Abstract
Sleep is crucial for brain development. Sleep disturbances are prevalent in children with autism spectrum disorder (ASD). Strikingly, these sleep problems are positively correlated with the severity of ASD core symptoms such as deficits in social skills and stereotypic behavior, indicating that sleep problems and the behavioral characteristics of ASD may be related. In this review, we will discuss sleep disturbances in children with ASD and highlight mouse models to study sleep disturbances and behavioral phenotypes in ASD. In addition, we will review neuromodulators controlling sleep and wakefulness and how these neuromodulatory systems are disrupted in animal models and patients with ASD. Lastly, we will address how the therapeutic interventions for patients with ASD improve various aspects of sleep. Together, gaining mechanistic insights into the neural mechanisms underlying sleep disturbances in children with ASD will help us to develop better therapeutic interventions.
Highlights
-
•
Sleep disturbances are prevalent in children with ASD and are positively correlated with the severity of ASD core symptoms.
-
•
Mouse models are valid tools to understand the mechanisms underlying sleep disturbances and related symptoms in ASD.
-
•
Elucidating neuromodulatory mechanisms underlying sleep problems in ASD will offer insights to novel therapeutic strategies.
1. Sleep disturbances in children with ASD
ASD occurs in approximately 1% of children (Elsabbagh et al., 2012; Kim et al., 2011). About 40–80% of children with ASD suffer from sleep disturbances including delayed sleep onset, frequent night awakenings, and short sleep episodes (Richdale and Schreck, 2009; Souders et al., 2009). Their sleep problems occur at the same time as the development of ASD symptoms in early childhood (Verhoeff et al., 2018). Children with ASD show an increase in sleep problems throughout development, whereas typically developing (TD) children sleep better as they grow up (Richdale and Prior, 1995; Sivertsen et al., 2012; Verhoeff et al., 2018).
Sleep questionnaires, such as the Children's Sleep Habits Questionnaire (CSHQ), are used in most studies to identify sleep disturbances in ASD patients. This questionnaire specifically has questions categorized into eight sections: (1) Bedtime resistance; (2) Sleep onset delay; (3) Sleep duration; (4) Sleep anxiety; (5) Night wakings; (6) Parasomnias; (7) Sleep disordered breathing; and (8) Daytime sleepiness. Children with ASD were reported to have greater bedtime resistance, delayed sleep onset, shorter sleep duration, sleep anxiety, increased sleep fragmentation, parasomnias, and early morning awakenings (Chen et al., 2021; Giannotti et al., 2008; Hodge et al., 2014; Honomichl et al., 2002; Irwanto et al., 2016; May et al., 2015; Mazurek and Petroski, 2015; Tudor et al., 2015; van der Heijden et al., 2018; Wang et al., 2016). Other sleep questionnaires and subjective measurements of sleep patterns such as sleep diaries reported similar sleep disturbances (Anders et al., 2012; Cotton and Richdale, 2006; Dominick et al., 2007; Gail Williams et al., 2004; Goldman et al., 2012; Hirata et al., 2016; Humphreys et al., 2014; Krakowiak et al., 2008; Liu et al., 2006; Mutluer et al., 2016; Patzold et al., 1998; Richdale and Prior, 1995; Schreck et al., 2004; Sivertsen et al., 2012; Taira et al., 1998; van der Heijden et al., 2018). Objective sleep measures such as actigraphy or polysomnography are more informative to determine the exact sleep problems. Comparative studies, using a mixture of both sleep questionnaires and polysomnography/actigraphy, have identified symptoms such as early morning awakenings, multiple night awakenings, variations in total sleep time, rapid eye movement (REM) sleep latency, insomnias, delayed sleep onset, daytime sleepiness and decreased sleep efficiency in children with ASD compared with TD children of the same age (Aathira et al., 2017; Allik et al., 2006; Baker et al., 2013; Buckley et al., 2010; Carmassi et al., 2019; Diomedi et al., 1999; Elia et al., 2000; Fletcher et al., 2017; Giannotti et al., 2011; Godbout et al., 2000; Goldman et al., 2009; Goodlin-Jones et al., 2008; Hering et al., 1999; Malow et al., 2006; Miano et al., 2007; Richdale et al., 2014; Takase et al., 1998; Veatch et al., 2016; Wiggs and Stores, 2004). Children with ASD were also shown to exhibit significantly lower slow-wave activity following sleep onset suggesting that dysregulation of sleep homeostasis may contribute to their sleep disturbances (Arazi et al., 2020). Other factors also contribute to sleep problems in children with ASD including an evening chronotype, poor sleep hygiene, medication use, family history of sleep problems as well as comorbid disorders and symptoms such as epilepsy, attention-deficit/hyperactivity disorder (ADHD), asthma, anxiety and depression symptoms, poor growth, poor vision, suppressed appetite, and upper respiratory problems (Gail Williams et al., 2004; Hirata et al., 2016; Liu et al., 2006; van der Heijden et al., 2018).
Sleep disturbances can worsen ASD symptoms (Anders et al., 2012; Goldman et al., 2009; MacDuffie et al., 2020a, MacDuffie et al., 2020b; Richdale et al., 2014; Schreck et al., 2004). However, the causal relationship between sleep problems and the related symptoms is not well understood. Children with ASD who are good sleepers generally did not differ from TD peers in their sleep architecture and displayed fewer affective problems and better social interactions compared with bad sleepers with ASD (Malow et al., 2006). On the other hand, children with ASD who are bad sleepers displayed more severe behavioral symptoms such as inattention, hyperactivity, repetitive behaviors and problems in sociability compared with good sleepers with ASD (Aathira et al., 2017; Goldman et al., 2009). The sleep problems in children with ASD were shown to be decreased when their anxiety, as reported by the parents, was low (Fletcher et al., 2017). Furthermore, sleep problems and sensory sensitivities in ASD children are positively correlated (Linke et al., 2021). Increased sleep latency was shown to be associated with an over-connectivity between the thalamus and auditory cortex measured during natural sleep in magnetic resonance imaging (MRI), and the activity of the auditory cortex was elevated in children with ASD suggesting an abnormal thalamocortical functional connectivity, potentially contributing to sleep problems and sensory sensitivities in ASD (Linke et al., 2021). ASD patients display disrupted coordination between slow oscillations and sleep spindles in the electroencephalogram (EEG) during stage 2 (N2) non-REM (NREM) sleep, and a spindle density deficiency during N3 NREM sleep in polysomnography recordings, suggesting abnormal thalamocortical interactions and thalamic reticular nucleus dysfunction in ASD patients, which consequently influences memory consolidation during sleep (Mylonas et al., 2022).
Taken together, sleep disturbances during development may have a long-lasting impact on behaviors in children. Sleep problems in children with ASD are associated with increased autism scores such as deficits in social skills and stereotypic behavior, indicating that sleep problems and ASD symptoms may be related. Longitudinal studies to examine how sleep patterns change over time in children with ASD, and how such changes are related to the other behaviors will be important to understand the link between sleep and ASD symptoms throughout development.
2. Sleep disturbances in mouse models of ASD
Animal models provide useful tools to investigate the causal role of genetic mutations and environmental factors in symptoms of ASD (Crawley, 2012). A variety of mouse models incorporating genetic abnormalities associated with ASD patients have been generated and used to understand the mechanisms underlying ASD symptoms and to test pharmacological targets to treat core symptoms of ASD. About 10–20% of patients with ASD have an identified genetic etiology such as chromosomal rearrangements, mendelian disorders (Fragile X syndrome, Rett syndrome), mutations in synaptic genes (that encode for SH3 and multiple ankyrin repeat domains protein 3 [Shank3] and synaptic Ras GTPase activating protein [SynGAP]) and copy number variations (such as 16p11.2 deletion) (Levy et al., 2011; Marshall et al., 2008; Pinto et al., 2010; Sanders et al., 2011; Sebat et al., 2007). Data from clinical cohorts with identified genetic etiology and transgenic animal models may provide key insights into the role of ASD-related genes in sleep pathology. Sleep disturbances in rodent models of ASD have been thoroughly reviewed in previously published papers (Doldur-Balli et al., 2022; Wintler et al., 2020). In this review, we will focus on sleep disturbances in 16p11.2 deletion, Syngap1, Shank3, and Fragile X messenger ribonucleoprotein (Fmr1) KO mouse models of ASD that have been well characterized to have sleep disturbances.
2.1. 16p11.2 deletion
The 16p11.2 copy number variation is observed in about 1% of ASD cases (Kumar et al., 2008; Weiss et al., 2008) and was shown to be associated with short sleep duration and frequent arousals during sleep (Hanson et al., 2010; Kamara et al., 2021; Tabet et al., 2012).
The 16p11.2 chromosomal region is highly conserved in the mouse chromosome (Blumenthal et al., 2014), and the copy number variation in this region was thus modeled in mice. Mice hemizygous for the 16p11.2 deletion (16p11.2+/−) exhibit hyperactivity, heightened anxiety, impaired learning and memory, reduced ultrasonic vocalizations, and sensory and motor abnormalities (Angelakos et al., 2017; Arbogast et al., 2016; Grissom et al., 2018; Horev et al., 2011; Lynch et al., 2020; Portmann et al., 2014; Pucilowska et al., 2018; Tian et al., 2015; Yang et al., 2015a, Yang et al., 2015b). 16p11.2+/− deletion in male mice is associated with sleep deficits including reduced NREM sleep (Angelakos et al., 2017; Lu et al., 2018) and longer wake bout duration, while no differences were found in female 16p11.2+/− mice (Angelakos et al., 2017). Another study showed that REM sleep is reduced in male 16p11.2+/− mice due to attenuated initiation and maintenance of REM sleep episodes (Lu et al., 2018). In 16p11.2+/− mice, whole-cell patch clamp recordings demonstrated that the excitability of GABAergic neurons in the ventral medulla (vM) projecting to the ventrolateral periaqueductal gray (vlPAG) is reduced (Lu et al., 2018). Given that the vM neurons and their projections to the vlPAG promote REM sleep (Weber et al., 2015), their reduced excitability may explain REM sleep impairments in 16p11.2+/− mice.
2.2. Syngap1 mutations
The synaptic Ras-GTPase-activating protein, SynGAP, is enriched in the postsynaptic density, and mutations in this gene are associated with abnormal dendritic spine maturation (Clement et al., 2012). Clinical studies have shown that SYNGAP1 mutations are closely associated with neurodevelopmental disorders. SYNGAP1 mutations are estimated to account for 0.5–1% of intellectual disability cases (Berryer et al., 2013; Deciphering Developmental Disorders Study, 2015; 2017). SYNGAP1 mutations also contribute to sleep problems, including initiating and maintaining sleep (Berryer et al., 2013; Parker et al., 2015; Prchalova et al., 2017; Vlaskamp et al., 2019). Sleep promoting therapeutics, discussed in detail below in section IV, such as melatonin, clonidine, or trazodone have been reported to improve sleep in patients with SYNGAP1 mutations (Vlaskamp et al., 2019).
Syngap1+/− mice exhibit reduced social novelty preference, cognitive deficits, susceptibility to seizures, increased locomotor activity, impaired memory, increased startle reflex, decreased sensitivity to pain, impaired motor function, and structural abnormalities in dendritic spines during development (Berryer et al., 2016; Clement et al., 2012; Guo et al., 2009; Muhia et al., 2010, 2012; Nakajima et al., 2019; Ozkan et al., 2014). Syngap1+/− mice were shown to spend less time in REM sleep compared with wild-type (WT) littermates (Angelakos and Abel, 2017). Following 6 h of sleep deprivation, NREM sleep was elevated in Syngap1+/− mice with a blunted increase in the delta power, suggesting an impaired homeostatic response following sleep deprivation (Angelakos and Abel, 2017). A higher baseline delta power in Syngap1+/− mice compared with WT controls may contribute to the blunted slow wave activity during the sleep rebound. Seizure activity is another symptom observed in clinical cohorts with SYNGAP1 mutations (Berryer et al., 2013; Carvill et al., 2013; Mignot et al., 2016; Parker et al., 2015; Sullivan et al., 2020; von Stülpnagel et al., 2015), which has been reproduced in Syngap1+/− mice (Sullivan et al., 2020). In Syngap1+/− mice, myoclonic seizures predominantly occur during NREM sleep at P60. From P60 to P120, the average seizure duration significantly increased due to the emergence of multiple seizure phenotypes. Furthermore, Syngap1+/− mice spent more time asleep during the light phase compared with the WT mice. Overall, Syngap1+/− mice exhibit a progressive worsening of sleep and epilepsy with age (Sullivan et al., 2020).
2.3. Shank3 mutations
The Shank gene family encodes scaffolding proteins located in the postsynaptic density of glutamatergic synapses, which are important for synaptic function and development (Sala et al., 2015). Mutations in the Shank gene family account for approximately 1% of ASD cases (Leblond et al., 2014). Disruptions in Shank genes, particularly Shank3 polymorphisms, are implicated in pathophysiological changes in neurodevelopment such as Phelan-McDermid syndrome and ASD in humans (Anderlid et al., 2002; Boccuto et al., 2013; Boeckers et al., 2002; Bonaglia et al., 2001; Bro et al., 2017; Costales and Kolevzon, 2015; Durand et al., 2007; Gauthier et al., 2009; Moessner et al., 2007; Naisbitt et al., 1999; Smith-Hicks et al., 2021; Soorya et al., 2013; Wilson et al., 2003) and social deficits and repetitive behaviors in mice (Peça et al., 2011). Phelan-McDermid syndrome patients carrying a Shank3 deletion exhibit delayed sleep onset and frequent night awakenings particularly during adolescence (Ingiosi et al., 2019).
Shank3ΔC/ΔC mice, whose exon 21 is deleted, spend less time asleep and have fragmented sleep during juvenile (P25-41), adolescent (P42-56) and adult stages compared with WT littermates (Ingiosi et al., 2019; Lord et al., 2022; Medina et al., 2022). During P23-59, Shank3ΔC/ΔC mice showed an increase in REM sleep due to an increased frequency of REM sleep episodes during the light phase relative to WT (Medina et al., 2022). Similar to Shank3ΔC/ΔC mice, a homozygous InsG3680 mutation in the Shank3 gene (Shank3 InsG3680 knock-in mice) (Zhou et al., 2016) caused a reduction of NREM sleep, especially during the light phase (Bian et al., 2022). While Shank3 heterozygotes (Shank3WT/ΔC) do not exhibit significant differences in their sleep architecture during juvenile and adolescent stages, sleep disruption during early development (P14-21) and post-adolescence (P56-63) impaired sociability and social novelty preference, respectively (Lord et al., 2022). Similarly, increasing NREM sleep in Shank3 InsG3680+/+ mice during this critical developmental period (P35-42) by the injection of flupirtine, a selective KCNQ2/KCNQ3 potassium channel opener, or opto- and chemogenetic inhibition of dopaminergic neurons in the ventral tegmental area (VTA) improved sleep and restored social novelty preference in adulthood (Bian et al., 2022). Therefore, sleep disruptions in Shank3WT/ΔC mice during development exacerbated social behavioral deficits, which could be mitigated through improved sleep.
2.4. Fmr1 knockout
Fragile X Syndrome (FXS) is caused by loss of function mutations in the Fragile X messenger ribonucleoprotein 1 gene (Fmr1) that encodes the RNA binding protein FMRP (O'Donnell and Warren, 2002; Verkerk et al., 1991). 2–5% of children with ASD have FXS (Hagerman et al., 2005; Kaufmann et al., 2004). FXS patients exhibit sleep disturbances such as frequent night waking, trouble falling asleep, delayed onset to REM sleep, disrupted cyclic alternating pattern, daytime sleepiness, restless sleep and sleep apnea (Kidd et al., 2014; Kronk et al., 2009, 2010; Miano et al., 2008; Musumeci et al., 1995; Richdale, 2003).
Fmr1 KO mice exhibit a reduction in REM sleep due to decreased frequency of REM sleep episodes during 1-h recordings at the light phase (Boone et al., 2018) while the overall time spent in REM sleep throughout the light and dark phase is unknown. Abnormal sleep architecture in Fmr1 KO mice may depend on the developmental stage of these mice. Fmr1 KO mice do not exhibit any differences in the amount of sleep compared with WT mice at P21 while they sleep less during the light phase at P70 and P180 (Saré et al., 2017), suggesting that sleep deficits may arise in adulthood. Furthermore, Fmr1 KO mice exhibit sensory hyperexcitability, susceptibility to seizures, decreased synaptic pruning in the hippocampus, cognitive impairments, changes in functional connectivity of hippocampal or neocortical circuits, and an abnormal size of the hippocampus (Berry-Kravis, 2002; Boone et al., 2018; Chen and Toth, 2001; Gibson et al., 2008; Gonçalves et al., 2013; Haberl et al., 2015; Hall et al., 2013; Hays et al., 2011; Hessl et al., 2004; Kates et al., 1997; Molnár and Kéri, 2014; Musumeci et al., 1988, 1999, 2000; Pfeiffer and Huber, 2007; Testa-Silva et al., 2012; Zhang et al., 2014). In line with previous studies describing aberrant hippocampal activity and impaired memory in Fmr1 KO mice and FXS patients (Haberl et al., 2015; Hall et al., 2013; Hessl et al., 2004; Kates et al., 1997; Molnár and Kéri, 2014; Pfeiffer and Huber, 2007; Seese et al., 2014; Testa-Silva et al., 2012), Fmr1 KO mice exhibit an increase in the duration and a lower peak frequency in sharp-wave ripples during NREM sleep and quiet wake (Boone et al., 2018), which may consequently impair memory consolidation (Ego-Stengel and Wilson, 2010; Girardeau et al., 2009; Jadhav et al., 2012).
Taken together, the neural mechanisms underlying sleep disturbances in these animal models of ASD remain to be investigated. Elucidating neural circuits and molecular mechanisms underlying sleep disturbances will help to improve sleep in ASD patients and further reveal whether improving sleep quality could be beneficial for attenuating related behavioral symptoms.
