) | 20 (76.9) | 139 (86.3) | |
| Unknown | 0 (0) | 0 (0) | 0 (0) | 3 (11.5) | 3 (1.9) | |
| Contraception, n (%) | 0.239 | |||||
| Nil | 25 (47.2) | 15 (32.6) | 11 (30.5) | 12 (46.1) | 63 (39.2) | |
| Condoms | 24 (45.3) | 21 (45.6) | 17 (47.2) | 7 (26.9) | 69 (42.8) | |
| Copper IUD + Ligation | 4 (7.5) | 9 (19.6) | 7 (19.4) | 5 (19.2) | 25 (15.5) | |
| Other oral hormonal contraception | 0 (0) | 1 (2.2) | 1 (2.8) | 2 (7.7) | 4 (2.5) | |
| Times of abortion | 1.2 (1.3, 0–5) | 1.4 (1.1,0–4) | 1.2 (2.0, 0–11) | 1.4 (1.2, 0–4) | 1.3 (1.4, 0–11) | 0.270 |
| Mean (SD, range) | ||||||
| Number of sexual partners | 2.6 | 2.0 | 2.2 | 3.3 | 2.4 | 0.161 |
| Mean (SD, range) | (4.7, 1–24) | (1.5, 1–8) | (1.5, 1–8) | (6.3,1–24) | (3.8, 1–24) | |
| Complications, n (%) | 4 (7.5) | 4 (8.7) | 4 (11.1) | 4 (15.4) | 16 (9.9) | 0.684 |
| Multifocal lesions, n (%) | 0 (0) | 8 (17.4) | 2 (5.5) | 0 (0) | 10 (6.2) | – |
| BV and STIs | NA | NA | NA | NA | NA | – |
| Lactobacillus profiles | 0.004 | |||||
| Lac-dominant, n (%) | 33 (62.3) | 30 (65.2) | 24 (66.7) | 7 (26.9) | 94 (58.4) | |
| Lac-depleted, n (%) | 19 (35.8) | 15 (32.6) | 12 (33.3) | 19 (73.1) | 65 (40.4) | |
| CSTs | 0.001 | |||||
| CST I, n (%) | 15 (28.3) | 9 (19.5) | 5 (13.9) | 0 | 29 (18) | |
| CST III, n (%) | 18 (34) | 22 (47.8) | 19 (52.8) | 7 (26.9) | 66 (41) | |
| CST IV, n (%) | 18 (34) | 12 (26.1) | 12 (33.3) | 19 (73.1) | 61 (37.9) | |
| CST V, n (%) | 1 (1.9) | 2 (4.3) | 0 | 0 | 3 (1.9) | |
Fisher's exact test and Kruskal-Wallis (with Dunn's correction) test were used as appropriate for the comparison of the variables between different groups (NT, LSIL, HSIL and CC). NT, normal tissue; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; CC, cervical cancer. BV, bacterial vaginosis; STIs, sexually transmitted infections; NA, not available.
2.2. Sample collection
Vaginal swabs were collected upon clinic visit, and were immersed in virus transport medium (Yocon, Beijing, China) immediately. Samples were first stored at 4 °C and then transferred to −80 °C within 24 h. A total of 161 swabs were collected.
2.3. Vaginal virome-sample processing, library construction and sequencing
The enrichment of viral particles was performed as previously described (Li et al., 2019; Li et al., 2021) with a few modifications. Briefly, vaginal swab was resuspended in 400 μL PBS, and the suspension was homogenized with ceramic beads beating twice at 30 Hz for 30 s each on a Tissuelyser-24 (Shanghai Jing Xin), with a 2-min interval between bead-beating cycles. A negative control sample (the same PBS used for swab suspension) was also performed in parallel to monitor potential background contaminations. The suspension was centrifuged at 12,000×g for 10 min. Supernatant was passed through a 0.45 μm sterile filter to reduce background noise (Costar Spin-X centrifuge tube filters, Corning, USA). Filtrates were incubated with a cocktail of nucleases for 2 h at 37 °C. The reaction was terminated with 30 mmol/L EDTA at 65 °C for 10 min. Both DNA and RNA were extracted using QIAamp MinElute virus kit (Qiagen, Germany), and then amplified using random RT-PCR as described (Li et al., 2020a; Li et al., 2020b). Briefly, reverse transcription was performed with the following primer, 5′GCCGACTAATGCGTAGTCNNNNNNNNN-3′, and the second strand synthesis was performed using Klenow Fragment DNA polymerase (New England Biolabs, Massachusetts, USA). Both cDNA and DNA were then amplified using AmpliTaq Gold DNA polymerase. DNA libraries were prepared using the transposon-based Nextera XT Sample Preparation Kit and were sequenced on the Illumina Novaseq platform (Illumina, USA) with 2 × 150-bp paired reads.
2.4. Virome bioinformatic analyses
Sequencing data were analyzed as previously described (Zhao et al., 2017; Li et al., 2022). The metagenomic sequencing data were first filtered by Trimmomatic v.0.38 (Bolger et al., 2014) by removing adaptors and low-quality sequences. Human sequences (using HG38 database) were subtracted from the data using Bowtie2 v.2.3.4.3 (Langmead and Salzberg, 2012). Remaining high-quality reads were subjected to de-novo assembly using Megahit v.1.1.3 (Li et al., 2015). Assembled contigs, as well as singlets, were mapped against the viral nucleic acid and protein database using BLAST 2.11.0+ (E < 10−10) and BLASTx (E < 10−5) (DIAMOND v.0.9.24) (Buchfink et al., 2015), respectively. All the viral hit candidates were then searched against the NCBI nonredundant nt and nr database to further identify sequences that have higher similarity to non-viral sequences. To reduce potential cross-library contamination (Geoghegan et al., 2021), viruses with a read count less than 0.1% of the highest count for that virus among the other libraries were removed from subsequent analyses. Viral abundance of each sample was calculated by reads per million (RPM) of total clean reads.
2.5. Vaginal bacterial microbiome-sample processing, library construction, sequencing and analyses
Total DNA was extracted from the swabs using TIANGEN bacterial DNA Kit (Tiangen Biotech Co., Ltd, China). The library was generated as previously described (Xu et al., 2021). Briefly, the V4 hypervariable region (515–806 nt) of 16S rRNA gene was amplified by two rounds of PCR. The amplicon libraries were purified and sequenced using the Illumina Miseq platform by Novogene Co., Ltd. The NGS data were first filtered by Trimmomatic v.0.39 to remove adaptors and low-quality sequences (SLIDINGWINDOW:5:20 MINLEN:50). Reads were merged using Vsearch v.2.18 and demultiplexed by Fastx_toolkit. DADA2 was used to quality filtering, dereplication, denoising with default settings. All the sequences were truncated to 250 bp using QIIME 2 platform (https://qiime2.org). The taxonomic classification of amplicon sequence variants (ASVs) representative sequences was performed using the Naive Bayesian Classifier algorithm based on the Silva database at genus level (Quast et al., 2013). The bacterial microbiome composition at the genus level was analyzed using the Statistical Analysis of Metagenomic Profiles (STAMP) package (v.2.1.3) (Parks and Beiko, 2010). The vaginal Lactobacillus community state types (CSTs) I–V were determined as previous described (Ravel et al., 2011). Based on the hierarchical clustering analysis (HCA) of bacterial microbiome, samples with a Lactobacillus relative abundance of > 65.7% were classified as Lactobacillus-dominant (lac-dominant), and samples with a Lactobacillus relative abundance of < 65.7% were classified as Lactobacillus-depleted (lac-depleted).