3. Neuromodulators implicated in sleep disturbances in ASD
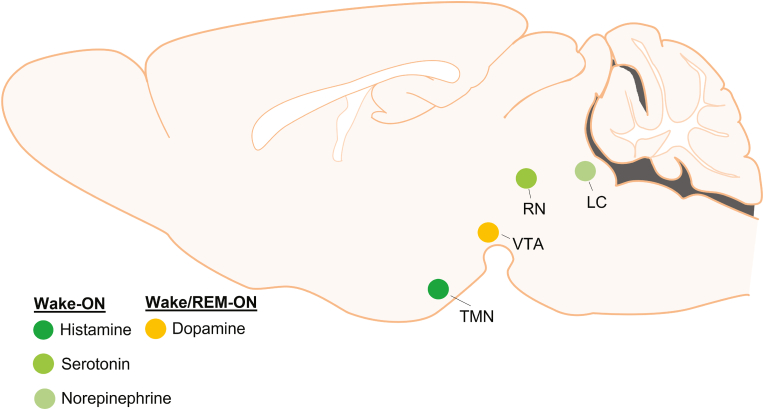
Sleep, wakefulness and transitions between them are regulated by reciprocal interactions between sleep- and wake-regulatory neurons that are distributed throughout the brain (Saper and Fuller, 2017; Scammell et al., 2017; Weber and Dan, 2016). Nuclei regulating sleep comprise the preoptic area (POA), including the ventrolateral and median preoptic area, the lateral hypothalamus, and the parafacial zone in the brainstem (Anaclet et al., 2014; Kroeger et al., 2018; Sherin et al., 1996; Suntsova et al., 2002; Szymusiak et al., 1998; Takahashi et al., 2009). Conversely, wakefulness is thought to be regulated by arousal centers such as the histaminergic tuberomammillary nucleus (TMN), serotonergic dorsal raphe nuclei (DRN), the dopaminergic ventral tegmental area (VTA), and the noradrenergic locus coeruleus (LC) (Fig. 1), many of which innervate and inhibit sleep-active nuclei. While the interactions of these brain regions control sleep and wakefulness, the release of melatonin from the pineal gland regulates the timing of sleep onset.
Fig. 1.
Arousal centers in the brain
Wakefulness is regulated by arousal centers such as the tuberomammillary nucleus (TMN), ventral tegmental area (VTA), raphe nuclei (RN) and locus coeruleus (LC) that synthesize unique monoamine neurotransmitters including histamine, dopamine, serotonin and norepinephrine. Unlike the other neurotransmitters, dopamine neurons are active during wake and REM sleep, whereas others are active wake.
Understanding the neural circuit mechanisms underlying sleep and wake regulation and how they become dysfunctional in children with ASD will provide invaluable insights to discover novel therapeutic strategies to improve sleep and the quality of life in patients and their families. The following sections will highlight neuromodulatory systems controlling sleep and wakefulness, in particular norepinephrine, serotonin, dopamine, histamine, and melatonin, and how they are impaired in animal models and patients with ASD. Table 1 summarizes the mechanisms of action and effects on sleep for selected receptors that we discuss in this section.
Table 1.
Ligands of G protein-coupled receptors and their effect on sleep.
| Receptor | Downstream Effector | Ligand | Action | Effect on Sleep | Reference |
|---|---|---|---|---|---|
| Melatonin Receptor | |||||
| MT2 receptor | Gi | UCM924 | Partial Agonist | Reduced NREM latency and increased NREM episode duration in rats | Ochoa-Sanchez et al. (2014) |
| IIK7 | Full Agonist | Reduced NREM latency and increased NREM episode duration in rats | Fisher and Sugden (2009) | ||
| Norepinephrine Receptors | |||||
| α2-adrenoceptor family | Gi | Clonidine | Partial agonist | Reduced REM; Increased NREM delta power sleep in cats | Crochet and Sakai (1999) |
| Reduced REM sleep in cats | Tononi et al. (1991) | ||||
| Reduced REM sleep in humans | Nicholson and Pascoe (1991) | ||||
| Reduced REM sleep in humans | Spiegel and DeVos (1980) | ||||
| Detomidine | Full Agonist | Reduced REM sleep in rats; increased NREM delta power | Hayat et al. (2020) | ||
| Serotonin Receptors | |||||
| 5-HT6 | Gs | WAY-208466 | Full Agonist | Increased wakefulness, reduced NREM and REM in rats (systemic); Reduced REM in rats (intracranial into DRN) |
Monti et al. (2013) |
| 5-HT2 family | Gq | RO-600175 | Full Agonist | Increased wakefulness; reduced NREM and REM sleep in rats (intracranial into DRN) | Monti and Jantos (2015) |
| Ritanserin | Antagonist | Increased NREM sleep in rats | Dugovic and Wauquier (1987) | ||
| Increased NREM sleep in humans | Idzikowski et al. (1986) | ||||
| Increased wakefulness in cats | Sommerfelt and Ursin (1993) | ||||
| 5-HT1A | Gi | 8-OH-DPAT | Full agonist | Increased wakefulness, reduced NREM and REM sleep in rats (systemic) | Dzoljic et al. (1992) |
| Increased NREM sleep, reduced wakefulness in rats (intracranial into DRN) | Monti and Jantos (1992) | ||||
| Increased wakefulness, reduced NREM and REM sleep in rats (systemic) Increased REM sleep in rats (intracranial into DRN) |
Bjorvatn et al. (1997) | ||||
| Ipsapirone | Partial agonist | Increased wakefulness, reduced NREM and REM sleep in rats (systemic) | Monti et al. (1995) | ||
| Increased wakefulness, reduced REM sleep in rats | Tissier et al. (1993) | ||||
| Reduced REM sleep in humans | Driver et al. (1995) | ||||
| Increased NREM EEG delta power in humans | Seifritz et al. (1996) | ||||
| Buspirone | Partial agonist | Increased wakefulness, reduced NREM and REM sleep in rats (systemic) | Monti et al. (1995) | ||
| Increased wakefulness in rats at 3 mg/kg (systemic) Increased wakefulness, abolished REM in rats at 10 mg/kg (systemic) |
Lerman et al. (1986) | ||||
| Gepirone | Partial agonist | Increased wakefulness, reduced NREM and REM sleep in rats (systemic) | Monti et al. (1995) | ||
| Histamine Receptors | |||||
| H1 | Gq | Doxepin | Antagonist | Increased NREM sleep in mice | Y.-Q. Wang et al. (2015) |
| Diphenhydramine | Antagonist | Increased NREM sleep in mice | Y.-Q. Wang et al. (2015) | ||
| Triprolidine | Antagonist | Increased NREM sleep in mice | Parmentier et al. (2016) | ||
| H3 | Gi | Pitolisant | Inverse agonist | Prevents excessive daytime sleepiness in narcoleptic patients | Dauvilliers et al. (2019) |
3.1. Melatonin
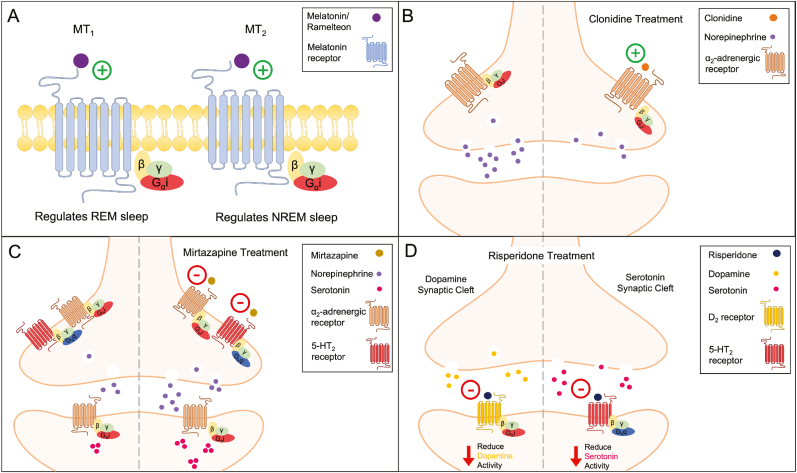
Melatonin is a neurohormone synthesized and released in the pineal gland that regulates circadian rhythms of sleep-wake timing and body temperature. Melatonin is secreted in a circadian fashion and is entrained by light exposure: secretion increases at the start of the evening, peaks in the middle of the night (around 3:00 a.m.), and decreases during the early morning (Grivas and Savvidou, 2007). The hypnotic effects of melatonin are thought to occur through activation of the melatonin (MT) receptors: activation of the MT1 receptors is implicated in the regulation of REM sleep and MT2 receptors are involved in regulating NREM sleep (Fisher and Sugden, 2009; Gobbi and Comai, 2019; Ochoa-Sanchez et al., 2011) (Fig. 2A). In particular, knockout mice lacking the MT1 receptors displayed reductions in REM sleep and theta power in the EEG during REM sleep, whereas MT2 receptor knockout mice displayed decreased NREM sleep and delta power in the EEG during NREM sleep (Comai et al., 2013). Furthermore, systemic injections of the selective MT2 receptor partial agonist, UCM924, or agonist, IIK7, in rats reduced the latency to NREM sleep and increased the duration of NREM sleep episodes (Fisher and Sugden, 2009; Ochoa-Sanchez et al., 2014). MT2 receptors are located in NREM sleep-active regions, including the reticular thalamus and preoptic areas, which could explain their role in NREM sleep regulation (Lacoste et al., 2015; Ochoa-Sanchez et al., 2011). Nonetheless, the exact circuit mechanisms by which melatonin promotes different aspects of sleep remain to be elucidated (Gobbi and Comai, 2019).
Fig. 2.
Mechanisms of Action for Sleep Therapeutics
A. Melatonin and Ramelteon are agonists (plus) on melatonin (MT) receptors. Activation of the Gi coupled MT receptors promotes sleep, with distinct activation of the different subtypes promoting either REM or NREM sleep.
B. Clonidine is a partial agonist (plus) on α2-adrenergic receptors. Activation of these Gi coupled auto-receptors reduces norepinephrine from the synapse.
C. Mirtazapine is an antagonist (negative) on α2-adrenergic and 5-HT2 receptors. Inhibition of the α2-adrenergic auto-receptors increases norepinephrine release from the synapse. In turn, this increase in norepinephrine activates postsynaptic α2-adrenergic receptors on serotonin neurons resulting in elevated serotonin levels.
D. Risperidone is an antagonist (negative) on D2 and 5-HT2 receptors. Inhibition of both Gi coupled receptors reduces dopamine and serotonin levels.
Numerous studies indicate that patients with ASD have abnormal levels of melatonin throughout the day, compared with peers, which may contribute to sleep disturbances (Goldman et al., 2014; Melke et al., 2008; Rossignol and Frye, 2011; Tordjman et al., 2005; Veatch et al., 2015b). In particular, one study reported that adult FXS patients have decreased levels of melatonin (O'hare et al., 1986), which is in contrast to another study reporting increased levels of melatonin in children and adolescence with FXS (Gould et al., 2000). A potential cause for these abnormal levels of melatonin in patients with ASD could be due to mutations of key enzymes in the biosynthesis pathway of melatonin. A genetic screening study demonstrated that the transcript level of acetylserotonin methyltransferase, the last enzyme in the synthesis of melatonin, is reduced in patients with ASD (Melke et al., 2008). Furthermore, patients with ASD demonstrate lower daytime and nocturnal excretion of 6-sulfatoxymelatonin, the main metabolite of melatonin in urine (Tordjman et al., 2005, 2012). An additional cause for these alterations in melatonin level could also be from differences in secretion. It was shown that low melatonin levels observed in ASD patients were positively correlated with decreased volume of the pineal gland (Maruani et al., 2019). Dim light melatonin onset (DLMO) refers to the time in which salivary concentrations of melatonin start to rise, typically occurring 2–3 h before sleep onset (Burgess and Fogg, 2008; Pandi-Perumal et al., 2007; Yavuz-Kodat et al., 2020). Previous studies of patients with ASD did not find differences in DLMO (Goldman et al., 2017), whereas one study found more variability in patients with ASD compared to TD peers (Baker et al., 2017). These findings are in contrast to a more recent study demonstrating that patients with ASD had delayed DLMO (Martinez-Cayuelas et al., 2023). These discrepant results could be due to the inclusion criteria of participants. The former studies focused on adults and adolescents who were taking medications whereas the latter study investigated DLMO in children and adolescents without pharmacological treatment for ASD symptoms. Nonetheless, it remains to be investigated whether abnormal levels of melatonin are caused by impairment in the biosynthesis and/or secretion of melatonin throughout development in patients with ASD.
3.2. Norepinephrine
The locus coeruleus (LC) is the primary site for the synthesis of norepinephrine (also known as noradrenaline) in the brain and its neurons project throughout the brain (Plummer et al., 2020; Waterhouse et al., 2022). Noradrenergic LC neurons are well characterized for their roles in regulating arousal and related behaviors (Poe et al., 2020). They are most active during wakefulness, particularly in response to salient, novel, or stressful stimuli, less active during NREM sleep and silent during REM sleep (Antila et al., 2022; Aston-Jones and Bloom, 1981; Aston-Jones and Cohen, 2005; Berridge et al., 2012; Bouret and Sara, 2004; Chandler et al., 2019; Clayton et al., 2004; Eschenko and Sara, 2008; Jacobs, 1986; Kjaerby et al., 2022; McCall et al., 2015; Osorio-Forero et al., 2021; Poe et al., 2020; Rajkowski et al., 1994; Rasmussen et al., 1986; Sara, 2009; Valentino and Van Bockstaele, 2008; Vankov et al., 1995; Zitnik, 2016).
Recent studies have identified the role of noradrenergic LC neurons in regulating the sleep microarchitecture (Antila et al., 2022; Kjaerby et al., 2022; Osorio-Forero et al., 2021). During NREM sleep, the noradrenergic LC neurons are rhythmically activated in synchrony with an infraslow (∼minute) rhythm in the spindle band (10–15 Hz) of the EEG (Lecci et al., 2017), and their activation is accompanied by microarousals (MAs) and suppression of sleep spindles (Antila et al., 2022; Aston-Jones and Bloom, 1981; Kjaerby et al., 2022; Swift et al., 2018). Opto- or chemogenetic activation of the noradrenergic LC neurons leads to transitions from NREM sleep to arousals, as well as a suppression of sleep spindles and REM sleep (Antila et al., 2022; Carter et al., 2010; Kjaerby et al., 2022; Liang et al., 2021; Osorio-Forero et al., 2021; Swift et al., 2018; Yamaguchi et al., 2018). In contrast, opto- or chemogenetic inhibition of noradrenergic LC neurons increase NREM sleep and sleep spindles (Carter et al., 2010; Kjaerby et al., 2022; Liang et al., 2021; Osorio-Forero et al., 2021; Yamaguchi et al., 2018). Injection of α2-adrenergic receptor agonists, including clonidine and detomidine, led to a decrease in REM sleep in cats (Crochet and Sakai, 1999; Tononi et al., 1991), rats (Hayat et al., 2020), and humans (Nicholson and Pascoe, 1991; Spiegel and DeVos, 1980) as well as an increase in delta power during NREM sleep (Crochet and Sakai, 1999; Hayat et al., 2020; Tononi et al., 1991). These findings demonstrate a significant role of the LC in regulating arousal as well as the sleep microarchitecture.
The noradrenergic system is thought to be dysregulated in patients with ASD (London, 2018). Serum norepinephrine levels are higher in ASD compared with TD children (Lake et al., 1977). Furthermore, dysregulation of the noradrenergic system has been shown to modulate attentional deficits in children with ASD (Bast et al., 2018). In particular, nighttime awakenings, delayed sleep onset, and insomnia were attenuated after clonidine administration (further described in section IV) indicating that heightened noradrenergic signaling may contribute to sleep problems in children with ASD (Ingrassia and Turk, 2005; Ming et al., 2008; Schnoes et al., 2006). Notably, a decrease in sleep spindle activity has been reported in ASD. Polysomnography studies show that adolescents with ASD exhibit fewer sleep spindles during N2 NREM sleep (Limoges et al., 2005), a lower amplitude and intensity of fast sleep spindles (Merikanto et al., 2019), and disrupted coordination between cortical slow-oscillations and sleep spindles, which is a measure of thalamocortical communication (Mylonas et al., 2022). Given the important role of the LC in regulating spindles, it remains to be determined whether the decrease in sleep spindles in children with ASD is due to elevated noradrenaline levels. In 16p11.2 ± mouse model of ASD, innervation of noradrenergic fibers is significantly reduced in the motor cortex, and pharmacogenetic activation of LC-NE neurons was shown to rescue their delayed motor learning (Yin et al., 2021). It remains to be investigated whether changes in noradrenergic signaling contribute to sleep disturbances in 16p11.2 deletion mice. These findings provide insights into a potential role of the LC noradrenergic system in regulating sleep problems and related symptoms in children and animal models with ASD.
3.3. Serotonin
The largest group of serotonergic neurons is found in the dorsal raphe nucleus (DRN). Early electrophysiology studies in cats demonstrated that most serotonergic DRN neurons exhibit the highest activity during wakefulness and become less active during NREM sleep and nearly silent during REM sleep (Cespuglio et al., 1981; Lydic et al., 1987; McGinty and Harper, 1976; Puizillout et al., 1979; Trulson and Jacobs, 1979). Microdialysis studies in freely moving rats and cats showed that serotonin levels in the brain are highest during waking, lower during NREM sleep, and lowest during REM sleep (Portas et al., 1996, 1998; Portas and McCarley, 1994). These findings have been confirmed by fiber photometry recordings (Oikonomou et al., 2019). Pharmacological manipulations of the serotonin system have demonstrated a direct role of serotonin in sleep and wakefulness (full review Portas and Grønli, 2008, summarized in Table 1). Systemic or intracranial injections of the serotonin receptor 6 (5-HT6) agonist WAY-208466 or 5-HT2C receptor agonist RO-600175 into the DRN of rats increased wakefulness and reduced NREM and REM sleep (Monti et al., 2013; Monti and Jantos, 2015). In contrast, ritanserin, an antagonist of 5-HT2 receptors, promoted sleep in both humans and rats, but induced wakefulness in cats (Dugovic and Wauquier, 1987; Idzikowski et al., 1986; Sommerfelt and Ursin, 1993). Agonists of 5-HT1A receptors decreased wakefulness when injected into the DRN, but increased wakefulness when injected systemically. These conflicting results could be due to differences in the receptor expression between the various species or the delivery method of drugs (systemic versus intracranial), given that a variety of serotonin receptors are broadly distributed in the gastrointestinal tract (Mawe and Hoffman, 2013). Optogenetic manipulation and in vivo electrophysiological recordings were used to investigate the role of serotonin neurons in regulating sleep and wakefulness. Long term (1 h) optogenetic stimulation of serotonergic DRN neurons reduced NREM sleep and increased wakefulness (Ito et al., 2013). Serotonergic DRN neurons have different firing patterns, including tonic (∼1–6 Hz) (McGinty and Harper, 1976) and burst (up to ∼30 Hz) activity depending on whether animals are exposed to rewards, aversive stimuli, or motor tasks (Cohen et al., 2015; Liu et al., 2014; Schweimer and Ungless, 2010; Veasey et al., 1995). Tonic optogenetic stimulation (3 Hz) of serotonergic DRN neurons in mice enhanced NREM sleep, whereas burst stimulation (25 Hz) increased wakefulness. Both stimulation protocols reduced REM sleep (Oikonomou et al., 2019). These findings demonstrate that serotonergic DRN neurons have distinct effects on sleep and wakefulness depending on their activity pattern.
Many studies suggest a link between serotonin and ASD (Muller et al., 2016). Elevated levels of serotonin in the blood were observed in ASD patients (Schain and Freedman, 1961). Hyperserotonemia, a condition in which blood serotonin levels are above the 95th percentile of normal levels, is estimated to be present in more than 25–30% of the ASD population (Gabriele et al., 2014; Hranilovic et al., 2007; Mulder et al., 2004). Compared with siblings, patients with ASD have elevated levels of serotonin in the dentate nucleus of the cerebellum and lower levels in the thalamus and frontal cortex (Chugani et al., 1997). Postmortem studies found that both 5-HT2A and 5-HT1A receptors along with the serotonin transporter, SERT, have decreased affinity for serotonin binding in ASD patients (Makkonen et al., 2008; Nakamura et al., 2010; Oblak et al., 2013). Furthermore, genetic studies in patients with ASD have identified polymorphisms in genes involved in the synthesis of serotonin, serotonin transporters, and serotonin receptors (Anderson et al., 2009; Devlin et al., 2005). A study in mice further supports these findings: The SERT amino acid variant, Ala56, induced hyperserotonemia in mice and increased repetitive behavior while reducing social behavior (Veenstra-VanderWeele et al., 2012). Recent studies have begun linking genetic mutations of the serotonin system with sleep disturbances in ASD patients and hyperserotonemia, suggesting a role of serotonin in sleep problems of ASD. Large-scale exome sequencing data show that mutations in the genes, chromodomain helicase DNA binding protein-8 (CHD8) and protein-7 (CHD7) are leading risk factors for ASD, and individuals with mutations in these genes suffer from difficulties with sleep initiation and maintenance (Bernier et al., 2014; Coll-Tané et al., 2021; Cotney et al., 2015; Satterstrom et al., 2020; Stessman et al., 2017). Knock-down of the CHD8/CHD7 ortholog, kismet, in Drosophila decreased sleep maintenance and led to a hyperserotonemia state, both of which are phenotypes that are also observed in patients with ASD (Coll-Tané et al., 2021). Furthermore, kismet mutants treated with α-methyl-DL-tryptophan (αMTP), a serotonin synthesis inhibitor, throughout development rescued sleep fragmentation (Coll-Tané et al., 2021). Shank3 deficient mice have higher mRNA levels of SERT in the hippocampus, hypothalamus, and frontal cortex at P5. In adult mice, the expression of SERT is significantly higher than WT mice in the hippocampus but significantly lower in the frontal cortex (Bukatova et al., 2021), suggesting altered serotonergic neurotransmission occurs during development in Shank3 deficient mice. Furthermore, in 16p11.2 deletion mice, dysregulation of the serotonergic system was shown to contribute to hyperactivity, reduced sociability and coping response to acute stress (Mitchell et al., 2020; Panzini et al., 2017). The role of serotonergic transmission in sleep disturbances in these animal models remains to be investigated. Together, these results provide important insights into our understanding of the role of the serotonergic system in sleep problems of ASD.
3.4. Dopamine
Dopamine is a neurotransmitter produced mainly in the ventral tegmental area (VTA) and substantia nigra (SN) (Björklund and Dunnett, 2007). Initial electrophysiological recordings in cats and rats demonstrated that the activity of dopaminergic neurons in the VTA and SN is highest during periods of active wakefulness and lower during sleep (Miller et al., 1983; Steinfels et al., 1983; Trulson et al., 1981; Trulson and Preussler, 1984). Genetically modified mice with a deletion of the dopamine transporter (DAT) gene show a reduction in NREM sleep and an increase in wakefulness (Wisor et al., 2001). More recent studies have demonstrated that dopaminergic neurons play an important role in regulating REM sleep (Hasegawa et al., 2022; Monti and Monti, 2007). Acute pharmacological depletion of dopamine levels suppressed NREM sleep and abolished REM sleep (Dzirasa et al., 2006). More recent electrophysiological recordings of dopaminergic VTA neurons demonstrated a burst firing activity during REM sleep (Dahan et al., 2007). These findings were further supported by fiber photometry recordings, which demonstrated that dopaminergic VTA neurons are most active during REM sleep, less active during wakefulness and least active during NREM sleep (Eban-Rothschild et al., 2016). Chemogenetic activation of dopaminergic VTA neurons promoted sustained wakefulness while optogenetic activation induced a rapid transition from sleep to wakefulness (Eban-Rothschild et al., 2016; Oishi et al., 2017; Sun et al., 2017). In contrast, chemogenetic inhibition of dopaminergic VTA neurons promoted sleep (Eban-Rothschild et al., 2016). The dopaminergic VTA neurons regulate REM sleep through various projection targets including the nucleus accumbens (NAc), prefrontal cortex (PFC), and basolateral amygdala (BLA). Using microdialysis in freely moving rats, it was demonstrated that extracellular dopamine levels increased in the NAc and PFC during REM sleep and wake; whereas other neurotransmitters including norepinephrine and glutamate only slightly increased during wake (Léna et al., 2005). Moreover, a recent study using a genetically encoded dopamine sensor demonstrated an increase of dopamine in the NAc during REM sleep but not in the PFC (Hasegawa et al., 2022). Furthermore, dopamine levels in the BLA began to increase prior to the onset of REM sleep and optogenetic activation of VTA dopaminergic projections to the BLA during NREM sleep promoted REM sleep, suggesting that dopaminergic transmission in the BLA plays a pivotal role in transitions to REM sleep (Hasegawa et al., 2022). Lastly, it has been demonstrated that NREM to REM sleep transitions are facilitated by BLA neurons containing the dopamine D2 receptors, as optogenetic inhibition of these neurons increased REM sleep initiation (Hasegawa et al., 2022).
The dopaminergic system is believed to be highly associated with ASD, in particular social deficits (Pavăl, 2017). Human studies demonstrated that genetic mutations of the dopaminergic system, including DAT (Bowton et al., 2014; Hamilton et al., 2013), dopamine receptors (de Krom et al., 2009; Hettinger et al., 2008; Mariggiò et al., 2021), and enzymes of dopamine biosynthesis (Nguyen et al., 2014), are implicated in ASD. Mice with increased neurotransmission of dopamine in the dorsal striatum due to suppression of DAT within the SN promotes autistic-like behaviors such as sociability deficits and repetitive behaviors (Lee et al., 2018). Clinical studies using positron emission tomographic (PET) scanning demonstrated a lower dopaminergic activity in the medial prefrontal cortex of children with ASD suggesting dysfunction of the mesocorticolimbic dopaminergic system, a circuit involved in reward processing (Ernst et al., 1997). Furthermore, a functional MRI (fMRI) study demonstrated that the mesocorticolimbic dopamine system is less active in response to monetary incentives in patients with ASD (Dichter et al., 2012a; Dichter et al., 2012b). Many studies support that the dopaminergic system contributes to sleep disturbances in ASD. The gene SLC6A3, which encodes DAT, is a risk gene for both ASD and sleep disorders (Abel et al., 2020). Additionally, restless leg syndrome, often a comorbidity with ASD that may contribute to insomnia, was found to be associated with decreased levels of D2 receptors in the putamen (Kanney et al., 2020; Veatch et al., 2015a). Recently, it has been shown that the dopaminergic system plays a critical role in the development of social interactions in mice as disruption of sleep during adolescence in mice reduces social novelty preference and novelty-dependent responses in dopaminergic VTA neurons in adulthood (Bian et al., 2022). Furthermore, social deficits in Shank3 mutant mice were rescued if NREM sleep was increased either by injections of flupirtine, a selective KCNQ2/KCNQ3 potassium channel opener, or chemogenetic inhibition of dopaminergic VTA neurons during adolescence (Bian et al., 2022). These findings provide an important insight into how dopamine contributes to shape sleep and social interactions during adolescence and how the dopaminergic system becomes dysfunctional in the Shank3 mutant mouse model of ASD.
3.5. Histamine
Histamine is a wake-promoting neurotransmitter found primarily in neurons of the tuberomammillary nucleus (TMN) (Panula et al., 1984; Watanabe et al., 1983, 1984). In mice, histaminergic TMN neurons are most active during wakefulness, in particular during active wake, less active during drowsy states and NREM sleep, and silent during REM sleep (John et al., 2004; Takahashi et al., 2006). Pharmacological studies on histamine receptors also support a role of histamine in wakefulness. Histamine H1 receptor antagonists such as doxepin, diphenhydramine, and triprolidine have been shown to promote NREM sleep in mice (Parmentier et al., 2016; Wang et al., 2015). Pitolisant, an inverse agonist for the histamine H3 auto-receptor, is used to prevent excessive daytime sleepiness in narcoleptic adult patients due to its unique mechanism of action which increase synthesis and release of histamine (Dauvilliers et al., 2013, 2019; Syed, 2016; Szakacs et al., 2017). Chemogenetic activation of histaminergic neurons promotes wakefulness (Yu et al., 2015), while optogenetic inhibition increases NREM sleep (Fujita et al., 2017).
Previous studies suggest that the histaminergic system is dysregulated in ASD. Postmortem brains from patients with ASD showed altered gene expression of histamine N-methyltransferase (HMT), an enzyme involved in the metabolism of histamine, and histamine receptors (H1,H2, and H3) in the dorsolateral prefrontal cortex (Wright et al., 2017). A recent study reported higher plasma histamine levels in children with ASD compared with TD peers (Rashaid et al., 2022). In a rodent model of ASD and children with ASD, histamine antagonists alleviated ASD symptoms, which further supports a link between histamine and ASD. Specifically, ciproxifan, an H3 antagonist ameliorated social interaction deficits and repetitive behaviors observed in the valproic acid-induced mouse model of ASD (Baronio et al., 2015; Taheri et al., 2022). Furthermore, famotidine, an H2 receptor antagonist, and cyproheptadine, an H1 receptor inverse agonist, have been reported to alleviate irritability, lethargy, hyperactivity, and social withdrawal in children with ASD (Akhondzadeh et al., 2004; Linday et al., 2001). While the role of histamine signaling in sleep disturbances of ASD patients or animal models has not been investigated yet, histamine receptor antagonists have been shown to reduce hyperactivity in mouse models of ASD. Pitolisant reduced hyperactivity (Meyza and Blanchard, 2017), while ciproxifan had no effect (Molenhuis et al., 2022) in the BTBR T + Itpr3tf/J mouse model of ASD that exhibits reduced social interactions and repetitive behaviors (Babineau et al., 2013; McFarlane et al., 2008; Silverman et al., 2010). However, in a valproic acid-induced ASD mouse model that was shown to exhibit sleep disturbances (Cusmano and Mong, 2014; Tsujino et al., 2007), DL77, an H3 receptor antagonist, had no effect on hyperactivity (Eissa et al., 2018). Nonetheless, while these studies suggest that antagonizing histamine neurotransmission can improve autism-like phenotypes in these mouse models of ASD, it remains unknown whether the quality of sleep in these mice is also improved.
4. Therapeutics for sleep disturbances in ASD
To date, there are no therapeutic sleeping aids approved by the FDA to attenuate sleep disturbances in children with ASD. The neural mechanisms underlying sleep disturbances in children with ASD are not fully understood, making it challenging to develop targeted therapeutics. In addition, careful considerations on dosing, tolerance, and adverse side effects need to be considered in developing therapeutics for pediatric patient populations. Nonetheless, a common side-effect of therapeutics used to treat stereotypic ASD behavior is sedation. These include FDA approved therapeutics for ADHD, depression, anxiety, irritability and aggression, and none of these drugs are approved for improving sleep. Additionally, sleep promoting agents have been prescribed off-label for this population. It is estimated that 38% of pediatricians recommend sleep therapeutics to children with ASD that experience insomnia, sleep onset delay, or night awakenings (Owens et al., 2003). Behavioral interventions such as education on sleep hygiene and positive reinforcement for sleep behavior are also recommended to be used as a first-line approach prior to pharmacological interventions to treat sleep disturbances in children with ASD (Malow et al., 2012, Malow et al., 2012). The following sections and Table 2 summarize pharmacological approaches to attenuate sleep disturbances in pediatric patients with ASD. Many of these studies are case studies that rely on anecdotal accounts from parents or patients, and are complicated further by different therapeutic regimens to treat ASD symptoms. Given the lack of rigorous controlled clinical trials, careful considerations are required to interpret the findings from these case studies.
Table 2.
Therapeutics for sleep disturbances in ASD.
| Ligand | Primary Target (s) | Type | Action | Participants | Effect on Sleep | Reference |
|---|---|---|---|---|---|---|
| Melatonin Agonists | ||||||
| Melatonin | MT1 receptor MT2 receptor |
Agonist | Full Agonist | n = 107; 2–18 year olds with ASD | Improved sleep in 60% of participants | Andersen et al. (2008) |
| n = 146; 3–15 year olds with NDD, including ASD | Improved total sleep time, reduced sleep latency | Gringras et al. (2012) | ||||
| n = 24; 3–10 year olds with ASD, AS, PDD, PDD-NOS | Reduced sleep latency | Malow et al. (2012) | ||||
| n = 22; 3–16 year olds with ASD | Reduced sleep latency, improved sleep duration; no reduction in night awakenings | Wright et al. (2011) | ||||
| n = 12; 2–15 year olds with ASD, FXS, ASD + FXS, FX premutation | Reduced sleep latency, improved sleep duration, no difference in night awakenings, earlier bedtimes | Wirojanan et al. (2009) | ||||
| n = 11*; 4–16 year olds with ASD | Reduced sleep latency, improved total sleep time; reduced number of night awakenings. *Discontinued at 7 participants when discovered placebo capsules were empty |
Garstang and Wallis, 2006 | ||||
| n = 9; 2–11 year olds with ASD | Reduced sleep latency, improved total sleep duration | Gupta and Hutchins (2005) | ||||
| n = 9; 4–17 year olds with RS | Reduced sleep latency, improved total sleep time, sleep efficiency | McArthur and Budden (1998) | ||||
| n = 6; 19–52 year olds with ASD | Reduced sleep latency, night awakenings; increased total sleep time | Galli-Carminati et al. (2009) | ||||
| n = 2; 7, 13 year old with RS | Reduced sleep latency, improved sleep maintenance | Miyamoto et al. (1999) | ||||
| n = 1; a 17 year old with AS | Improved sleep maintenance; minimized night awakenings | Horrigan and Barnhill (1997) | ||||
| n = 1; a 10 year old with RS | Reduced sleep latency; reduced night awakenings | Yamashita et al. (1999) | ||||
| n = 1; a 14 year old with ASD | Improved sleep duration, daily sleep-wake rhythm | Hayashi (2000) | ||||
| n = 1, a 12 year old with AS | Reduced sleep latency; reduced night terrors, night awakenings | Bottanelli et al. (2004) | ||||
| Melatonin-CR | MT1 receptor MT2 receptor |
Agonist | Full Agonist | n = 160, 4–10 year olds with ASD | Reduced sleep latency, improved total sleep time; reduced night awakenings | Cortesi et al. (2012) |
| n = 50, 2–18 year olds with NDD including ASD (cross-over trial) | Improved total sleep duration, reduced sleep latency | Wasdell et al. (2008) | ||||
| n = 47, 2–18 year olds with NDD including ASD (open-label phase) | Improved sleep efficiency, longer sleep episodes | Wasdell et al. (2008) | ||||
| n = 25; 2–9 year olds with ASD | Improved sleep onset, sleep duration, night-time awakenings, parasomnias and daytime sleepiness | Giannotti et al. (2006) | ||||
| Melatonin-PedPRM | MT1 receptor MT2 receptor |
Agonist | Full Agonist | n = 125, 2–17 year olds with ASD (96.8%) or SMS (3.2%) | Improved total sleep time, reduced sleep latency; reduced sleep disturbances | Gringras et al. (2017) |
| n = 125, 2–17 year olds with ASD (96.8%) or SMS (3.2%) | Improved sleep duration, reduced sleep latency | Schroder et al. (2019) | ||||
| n = 95, 2–17 year olds with ASD, neurogenic disorders | Improved total sleep time, reduced sleep latency, improved sleep quality; reduced night awakenings | Maras et al. (2018) | ||||
| n = 80, 2–17 year olds with ASD (96%) or SMS (4%) | Improved sleep disturbances, quality of sleep, caregiver satisfaction with children sleep patterns | Malow et al. (2021) | ||||
| Ramelteon | MT1 receptor MT2 receptor |
Agonist | Full Agonist | n = 3; 9–12 year olds with ASD | Improved latency to persistent sleep, total sleep time | Kawabe et al. (2014) |
| n = 2; 7, 18 year olds with ASD | Reduced sleep latency, improved sleep maintenance; reduced night awakenings | Stigler et al. (2006) | ||||
| α-Agonists | ||||||
| Clonidine | α2A-adrenoreceptor α2B-adrenoreceptor |
Agonist | Partial agonist | n = 19; 4–16 year olds with ASD, PDD, AS | Reduced sleep latency; reduced night awakenings | Ming et al. (2008) |
| n = 6; 6–14 year olds with ASD, PDD | Reduced sleep latency; Increased total sleep duration; reduced night awakenings | Ingrassia and Turk (2005) | ||||
| Guanfacine | α2A-adrenoreceptor α2B-adrenoreceptor α2C-adrenoreceptor |
Agonist Agonist Agonist |
Partial agonist Full Agonist Partial agonist |
n = 80; 3–18 years old with ASD, AS, PDD-NOS | Improved insomnia in 24% of patients | Posey et al. (2004) |
| Guanfacine-ER | α2A-adrenoreceptor α2B-adrenoreceptor α2C-adrenoreceptor |
Agonist Agonist Agonist |
Partial agonist Full Agonist Partial agonist |
n = 62; 5–14 year olds with ASD | No effect | Politte et al. (2018) |
| n = 1; 10 year old with ASD | Reduced sleep latency, improved sleep maintenance | Propper (2018) | ||||
| Antihistamines | ||||||
| Niaprazine | H1 receptor 5-HT2 receptor α1-adrenoreceptor |
Antagonist | – | n = 25; 2–20 year olds with ASD | Improved sleep disorders in 52% of patients | Rossi et al. (1999) |
| Antidepressants | ||||||
| Mirtazapine | α2C-adrenoreceptor α2A-adrenoreceptor α2B-adrenoreceptor 5-HT2C receptor 5-HT2A receptor |
Antagonist | – | n = 26; 3–23 year olds with ASD, AS, RS, PDD | Improved sleep quality | Posey et al. (2001) |
| n = 26; 5–17 year olds with ASD | No reduction in problematic sleep habits compared to placebo | McDougle et al. (2022) | ||||
| n = 1; 13 year old with ASD | Improved sleep | Nguyen and Murphy (2001) | ||||
| n = 1; 13 year old with ASD | Reduced sleep latency | Coskun and Mukaddes (2008) | ||||
| n = 1; 11 year old with ASD | Reduced sleep problems (combined with Aripiprazole) | Akbas and Akca (2018) | ||||
| n = 1; 15 year old with ASD | Improved sleep | Naguy et al. (2019) | ||||
| Trazodone | 5-HT2A receptor 5-HT2C receptor |
Antagonist | – | n = 1; 8 year old with ASD | Improved sleep | Rapin, 2001 |
| 1 year follow-up of Rapin (2001) | Continued to help with sleep | Parker and Hartman (2002) | ||||
| n = 1; 9 year old with AS | Reduced sleep latency | Bloomfield et al. (2015) | ||||
| Fluoxetine | SERT | Inhibitor | Inhibition | n = 1; 16 year old with ASD | Reduced insomnia | Cawkwell et al. (2016) |
| Fluvoxamine | SERT | Inhibitor | Inhibition | n = 1; 8 year old with AS | Reduced sleep disturbances | Furusho et al. (2001) |
| Paroxetine | SERT | Inhibitor | Inhibition | n = 1; 7 year old with ASD/PDD | Improved sleep | Posey et al. (1999) |
| Amitriptyline | H1 receptor M4 receptor M2 receptor M3 receptor M5 receptor SERT NET |
Antagonist Inhibitor |
– Inhibition |
n = 1; 6 year old with ASD | Improved sleep | Pollard and Prendergast (2004) |
| Antipsychotics | ||||||
| Risperidone | 5-HT2A receptor D2 receptor 5-HT1D receptor 5-HT1B receptor |
Antagonist | Inverse agonist – – – |
n = 56; 5–17 year olds with ASD | Improved sleep quality | Kent et al. (2013) |
| n = 23; 3–13 year olds with ASD, PDD-NOS, late-onset ASD | Improved sleep quality and/or duration | Capone et al. (2008) | ||||
| n = 11; 6–34 year olds with ASD | Reduced sleep latency, improved staying asleep | Horrigan and Barnhill (1997) | ||||
| n = 11; 7–17 year olds with ASD, PDD-NOS | Reduced sleep disturbances | Zuddas et al., 2000 | ||||
| n = 6; 3–13 year olds with ASD | Reduced sleep disturbances | Vercellino et al. (2001) | ||||
| n = 1; 16 year old with ASD | Upon withdrawal of risperidone, sleep worsened | Feroz-Nainar et al. (2006) | ||||
| n = 1; 10 year old with ASD | Improved sleeping throughout the night | Ivanov et al. (2006) | ||||
| n = 1; 3 year old with PDD | Improved sleep; reduced night awakenings | Doan (1998) | ||||
| n = 1; 5 year old with ASD | Improved sleep | Demb (1996) | ||||
| Aripiprazole | D2 receptor | Agonist | Partial Agonist | n = 32; 5–19 year olds with ASD | Improved sleep in 4/9 with sleep disorders | Valicenti-McDermott and Demb (2006) |
| n = 5; 5–18 year olds with PDD | Improved sleep | Stigler et al. (2004) | ||||
| n = 1; 11 year old with ASD | Reduced sleep problems (combined with Mirtazapine) | Akbas and Akca (2018) | ||||
| Quetiapine | D2 receptor 5-HT2A receptor |
Antagonist | – | n = 11; 13–17 year olds with ASD | Improved sleep disturbances, sleep quality | Golubchik et al. (2011) |
| n = 1; 16 year old with PDD-NOS | Improved sleep during manic state | Vijapura et al. (2014) | ||||
| n = 1; 17 year old with ASD | Improved sleep | Tufan and Kutlu (2009) | ||||
| Ziprasidone | 5-HT2A receptor D2 receptor 5-HT2C receptor |
Antagonist | – – Inverse agonist |
n = 1; 7 year old with ASD | Improved sleep pattern | Goforth and Rao, 2003 |
| Olanzapine | 5-HT2A receptor D2 receptor 5-HT2C receptor |
Antagonist | – – Inverse agonist |
n = 1; 6 year old with ASD | Improved sleep | Hergüner (2010) |
AS: Asperger's Syndrome; PDD: Pervasive Developmental Disorders; PDD-NOS: PDD-Not Otherwise Specified; FXS: Fragile-X Syndrome; RS: Rett-Syndrome; NDD: Neurodevelopmental Disorders; CR: Controlled-Release; PedPRM: Pediatric Prolonged Release Melatonin; SMS: Smith-Magenis Syndrome; ER: Extended Release; M# receptor: Acetylcholine receptor; D# receptor: Dopamine receptor.