2.6. Phylogenetic analyses
For the phylogenetic analyses of anellovirus and papillomavirus, all the viral sequences annotated as Anelloviridae and Papillomaviridae were first extracted and assembled into contigs. ORFs of the contigs longer than 1500 bp were extracted using NCBI's ORF Finder tool under the “any sense codon” option. The ORF1 region of Anelloviridae and the L1 region of Papillomaviridae were further identified and curated by aligning against an in-house database containing the ORF1 region of anellovirus reference sequences and the L1 region of papillomavirus reference sequences, respectively. Viral nucleic acid sequences were aligned using MAFFT (Katoh et al., 2019). Phylogenetic trees were inferred using the maximum likelihood method with IQ-Tree (Minh et al., 2020). Modelfinder was used to determine the best substitution models. Phylogenetic trees based on nucleotide sequences were generated using the bootstrap method (1000 times). All the sequences used in the phylogenetic analyses were deposited in Genbank under the accession numbers OP721120–OP721159 (anellovirus) and OP721160–OP721243 (papillomavirus).
2.7. Detection of anellovirus and papillomavirus with qPCR methods
Anellovirus was detected using a SYBR qPCR method. Primers targeting the conserved region of anellovirus were used (Thijssen et al., 2020; Li et al., 2022). Anellovirus copy number in each sample was calculated based on a standard curve constructed by plasmid standards. qPCR program was as follows: 95 °C for 1 min and 30 s, forty cycles at 95 °C for 15 s, and 63 °C for 1 min. HPV was detected by BioPerfectus HPV Genotyping Real Time PCR kit (BioPerfectus Tech-nologies, Taizhou, China), which covers 13 high risk HPV types (HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, and -68) and 8 low risk HPV types (HPV-26, -53, -66, -73, -82, -6, -11, and -81).
2.8. Alpha- and beta-diversities of the vaginal viral community
The alpha diversity was calculated by the Shannon diversity index (H = −Σ(pi log(pi)) and chao richness score, which measures species diversity and relative abundance, and computes the number of species in a community, respectively. The beta diversity of viral community was indicated by PCoA analyses based on Bray-Curtis distances. All indexes were calculated at species level or genotypes (HPV).
2.9. Co-occurrence network analyses
Co-occurrence networks of the viral genus/family were analyzed in different groups. Rho > |0.6| and P < 0.05 (Spearman correlation coefficient) were used as cutoff for including edges into the graph. Co-occurrence network of correlated viral taxa was visualized by Gephi v.0.9.2.
2.10. Statistics
Continuous variables between groups were compared by the nonparametric Mann-Whitney U test or Kruskal-Wallis Test with Dunn's correction. Frequencies were compared by Fisher's exact test. Statistical significance for principal coordinate analyses (PCoA) was determined by PERMANOVA testing. The correlations between the qPCR and metagenomic sequencing (RPM), age and anellovirus or HPV abundance were calculated by Spearman's correlation. Viral abundance was shown as the reads per million (RPM). LEfSe analysis was performed to identity discriminative vaginal viral features (Segata et al., 2011). The linear discriminant analysis (LDA) was used to analyze specific taxa among different groups based on the relative abundance of each taxon. A logarithmic LDA score of >4 was used to determine discriminative features. All statistical analyses were performed using Rstudio v.3.8, Graphpad Prism 8 and SPSS 26.0.
3. Results
3.1. Demographics and clinical characteristics
Demographic, clinical characteristics and vaginal Lactobacillus profiles of the cohort are listed in Table 1. The vaginal swabs from 53 women with NT, 46 with LSIL, 36 with HSIL and 26 with CC were subjected to viral metagenomic sequencing and 16s sequencing. The mean age of all women is 41.2 years old, and women with CC were significantly older than those in other groups (P = 0.005). There was no significant difference in douching habits, contraception methods, abortion history, sexual partners and clinical complications among different groups. Multifocal lesions were only detected in 6.2% of all individuals (8 and 2 women from LSIL and HSIL groups, respectively). In order to analyze the association between virome alterations and the Lactobacillus profiles, samples were classified as either lac-dominant or lac-depleted, and different CSTs (Table 1) as described in the method.
3.2. Overview of the vaginal virome
In total, 1526 million clean reads were generated. After quality control and annotation, 367 million reads were recognized as viral sequences. Overall, seven families of human eukaryotic viruses were detected in the vaginal swabs (Fig. 1 and Supplementary Fig. S1), including Papillomaviridae, Anelloviridae, Herpesviridae, Polyomaviridae, Adenoviridae, Retroviridae and Hepadnaviridae. At least one eukaryotic virus was detected in 90.7% of the samples. Papillomaviruses (HPV) and anelloviruses were the most commonly detected viruses, which were present in 78.3% and 69.6% of women, respectively. They were also the most abundant viruses, accounting for more than 90% of the total eukaryotic viral reads (Fig. 1B). Other five viral families were detected at low prevalence (0.6%–7.4%). Bacteriophages were present in all samples, with Siphoviridae, Myoviridae and other Caudovirales being the most abundant phages (Fig. 1C).
Fig. 1.
The vaginal virome of the studied cohort. A Overall eukaryotic viruses detected in the vaginal swab of each patient. Each patient is represented in a column, and all the eukaryotic viruses are highlighted according to their relative abundance. Different disease status (NT, n = 53; LSIL, n = 46; HSIL, n = 36; CC, n = 26), Lactobacillus abundance and CSTs of each woman are shown at the top. Relative abundance of eukaryotic viral families (B) and prokaryotic (bacteriophages) viral families (C) in different disease groups. NT, normal tissue; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; CC, cervical cancer; CSTs, community state types.
3.3. Vaginal virome with different cervical lesion severity and bacterial microbiome profiles
We first investigated the relationship between vaginal eukaryotic virome and different cervical lesion severity (Fig. 2A–D). Viral abundance was significantly higher in the LSIL group (P < 0.001) than the NT and HSIL groups. Women with CC showed a similar trend, but no significance was reached (P = 0.054 and 0.082) (Fig. 2A). Shannon diversity of the vaginal virome was highest in the CC group, and viral richness was significantly higher in both the LSIL and CC groups than the NT and HSIL groups (P < 0.01) (Fig. 2B and C). Principal coordinate analyses (PCoA) showed that the virome community of the CC group is significantly separated from the NT, LSIL and HSIL groups that shared similar virome community (PERMANOVA, adjusted P = 0.015, 0.024, 0.034 respectively, Fig. 2D). LEfSe analyses revealed that specific HPV types were associated with the LSIL and HSIL groups, and two anellovirus species were associated with the CC group (Fig. 2E). Since the Lactobacillus profile is associated with cervical lesion severity and CC, samples were classified as either lac-dominant or lac-depleted, and different CSTs (Table 1) as described in the method, and the relationship between the vaginal eukaryotic virome and different Lactobacillus profiles was investigated (Fig. 2F–M). The virome community was significantly separated between the lac-dominant and lac-depleted groups (PERMANOVA, P = 0.009) (Fig. 2I), and slightly higher viral abundance and diversity indexes were observed in the lac-depleted group than the lac-dominant group (P > 0.05) (Fig. 2F–H). The virome community was also significantly separated among CSTs I, III and IV (PERMANOVA, adjusted P = 0.003, 0.032 and 0.035 respectively, Fig. 2M). We found significantly higher viral abundance in the CSTs III (P = 0.017) and IV (P = 0.004) groups, and only a slightly higher richness was observed in CST IV compared to CST I (P = 0.054) (Fig. 2J–L).