4.1. Melatonin analogues
Despite melatonin being a natural supplement that is widely used with low safety concerns, it is not approved by the FDA. Many pediatricians and child psychiatrists recommend melatonin for sleep problems in children, especially with ASD (Bramble and Feehan, 2005). Numerous studies indicate that melatonin improves sleep quality in ASD children by reducing the latency to sleep onset (Aathira et al., 2017; Galli-Carminati et al., 2009; Garstang and Wallis, 2006; Gringras et al., 2012; Gupta and Hutchins, 2005; Jan et al., 2004; Malow et al., 2012; McArthur and Budden, 1998; Miyamoto et al., 1999; Wirojanan et al., 2009; Wright et al., 2011; Yamashita et al., 1999), increasing total sleep duration (Galli-Carminati et al., 2009; Garstang and Wallis, 2006; Gringras et al., 2012; Gupta and Hutchins, 2005; Hayashi, 2000; McArthur and Budden, 1998; Wirojanan et al., 2009; Wright et al., 2011) and decreasing night awakenings (Galli-Carminati et al., 2009; Garstang and Wallis, 2006; Horrigan and Barnhill, 1997; Jan et al., 2004; Yamashita et al., 1999) (Table 2). In addition, controlled-release melatonin has also been successful in reducing sleep disturbances in ASD children. Specifically, this novel therapeutic strategy closely mimics the circadian rhythm of melatonin secretion, with a low dose immediately released and a higher dose released hours later. This type of dosing strategy has been shown to improve sleep onset and total sleep duration while decreasing the number of night awakenings (Cortesi et al., 2012; Giannotti et al., 2006; Wasdell et al., 2008). Studies have also investigated the efficacy and safety of a pediatric prolonged release-melatonin (PedPRM) formulation for children with ASD. PedPRM has been shown to significantly reduce sleep latency and enhance total sleep time, by increasing the duration of sleep episodes and decreasing brief awakenings (Gringras et al., 2017; Schroder et al., 2019). Moreover, long-term treatment of PedPRM (104 weeks) was effective in improving sleep and was well tolerated in pediatric ASD patients with no adverse effect on growth and development (Malow et al., 2021; Maras et al., 2018). In addition to improving sleep, children's disruptive behavior was reduced and the caregivers' quality of life was improved in the PedPRM treated group compared with the placebo-group (Schroder et al., 2019). Ramelteon, an analogue of melatonin that was developed to treat insomnia, was also effective in reducing the latency to sleep and night awakenings while increasing total sleep time in children with ASD (Kawabe et al., 2014; Stigler et al., 2006).
4.2. α2-adrenergic agonists
The α2-adrenergic receptors are auto-receptors that can be located both on the presynapse or postsynapse (Molinoff, 1984). When norepinephrine is released it can bind to presynaptic α2-adrenergic receptors to inhibit further release of norepinephrine (i.e. negative feedback). Agonists of the α2-adrenergic receptors are sympathomimetic agents often used to promote sedation, muscle relaxation, and as an analgesics (Khan et al., 1999). Agonists of α2-adrenergic receptors, such as clonidine (Fig. 2B) and guanfacine, have been used to manage the symptoms of attention deficit hyperactivity disorder (ADHD), which is often a comorbidity with ASD (Connor et al., 2010; Fankhauser et al., 1992; Hazell, 2007; Jaselskis et al., 1992; Sallee et al., 2009; Wilens et al., 2012). The use of α2-adrenergic agonists in ASD children and adolescents has increased to improve sleep and anxiety symptoms (Fiks et al., 2015).
Clonidine, an anti-hypertensive therapeutic, is the most used medication within this drug class for treating sleep disturbances, including children with ASD (Schnoes et al., 2006) (Table 2). Clonidine decreased the time to fall asleep and helped children with neurodevelopmental disorders, including ASD, pervasive developmental disorders (PDDs), and Asperger's syndrome (AS) to stay asleep (Ingrassia and Turk, 2005; Ming et al., 2008). Clonidine is generally well tolerated in children with few adverse side effects such as REM sleep suppression (Pelayo and Dubik, 2008).
The other α2-adrenergic agonist that has been shown to improve sleep in children with ASD is guanfacine (Politte et al., 2018; Posey et al., 2004; Propper, 2018). Guanfacine is a highly selective agonist of the α2A-adrenergic receptor that is used to improve attention in children with ADHD. In terms of its effect on improving sleep in children with ASD, guanfacine treatment led to mixed results. A retrospective study of children with neurodevelopmental disorders indicated that guanfacine attenuated insomnia in 24% of patients, in line with a case study reporting reduced sleep latency and enhanced maintenance (Posey et al., 2004; Propper, 2018). However, a placebo-controlled study did not find an improvement in sleep habits in children with ASD (Politte et al., 2018).
4.3. Antihistamines
First-generation antihistamines were primarily designed to treat allergic rhinitis and other allergies that induce atopic eczema or from food and insect stings. However, a common side-effect of these drugs is sedation due to their ability to readily cross the blood-brain barrier and inhibit H1 receptors in the CNS (Slater et al., 1999). For this reason, many of the common first-generation antihistamines have been repurposed for nighttime sleeping aids. In general, antihistamines are the most common drugs recommended by pediatricians to treat sleep disorders within the pediatric population (Owens et al., 2003; Schnoes et al., 2006).
Diphenhydramine is the most widely used non-prescription antihistamine for sleep disorders. However, the use of diphenhydramine to treat sleep disturbances led to mixed results. A study has shown that diphenhydramine reduced sleep latency, the number of nighttime awakenings, and slightly increased sleep duration in children with sleep disorders (Russo et al., 1976), while more recent studies have demonstrated that it is not effective in treating sleep disturbances (Merenstein et al., 2006; Paul et al., 2004). Nonetheless, the efficacy of diphenhydramine in improving sleep of children with ASD remains to be tested.
Niaprazine has been shown to be effective in treating sleep disturbances of children and adolescents (Montanari et al., 1992; Ottaviano et al., 1991). In particular, niaprazine improved sleep in 52% of children and adolescents with ASD suffering from sleep disorders (Rossi et al., 1999) (Table 2). However, the exact mechanism by which niaprazine promotes sleep is not clear. Although originally thought to act as an antihistamine, niaprazine has been shown to have only a low binding affinity to H1 receptors and is instead more potent as an antagonist of 5-HT2 receptors and α1-adrenoceptors, which may contribute to its sleep-promoting effects (Scherman et al., 1988).
4.4. Antidepressants
It is estimated that 50–69% of children and adolescents with ASD display symptoms of anxiety (Kerns et al., 2021; McDougle et al., 2022). A common treatment regimen for children with ASD to attenuate anxiety symptoms and repetitive behaviors includes the use of antidepressants (McDougle et al., 2022; Posey et al., 2001). Within some classes of antidepressant drugs, sedation is often a common side effect and therefore while not explicitly prescribed for sleep disturbances, antidepressants have shown promising effects in improving sleep disturbances. The effect of antidepressants in promoting sleep may be mediated by the serotoninergic system or a combination of serotonin and norepinephrine (Relia and Ekambaram, 2018).
Mirtazapine is an antidepressant that has been shown to improve sleep in children with ASD. Mirtazapine produces sedative and anxiolytic effects by increasing both norepinephrine and serotonin levels. Specifically, mirtazapine blocks 5-HT2 and α2-adrenergic receptors, leading to increased norepinephrine levels. Norepinephrine in turn activates α2-adrenergic receptors on serotonergic neurons resulting in elevated serotonin levels via disinhibition (Haddjeri et al., 1995; Schatzberg and DeBattista, 2019a) (Fig. 2C). An open-label study and several case reports have suggested that mirtazapine may be beneficial for treating sleep disturbances in children with ASD (Akbas and Akca, 2018; Coskun and Mukaddes, 2008; Naguy et al., 2019; Nguyen and Murphy, 2001; Posey et al., 2001). However, a recent randomized double-blind placebo-controlled study assessed sleep habits with the CSHQ in children with ASD and demonstrated that both mirtazapine and placebo groups reduced sleep problems (McDougle et al., 2022). Nonetheless, it is unclear if these improvements in sleep are indirectly mediated by the drug's effect on managing other behavioral symptoms such as anxiety or a direct effect on attenuating sleep disturbances.
Limited data also exists from clinical observations in case reports on the use of other antidepressants to improve sleep in children with ASD (Table 2). Low-doses of trazodone, a 5-HT2A and 5-HT2C antagonist, was shown to relieve symptoms of insomnia, in particular with individuals experiencing depression (Kasper et al., 2005; Munizza et al., 2006; Nierenberg et al., 1994). A case report detailed that trazodone improved sleep in a child with ASD and this improvement was maintained after a one year follow-up (Parker and Hartman, 2002; Rapin, 2001). Another case report demonstrated trazodone decreased sleep latency in a child with AS (Bloomfield et al., 2015). Trazodone has been reported to improve sleep in children with Angelman syndrome, a neurodevelopmental disorder that has high comorbidity and is often misdiagnosed as ASD (Pereira et al., 2020). Selective serotonin reuptake inhibitors (SSRIs) antidepressants that inhibit the serotonin transporter, SERT, such as fluoxetine, fluvoxamine, and paroxetine improved sleep in children with neurodevelopmental disorders (Cawkwell et al., 2016; Furusho et al., 2001; Posey et al., 2006). Lastly, a case report suggested that amitriptyline improved sleep in a child with ASD (Pollard and Prendergast, 2004), but the exact mechanism is unclear given that this therapeutic inhibits both SERT and H1 receptors, among other targets (full list of targets in Table 2).
4.5. Antipsychotics
Given that symptoms in children with ASD include irritability, aggression, and self-injurious behavior, antipsychotics are often prescribed as an intervention to ameliorate these behaviors (Posey et al., 2008). While often prescribed for these behavioral symptoms, a secondary outcome is improved sleep. Antipsychotics are divided into typical and atypical; the former first-generation agents have an increased risk of tardive dyskinesia even after discontinuation of medication and were therefore replaced by atypical antipsychotics (Schatzberg and DeBattista, 2019a, Schatzberg and DeBattista, 2019b). Nonetheless, both groups of antipsychotics generally block the brain's dopaminergic system, particularly by antagonizing D2 receptors. This may lead to an increase in sleep, as D2 receptor knock-out mice sleep more, a phenotype that is mimicked in WT mice treated with the selective D2 receptor antagonist, raclopride (Qu et al., 2010).
Risperidone, which is used for the treatment of irritability in children and adolescents with ASD, has also been demonstrated to improve sleep. Risperidone is an antagonist of D2 and 5-HT receptors, including 5-HT2A, 5-HT1D, and 5-HT1B (Schotte et al., 1996) (Fig. 2D). Risperidone shares a similar pharmacological mechanism of action as mirtazapine, but also blocks the dopamine system, making it challenging to fully understand the sleep-promoting properties. Risperidone treatment in children with ASD was shown to decrease latency to fall asleep and increase the amount of sleep (Capone et al., 2008; Kent et al., 2013), which was further corroborated by other studies showing that risperidone attenuated sleep disturbances and reduced night awakenings (Demb, 1996; Doan, 1998; Horrigan and Barnhill, 1997; Ivanov et al., 2006; Vercellino et al., 2001; Zuddas et al., 2000). A case report noted that withdrawal of risperidone worsened sleep in a child with ASD, further supporting the effect of risperidone in improving sleep (Feroz-Nainar et al., 2006).
Aripiprazole, unlike other atypical antipsychotics, is a partial agonist of D2 receptors (Davies et al., 2004; Kikuchi et al., 1995) and used for the treatment of irritability in children with ASD. Studies suggest that aripiprazole can improve sleep in children with neurodevelopmental disorders (Stigler et al., 2004; Valicenti-McDermott and Demb, 2006). One case report demonstrated that aripiprazole in combination with mirtazapine reduced sleep problems in a child with ASD (Akbas and Akca, 2018). Finally, one study demonstrated that switching medication from risperidone to aripiprazole reduced daytime sleepiness in a few children with PDD, suggesting aripiprazole may be a suitable alternative if risperidone induces daytime sleepiness (Ishitobi et al., 2012).
The effects of atypical antipsychotics including quetiapine, ziprasidone, and olanzapine have been reported to improve sleep in children with ASD. Quetiapine, a D2 and 5-HT2A antagonist (Richelson and Souder, 2000), was shown to improve sleep quality in children with ASD (Golubchik et al., 2011). Additional case reports also indicate that quetiapine improved sleep in a child with ASD as well as a child with PDD-NOS during a manic state (Tufan and Kutlu, 2009; Vijapura et al., 2014). Lastly, ziprasidone and olanzapine, both of which are antagonists of 5-HT2A, D2, and 5-HT2C (Richelson and Souder, 2000), have been demonstrated to improve sleep in separate case reports (Hergüner, 2010; Goforth and Rao, 2003). While antipsychotics have been demonstrated to attenuate irritability and agitation in children with ASD, adverse side-effects need to be taken into consideration. A study found that compared with risperidone and aripiprazole, olanzapine treatment leads to more frequent side-effects in children with ASD, including 75% of participants experiencing sleepiness/sedation (Tural Hesapcioglu et al., 2020).
5. Conclusions
40–80% of children with ASD suffer from sleep problems and sleep disturbances have been suggested as a potential predictor of worsening core symptoms of ASD. Because ASD is a complicated biological puzzle that affects many different parts of children's brains, including the arousal and sleep centers detailed above, it is challenging to understand and treat ASD in its entirety. Importantly, good quality sleep alleviates ASD related psychopathologies. Uncovering the causal link between sleep quality and ASD symptoms using state-of-the-art systems neuroscience techniques combined with neuropharmacological approach in mouse models of ASD will elucidate the neural circuits and molecular mechanisms underlying sleep disturbances. To date, there are no therapeutic sleeping aids approved by the FDA to attenuate sleep disturbances in children with ASD. Leveraging the obtained findings to develop novel therapies to treat sleep disorders in turn may offer relief from ASD core symptoms and improve developmental trajectories. Furthermore, these research efforts will raise awareness of the importance of treating sleep disorders in children with ASD, and will transform behavioral therapies combined with drug treatment to enhance the overall treatment of ASD and thus improve the quality of life in patients and their families.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Franz Weber for proofreading the manuscript. This work was supported by the National Institute of Neurological Disorders and Stroke (R01-NS-110865), the Whitehall Foundation, Alfred P. Sloan Foundation, a NARSAD Young Investigator grant, Simons Foundation Pilot Award, Eagle Autism Challenge Pilot Grant, The McCabe Fund Award, The Hartwell Individual Biomedical Research Award to S. C. and the National Institute of Neurological Disorders and Strokes individual F31 fellowship (NS118963-01A1) to J.M. We thank the members from Chung and Weber labs for helpful discussion. Figures were created with Motifolio Illustration Toolkit-Neuroscience.
Handling Editor: Mark R. Opp
References
- Aathira R., Gulati S., Tripathi M., Shukla G., Chakrabarty B., Sapra S., Dang N., Gupta A., Kabra M., Pandey R.M. Prevalence of sleep abnormalities in Indian children with autism spectrum disorder: a cross-sectional study. Pediatr. Neurol. 2017;74:62–67. doi: 10.1016/j.pediatrneurol.2017.05.019. [DOI] [PubMed] [Google Scholar]
- Abel E.A., Schwichtenberg A.J., Mannin O.R., Marceau K. Brief report: a gene enrichment approach applied to sleep and autism. J. Autism Dev. Disord. 2020;50(5):1834–1840. doi: 10.1007/s10803-019-03921-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbas B., Akca O.F. Treatment of a child with autism spectrum disorder and food refusal due to restricted and repetitive behaviors. J. Child Adolesc. Psychopharmacol. 2018;28(5):364–365. doi: 10.1089/cap.2017.0168. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S., Erfani S., Mohammadi M.R., Tehrani-Doost M., Amini H., Gudarzi S.S., Yasamy M.T. Cyproheptadine in the treatment of autistic disorder: a double-blind placebo-controlled trial. J. Clin. Pharm. Therapeut. 2004;29(2):145–150. doi: 10.1111/j.1365-2710.2004.00546.x. [DOI] [PubMed] [Google Scholar]
- Allik H., Larsson J.-O., Smedje H. Sleep patterns of school-age children with Asperger syndrome or high-functioning autism. J. Autism Dev. Disord. 2006;36(5):585–595. doi: 10.1007/s10803-006-0099-9. [DOI] [PubMed] [Google Scholar]
- Anaclet C., Ferrari L., Arrigoni E., Bass C.E., Saper C.B., Lu J., Fuller P.M. The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat. Neurosci. 2014;17(9):1217–1224. doi: 10.1038/nn.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderlid B.-M., Schoumans J., Annerén G., Tapia-Paez I., Dumanski J., Blennow E., Nordenskjöld M. FISH-mapping of a 100-kb terminal 22q13 deletion. Hum. Genet. 2002;110(5):439–443. doi: 10.1007/s00439-002-0713-7. [DOI] [PubMed] [Google Scholar]
- Anders T., Iosif A.-M., Schwichtenberg A.J., Tang K., Goodlin-Jones B. Sleep and daytime functioning: a short-term longitudinal study of three preschool-age comparison groups. Am. J. Intellect. Dev. Disabil. 2012;117(4):275–290. doi: 10.1352/1944-7558-117.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen I.M., Kaczmarska J., McGrew S.G., Malow B.A. Melatonin for insomnia in children with autism spectrum disorders. J. Child Neurol. 2008;23(5):482–485. doi: 10.1177/0883073807309783. [DOI] [PubMed] [Google Scholar]
- Anderson B.M., Schnetz-Boutaud N.C., Bartlett J., Wotawa A.M., Wright H.H., Abramson R.K., Cuccaro M.L., Gilbert J.R., Pericak-Vance M.A., Haines J.L. Examination of association of genes in the serotonin system to autism. Neurogenetics. 2009;10(3):209–216. doi: 10.1007/s10048-009-0171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelakos, C., & Abel, T. (Unpublished results). SLEEP AND ACTIVITY PROBLEMS IN MOUSE MODELS OF NEURODEVELOPMENTAL DISORDERS. University of Pennsylvania.
- Angelakos C.C., Watson A.J., O'Brien W.T., Krainock K.S., Nickl-Jockschat T., Abel T. Hyperactivity and male-specific sleep deficits in the 16p11.2 deletion mouse model of autism. Autism Res.: Official Journal of the International Society for Autism Research. 2017;10(4):572–584. doi: 10.1002/aur.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antila H., Kwak I., Choi A., Pisciotti A., Covarrubias I., Baik J., Eisch A., Thomas S., Weber F., Chung S. A noradrenergic-hypothalamic neural substrate for stress-induced sleep disturbances. Proc. Natl. Acad. Sci. USA. 2022;119(45) doi: 10.1073/pnas.2123528119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arazi A., Meiri G., Danan D., Michaelovski A., Flusser H., Menashe I., Tarasiuk A., Dinstein I. Reduced sleep pressure in young children with autism. Sleep. 2020;43(6):zsz309. doi: 10.1093/sleep/zsz309. [DOI] [PubMed] [Google Scholar]
- Arbogast T., Ouagazzal A.-M., Chevalier C., Kopanitsa M., Afinowi N., Migliavacca E., Cowling B.S., Birling M.-C., Champy M.-F., Reymond A., Herault Y. Reciprocal effects on neurocognitive and metabolic phenotypes in mouse models of 16p11.2 deletion and duplication syndromes. PLoS Genet. 2016;12(2) doi: 10.1371/journal.pgen.1005709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G., Bloom F.E. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci.: The Official Journal of the Society for Neuroscience. 1981;1(8):876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G., Cohen J.D. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J. Comp. Neurol. 2005;493(1):99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Babineau B.A., Yang M., Berman R.F., Crawley J.N. Low home cage social behaviors in BTBR T+tf/J mice during juvenile development. Physiol. Behav. 2013;114(115):49–54. doi: 10.1016/j.physbeh.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E., Richdale A., Short M., Gradisar M. An investigation of sleep patterns in adolescents with high-functioning autism spectrum disorder compared with typically developing adolescents. Dev. Neurorehabil. 2013;16(3):155–165. doi: 10.3109/17518423.2013.765518. [DOI] [PubMed] [Google Scholar]
- Baker E.K., Richdale A.L., Hazi A., Prendergast L.A. Assessing the dim light melatonin onset in adults with autism spectrum disorder and No comorbid intellectual disability. J. Autism Dev. Disord. 2017;47(7):2120–2137. doi: 10.1007/s10803-017-3122-4. [DOI] [PubMed] [Google Scholar]
- Baronio D., Castro K., Gonchoroski T., de Melo G.M., Nunes G.D.F., Bambini-Junior V., Gottfried C., Riesgo R. Effects of an H3R antagonist on the animal model of autism induced by prenatal exposure to valproic acid. PLoS One. 2015;10(1) doi: 10.1371/journal.pone.0116363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast N., Poustka L., Freitag C.M. The locus coeruleus-norepinephrine system as pacemaker of attention—a developmental mechanism of derailed attentional function in autism spectrum disorder. Eur. J. Neurosci. 2018;47(2):115–125. doi: 10.1111/ejn.13795. [DOI] [PubMed] [Google Scholar]
- Bernier R., Golzio C., Xiong B., Stessman H.A., Coe B.P., Penn O., Witherspoon K., Gerdts J., Baker C., Vulto-van Silfhout A.T., Schuurs-Hoeijmakers J.H., Fichera M., Bosco P., Buono S., Alberti A., Failla P., Peeters H., Steyaert J., Vissers L.E.L.M., et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell. 2014;158(2):263–276. doi: 10.1016/j.cell.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge C.W., Schmeichel B.E., España R.A. Noradrenergic modulation of wakefulness/arousal. Sleep Med. Rev. 2012;16(2):187–197. doi: 10.1016/j.smrv.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E. Epilepsy in fragile X syndrome. Dev. Med. Child Neurol. 2002;44(11):724–728. doi: 10.1017/s0012162201002833. [DOI] [PubMed] [Google Scholar]
- Berryer M.H., Hamdan F.F., Klitten L.L., Møller R.S., Carmant L., Schwartzentruber J., Patry L., Dobrzeniecka S., Rochefort D., Neugnot-Cerioli M., Lacaille J.-C., Niu Z., Eng C.M., Yang Y., Palardy S., Belhumeur C., Rouleau G.A., Tommerup N., Immken L., et al. Mutations in SYNGAP1 cause intellectual disability, autism, and a specific form of epilepsy by inducing haploinsufficiency. Hum. Mutat. 2013;34(2):385–394. doi: 10.1002/humu.22248. [DOI] [PubMed] [Google Scholar]
- Berryer M.H., Chattopadhyaya B., Xing P., Riebe I., Bosoi C., Sanon N., Antoine-Bertrand J., Lévesque M., Avoli M., Hamdan F.F., Carmant L., Lamarche-Vane N., Lacaille J.-C., Michaud J.L., Di Cristo G. Decrease of SYNGAP1 in GABAergic cells impairs inhibitory synapse connectivity, synaptic inhibition and cognitive function. Nat. Commun. 2016;7(1) doi: 10.1038/ncomms13340. Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian W.-J., Brewer C.L., Kauer J.A., de Lecea L. Adolescent sleep shapes social novelty preference in mice. Nat. Neurosci. 2022;25(7):912–923. doi: 10.1038/s41593-022-01076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund A., Dunnett S.B. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30(5):194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Bjorvatn B., Fagerland S., Eid T., Ursin R. Sleep/waking effects of a selective 5-HT1A receptor agonist given systemically as well as perfused in the dorsal raphe nucleus in rats. Brain Res. 1997;770(1–2):81–88. doi: 10.1016/s0006-8993(97)00758-0. [DOI] [PubMed] [Google Scholar]
- Bloomfield K., Pulliam E., Schultz E., Ward W.L. Russian adoption: a case study with mixed clinical presentation. Clin. Pediatr. 2015;54(11):1123–1124. doi: 10.1177/0009922815576886. [DOI] [PubMed] [Google Scholar]
- Blumenthal I., Ragavendran A., Erdin S., Klei L., Sugathan A., Guide J.R., Manavalan P., Zhou J.Q., Wheeler V.C., Levin J.Z., Ernst C., Roeder K., Devlin B., Gusella J.F., Talkowski M.E. Transcriptional consequences of 16p11.2 deletion and duplication in mouse cortex and multiplex autism families. Am. J. Hum. Genet. 2014;94(6):870–883. doi: 10.1016/j.ajhg.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccuto L., Lauri M., Sarasua S.M., Skinner C.D., Buccella D., Dwivedi A., Orteschi D., Collins J.S., Zollino M., Visconti P., Dupont B., Tiziano D., Schroer R.J., Neri G., Stevenson R.E., Gurrieri F., Schwartz C.E. Prevalence of SHANK3 variants in patients with different subtypes of autism spectrum disorders. Eur. J. Hum. Genet.: EJHG (Eur. J. Hum. Genet.) 2013;21(3):310–316. doi: 10.1038/ejhg.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckers T.M., Bockmann J., Kreutz M.R., Gundelfinger E.D. ProSAP/Shank proteins—a family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. J. Neurochem. 2002;81(5):903–910. doi: 10.1046/j.1471-4159.2002.00931.x. [DOI] [PubMed] [Google Scholar]
- Bonaglia M.C., Giorda R., Borgatti R., Felisari G., Gagliardi C., Selicorni A., Zuffardi O. Disruption of the ProSAP2 gene in a t(12;22)(q24.1;q13.3) is associated with the 22q13.3 deletion syndrome. Am. J. Hum. Genet. 2001;69(2):261–268. doi: 10.1086/321293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone C.E., Davoudi H., Harrold J.B., Foster D.J. Abnormal sleep architecture and hippocampal circuit dysfunction in a mouse model of fragile X syndrome. Neuroscience. 2018;384:275–289. doi: 10.1016/j.neuroscience.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Bottanelli M., Rubini G., Venturelli V., Cosentino A., Rossato G., Vicentini S., Romito S., Rizzuto N., Bertolasi L. Weight and height gain after intrathecal baclofen pump implantation in children with spastic tetraparesis. Dev. Med. Child Neurol. 2004;46(11):788–789. doi: 10.1017/s0012162204221351. [DOI] [PubMed] [Google Scholar]
- Bouret S., Sara S.J. Reward expectation, orientation of attention and locus coeruleus-medial frontal cortex interplay during learning. Eur. J. Neurosci. 2004;20(3):791–802. doi: 10.1111/j.1460-9568.2004.03526.x. [DOI] [PubMed] [Google Scholar]
- Bowton E., Saunders C., Reddy I.A., Campbell N.G., Hamilton P.J., Henry L.K., Coon H., Sakrikar D., Veenstra-VanderWeele J.M., Blakely R.D., Sutcliffe J., Matthies H.J.G., Erreger K., Galli A. SLC6A3 coding variant Ala559Val found in two autism probands alters dopamine transporter function and trafficking. Transl. Psychiatry. 2014;4(10):e464. doi: 10.1038/tp.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramble D., Feehan C. Psychiatrists' use of melatonin with children. Child Adolesc. Ment. Health. 2005;10(3):145–149. doi: 10.1111/j.1475-3588.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- Bro D., O'Hara R., Primeau M., Hanson-Kahn A., Hallmayer J., Bernstein J.A. Sleep disturbances in individuals with phelan-McDermid syndrome: correlation with caregivers' sleep quality and daytime functioning. Sleep. 2017;40(2) doi: 10.1093/sleep/zsw062. [DOI] [PubMed] [Google Scholar]
- Buckley A.W., Rodriguez A.J., Jennison K., Buckley J., Thurm A., Sato S., Swedo S. Rapid eye movement sleep percentage in children with autism compared with children with developmental delay and typical development. Arch. Pediatr. Adolesc. Med. 2010;164(11):1032–1037. doi: 10.1001/archpediatrics.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukatova S., Renczes E., Reichova A., Filo J., Sadlonova A., Mravec B., Ostatnikova D., Bakos J., Bacova Z. Shank3 deficiency is associated with altered profile of neurotransmission markers in pups and adult mice. Neurochem. Res. 2021;46(12):3342–3355. doi: 10.1007/s11064-021-03435-6. [DOI] [PubMed] [Google Scholar]
- Burgess H.J., Fogg L.F. Individual differences in the amount and timing of salivary melatonin secretion. PLoS One. 2008;3(8):e3055. doi: 10.1371/journal.pone.0003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone G.T., Goyal P., Grados M., Smith B., Kammann H. Risperidone use in children with Down syndrome, severe intellectual disability, and comorbid autistic spectrum disorders: a naturalistic study. J. Dev. Behav. Pediatr.: JDBP (J. Dev. Behav. Pediatr.) 2008;29(2):106–116. doi: 10.1097/DBP.0b013e318165c100. [DOI] [PubMed] [Google Scholar]
- Carmassi C., Palagini L., Caruso D., Masci I., Nobili L., Vita A., Dell'Osso L. Systematic review of sleep disturbances and circadian sleep desynchronization in autism spectrum disorder: toward an integrative model of a self-reinforcing loop. Front. Psychiatr. 2019;10:366. doi: 10.3389/fpsyt.2019.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M.E., Yizhar O., Chikahisa S., Nguyen H., Adamantidis A., Nishino S., Deisseroth K., de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 2010;13(12) doi: 10.1038/nn.2682. Article 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvill G.L., Heavin S.B., Yendle S.C., McMahon J.M., O'Roak B.J., Cook J., Khan A., Dorschner M.O., Weaver M., Calvert S., Malone S., Wallace G., Stanley T., Bye A.M.E., Bleasel A., Howell K.B., Kivity S., Mackay M.T., Rodriguez-Casero V., et al. Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP1. Nat. Genet. 2013;45(7):825–830. doi: 10.1038/ng.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawkwell P., Lawler A., Maneta E., Coffey B.J. Staying up at night: overlapping bipolar and obsessive-compulsive disorder symptoms in an adolescent with autism spectrum disorder. J. Child Adolesc. Psychopharmacol. 2016;26(1):74–77. doi: 10.1089/cap.2016.29100.bjc. [DOI] [PubMed] [Google Scholar]
- Cespuglio R., Faradji H., Gomez M.E., Jouvet M. Single unit recordings in the nuclei raphe dorsalis and magnus during the sleep-waking cycle of semi-chronic prepared cats. Neurosci. Lett. 1981;24(2):133–138. doi: 10.1016/0304-3940(81)90236-6. [DOI] [PubMed] [Google Scholar]
- Chandler D.J., Jensen P., McCall J.G., Pickering A.E., Schwarz L.A., Totah N.K. Redefining noradrenergic neuromodulation of behavior: impacts of a modular locus coeruleus architecture. J. Neurosci.: The Official Journal of the Society for Neuroscience. 2019;39(42):8239–8249. doi: 10.1523/JNEUROSCI.1164-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Toth M. Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience. 2001;103(4):1043–1050. doi: 10.1016/s0306-4522(01)00036-7. [DOI] [PubMed] [Google Scholar]
- Chen H., Yang T., Chen J., Chen L., Dai Y., Zhang J., Li L., Jia F., Wu L., Hao Y., Ke X., Yi M., Hong Q., Chen J., Fang S., Wang Y., Wang Q., Jin C., Li T. Sleep problems in children with autism spectrum disorder: a multicenter survey. BMC Psychiatr. 2021;21:406. doi: 10.1186/s12888-021-03405-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani D.C., Muzik O., Rothermel R., Behen M., Chakraborty P., Mangner T., da Silva E.A., Chugani H.T. Altered serotonin synthesis in the dentatothalamocortical pathway in autistic boys. Ann. Neurol. 1997;42(4):666–669. doi: 10.1002/ana.410420420. [DOI] [PubMed] [Google Scholar]
- Clayton E.C., Rajkowski J., Cohen J.D., Aston-Jones G. Phasic activation of monkey locus ceruleus neurons by simple decisions in a forced-choice task. J. Neurosci.: The Official Journal of the Society for Neuroscience. 2004;24(44):9914–9920. doi: 10.1523/JNEUROSCI.2446-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement J.P., Aceti M., Creson T.K., Ozkan E.D., Shi Y., Reish N.J., Almonte A.G., Miller B.H., Wiltgen B.J., Miller C.A., Xu X., Rumbaugh G. Pathogenic SYNGAP1 mutations impair cognitive development by disrupting maturation of dendritic spine synapses. Cell. 2012;151(4):709–723. doi: 10.1016/j.cell.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.Y., Amoroso M.W., Uchida N. Serotonergic neurons signal reward and punishment on multiple timescales. Elife. 2015;4 doi: 10.7554/eLife.06346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll-Tané M., Gong N.N., Belfer S.J., van Renssen L.V., Kurtz-Nelson E.C., Szuperak M., Eidhof I., van Reijmersdal B., Terwindt I., Durkin J., Verheij M.M.M., Kim C.N., Hudac C.M., Nowakowski T.J., Bernier R.A., Pillen S., Earl R.K., Eichler E.E., Kleefstra T., et al. The CHD8/CHD7/Kismet family links blood-brain barrier glia and serotonin to ASD-associated sleep defects. Sci. Adv. 2021;7(23):eabe2626. doi: 10.1126/sciadv.abe2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai S., Ochoa-Sanchez R., Gobbi G. Sleep–wake characterization of double MT1/MT2 receptor knockout mice and comparison with MT1 and MT2 receptor knockout mice. Behav. Brain Res. 2013;243:231–238. doi: 10.1016/j.bbr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Connor D.F., Findling R.L., Kollins S.H., Sallee F., López F.A., Lyne A., Tremblay G. Effects of guanfacine extended release on oppositional symptoms in children aged 6-12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a randomized, double-blind, placebo-controlled trial. CNS Drugs. 2010;24(9):755–768. doi: 10.2165/11537790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Cortesi F., Giannotti F., Sebastiani T., Panunzi S., Valente D. Controlled-release melatonin, singly and combined with cognitive behavioural therapy, for persistent insomnia in children with autism spectrum disorders: a randomized placebo-controlled trial. J. Sleep Res. 2012;21(6):700–709. doi: 10.1111/j.1365-2869.2012.01021.x. [DOI] [PubMed] [Google Scholar]