Fig. 2.
Vaginal eukaryotic virome of women with different disease status (NT, n = 53; LSIL, n = 46; HSIL, n = 36; CC, n = 26) and Lactobacillus profiles (lac-dominant, n = 94; lac-depleted, n = 65; CST I, n = 29, CST III, n = 66, CST IV, n = 61). Relative abundance (A), Shannon diversity (B), Richness (C), PCoA plot based on Bray-Curtis distances (D) of eukaryotic virome in different disease groups. LDA linear discriminant analysis (LEfSe) performed to display discriminant viruses between disease groups (E). Relative abundance (F), Shannon diversity (G), Richness (H), PCoA plot (I) of eukaryotic virome in lac-dominant and lac-depleted groups. Red and green circle in panel (I) represents lac-depleted and lac-dominant groups, respectively. Relative abundance (J), Shannon diversity (K), Richness (L), PCoA plot (M) of eukaryotic virome in different CTS groups. Statistical significance was assessed by Mann-Whitney test between two groups, and Kruskal-Wallis Test with Dunn's correction among multiple groups. Significance of beta diversity was analyzed by PERMANOVA (adjusted by the Benjamini and Hochberg method). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001; ns, not significant. NT, normal tissue; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; CC, cervical cancer; CSTs, community state types.
3.4. Anellovirus
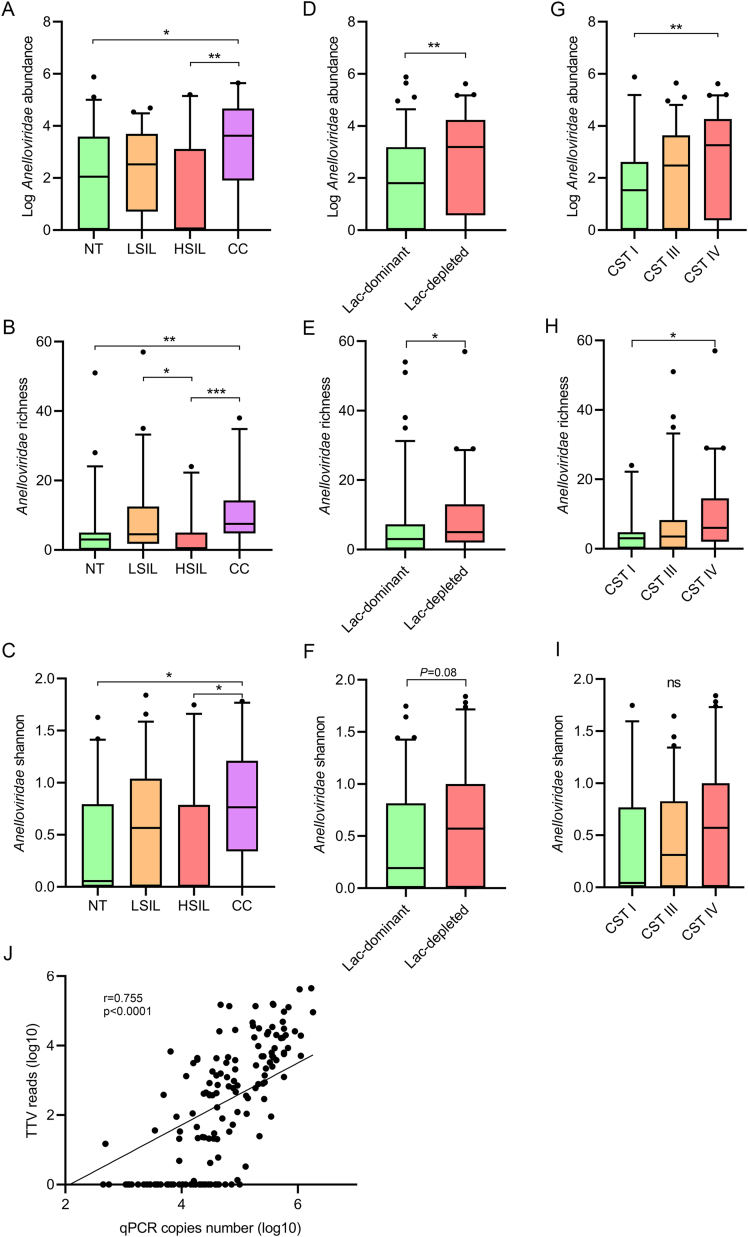
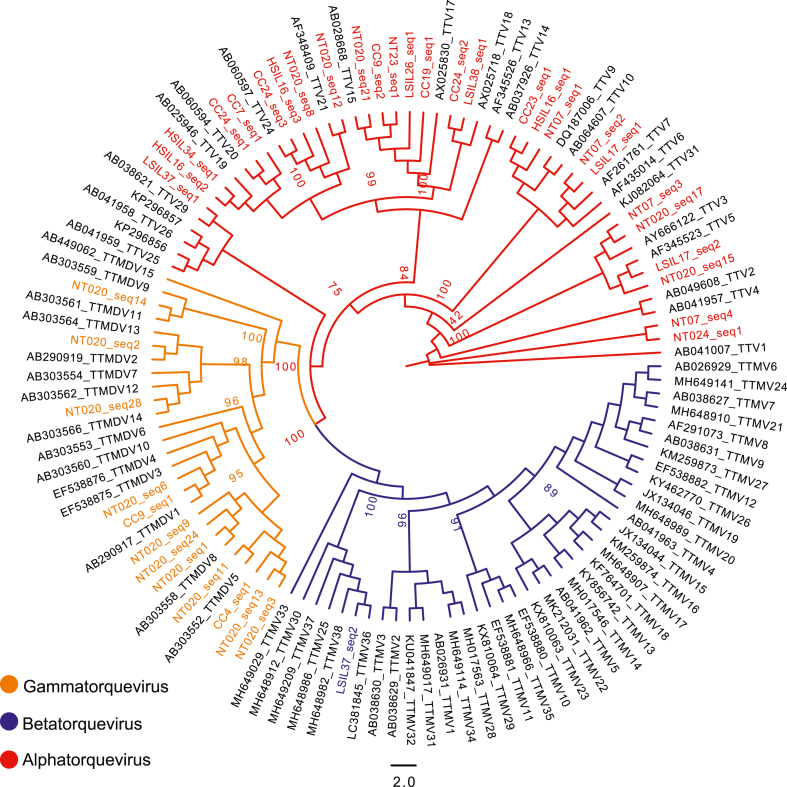
Since anellovirus was one of the most abundant and prevalent viruses in women's vagina, we further analyzed vaginal anellome in different groups (Fig. 3). The CC and LSIL groups had substantially higher anellovirus abundance, richness and Shannon diversity than the other two groups, with the highest level in the CC group (Fig. 3A–C). In addition, substantially higher anellovirus abundance and richness were detected in the lac-depleted group (P < 0.05) than the lac-dominant group (Fig. 3D and E). Anellovirus Shannon diversity was also higher in lac-depleted group, but no significance was reached (P = 0.08) (Fig. 3F). Higher anellovirus abundance and richness were detected in the CST III (P > 0.05) and IV (P < 0.05) groups than the CST I group (Fig. 3G and H). No significant difference of anellovirus Shannon diversity was found between CSTs (Fig. 3I). We further performed a qPCR assay to validate the anellovirus abundance of NGS, and a strong positive correlation was found between the number of reads and the qPCR viral loads (Fig. 3J, Spearman's correlation r = 0.755, P < 0.0001). All three genera of anelloviruses, as well as some unclassified anelloviruses were detected in women's vagina (Fig. 4, and Supplementary Fig. S2), and gammatorquevirus and unclassified anelloviruses mainly contributed to higher anellovirus abundance in the CC group. Slightly higher age of the CC group did not contribute to the higher anellovirus abundance since anellovirus abundance was not significantly associated with age (Supplementary Fig. S3).