- Coskun M., Mukaddes N.M. Mirtazapine treatment in a subject with autistic disorder and fetishism. J. Child Adolesc. Psychopharmacol. 2008;18(2):206–209. doi: 10.1089/cap.2007.0014. [DOI] [PubMed] [Google Scholar]
- Costales J.L., Kolevzon A. Phelan-McDermid syndrome and SHANK3: implications for treatment. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics. 2015;12(3):620–630. doi: 10.1007/s13311-015-0352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotney J., Muhle R.A., Sanders S.J., Liu L., Willsey A.J., Niu W., Liu W., Klei L., Lei J., Yin J., Reilly S.K., Tebbenkamp A.T., Bichsel C., Pletikos M., Sestan N., Roeder K., State M.W., Devlin B., Noonan J.P. The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nat. Commun. 2015;6:6404. doi: 10.1038/ncomms7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton S., Richdale A. Brief report: parental descriptions of sleep problems in children with autism, Down syndrome, and Prader-Willi syndrome. Res. Dev. Disabil. 2006;27(2):151–161. doi: 10.1016/j.ridd.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Crawley J.N. Translational animal models of autism and neurodevelopmental disorders. Dialogues Clin. Neurosci. 2012;14(3):293–305. doi: 10.31887/DCNS.2012.14.3/jcrawley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crochet S., Sakai K. Alpha-2 adrenoceptor mediated paradoxical (REM) sleep inhibition in the cat. Neuroreport. 1999;10(10):2199–2204. doi: 10.1097/00001756-199907130-00036. [DOI] [PubMed] [Google Scholar]
- Cusmano D.M., Mong J.A. Utero exposure to valproic acid changes sleep in juvenile rats: a model for sleep disturbances in autism. Sleep. 2014;37(9):1489–1499. doi: 10.5665/sleep.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan L., Astier B., Vautrelle N., Urbain N., Kocsis B., Chouvet G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2007;32(6):1232–1241. doi: 10.1038/sj.npp.1301251. [DOI] [PubMed] [Google Scholar]
- Dauvilliers Y., Bassetti C., Lammers G.J., Arnulf I., Mayer G., Rodenbeck A., Lehert P., Ding C.-L., Lecomte J.-M., Schwartz J.-C., HARMONY I study group Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. The Lancet. Neurology. 2013;12(11):1068–1075. doi: 10.1016/S1474-4422(13)70225-4. [DOI] [PubMed] [Google Scholar]
- Dauvilliers Y., Arnulf I., Szakacs Z., Leu-Semenescu S., Lecomte I., Scart-Gres C., Lecomte J.-M., Schwartz J.-C., HARMONY III study group Long-term use of pitolisant to treat patients with narcolepsy: harmony III Study. Sleep. 2019;42(11):zsz174. doi: 10.1093/sleep/zsz174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M.A., Sheffler D.J., Roth B.L. Aripiprazole: a novel atypical antipsychotic drug with a uniquely robust pharmacology. CNS Drug Rev. 2004;10(4):317–336. doi: 10.1111/j.1527-3458.2004.tb00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Krom M., Staal W.G., Ophoff R.A., Hendriks J., Buitelaar J., Franke B., de Jonge M.V., Bolton P., Collier D., Curran S., van Engeland H., van Ree J.M. A common variant in DRD3 receptor is associated with autism spectrum disorder. Biol. Psychiatr. 2009;65(7):625–630. doi: 10.1016/j.biopsych.2008.09.035. [DOI] [PubMed] [Google Scholar]
- Deciphering Developmental Disorders Study Large-scale discovery of novel genetic causes of developmental disorders. Nature. 2015;519(7542):223–228. doi: 10.1038/nature14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deciphering Developmental Disorders Study Prevalence and architecture of de novo mutations in developmental disorders. Nature. 2017;542(7642):433–438. doi: 10.1038/nature21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb H.B. Risperidone in young children with pervasive developmental disorders and other developmental disabilities. J. Child Adolesc. Psychopharmacol. 1996;6(1):79–80. doi: 10.1089/cap.1996.6.79. [DOI] [PubMed] [Google Scholar]
- Devlin B., Cook E.H., Coon H., Dawson G., Grigorenko E.L., McMahon W., Minshew N., Pauls D., Smith M., Spence M.A., Rodier P.M., Stodgell C., Schellenberg G.D., CPEA Genetics Network Autism and the serotonin transporter: the long and short of it. Mol. Psychiatr. 2005;10(12):1110–1116. doi: 10.1038/sj.mp.4001724. [DOI] [PubMed] [Google Scholar]
- Dichter G.S., Felder J.N., Green S.R., Rittenberg A.M., Sasson N.J., Bodfish J.W. Reward circuitry function in autism spectrum disorders. Soc. Cognit. Affect Neurosci. 2012;7(2):160–172. doi: 10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter G.S., Richey J.A., Rittenberg A.M., Sabatino A., Bodfish J.W. Reward circuitry function in autism during face anticipation and outcomes. J. Autism Dev. Disord. 2012;42(2):147–160. doi: 10.1007/s10803-011-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diomedi M., Curatolo P., Scalise A., Placidi F., Caretto F., Gigli G.L. Sleep abnormalities in mentally retarded autistic subjects: down's syndrome with mental retardation and normal subjects. Brain & Dev. 1999;21(8):548–553. doi: 10.1016/s0387-7604(99)00077-7. [DOI] [PubMed] [Google Scholar]
- Doan R.J. Risperidone for insomnia in PDDs. Canadian journal of psychiatry. Rev. Canad. Psychiatr. 1998;43(10):1050–1051. [PubMed] [Google Scholar]
- Doldur-Balli F., Imamura T., Veatch O.J., Gong N.N., Lim D.C., Hart M.P., Abel T., Kayser M.S., Brodkin E.S., Pack A.I. Synaptic dysfunction connects autism spectrum disorder and sleep disturbances: a perspective from studies in model organisms. Sleep Med. Rev. 2022;62 doi: 10.1016/j.smrv.2022.101595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominick K.C., Davis N.O., Lainhart J., Tager-Flusberg H., Folstein S. Atypical behaviors in children with autism and children with a history of language impairment. Res. Dev. Disabil. 2007;28(2):145–162. doi: 10.1016/j.ridd.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Driver H.S., Flanigan M.J., Bentley A.J., Luus H.G., Shapiro C.M., Mitchell D. The influence of ipsapirone, a 5-HT1A agonist, on sleep patterns of healthy subjects. Psychopharmacology. 1995;117(2):186–192. doi: 10.1007/BF02245186. [DOI] [PubMed] [Google Scholar]
- Dugovic C., Wauquier A. 5-HT2 receptors could be primarily involved in the regulation of slow-wave sleep in the rat. Eur. J. Pharmacol. 1987;137(1):145–146. doi: 10.1016/0014-2999(87)90196-8. [DOI] [PubMed] [Google Scholar]
- Durand C.M., Betancur C., Boeckers T.M., Bockmann J., Chaste P., Fauchereau F., Nygren G., Rastam M., Gillberg I.C., Anckarsäter H., Sponheim E., Goubran-Botros H., Delorme R., Chabane N., Mouren-Simeoni M.-C., de Mas P., Bieth E., Rogé B., Héron D., et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat. Genet. 2007;39(1):25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzirasa K., Ribeiro S., Costa R., Santos L.M., Lin S.-C., Grosmark A., Sotnikova T.D., Gainetdinov R.R., Caron M.G., Nicolelis M.A.L. Dopaminergic control of sleep-wake states. J. Neurosci.: The Official Journal of the Society for Neuroscience. 2006;26(41):10577–10589. doi: 10.1523/JNEUROSCI.1767-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzoljic M.R., Ukponmwan O.E., Saxena P.R. 5-HT1-like receptor agonists enhance wakefulness. Neuropharmacology. 1992;31(7):623–633. doi: 10.1016/0028-3908(92)90140-k. [DOI] [PubMed] [Google Scholar]
- Eban-Rothschild A., Rothschild G., Giardino W.J., Jones J.R., de Lecea L. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat. Neurosci. 2016;19(10):1356–1366. doi: 10.1038/nn.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ego-Stengel V., Wilson M.A. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20(1):1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissa N., Jayaprakash P., Azimullah S., Ojha S.K., Al-Houqani M., Jalal F.Y., Łażewska D., Kieć-Kononowicz K., Sadek B. The histamine H3R antagonist DL77 attenuates autistic behaviors in a prenatal valproic acid-induced mouse model of autism. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-31385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia M., Ferri R., Musumeci S.A., Del Gracco S., Bottitta M., Scuderi C., Miano G., Panerai S., Bertrand T., Grubar J.C. Sleep in subjects with autistic disorder: a neurophysiological and psychological study. Brain & Dev. 2000;22(2):88–92. doi: 10.1016/s0387-7604(99)00119-9. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M., Divan G., Koh Y.-J., Kim Y.S., Kauchali S., Marcín C., Montiel-Nava C., Patel V., Paula C.S., Wang C., Yasamy M.T., Fombonne E. Global prevalence of autism and other pervasive developmental disorders. Autism Res.: Official Journal of the International Society for Autism Research. 2012;5(3):160–179. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Zametkin A.J., Matochik J.A., Pascualvaca D., Cohen R.M. Low medial prefrontal dopaminergic activity in autistic children. Lancet (London, England) 1997;350(9078):638. doi: 10.1016/s0140-6736(05)63326-0. [DOI] [PubMed] [Google Scholar]
- Eschenko O., Sara S.J. Learning-dependent, transient increase of activity in noradrenergic neurons of locus coeruleus during slow wave sleep in the rat: brain stem-cortex interplay for memory consolidation? Cerebr. Cortex. 2008;18(11):2596–2603. doi: 10.1093/cercor/bhn020. [DOI] [PubMed] [Google Scholar]
- Fankhauser M.P., Karumanchi V.C., German M.L., Yates A., Karumanchi S.D. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J. Clin. Psychiatry. 1992;53(3):77–82. [PubMed] [Google Scholar]
- Feroz-Nainar C., Selvaraj P., Roy M. Risperidone induced oedema in a child with learning disability and autism. Autism: The International Journal of Research and Practice. 2006;10(3):308–310. doi: 10.1177/1362361306063302. [DOI] [PubMed] [Google Scholar]
- Fiks A.G., Mayne S.L., Song L., Steffes J., Liu W., McCarn B., Margolis B., Grimes A., Gotlieb E., Localio R., Ross M.E., Grundmeier R.W., Wasserman R., Leslie L.K. Changing patterns of alpha agonist medication use in children and adolescents 2009-2011. J. Child Adolesc. Psychopharmacol. 2015;25(4):362–367. doi: 10.1089/cap.2014.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S.P., Sugden D. Sleep-promoting action of IIK7, a selective MT2 melatonin receptor agonist in the rat. Neurosci. Lett. 2009;457(2):93–96. doi: 10.1016/j.neulet.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher F.E., Foster-Owens M.D., Conduit R., Rinehart N.J., Riby D.M., Cornish K.M. The developmental trajectory of parent-report and objective sleep profiles in autism spectrum disorder: associations with anxiety and bedtime routines. Autism: The International Journal of Research and Practice. 2017;21(4):493–503. doi: 10.1177/1362361316653365. [DOI] [PubMed] [Google Scholar]
- Fujita A., Bonnavion P., Wilson M.H., Mickelsen L.E., Bloit J., de Lecea L., Jackson A.C. Hypothalamic tuberomammillary nucleus neurons: electrophysiological diversity and essential role in arousal stability. J. Neurosci.: The Official Journal of the Society for Neuroscience. 2017;37(39):9574–9592. doi: 10.1523/JNEUROSCI.0580-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusho J., Matsuzaki K., Ichihashi I., Satoh H., Yamaguchi K., Kumagai K. Alleviation of sleep disturbance and repetitive behavior by a selective serotonin re-uptake inhibitor in a boy with Asperger's syndrome. Brain & Dev. 2001;23(2):135–137. doi: 10.1016/s0387-7604(01)00182-6. [DOI] [PubMed] [Google Scholar]
- Gabriele S., Sacco R., Persico A.M. Blood serotonin levels in autism spectrum disorder: a systematic review and meta-analysis. Eur. Neuropsychopharmacol: The Journal of the European College of Neuropsychopharmacology. 2014;24(6):919–929. doi: 10.1016/j.euroneuro.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Gail Williams P., Sears L.L., Allard A. Sleep problems in children with autism. J. Sleep Res. 2004;13(3):265–268. doi: 10.1111/j.1365-2869.2004.00405.x. [DOI] [PubMed] [Google Scholar]
- Galli-Carminati G., Deriaz N., Bertschy G. Melatonin in treatment of chronic sleep disorders in adults with autism: a retrospective study. Swiss Med. Wkly. 2009;139(19–20):293–296. doi: 10.4414/smw.2009.12342. [DOI] [PubMed] [Google Scholar]
- Garstang J., Wallis M. Randomized controlled trial of melatonin for children with autistic spectrum disorders and sleep problems. Child Care Health Dev. 2006;32(5):585–589. doi: 10.1111/j.1365-2214.2006.00616.x. [DOI] [PubMed] [Google Scholar]
- Gauthier J., Spiegelman D., Piton A., Lafrenière R.G., Laurent S., St-Onge J., Lapointe L., Hamdan F.F., Cossette P., Mottron L., Fombonne E., Joober R., Marineau C., Drapeau P., Rouleau G.A. Novel de novo SHANK3 mutation in autistic patients. Am. J. Med. Genet. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2009;150B(3):421–424. doi: 10.1002/ajmg.b.30822. [DOI] [PubMed] [Google Scholar]
- Giannotti F., Cortesi F., Cerquiglini A., Bernabei P. An open-label study of controlled-release melatonin in treatment of sleep disorders in children with autism. J. Autism Dev. Disord. 2006;36(6):741–752. doi: 10.1007/s10803-006-0116-z. [DOI] [PubMed] [Google Scholar]
- Giannotti F., Cortesi F., Cerquiglini A., Miraglia D., Vagnoni C., Sebastiani T., Bernabei P. An investigation of sleep characteristics, EEG abnormalities and epilepsy in developmentally regressed and non-regressed children with autism. J. Autism Dev. Disord. 2008;38(10):1888–1897. doi: 10.1007/s10803-008-0584-4. [DOI] [PubMed] [Google Scholar]
- Giannotti F., Cortesi F., Cerquiglini A., Vagnoni C., Valente D. Sleep in children with autism with and without autistic regression. J. Sleep Res. 2011;20(2):338–347. doi: 10.1111/j.1365-2869.2010.00882.x. [DOI] [PubMed] [Google Scholar]
- Gibson J.R., Bartley A.F., Hays S.A., Huber K.M. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J. Neurophysiol. 2008;100(5):2615–2626. doi: 10.1152/jn.90752.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardeau G., Benchenane K., Wiener S.I., Buzsáki G., Zugaro M.B. Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci. 2009;12(10):1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- Gobbi G., Comai S. Differential function of melatonin MT1 and MT2 receptors in REM and NREM sleep. Front. Endocrinol. 2019;10:87. doi: 10.3389/fendo.2019.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout R., Bergeron C., Limoges E., Stip E., Mottron L. A laboratory study of sleep in Asperger's syndrome. Neuroreport. 2000;11(1):127–130. doi: 10.1097/00001756-200001170-00025. [DOI] [PubMed] [Google Scholar]
- Goforth H.W., Rao M.S. Improvement in behaviour and attention in an autistic patient treated with ziprasidone. Aust. N. Z. J. Psychiatr. 2003;37(6):775–776. doi: 10.1080/j.1440-1614.2003.01279.x. [DOI] [PubMed] [Google Scholar]
- Goldman S.E., Surdyka K., Cuevas R., Adkins K., Wang L., Malow B.A. Defining the sleep phenotype in children with autism. Dev. Neuropsychol. 2009;34(5):560–573. doi: 10.1080/87565640903133509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S.E., Richdale A.L., Clemons T., Malow B.A. Parental sleep concerns in autism spectrum disorders: variations from childhood to adolescence. J. Autism Dev. Disord. 2012;42(4):531–538. doi: 10.1007/s10803-011-1270-5. [DOI] [PubMed] [Google Scholar]
- Goldman S.E., Adkins K.W., Calcutt M.W., Carter M.D., Goodpaster R.L., Wang L., Shi Y., Burgess H.J., Hachey D.L., Malow B.A. Melatonin in children with autism spectrum disorders: endogenous and pharmacokinetic profiles in relation to sleep. J. Autism Dev. Disord. 2014;44(10):2525–2535. doi: 10.1007/s10803-014-2123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S., Alder M., Burgess H., Corbett B., Hundley R., Wofford D., Fawkes D., Wang L., Laudenslager M., Malow B. Characterizing sleep in adolescents and adults with autism spectrum disorders. J. Autism Dev. Disord. 2017;47(6):1682–1695. doi: 10.1007/s10803-017-3089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubchik P., Sever J., Weizman A. Low-dose quetiapine for adolescents with autistic spectrum disorder and aggressive behavior: open-label trial. Clin. Neuropharmacol. 2011;34(6):216–219. doi: 10.1097/WNF.0b013e31823349ac. [DOI] [PubMed] [Google Scholar]
- Gonçalves J.T., Anstey J.E., Golshani P., Portera-Cailliau C. Circuit level defects in the developing neocortex of Fragile X mice. Nat. Neurosci. 2013;16(7):903–909. doi: 10.1038/nn.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlin-Jones B.L., Tang K., Liu J., Anders T.F. Sleep patterns in preschool-age children with autism, developmental delay, and typical development. J. Am. Acad. Child Adolesc. Psychiatr. 2008;47(8):930–938. doi: 10.1097/CHI.ObO13e3181799f7c. [DOI] [PubMed] [Google Scholar]
- Gould E.L., Loesch D.Z., Martin M.J., Hagerman R.J., Armstrong S.M., Huggins R.M. Melatonin profiles and sleep characteristics in boys with fragile X syndrome: a preliminary study. Am. J. Med. Genet. 2000;95(4):307–315. doi: 10.1002/1096-8628(20001211)95:4<307::AID-AJMG3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Gringras P., Gamble C., Jones A.P., Wiggs L., Williamson P.R., Sutcliffe A., Montgomery P., Whitehouse W.P., Choonara I., Allport T., Edmond A., Appleton R., MENDS Study Group. Melatonin for sleep problems in children with neurodevelopmental disorders: randomised double masked placebo controlled trial. Br. Med. J. 2012;345 doi: 10.1136/bmj.e6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringras P., Nir T., Breddy J., Frydman-Marom A., Findling R.L. Efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatr. 2017;56(11):948–957.e4. doi: 10.1016/j.jaac.2017.09.414. [DOI] [PubMed] [Google Scholar]
- Grissom N.M., McKee S.E., Schoch H., Bowman N., Havekes R., O'Brien W.T., Mahrt E., Siegel S., Commons K., Portfors C., Nickl-Jockschat T., Reyes T.M., Abel T. Male-specific deficits in natural reward learning in a mouse model of neurodevelopmental disorders. Mol. Psychiatr. 2018;23(3):544–555. doi: 10.1038/mp.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivas T.B., Savvidou O.D. Melatonin the “light of night” in human biology and adolescent idiopathic scoliosis. Scoliosis. 2007;2:6. doi: 10.1186/1748-7161-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Hamilton P.J., Reish N.J., Sweatt J.D., Miller C.A., Rumbaugh G. Reduced expression of the NMDA receptor-interacting protein SynGAP causes behavioral abnormalities that model symptoms of Schizophrenia. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2009;34(7):1659–1672. doi: 10.1038/npp.2008.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R., Hutchins J. Melatonin: a panacea for desperate parents? (Hype or truth) Arch. Dis. Child. 2005;90(9):986–987. doi: 10.1136/adc.2005.075218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberl M.G., Zerbi V., Veltien A., Ginger M., Heerschap A., Frick A. Structural-functional connectivity deficits of neocortical circuits in the Fmr1 (-/y) mouse model of autism. Sci. Adv. 2015;1(10) doi: 10.1126/sciadv.1500775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddjeri N., Blier P., de Montigny C. Noradrenergic modulation of central serotonergic neurotransmission: acute and long-term actions of mirtazapine. Int. Clin. Psychopharmacol. 1995;10(Suppl. 4):11–17. doi: 10.1097/00004850-199512004-00003. [DOI] [PubMed] [Google Scholar]
- Hagerman R.J., Ono M.Y., Hagerman P.J. Recent advances in fragile X: a model for autism and neurodegeneration. Curr. Opin. Psychiatr. 2005;18(5):490–496. doi: 10.1097/01.yco.0000179485.39520.b0. [DOI] [PubMed] [Google Scholar]
- Hall S.S., Jiang H., Reiss A.L., Greicius M.D. Identifying large-scale brain networks in fragile X syndrome. JAMA Psychiatr. 2013;70(11):1215–1223. doi: 10.1001/jamapsychiatry.2013.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton P.J., Campbell N.G., Sharma S., Erreger K., Herborg Hansen F., Saunders C., Belovich A.N., Sahai M.A., Cook E.H., Gether U., McHaourab H.S., Matthies H.J.G., Sutcliffe J.S., Galli A., NIH ARRA Autism Sequencing Consortium De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Mol. Psychiatr. 2013;18(12):1315–1323. doi: 10.1038/mp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson E., Nasir R.H., Fong A., Lian A., Hundley R., Shen Y., Wu B.-L., Holm I.A., Miller D.T., Clinicians, on B. of the 16p11 2 S. G Cognitive and behavioral characterization of 16p11.2 deletion syndrome. J. Dev. Behav. Pediatr. 2010;31(8):649–657. doi: 10.1097/DBP.0b013e3181ea50ed. [DOI] [PubMed] [Google Scholar]
- Hasegawa E., Miyasaka A., Sakurai K., Cherasse Y., Li Y., Sakurai T. Rapid eye movement sleep is initiated by basolateral amygdala dopamine signaling in mice. Science (New York, N.Y.) 2022;375(6584):994–1000. doi: 10.1126/science.abl6618. [DOI] [PubMed] [Google Scholar]
- Hayashi E. Effect of melatonin on sleep-wake rhythm: the sleep diary of an autistic male. Psychiatr. Clin. Neurosci. 2000;54(3):383–384. doi: 10.1046/j.1440-1819.2000.00725.x. [DOI] [PubMed] [Google Scholar]
- Hayat H., Regev N., Matosevich N., Sales A., Paredes-Rodriguez E., Krom A.J., Bergman L., Li Y., Lavigne M., Kremer E.J., Yizhar O., Pickering A.E., Nir Y. Locus coeruleus norepinephrine activity mediates sensory-evoked awakenings from sleep. Sci. Adv. 2020;6(15) doi: 10.1126/sciadv.aaz4232. eaaz4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays S.A., Huber K.M., Gibson J.R. Altered neocortical rhythmic activity states in Fmr1 KO mice are due to enhanced mGluR5 signaling and involve changes in excitatory circuitry. J. Neurosci.: The Official Journal of the Society for Neuroscience. 2011;31(40):14223–14234. doi: 10.1523/JNEUROSCI.3157-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell P. Drug therapy for attention-deficit/hyperactivity disorder-like symptoms in autistic disorder. J. Paediatr. Child Health. 2007;43(1–2):19–24. doi: 10.1111/j.1440-1754.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- Hergüner S. Excessive masturbation associated with olanzapine in a pediatric case. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2010;34(7):1349–1350. doi: 10.1016/j.pnpbp.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Hering E., Epstein R., Elroy S., Iancu D.R., Zelnik N. Sleep patterns in autistic children. J. Autism Dev. Disord. 1999;29(2):143–147. doi: 10.1023/a:1023092627223. [DOI] [PubMed] [Google Scholar]
- Hessl D., Rivera S.M., Reiss A.L. The neuroanatomy and neuroendocrinology of fragile X syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2004;10(1):17–24. doi: 10.1002/mrdd.20004. [DOI] [PubMed] [Google Scholar]
- Hettinger J.A., Liu X., Schwartz C.E., Michaelis R.C., Holden J.J.A. A DRD1 haplotype is associated with risk for autism spectrum disorders in male-only affected sib-pair families. Am. J. Med. Genet. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics. 2008;147B(5):628–636. doi: 10.1002/ajmg.b.30655. [DOI] [PubMed] [Google Scholar]
- Hirata I., Mohri I., Kato-Nishimura K., Tachibana M., Kuwada A., Kagitani-Shimono K., Ohno Y., Ozono K., Taniike M. Sleep problems are more frequent and associated with problematic behaviors in preschoolers with autism spectrum disorder. Res. Dev. Disabil. 2016;49–50:86–99. doi: 10.1016/j.ridd.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Hodge D., Carollo T.M., Lewin M., Hoffman C.D., Sweeney D.P. Sleep patterns in children with and without autism spectrum disorders: developmental comparisons. Res. Dev. Disabil. 2014;35(7):1631–1638. doi: 10.1016/j.ridd.2014.03.037. [DOI] [PubMed] [Google Scholar]
- Honomichl R.D., Goodlin-Jones B.L., Burnham M., Gaylor E., Anders T.F. Sleep patterns of children with pervasive developmental disorders. J. Autism Dev. Disord. 2002;32(6):553–561. doi: 10.1023/A:1021254914276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horev G., Ellegood J., Lerch J.P., Son Y.-E.E., Muthuswamy L., Vogel H., Krieger A.M., Buja A., Henkelman R.M., Wigler M., Mills A.A. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proc. Natl. Acad. Sci. U. S. A. 2011;108(41):17076–17081. doi: 10.1073/pnas.1114042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan J.P., Barnhill L.J. More on melatonin. J. Am. Acad. Child Adolesc. Psychiatr. 1997;36(8):1014. doi: 10.1097/00004583-199708000-00002. [DOI] [PubMed] [Google Scholar]
- Hranilovic D., Bujas-Petkovic Z., Vragovic R., Vuk T., Hock K., Jernej B. Hyperserotonemia in adults with autistic disorder. J. Autism Dev. Disord. 2007;37(10):1934–1940. doi: 10.1007/s10803-006-0324-6. [DOI] [PubMed] [Google Scholar]
- Humphreys J.S., Gringras P., Blair P.S., Scott N., Henderson J., Fleming P.J., Emond A.M. Sleep patterns in children with autistic spectrum disorders: a prospective cohort study. Arch. Dis. Child. 2014;99(2):114–118. doi: 10.1136/archdischild-2013-304083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzikowski C., Mills F.J., Glennard R. 5-Hydroxytryptamine-2 antagonist increases human slow wave sleep. Brain Res. 1986;378(1):164–168. doi: 10.1016/0006-8993(86)90299-4. [DOI] [PubMed] [Google Scholar]
- Ingiosi A.M., Schoch H., Wintler T., Singletary K.G., Righelli D., Roser L.G., Medina E., Risso D., Frank M.G., Peixoto L. Shank3 modulates sleep and expression of circadian transcription factors. Elife. 2019;8 doi: 10.7554/eLife.42819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingrassia A., Turk J. The use of clonidine for severe and intractable sleep problems in children with neurodevelopmental disorders—a case series. Eur. Child Adolesc. Psychiatr. 2005;14(1):34–40. doi: 10.1007/s00787-005-0424-4. [DOI] [PubMed] [Google Scholar]
- Irwanto Rehatta, N.M., Hartini S., Takada S. Sleep Problem of Children with Autistic Spectrum Disorder Assessed by Children Sleep Habits Questionnaire-Abbreviated in Indonesia and Japan. The Kobe Journal of Medical Sciences. 2016;62(2):E22–E26. [PMC free article] [PubMed] [Google Scholar]
- Ishitobi M., Hiratani M., Kosaka H., Takahashi T., Mizuno T., Asano M., Murata T., Tomoda A., Wada Y. Switching to aripiprazole in subjects with pervasive developmental disorders showing tolerability issues with risperidone. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2012;37(1):128–131. doi: 10.1016/j.pnpbp.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Ito H., Yanase M., Yamashita A., Kitabatake C., Hamada A., Suhara Y., Narita M., Ikegami D., Sakai H., Yamazaki M., Narita M. Analysis of sleep disorders under pain using an optogenetic tool: possible involvement of the activation of dorsal raphe nucleus-serotonergic neurons. Mol. Brain. 2013;6:59. doi: 10.1186/1756-6606-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I., Klein M., Green W.H., Coffey B. The challenges of psychopharmacological management of children with severe developmental disabilities. J. Child Adolesc. Psychopharmacol. 2006;16(6):793–799. doi: 10.1089/cap.2006.16.793. [DOI] [PubMed] [Google Scholar]
- Jacobs B.L. Single unit activity of locus coeruleus neurons in behaving animals. Prog. Neurobiol. 1986;27(2):183–194. doi: 10.1016/0301-0082(86)90008-0. [DOI] [PubMed] [Google Scholar]
- Jadhav S.P., Kemere C., German P.W., Frank L.M. Awake hippocampal sharp-wave ripples support spatial memory. Science (New York, N.Y.) 2012;336(6087):1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan J.E., Freeman R.D., Wasdell M.B., Bomben M.M. A child with severe night terrors and sleep-walking responds to melatonin therapy. Dev. Med. Child Neurol. 2004;46(11):789. doi: 10.1017/s0012162204231358. [DOI] [PubMed] [Google Scholar]
- Jaselskis C.A., Cook E.H., Fletcher K.E., Leventhal B.L. Clonidine treatment of hyperactive and impulsive children with autistic disorder. J. Clin. Psychopharmacol. 1992;12(5):322–327. [PubMed] [Google Scholar]
- John J., Wu M.-F., Boehmer L.N., Siegel J.M. Cataplexy-active neurons in the hypothalamus: implications for the role of histamine in sleep and waking behavior. Neuron. 2004;42(4):619–634. doi: 10.1016/s0896-6273(04)00247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamara D., De Boeck P., Lecavalier L., Neuhaus E., Beauchaine T.P. Characterizing sleep problems in 16p11.2 deletion and duplication. J. Autism Dev. Disord. 2021 doi: 10.1007/s10803-021-05311-2. [DOI] [PubMed] [Google Scholar]
- Kanney M.L., Durmer J.S., Trotti L.M., Leu R. Rethinking bedtime resistance in children with autism: is restless legs syndrome to blame? J. Clin. Sleep Med.: JCSM: Official Publication of the American Academy of Sleep Medicine. 2020;16(12):2029–2035. doi: 10.5664/jcsm.8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper S., Olivieri L., Di Loreto G., Dionisio P. A comparative, randomised, double-blind study of trazodone prolonged-release and paroxetine in the treatment of patients with major depressive disorder. Curr. Med. Res. Opin. 2005;21(8):1139–1146. doi: 10.1185/030079905X53243. [DOI] [PubMed] [Google Scholar]
- Kates W.R., Abrams M.T., Kaufmann W.E., Breiter S.N., Reiss A.L. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatr. Res. Neuroimaging. 1997;75(1):31–48. doi: 10.1016/S0925-4927(97)00019-X. [DOI] [PubMed] [Google Scholar]
- Kaufmann W.E., Cortell R., Kau A.S.M., Bukelis I., Tierney E., Gray R.M., Cox C., Capone G.T., Stanard P. Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. Am. J. Med. Genet. 2004;129A(3):225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Kawabe K., Horiuchi F., Oka Y., Ueno S.-I. The melatonin receptor agonist ramelteon effectively treats insomnia and behavioral symptoms in autistic disorder. Case Reports in Psychiatry. 2014 doi: 10.1155/2014/561071. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent J.M., Hough D., Singh J., Karcher K., Pandina G. An open-label extension study of the safety and efficacy of risperidone in children and adolescents with autistic disorder. J. Child Adolesc. Psychopharmacol. 2013;23(10):676–686. doi: 10.1089/cap.2012.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns C.M., Winder-Patel B., Iosif A.M., Nordahl C.W., Heath B., Solomon M., Amaral D.G. Clinically significant anxiety in children with autism spectrum disorder and varied intellectual functioning. J. Clin. Child Adolesc. Psychol.: The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division. 2021;50(6):780–795. doi: 10.1080/15374416.2019.1703712. 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan Z.P., Ferguson C.N., Jones R.M. alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54(2):146–165. doi: 10.1046/j.1365-2044.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- Kidd S.A., Lachiewicz A., Barbouth D., Blitz R.K., Delahunty C., McBrien D., Visootsak J., Berry-Kravis E. Fragile X syndrome: a review of associated medical problems. Pediatrics. 2014;134(5):995–1005. doi: 10.1542/peds.2013-4301. [DOI] [PubMed] [Google Scholar]
- Kikuchi T., Tottori K., Uwahodo Y., Hirose T., Miwa T., Oshiro Y., Morita S. 7-(4-[4-(2,3-Dichlorophenyl)-1-piperazinyl]butyloxy)-3,4-dihydro-2(1H)-quinolinone (OPC-14597), a new putative antipsychotic drug with both presynaptic dopamine autoreceptor agonistic activity and postsynaptic D2 receptor antagonistic activity. J. Pharmacol. Exp. Therapeut. 1995;274(1):329–336. [PubMed] [Google Scholar]
- Kim Y.S., Leventhal B.L., Koh Y.-J., Fombonne E., Laska E., Lim E.-C., Cheon K.-A., Kim S.-J., Kim Y.-K., Lee H., Song D.-H., Grinker R.R. Prevalence of autism spectrum disorders in a total population sample. Am. J. Psychiatr. 2011;168(9):904–912. doi: 10.1176/appi.ajp.2011.10101532. [DOI] [PubMed] [Google Scholar]
- Kjaerby C., Andersen M., Hauglund N., Untiet V., Dall C., Sigurdsson B., Ding F., Feng J., Li Y., Weikop P., Hirase H., Nedergaard M. Memory-enhancing properties of sleep depend on the oscillatory amplitude of norepinephrine. Nat. Neurosci. 2022;25(8):1059–1070. doi: 10.1038/s41593-022-01102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak P., Goodlin-Jones B., Hertz-Picciotto I., Croen L.A., Hansen R.L. Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: a population-based study. J. Sleep Res. 2008;17(2):197–206. doi: 10.1111/j.1365-2869.2008.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger D., Absi G., Gagliardi C., Bandaru S.S., Madara J.C., Ferrari L.L., Arrigoni E., Münzberg H., Scammell T.E., Saper C.B., Vetrivelan R. Galanin neurons in the ventrolateral preoptic area promote sleep and heat loss in mice. Nat. Commun. 2018;9(1):4129. doi: 10.1038/s41467-018-06590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronk R., Dahl R., Noll R. Caregiver reports of sleep problems on a convenience sample of children with fragile X syndrome. Am. J. Intellect. Dev. Disabil. 2009;114(6):383–392. doi: 10.1352/1944-7588-114.6.383. [DOI] [PubMed] [Google Scholar]
- Kronk R., Bishop E.E., Raspa M., Bickel J.O., Mandel D.A., Bailey D.B. Prevalence, nature, and correlates of sleep problems among children with fragile X syndrome based on a large scale parent survey. Sleep. 2010;33(5):679–687. doi: 10.1093/sleep/33.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R.A., KaraMohamed S., Sudi J., Conrad D.F., Brune C., Badner J.A., Gilliam T.C., Nowak N.J., Cook E.H., Dobyns W.B., Christian S.L. Recurrent 16p11.2 microdeletions in autism. Hum. Mol. Genet. 2008;17(4):628–638. doi: 10.1093/hmg/ddm376. [DOI] [PubMed] [Google Scholar]
- Lacoste B., Angeloni D., Dominguez-Lopez S., Calderoni S., Mauro A., Fraschini F., Descarries L., Gobbi G. Anatomical and cellular localization of melatonin MT1 and MT2 receptors in the adult rat brain. J. Pineal Res. 2015;58(4):397–417. doi: 10.1111/jpi.12224. [DOI] [PubMed] [Google Scholar]
- Lake C.R., Ziegler M.G., Murphy D.L. Increased norepinephrine levels and decreased dopamine-beta-hydroxylase activity in primary autism. Arch. Gen. Psychiatr. 1977;34(5):553–556. doi: 10.1001/archpsyc.1977.01770170063005. [DOI] [PubMed] [Google Scholar]
- Leblond C.S., Nava C., Polge A., Gauthier J., Huguet G., Lumbroso S., Giuliano F., Stordeur C., Depienne C., Mouzat K., Pinto D., Howe J., Lemière N., Durand C.M., Guibert J., Ey E., Toro R., Peyre H., Mathieu A., et al. Meta-analysis of shank mutations in autism spectrum disorders: a gradient of severity in cognitive impairments. PLoS Genet. 2014;10(9) doi: 10.1371/journal.pgen.1004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecci S., Fernandez L.M.J., Weber F.D., Cardis R., Chatton J.-Y., Born J., Lüthi A. Coordinated infraslow neural and cardiac oscillations mark fragility and offline periods in mammalian sleep. Sci. Adv. 2017;3(2) doi: 10.1126/sciadv.1602026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Kim H., Kim J.-E., Park J.-Y., Choi J., Lee J.-E., Lee E.-H., Han P.-L. Excessive D1 dopamine receptor activation in the dorsal striatum promotes autistic-like behaviors. Mol. Neurobiol. 2018;55(7):5658–5671. doi: 10.1007/s12035-017-0770-5. [DOI] [PubMed] [Google Scholar]