Fig. 3.
The association of Anelloviridae with disease status and Lactobacillus profiles. Relative abundance and richness/Shannon of anelloviruses are compared and shown for different disease groups (A, B, C), lac-dominant and lac-depleted groups (D, E, F) and CSTs (G, H, I). Spearman's correlations between NGS abundance (RPM) and qPCR viral load (copy numbers) of anelloviruses (J). Statistical significance was assessed by Mann-Whitney test between two groups, and Kruskal-Wallis Test with Dunn's correction among multiple groups. The number of individuals in each group is same to that in Figs. 1 and 2. NT, normal tissue; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; CC, cervical cancer; CSTs, community state types.
Fig. 4.
Maximum-likelihood phylogenetic tree (GTR + F + R7 model) of anellovirus ORF1 sequences. Sequences identified in this study are highlighted with different colors.
3.5. Papillomavirus
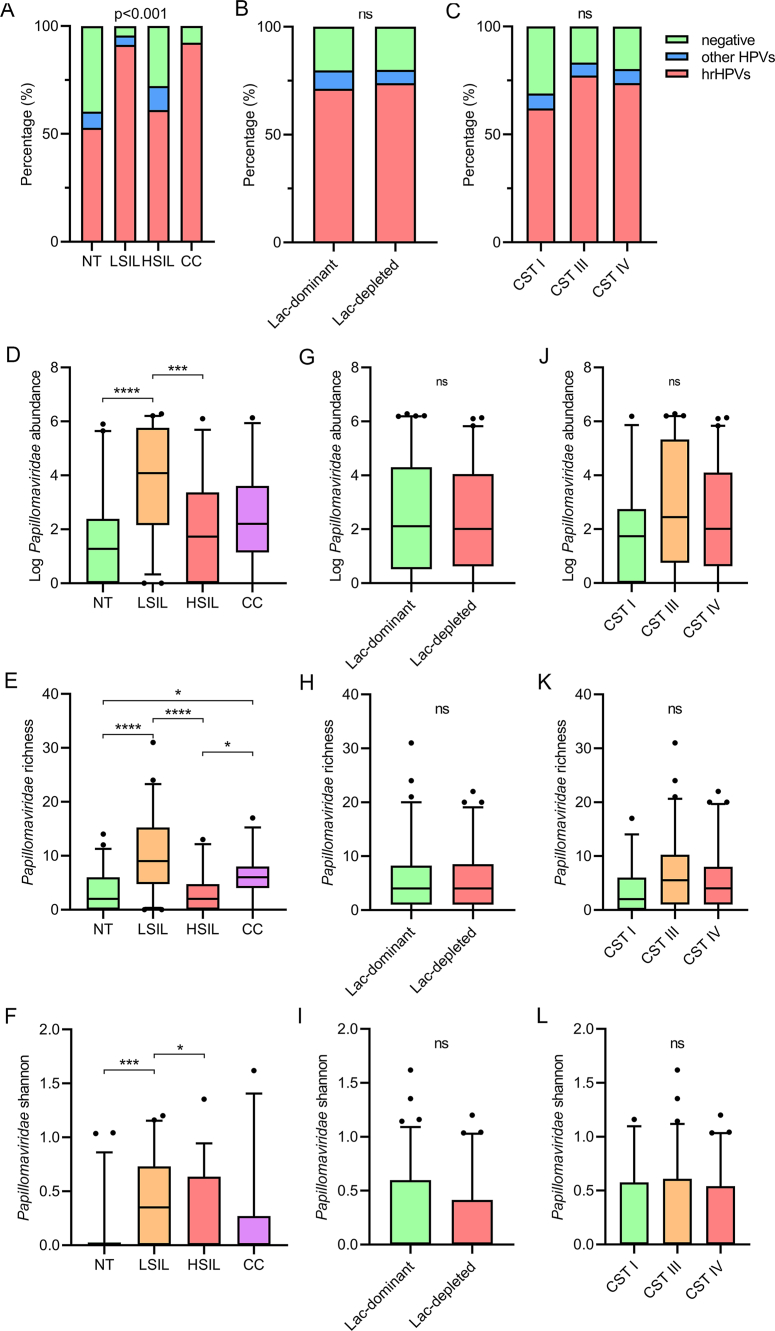
HPV was the second most abundant eukaryotic virus in women's vagina. The unbiased NGS method detected 65 HPV genotypes in 77.6% women, covering all 21 genotypes that were detected by qPCR method plus 44 additional genotypes (Supplementary Table S1). The agreement for overall HPV positivity between the two methods was 80.1% (73.3% by qPCR kit and 77.6% by NGS). The most prevalent hrHPVs by NGS were HPV 58 (37.3%), 51 (28.6%), 56 (22.4%), 52 (22.4%) and 16 (21.1%) (Supplementary Fig. S4). The hrHPVs positivity rates were significantly higher in LSIL and CC groups and were not associated with Lactobacillus profiles (Fig. 5A–C). Most of the HPVs were alphapapillomavirus, followed by gammapapillomavirus (Fig. 6), and one potential novel gammapapillomavirus genotype (with 86% identity to HPV226) was identified from a woman with no tissue lesions (Supplementary Fig. S5).
Fig. 5.
The association of Papillomaviridae with different disease status. The frequencies of high-risk HPVs (hrHPVs) are shown in disease groups (A), lac-dominant and lac-depleted groups (B) and CSTs (C). Relative abundance and richness/Shannon of papillomaviruses are compared and shown for different disease groups (D, E, F), lac-dominant and lac-depleted groups (G, H, I) and CSTs (J, K, L). Statistical significance was assessed by Mann-Whitney test between two groups; the differences between groups in panel A–C were compared using Fisher's exact test (adjusted by the Benjamini and Hochberg method) and Kruskal-Wallis Test with Dunn's correction among multiple groups in panel D–L. NT, normal tissue; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; CC, cervical cancer; CSTs, community state types.
Fig. 6.
Maximum-likelihood phylogenetic tree (GTR + F + R10 model) of papillomavirus full length L1 sequences. Sequences identified in this study are highlighted with different colors.
Women with LSIL had significantly higher HPV abundance than those with NT and HSIL, and both the LSIL and CC groups had significantly higher HPV richness and Shannon diversity than the other two groups, with LSIL showing the highest levels (Fig. 5D–F). No significant difference was found in HPV abundance, richness and Shannon diversity between the Lac-dominant and Lac-depleted groups (Fig. 5G–I). Slightly higher HPV abundance and richness were found in CST III and IV groups as compared to those in CST I, but no significance was reached (Fig. 4J and K). No difference of HPV Shannon diversity was found between CSTs (Fig. 4L). Similar to the observation in anellovirus abundance, age did not contribute to the higher HPV abundance in the LISL and CC groups (Supplementary Fig. S6).
3.6. The interactions of virome in different cervical disease severity
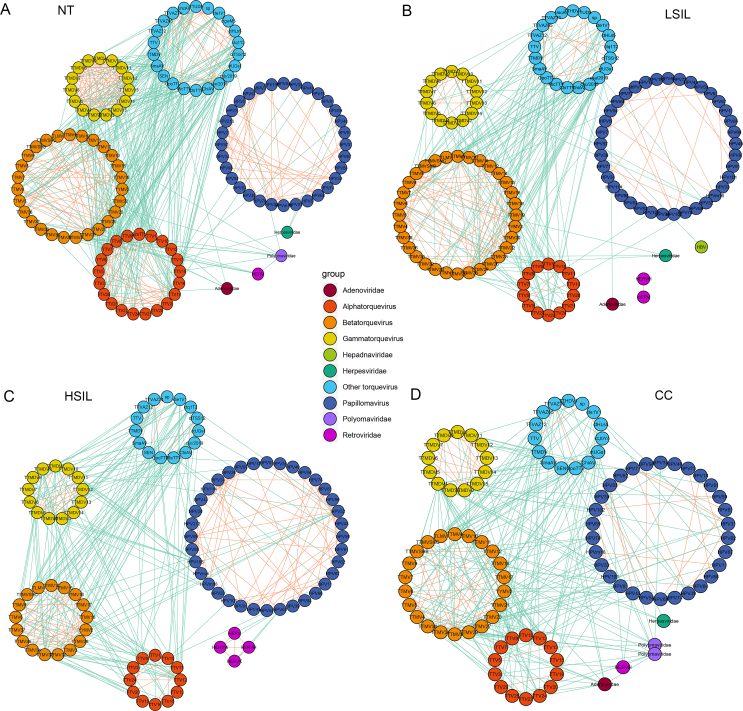
To reveal potential correlations among different viruses, the co-occurrence networks of virome from NT, LSIL, HSIL, and CC groups were compared (Fig. 7). The vast majority of the viral correlations occurred within the same viral family (mainly Anelloviridae and Papillomaviridae). The correlations between Anelloviridae and Papillomaviridae were rarely observed in the NT group (Fig. 7A), and correlations between the two viral families increased along with the aggravation of cervical disease severity from LISL to CC (Fig. 7B–D and Supplementary Fig. S7). In particular, a substantially increased network complexity was observed in the HSIL and CC groups, as more co-occurrence connections were detected between different viral taxa (Supplementary Figs. S7B–C, P < 0.001, Fisher's exact test).
Fig. 7.
Co-occurrence networks of eukaryotic viruses in the vaginal tract of women with different disease status. A NT, normal tissue; B LSIL, low-grade squamous intraepithelial lesion; C HSIL, high-grade squamous intraepithelial lesion; D CC, cervical cancer. Community clusters are grouped by viral genus (alphatorquevirus, betatorquevirus, gammatorquevirus and other anelloviruses) or family. Nodes represent core viral species (anelloviruses and papillomaviruses) or families (adenoviridae, herpesviridae, hepadnaviridae, polyomaviridae and retroviridae). Edges represent correlations, with orange and green color showing the correlations within the same viral taxa (same anellovirus genus or same papillomaviridae) and between different viral taxa (anellovirus genus and other viral families), respectively.
4. Discussion
We revealed that the eukaryotic virome was altered in the vagina of women with different levels of cervical lesions or cancer. The altered virome was mainly characterized by increased abundance and diversity of both Anelloviridae and Papillomaviridae in women with LISL and CC. In particular, higher anellovirus abundance was significantly associated with CC, as well as non-protective vaginal bacteria profiles (e.g., lac-depleted and CST IV). Furthermore, increased correlations between Anelloviridae and Papillomaviridae occurred in the women with increased cervical disease severity from LISL to CC. These results suggest that the vaginal virome may be involved in the development of various cervical diseases, especially CC.
Even though we investigated the vaginal virome using a method targeting both DNA and RNA viruses (Siqueira et al., 2018; da Costa et al., 2021; Li et al., 2021), the overall vaginal viral types are mainly DNA viruses, which are similar to previous studies (Madere and Monaco, 2022), with Papillomaviridae and Anelloviridae being the most common eukaryotic viruses and Caudovirales being the dominant bacteriophages. Higher eukaryotic viral abundance and richness were associated different cervical lesion severity or CC status, and this was consistent with the few studies investigating the vaginal virome in the context of women genital health. For example, higher eukaryotic viral diversity was significantly associated with preterm birth and reproductive outcomes in asymptomatic women (Wylie et al., 2018; Eskew et al., 2020). In women with BV, more eukaryotic viruses were detected (Zhang et al., 2021), and bacteriophages profiles were associated with bacterial community and BV status (Jakobsen et al., 2020). A recent study of the virome in HIV-1/HPV co-infected women reported that a higher abundance and diversity of HPV was associated with premalignant cervical lesions (LSIL and HSIL) (Siqueira et al., 2019). These data indicate that vaginal virome may play an unnoticed role in the local health and disease status.
Different from anellovirus, we found that HPV abundance and diversity were highest in LSIL, followed by CC. HPV is often detected extra-chromosomally in benign and low-grade lesions, and the integrated HPV (or persistent HPV infection) highly correlates with the severity of cervical disease (Klaes et al., 1999; Hudelist et al., 2004; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2008). This means a more active replication of HPVs at LSIL stage rather than HISL. Besides, because the samples and methods in this study mainly capture cell-free viral particles rather than the cells, the integrated HPVs have relatively low possibility to be detected. These may explain why the higher HPV abundance was observed in the LSIL group than in the HISL and CC groups. Similar to a recent study (Kaelin et al., 2022), we found no significant difference in HPV abundance and diversity by the Lactobacillus profile. This indicates that other virome alterations (such as anellovirus) other than HPV may better explain the changes in the vaginal Lactobacillus profile.
Anelloviruses are ubiquitous in human population and different anatomical sites (Tozetto-Mendoza et al., 2020; Taylo et al., 2022). Even though no convincing evidence links anelloviruses with particular disease types, they are believed to interact with the host immune system (Kaczorowska and van der Hoek, 2020). We found that vaginal anelloviruses were highly associated with CC and the Lactobacillus profiles, which were consistent with two recent studies (Kaelin et al., 2022; Tozetto-Mendoza et al., 2022). In these studies, they found that higher anellovirus abundance was associated with the genital inflammation and lower levels of Lactobacillus. Vaginal anellovirus abundance also negatively correlated with CD4+ T-cell count (Siqueira et al., 2019), highlighting possible interactions of them with our local immune response. The co-infection of multiple anelloviruses, as well as with other viruses are common in different body compartments, such as blood, respiratory tract, semen and vagina (Siqueira et al., 2019; Kaczorowska and van der Hoek, 2020; Liu et al., 2021; Li et al., 2022). In particular, the co-infection of anellovirus and HPV was found to be associated with increased laryngeal tumor progression (Szládek et al., 2005). Increased co-occurrence of anelloviruses with HPVs and other viruses was found to be associated with the lesion severity and CC; these data suggest that different anellovirus compositions and their cross-interactions with HPVs may involve in the HPV persistence, microenvironment change and progression to CC. Considering the widely reported association between anellovirus and the immune status, our findings suggest that anellovirus may be a potential indicator of the local vaginal immune and/or disease status. Whether the change in anellovirus composition is the result or cause of the altered vaginal microenvironment, such as HPV persistence and disease progression, needs to be further investigated.
This study further highlights the power of metagenomic sequencing for the diagnosis of viruses in clinic samples, as well as the mining of specific viral types or groups that may be associated with different disease conditions. Even though further works in a larger cohort with longitudinal samples are needed, our exploratory results indicate that, similar to the bacterial microbiome, specific viral signatures, especially the commensal anelloviruses that are often considered harmless and are neglected in clinical settings, could be linked with women's vaginal health. Anelloviruses are sensitive to host immune status, and their abundance are related to many disease conditions (Kaczorowska and van der Hoek, 2020; Liu et al., 2021; Hoek et al., 2022; Kaelin et al., 2022; Li et al., 2022; Redondo et al., 2022). Even though, it's not clear whether the high anellovirus abundance, diversity, as well as its correlation with HPVs in CC was the cause or result of different disease conditions, these data indicate a potential use of these commensal viruses to predict disease progression. For example, it was recently shown that a higher anellovirus abundance was associated with higher risk of the subsequent acquiring of bloodborne pathogen in drug users (Kandathil et al., 2021). Thus, with more evidence acquired in future studies, the early surveillance of the anellovirus traits may be used as an alternative adjunct biomarker to evaluate and identify women that are at higher risk of the development of cervical diseases.
Currently, limited knowledge is available about the role of the virome in women's genital health. The results of our study together with previous data suggest an important role of specific vaginal virome profiles in different cervical disease status. However, there are several unanswered questions could be considered in future studies. First, inflammation and local immunity are associated with HPV persistence and CC development (Mantovani et al., 2008), and immunocompromised or immunosuppressed status is the main risk factor for the development of cervical cancer (Graham, 2017). Anelloviruses are thought to be efficiently controlled by host immune system, and their blooms in both diversity and abundance are often associated with immune suppression (De Vlaminck et al., 2013; Fernández-Ruiz et al., 2019; Thijssen et al., 2020) or other viral infections (e.g., HIV-1 and HCV) (Li et al., 2022). Thus, it would be important to consider local host response such as cytokine or immune cells levels in the association with viral signatures and disease status. Besides, whether anelloviruses will influence HPV replication or persistence, including the expression of oncoproteins E6 and E7, which are key factors for progression to malignancy (Graham, 2017), could be investigated. Second, most HPV infections or vaginal lesions could regress spontaneously without clinical interventions (Graham, 2017). It would be interesting to investigate whether the virome that associate with certain disease status will restore or change along with the disease regression. This will further strengthen the associations between the specific virome and CC progression. Third, as the most abundant but often neglected part of the human virome, the dynamics of anelloviruses in both healthy women and along with the progression of CC could be monitored, and whether women with high levels of anelloviruses and/or coinfections of complex aneloviruses and HPVs will have higher risk for severe lesion and CC progression need to be investigated. These data will help to find novel diagnostic adjunct biomarkers or prevention strategies of CC.
This study has several limitations. First, this is a cross-sectional study. The cohort and sampling strategy don't reflect actual disease progression, and longitudinal samples, including cervical swabs and blood from different disease status of each woman will help us to understand the dynamic changes of the virome and their association with disease progression. Second, larger cohorts from multiple centers should be considered in future studies to exclude potential sampling or individual bias. Third, phages are one of the most abundant and important components of the human virome, and they could influence the bacterial microbiome, as well as host immunity and disease conditions, including the women's genital health (Madere and Monaco, 2022). This study preferentially focused on the eukaryotic viruses, and the role of prokaryotic virome in vaginal diseases deserves to be further investigated using an improved bioinformatic pipeline dedicated to phages.
5. Conclusions
The vaginal eukaryotic virome compositions are associated with different levels of lesion severity. Both the abundance and diversity of anelloviruses and papillomaviruses are associated with LSIL and CC. Besides, an altered viral correlation network, especially that between anelloviruses and papillomaviruses, was related to women with high lesion severity. These data indicate that the vaginal eukaryotic viral communities may play a critical role in maintaining a healthy microenvironment and influencing CC progression, and anellovirus may be used as a potential adjunct biomarker to predict the risk of developing to persistent HPV infection and/or CC.
Data availability
The short-read sequencing data are available at the NCBI Sequence Read Archive (SRA) under BioProject number PRJNA865010.
Ethics statement
Sample collection was approved by the Ethics Committees of Obstetrics and Gynecology Hospital of Fudan University. Written informed consents were obtained before sample collection.
Author contributions
Yanpeng Li: conceptualization, formal analysis, data curation, writing-original draft. Le Cao: formal analysis, methodology. Xiao Han: investigation, resources. Yingying Ma: methodology. Yanmei Liu: investigation, resources. Shujun Gao: conceptualization, resources, data curation, writing-review & editing, supervision. Chiyu Zhang: conceptualization, data curation, writing-review & editing, supervision.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This study was supported by the startup funding from Shanghai Public Health Clinical Center (to CZ). We thank all the patients who participated in the study and the nurses who contributed to the sample collections.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virs.2022.12.004.
Contributor Information
Shujun Gao, Email: 031108282@fudan.edu.cn.
Chiyu Zhang, Email: chiyu_zhang1999@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Audirac-Chalifour A., Torres-Poveda K., Bahena-Román M., Téllez-Sosa J., Martínez-Barnetche J., Cortina-Ceballos B., López-Estrada G., Delgado-Romero K., Burguete-García A.I., Cantú D., García-Carrancá A., Madrid-Marina V. Cervical microbiome and cytokine profile at various stages of cervical cancer: a pilot study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchfink B., Xie C., Huson D.H. Fast and sensitive protein alignment using diamond. Nat. Methods. 2015;12:59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- Cao Z., Sugimura N., Burgermeister E., Ebert M.P., Zuo T., Lan P. The gut virome: a new microbiome component in health and disease. EBioMedicine. 2022;81 doi: 10.1016/j.ebiom.2022.104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D., Daszak P., Wolfe N.D., Gao G.F., Morel C.M., Morzaria S., Pablos-Méndez A., Tomori O., Mazet J.A.K. The global virome project. Science. 2018;359:872–874. doi: 10.1126/science.aap7463. [DOI] [PubMed] [Google Scholar]
- Castanheira C.P., Sallas M.L., Nunes R.A.L., Lorenzi N.P.C., Termini L. Microbiome and cervical cancer. Pathobiology. 2021;88:187–197. doi: 10.1159/000511477. [DOI] [PubMed] [Google Scholar]
- Castellsagué X. Natural history and epidemiology of hpv infection and cervical cancer. Gynecol. Oncol. 2008;110:S4–S7. doi: 10.1016/j.ygyno.2008.07.045. [DOI] [PubMed] [Google Scholar]
- Champer M., Wong A.M., Champer J., Brito I.L., Messer P.W., Hou J.Y., Wright J.D. The role of the vaginal microbiome in gynaecological cancer. Bjog. 2018;125:309–315. doi: 10.1111/1471-0528.14631. [DOI] [PubMed] [Google Scholar]
- Chase D., Goulder A., Zenhausern F., Monk B., Herbst-Kralovetz M. The vaginal and gastrointestinal microbiomes in gynecologic cancers: a review of applications in etiology, symptoms and treatment. Gynecol. Oncol. 2015;138:190–200. doi: 10.1016/j.ygyno.2015.04.036. [DOI] [PubMed] [Google Scholar]
- Curty G., de Carvalho P.S., Soares M.A. The role of the cervicovaginal microbiome on the genesis and as a biomarker of premalignant cervical intraepithelial neoplasia and invasive cervical cancer. Int. J. Mol. Sci. 2019;21:222. doi: 10.3390/ijms21010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa A.C., Moron A.F., Forney L.J., Linhares I.M., Sabino E., Costa S.F., Mendes-Correa M.C., Witkin S.S. Identification of bacteriophages in the vagina of pregnant women: a descriptive study. Bjog. 2021;128:976–982. doi: 10.1111/1471-0528.16528. [DOI] [PubMed] [Google Scholar]
- De Vlaminck I., Khush K.K., Strehl C., Kohli B., Luikart H., Neff N.F., Okamoto J., Snyder T.M., Cornfield D.N., Nicolls M.R., Weill D., Bernstein D., Valantine H.A., Quake S.R. Temporal response of the human virome to immunosuppression and antiviral therapy. Cell. 2013;155:1178–1187. doi: 10.1016/j.cell.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskew A.M., Stout M.J., Bedrick B.S., Riley J.K., Omurtag K.R., Jimenez P.T., Odem R.R., Ratts V.S., Keller S.L., Jungheim E.S., Wylie K.M. Association of the eukaryotic vaginal virome with prophylactic antibiotic exposure and reproductive outcomes in a subfertile population undergoing in vitro fertilisation: a prospective exploratory study. Bjog. 2020;127:208–216. doi: 10.1111/1471-0528.15951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ruiz M., Albert E., Giménez E., Ruiz-Merlo T., Parra P., López-Medrano F., San Juan R., Polanco N., Andrés A., Navarro D., Aguado J.M. Monitoring of alphatorquevirus DNA levels for the prediction of immunosuppression-related complications after kidney transplantation. Am. J. Transplant. 2019;19:1139–1149. doi: 10.1111/ajt.15145. [DOI] [PubMed] [Google Scholar]
- Gardella B., Pasquali M.F., La Verde M., Cianci S., Torella M., Dominoni M. The complex interplay between vaginal microbiota, hpv infection, and immunological microenvironment in cervical intraepithelial neoplasia: a literature review. Int. J. Mol. Sci. 2022;23:7174. doi: 10.3390/ijms23137174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoghegan J.L., Di Giallonardo F., Wille M., Ortiz-Baez A.S., Costa V.A., Ghaly T., Mifsud J.C.O., Turnbull O.M.H., Bellwood D.R., Williamson J.E., Holmes E.C. Virome composition in marine fish revealed by meta-transcriptomics. Virus Evol. 2021;7 doi: 10.1093/ve/veab005. veab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J.A., Blaser M.J., Caporaso J.G., Jansson J.K., Lynch S.V., Knight R. Current understanding of the human microbiome. Nat. Med. 2018;24:392–400. doi: 10.1038/nm.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham S.V. The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clin Sci (Lond) 2017;131:2201–2221. doi: 10.1042/CS20160786. [DOI] [PubMed] [Google Scholar]
- Hoek R.A., Verschuuren E.A., de Vries R.D., Vonk J.M., van Baarle D., van der Heiden M., van Gemert J.P., Gore E.J., Niesters H.G., Erasmus M., Hellemons M.E., Scherbeijn S.M., Wijbenga N., Mahtab E.A.F., GeurtsvanKessel C.H., Buter C.V.L. High torque tenovirus (ttv) load before first vaccine dose is associated with poor serological response to covid-19 vaccination in lung transplant recipients. J. Heart Lung Transplant. 2022;41:765–772. doi: 10.1016/j.healun.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudelist G., Manavi M., Pischinger K.I., Watkins-Riedel T., Singer C.F., Kubista E., Czerwenka K.F. Physical state and expression of hpv DNA in benign and dysplastic cervical tissue: different levels of viral integration are correlated with lesion grade. Gynecol. Oncol. 2004;92:873–880. doi: 10.1016/j.ygyno.2003.11.035. [DOI] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans IARC monographs on the evaluation of carcinogenic risks to humans. Volume 97. 1,3-butadiene, ethylene oxide and vinyl halides (vinyl fluoride, vinyl chloride and vinyl bromide) IARC Monogr. Eval. Carcinog. Risks Hum. 2008;97:3–471. [PMC free article] [PubMed] [Google Scholar]
- Jakobsen R.R., Haahr T., Humaidan P., Jensen J.S., Kot W.P., Castro-Mejia J.L., Deng L., Leser T.D., Nielsen D.S. Characterization of the vaginal DNA virome in health and dysbiosis. Viruses. 2020;12:1143. doi: 10.3390/v12101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowska J., van der Hoek L. Human anelloviruses: diverse, omnipresent and commensal members of the virome. FEMS Microbiol. Rev. 2020;44:305–313. doi: 10.1093/femsre/fuaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin E.A., Skidmore P.T., Łaniewski P., Holland L.A., Chase D.M., Herbst-Kralovetz M.M., Lim E.S. Cervicovaginal DNA virome alterations are associated with genital inflammation and microbiota composition. mSystems. 2022;7 doi: 10.1128/msystems.00064-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandathil A.J., Cox A.L., Page K., Mohr D., Razaghi R., Ghanem K.G., Tuddenham S.A., Hsieh Y.H., Evans J.L., Coller K.E., Timp W., Celentano D.D., Ray S.C., Thomas D.L. Plasma virome and the risk of blood-borne infection in persons with substance use disorder. Nat. Commun. 2021;12:6909. doi: 10.1038/s41467-021-26980-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Rozewicki J., Yamada K.D. Mafft online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings Bioinf. 2019;20:1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaes R., Woerner S.M., Ridder R., Wentzensen N., Duerst M., Schneider A., Lotz B., Melsheimer P., von Knebel Doeberitz M. Detection of high-risk cervical intraepithelial neoplasia and cervical cancer by amplification of transcripts derived from integrated papillomavirus oncogenes. Cancer Res. 1999;59:6132–6136. [PubMed] [Google Scholar]
- Kyrgiou M., Mitra A., Moscicki A.B. Does the vaginal microbiota play a role in the development of cervical cancer? Transl. Res. 2017;179:168–182. doi: 10.1016/j.trsl.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., Salzberg S.L. Fast gapped-read alignment with bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łaniewski P., Ilhan Z.E., Herbst-Kralovetz M.M. The microbiome and gynaecological cancer development, prevention and therapy. Nat. Rev. Urol. 2020;17:232–250. doi: 10.1038/s41585-020-0286-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Liu C.M., Luo R., Sadakane K., Lam T.W. Megahit: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de bruijn graph. Bioinformatics. 2015;31:1674–1676. doi: 10.1093/bioinformatics/btv033. [DOI] [PubMed] [Google Scholar]
- Li Y., Altan E., Reyes G., Halstead B., Deng X., Delwart E. Virome of bat guano from nine northern California roosts. J. Virol. 2021;95:e01713–e01720. doi: 10.1128/JVI.01713-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Altan E., Pilcher C., Hartogensis W., Hecht F.M., Deng X., Delwart E. Semen virome of men with hiv on or off antiretroviral treatment. AIDS. 2020;34:827–832. doi: 10.1097/QAD.0000000000002497. [DOI] [PubMed] [Google Scholar]
- Li Y., Gordon E., Idle A., Altan E., Seguin M.A., Estrada M., Deng X., Delwart E. Virome of a feline outbreak of diarrhea and vomiting includes bocaviruses and a novel chapparvovirus. Viruses. 2020;12:506. doi: 10.3390/v12050506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Cao L., Ye M., Xu R., Chen X., Ma Y., Tian R.R., Liu F.L., Zhang P., Kuang Y.Q., Zheng Y.T., Zhang C. Plasma virome reveals blooms and transmission of anellovirus in intravenous drug users with hiv-1, hcv, and/or hbv infections. Microbiol. Spectr. 2022;10 doi: 10.1128/spectrum.01447-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Fu X., Ma J., Zhang J., Hu Y., Dong W., Wan Z., Li Q., Kuang Y.Q., Lan K., Jin X., Wang J.H., Zhang C. Altered respiratory virome and serum cytokine profile associated with recurrent respiratory tract infections in children. Nat. Commun. 2019;10:2288. doi: 10.1038/s41467-019-10294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G., Bushman F.D. The human virome: assembly, composition and host interactions. Nat. Rev. Microbiol. 2021;19:514–527. doi: 10.1038/s41579-021-00536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Li Y., Xu R., Zhang Y., Zheng C., Wan Z., Li H., Yang Z., Jin X., Hao P., Zhao J., Zhang C. Hiv-1 infection alters the viral composition of plasma in men who have sex with men. mSphere. 2021;6 doi: 10.1128/mSphere.00081-21. e00081-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madere F.S., Monaco C.L. The female reproductive tract virome: understanding the dynamic role of viruses in gynecological health and disease. Curr Opin Virol. 2022;52:15–23. doi: 10.1016/j.coviro.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Minh B.Q., Schmidt H.A., Chernomor O., Schrempf D., Woodhams M.D., von Haeseler A., Lanfear R. Iq-tree 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020;37:1530–1534. doi: 10.1093/molbev/msaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A., MacIntyre D.A., Marchesi J.R., Lee Y.S., Bennett P.R., Kyrgiou M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome. 2016;4:58. doi: 10.1186/s40168-016-0203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A., MacIntyre D.A., Lee Y.S., Smith A., Marchesi J.R., Lehne B., Bhatia R., Lyons D., Paraskevaidis E., Li J.V., Holmes E., Nicholson J.K., Bennett P.R., Kyrgiou M. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci. Rep. 2015;5 doi: 10.1038/srep16865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman J.M., Handley S.A., Baldridge M.T., Droit L., Liu C.Y., Keller B.C., Kambal A., Monaco C.L., Zhao G., Fleshner P., Stappenbeck T.S., McGovern D.P., Keshavarzian A., Mutlu E.A., Sauk J., Gevers D., Xavier R.J., Wang D., Parkes M., Virgin H.W. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell. 2015;160:447–460. doi: 10.1016/j.cell.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D.H., Beiko R.G. Identifying biologically relevant differences between metagenomic communities. Bioinformatics. 2010;26:715–721. doi: 10.1093/bioinformatics/btq041. [DOI] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glockner F.O. The silva ribosomal rna gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel J., Gajer P., Abdo Z., Schneider G.M., Koenig S.S., McCulle S.L., Karlebach S., Gorle R., Russell J., Tacket C.O., Brotman R.M., Davis C.C., Ault K., Peralta L., Forney L.J. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U. S. A. 2011;108(Suppl. 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo N., Navarro D., Aguado J.M., Fernández-Ruiz M. Viruses, friends, and foes: the case of torque teno virus and the net state of immunosuppression. Transpl. Infect. Dis. 2022;24 doi: 10.1111/tid.13778. [DOI] [PubMed] [Google Scholar]
- Ren N.J., Wang S., Shi C.Y., Yu P., Zhao L., Huang D.D., Ma H.X., Xiao S.Q., Wang F., Yuan Z.M., Xia H. Dynamic surveillance of mosquitoes and their viromes in Wuhan during 2020. Zoonoses. 2021;1:8. [Google Scholar]
- Schiffman M., Wentzensen N., Perkins R.B., Guido R.S. An introduction to the 2019 asccp risk-based management consensus guidelines. J. Low. Genit. Tract Dis. 2020;24:87–89. doi: 10.1097/LGT.0000000000000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira J.D., Dominguez-Bello M.G., Contreras M., Lander O., Caballero-Arias H., Xutao D., Noya-Alarcon O., Delwart E. Complex virome in feces from amerindian children in isolated amazonian villages. Nat. Commun. 2018;9:4270. doi: 10.1038/s41467-018-06502-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira J.D., Curty G., Xutao D., Hofer C.B., Machado E.S., Seuánez H.N., Soares M.A., Delwart E., Soares E.A. Composite analysis of the virome and bacteriome of hiv/hpv co-infected women reveals proxies for immunodeficiency. Viruses. 2019;11:422. doi: 10.3390/v11050422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Szládek G., Juhász A., Kardos G., Szoke K., Major T., Sziklai I., Tar I., Márton I., Kónya J., Gergely L., Szarka K. High co-prevalence of genogroup 1 tt virus and human papillomavirus is associated with poor clinical outcome of laryngeal carcinoma. J. Clin. Pathol. 2005;58:402–405. doi: 10.1136/jcp.2004.022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylo L.J., Keeler E.L., Bushman F.D., Collman R.G. The enigmatic roles of anelloviridae and redondoviridae in humans. Curr Opin Virol. 2022;55 doi: 10.1016/j.coviro.2022.101248. [DOI] [PubMed] [Google Scholar]
- Thijssen M., Tacke F., Beller L., Deboutte W., Yinda K.C., Nevens F., Laleman W., Van Ranst M., Pourkarim M.R. Clinical relevance of plasma virome dynamics in liver transplant recipients. EBioMedicine. 2020;60 doi: 10.1016/j.ebiom.2020.103009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozetto-Mendoza T.R., Mendes-Correa M.C., Moron A.F., Forney L.J., Linhares I.M., Ribeiro da Silva A., Jr., Honorato L., Witkin S.S. The vaginal torquetenovirus titer varies with vaginal microbiota composition in pregnant women. PLoS One. 2022;17 doi: 10.1371/journal.pone.0262672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozetto-Mendoza T.R., Bongiovanni A.M., Minis E., Linhares I.M., Boester A., Freire W.S., Lima S.H., de Oliveira N.P.G., Mendes-Correa M.C., Forney L.J., Witkin S.S. Torquetenovirus titer in vaginal secretions from pregnant and postpartum women: association with absence of lactobacillus crispatus and levels of lactic acid and matrix metalloproteinase-8. Reprod. Sci. 2020;27:2075–2081. doi: 10.1007/s43032-020-00227-1. [DOI] [PubMed] [Google Scholar]
- Usyk M., Zolnik C.P., Castle P.E., Porras C., Herrero R., Gradissimo A., Gonzalez P., Safaeian M., Schiffman M., Burk R.D. Cervicovaginal microbiome and natural history of hpv in a longitudinal study. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H.W. The virome in mammalian physiology and disease. Cell. 2014;157:142–150. doi: 10.1016/j.cell.2014.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie K.M., Wylie T.N., Cahill A.G., Macones G.A., Tuuli M.G., Stout M.J. The vaginal eukaryotic DNA virome and preterm birth. Am. J. Obstet. Gynecol. 2018;219 doi: 10.1016/j.ajog.2018.04.048. 189.e181-189.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., Lu R., Zhang T., Wu Q., Cai W., Han X., Wan Z., Jin X., Zhang Z., Zhang C. Temporal association between human upper respiratory and gut bacterial microbiomes during the course of covid-19 in adults. Commun Biol. 2021;4:240. doi: 10.1038/s42003-021-01796-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.T., Wang H., Wu H.S., Zeng J., Yang Y. Comparison of viromes in vaginal secretion from pregnant women with and without vaginitis. Virol. J. 2021;18:11. doi: 10.1186/s12985-020-01482-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Xu X., Yu L., Shi X., Min M., Xiong L., Pan J., Zhang Y., Liu P., Wu G., Gao G. Vaginal microbiota changes caused by hpv infection in Chinese women. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.814668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Wu G., Lim E.S., Droit L., Krishnamurthy S., Barouch D.H., Virgin H.W., Wang D. Virusseeker, a computational pipeline for virus discovery and virome composition analysis. Virology. 2017;503:21–30. doi: 10.1016/j.virol.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The short-read sequencing data are available at the NCBI Sequence Read Archive (SRA) under BioProject number PRJNA865010.