- Léna I., Parrot S., Deschaux O., Muffat-Joly S., Sauvinet V., Renaud B., Suaud-Chagny M.-F., Gottesmann C. Variations in extracellular levels of dopamine, noradrenaline, glutamate, and aspartate across the sleep—wake cycle in the medial prefrontal cortex and nucleus accumbens of freely moving rats. J. Neurosci. Res. 2005;81(6):891–899. doi: 10.1002/jnr.20602. [DOI] [PubMed] [Google Scholar]
- Lerman J.A., Kaitin K.I., Dement W.C., Peroutka S.J. The effects of buspirone on sleep in the rat. Neurosci. Lett. 1986;72(1):64–68. doi: 10.1016/0304-3940(86)90619-1. [DOI] [PubMed] [Google Scholar]
- Levy D., Ronemus M., Yamrom B., Lee Y., Leotta A., Kendall J., Marks S., Lakshmi B., Pai D., Ye K., Buja A., Krieger A., Yoon S., Troge J., Rodgers L., Iossifov I., Wigler M. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70(5):886–897. doi: 10.1016/j.neuron.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Liang Y., Shi W., Xiang A., Hu D., Wang L., Zhang L. The NAergic locus coeruleus-ventrolateral preoptic area neural circuit mediates rapid arousal from sleep. Curr. Biol. 2021;31(17):3729–3742.e5. doi: 10.1016/j.cub.2021.06.031. [DOI] [PubMed] [Google Scholar]
- Limoges E., Mottron L., Bolduc C., Berthiaume C., Godbout R. Atypical sleep architecture and the autism phenotype. Brain: J. Neurol. 2005;128(Pt 5):1049–1061. doi: 10.1093/brain/awh425. [DOI] [PubMed] [Google Scholar]
- Linday L.A., Tsiouris J.A., Cohen I.L., Shindledecker R., DeCresce R. Famotidine treatment of children with autistic spectrum disorders: pilot research using single subject research design. J. Neural. Transm. 2001;108(5):593–611. doi: 10.1007/s007020170059. [DOI] [PubMed] [Google Scholar]
- Linke A.C., Chen B., Olson L., Ibarra C., Fong C., Reynolds S., Apostol M., Kinnear M., Müller R.-A., Fishman I. Sleep problems in preschoolers with autism spectrum disorder are associated with sensory sensitivities and thalamocortical overconnectivity. Biological psychiatry. Cognitive Neuroscience and Neuroimaging. 2021;(21):S2451–S9022. doi: 10.1016/j.bpsc.2021.07.008. 00202-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Hubbard J.A., Fabes R.A., Adam J.B. Sleep disturbances and correlates of children with autism spectrum disorders. Child Psychiatr. Hum. Dev. 2006;37(2):179–191. doi: 10.1007/s10578-006-0028-3. [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhou J., Li Y., Hu F., Lu Y., Ma M., Feng Q., Zhang J.-E., Wang D., Zeng J., Bao J., Kim J.-Y., Chen Z.-F., El Mestikawy S., Luo M. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron. 2014;81(6):1360–1374. doi: 10.1016/j.neuron.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London E.B. Neuromodulation and a reconceptualization of autism spectrum disorders: using the locus coeruleus functioning as an exemplar. Front. Neurol. 2018;9:1120. doi: 10.3389/fneur.2018.01120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J.S., Gay S.M., Harper K.M., Nikolova V.D., Smith K.M., Moy S.S., Diering G.H. Early life sleep disruption potentiates lasting sex-specific changes in behavior in genetically vulnerable Shank3 heterozygous autism model mice. Mol. Autism. 2022;13(1):35. doi: 10.1186/s13229-022-00514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H.-C., Pollack H., Lefante J.J., Mills A.A., Tian D. Altered sleep architecture, rapid eye movement sleep, and neural oscillation in a mouse model of human chromosome 16p11.2 microdeletion. Sleep. 2018;42(3):zsy253. doi: 10.1093/sleep/zsy253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydic R., McCarley R.W., Hobson J.A. Serotonin neurons and sleep. II. Time course of dorsal raphe discharge, PGO waves, and behavioral states. Arch. Ital. Biol. 1987;126(1):1–28. [PubMed] [Google Scholar]
- Lynch J.F., Ferri S.L., Angelakos C., Schoch H., Nickl-Jockschat T., Gonzalez A., O'Brien W.T., Abel T. Comprehensive behavioral phenotyping of a 16p11.2 del mouse model for neurodevelopmental disorders. Autism Res.: Official Journal of the International Society for Autism Research. 2020;13(10):1670–1684. doi: 10.1002/aur.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDuffie K.E., Munson J., Greenson J., Ward T.M., Rogers S.J., Dawson G., Estes A. Sleep problems and trajectories of restricted and repetitive behaviors in children with neurodevelopmental disabilities. J. Autism Dev. Disord. 2020;50(11):3844–3856. doi: 10.1007/s10803-020-04438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDuffie K.E., Shen M.D., Dager S.R., Styner M.A., Kim S.H., Paterson S., Pandey J., St John T., Elison J.T., Wolff J.J., Swanson M.R., Botteron K.N., Zwaigenbaum L., Piven J., Estes A.M. Sleep onset problems and subcortical development in infants later diagnosed with autism spectrum disorder. Am. J. Psychiatr. 2020;177(6):518–525. doi: 10.1176/appi.ajp.2019.19060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkonen I., Riikonen R., Kokki H., Airaksinen M.M., Kuikka J.T. Serotonin and dopamine transporter binding in children with autism determined by SPECT. Dev. Med. Child Neurol. 2008;50(8):593–597. doi: 10.1111/j.1469-8749.2008.03027.x. [DOI] [PubMed] [Google Scholar]
- Malow B.A., Marzec M.L., McGrew S.G., Wang L., Henderson L.M., Stone W.L. Characterizing sleep in children with autism spectrum disorders: a multidimensional approach. Sleep. 2006;29(12):1563–1571. doi: 10.1093/sleep/29.12.1563. [DOI] [PubMed] [Google Scholar]
- Malow B.A., Byars K., Johnson K., Weiss S., Bernal P., Goldman S.E., Panzer R., Coury D.L., Glaze D.G., Sleep Committee of the Autism Treatment Network A practice pathway for the identification, evaluation, and management of insomnia in children and adolescents with autism spectrum disorders. Pediatrics. 2012;130(Suppl. 2):S106–S124. doi: 10.1542/peds.2012-0900I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malow B., Adkins K.W., McGrew S.G., Wang L., Goldman S.E., Fawkes D., Burnette C. Melatonin for sleep in children with autism: a controlled trial examining dose, tolerability, and outcomes. J. Autism Dev. Disord. 2012;42(8):1729–1737. doi: 10.1007/s10803-011-1418-3. author reply 1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malow B.A., Findling R.L., Schroder C.M., Maras A., Breddy J., Nir T., Zisapel N., Gringras P. Sleep, growth, and puberty after 2 Years of prolonged-release melatonin in children with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatr. 2021;60(2):252–261.e3. doi: 10.1016/j.jaac.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maras A., Schroder C.M., Malow B.A., Findling R.L., Breddy J., Nir T., Shahmoon S., Zisapel N., Gringras P. Long-term efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J. Child Adolesc. Psychopharmacol. 2018;28(10):699–710. doi: 10.1089/cap.2018.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariggiò M.A., Palumbi R., Vinella A., Laterza R., Petruzzelli M.G., Peschechera A., Gabellone A., Gentile O., Vincenti A., Margari L. DRD1 and DRD2 receptor polymorphisms: genetic neuromodulation of the dopaminergic system as a risk factor for ASD, ADHD and ASD/ADHD overlap. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.705890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C.R., Noor A., Vincent J.B., Lionel A.C., Feuk L., Skaug J., Shago M., Moessner R., Pinto D., Ren Y., Thiruvahindrapduram B., Fiebig A., Schreiber S., Friedman J., Ketelaars C.E.J., Vos Y.J., Ficicioglu C., Kirkpatrick S., Nicolson R., et al. Structural variation of chromosomes in autism spectrum disorder. Am. J. Hum. Genet. 2008;82(2):477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Cayuelas E., Gavela-Pérez T., Rodrigo-Moreno M., Losada-Del Pozo R., Moreno-Vinues B., Garces C., Soriano-Guillén L. Sleep problems, circadian rhythms, and their relation to behavioral difficulties in children and adolescents with autism spectrum disorder. J. Autism Dev. Disord. 2023:1–15. doi: 10.1007/s10803-023-05934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruani A., Dumas G., Beggiato A., Traut N., Peyre H., Cohen-Freoua A., Amsellem F., Elmaleh M., Germanaud D., Launay J.-M., Bourgeron T., Toro R., Delorme R. Morning plasma melatonin differences in autism: beyond the impact of pineal gland volume. Front. Psychiatr. 2019;10:11. doi: 10.3389/fpsyt.2019.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawe G.M., Hoffman J.M. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013;10(8):473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May T., Cornish K., Conduit R., Rajaratnam S.M.W., Rinehart N.J. Sleep in high-functioning children with autism: longitudinal developmental change and associations with behavior problems. Behav. Sleep Med. 2015;13(1):2–18. doi: 10.1080/15402002.2013.829064. [DOI] [PubMed] [Google Scholar]
- Mazurek M.O., Petroski G.F. Sleep problems in children with autism spectrum disorder: examining the contributions of sensory over-responsivity and anxiety. Sleep Med. 2015;16(2):270–279. doi: 10.1016/j.sleep.2014.11.006. [DOI] [PubMed] [Google Scholar]
- McArthur A.J., Budden S.S. Sleep dysfunction in Rett syndrome: a trial of exogenous melatonin treatment. Dev. Med. Child Neurol. 1998;40(3):186–192. doi: 10.1111/j.1469-8749.1998.tb15445.x. [DOI] [PubMed] [Google Scholar]
- McCall J.G., Al-Hasani R., Siuda E.R., Hong D.Y., Norris A.J., Ford C.P., Bruchas M.R. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87(3):605–620. doi: 10.1016/j.neuron.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle C.J., Thom R.P., Ravichandran C.T., Palumbo M.L., Politte L.C., Mullett J.E., Keary C.J., Erickson C.A., Stigler K.A., Mathieu-Frasier L., Posey D.J. A randomized double-blind, placebo-controlled pilot trial of mirtazapine for anxiety in children and adolescents with autism spectrum disorder. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2022;47(6):1263–1270. doi: 10.1038/s41386-022-01295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane H.G., Kusek G.K., Yang M., Phoenix J.L., Bolivar V.J., Crawley J.N. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Gene Brain Behav. 2008;7(2):152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- McGinty D.J., Harper R.M. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101(3):569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- Medina E., Schoch H., Ford K., Wintler T., Singletary K.G., Peixoto L. Shank3 influences mammalian sleep development. J. Neurosci. Res. 2022;100(12):2174–2186. doi: 10.1002/jnr.25119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melke J., Goubran Botros H., Chaste P., Betancur C., Nygren G., Anckarsäter H., Rastam M., Ståhlberg O., Gillberg I.C., Delorme R., Chabane N., Mouren-Simeoni M.-C., Fauchereau F., Durand C.M., Chevalier F., Drouot X., Collet C., Launay J.-M., Leboyer M., et al. Abnormal melatonin synthesis in autism spectrum disorders. Mol. Psychiatr. 2008;13(1):90–98. doi: 10.1038/sj.mp.4002016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merenstein D., Diener-West M., Halbower A.C., Krist A., Rubin H.R. The trial of infant response to diphenhydramine: the TIRED study--a randomized, controlled, patient-oriented trial. Arch. Pediatr. Adolesc. Med. 2006;160(7):707–712. doi: 10.1001/archpedi.160.7.707. [DOI] [PubMed] [Google Scholar]
- Merikanto I., Kuula L., Makkonen T., Salmela L., Räikkönen K., Pesonen A.-K. Autistic traits are associated with decreased activity of fast sleep spindles during adolescence. J. Clin. Sleep Med.: JCSM: Official Publication of the American Academy of Sleep Medicine. 2019;15(3):401–407. doi: 10.5664/jcsm.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyza K.Z., Blanchard D.C. The BTBR mouse model of idiopathic autism—current view on mechanisms. Neurosci. Biobehav. Rev. 2017;76(Pt A):99–110. doi: 10.1016/j.neubiorev.2016.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miano S., Bruni O., Elia M., Trovato A., Smerieri A., Verrillo E., Roccella M., Terzano M.G., Ferri R. Sleep in children with autistic spectrum disorder: a questionnaire and polysomnographic study. Sleep Med. 2007;9(1):64–70. doi: 10.1016/j.sleep.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Miano S., Bruni O., Elia M., Scifo L., Smerieri A., Trovato A., Verrillo E., Terzano M.G., Ferri R. Sleep phenotypes of intellectual disability: a polysomnographic evaluation in subjects with Down syndrome and Fragile-X syndrome. Clin. Neurophysiol.: Official Journal of the International Federation of Clinical Neurophysiology. 2008;119(6):1242–1247. doi: 10.1016/j.clinph.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Mignot C., von Stülpnagel C., Nava C., Ville D., Sanlaville D., Lesca G., Rastetter A., Gachet B., Marie Y., Korenke G.C., Borggraefe I., Hoffmann-Zacharska D., Szczepanik E., Rudzka-Dybała M., Yiş U., Çağlayan H., Isapof A., Marey I., Panagiotakaki E., et al. Genetic and neurodevelopmental spectrum of SYNGAP1-associated intellectual disability and epilepsy. J. Med. Genet. 2016;53(8):511–522. doi: 10.1136/jmedgenet-2015-103451. [DOI] [PubMed] [Google Scholar]
- Miller J.D., Farber J., Gatz P., Roffwarg H., German D.C. Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and walking in the rat. Brain Res. 1983;273(1):133–141. doi: 10.1016/0006-8993(83)91101-0. [DOI] [PubMed] [Google Scholar]
- Ming X., Gordon E., Kang N., Wagner G.C. Use of clonidine in children with autism spectrum disorders. Brain & Dev. 2008;30(7):454–460. doi: 10.1016/j.braindev.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Mitchell E.J., Thomson D.M., Openshaw R.L., Bristow G.C., Dawson N., Pratt J.A., Morris B.J. Drug-responsive autism phenotypes in the 16p11.2 deletion mouse model: a central role for gene-environment interactions. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-69130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto A., Oki J., Takahashi S., Okuno A. Serum melatonin kinetics and long-term melatonin treatment for sleep disorders in Rett syndrome. Brain & Dev. 1999;21(1):59–62. doi: 10.1016/s0387-7604(98)00072-2. [DOI] [PubMed] [Google Scholar]
- Moessner R., Marshall C.R., Sutcliffe J.S., Skaug J., Pinto D., Vincent J., Zwaigenbaum L., Fernandez B., Roberts W., Szatmari P., Scherer S.W. Contribution of SHANK3 mutations to autism spectrum disorder. Am. J. Hum. Genet. 2007;81(6):1289–1297. doi: 10.1086/522590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenhuis R.T., Hutten L., Kas M.J.H. Histamine H3 receptor antagonism modulates autism-like hyperactivity but not repetitive behaviors in BTBR T+Itpr3tf/J inbred mice. Pharmacol. Biochem. Behav. 2022;212 doi: 10.1016/j.pbb.2021.173304. [DOI] [PubMed] [Google Scholar]
- Molinoff P.B. Alpha- and beta-adrenergic receptor subtypes properties, distribution and regulation. Drugs. 1984;28(Suppl. 2):1–15. doi: 10.2165/00003495-198400282-00002. [DOI] [PubMed] [Google Scholar]
- Molnár K., Kéri S. Bigger is better and worse: on the intricate relationship between hippocampal size and memory. Neuropsychologia. 2014;56:73–78. doi: 10.1016/j.neuropsychologia.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Montanari G., Schiaulini P., Covre A., Steffan A., Furlanut M. Niaprazine vs chlordesmethyldiazepam in sleep disturbances in pediatric outpatients. Pharmacol. Res. 1992;25(Suppl. 1):83–84. doi: 10.1016/1043-6618(92)90551-l. [DOI] [PubMed] [Google Scholar]
- Monti J.M., Jantos H. Dose-dependent effects of the 5-HT1A receptor agonist 8-OH-DPAT on sleep and wakefulness in the rat. J. Sleep Res. 1992;1(3):169–175. doi: 10.1111/j.1365-2869.1992.tb00033.x. [DOI] [PubMed] [Google Scholar]
- Monti J.M., Jantos H. The effects of systemic administration and local microinjection into the central nervous system of the selective serotonin 5-HT2C receptor agonist RO-600175 on sleep and wakefulness in the rat. Behav. Pharmacol. 2015;26(5):418–426. doi: 10.1097/FBP.0000000000000142. [DOI] [PubMed] [Google Scholar]
- Monti J.M., Monti D. The involvement of dopamine in the modulation of sleep and waking. Sleep Med. Rev. 2007;11(2):113–133. doi: 10.1016/j.smrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Monti J.M., Jantos H., Silveira R., Reyes-Parada M., Scorza C. Sleep and waking in 5,7-DHT-lesioned or (-)-pindolol-pretreated rats after administration of buspirone, ipsapirone, or gepirone. Pharmacol. Biochem. Behav. 1995;52(2):305–312. doi: 10.1016/0091-3057(94)00414-e. [DOI] [PubMed] [Google Scholar]
- Monti J.M., Jantos H., Schechter L.E. The effects of systemic and local microinjection into the central nervous system of the selective serotonin 5-HT6 receptor agonist WAY-208466 on sleep and wakefulness in the rat. Behav. Brain Res. 2013;249:65–74. doi: 10.1016/j.bbr.2013.04.024. [DOI] [PubMed] [Google Scholar]
- Muhia M., Yee B.K., Feldon J., Markopoulos F., Knuesel I. Disruption of hippocampus-regulated behavioural and cognitive processes by heterozygous constitutive deletion of SynGAP. Eur. J. Neurosci. 2010;31(3):529–543. doi: 10.1111/j.1460-9568.2010.07079.x. [DOI] [PubMed] [Google Scholar]
- Muhia M., Willadt S., Yee B.K., Feldon J., Paterna J.-C., Schwendener S., Vogt K., Kennedy M.B., Knuesel I. Molecular and behavioral changes associated with adult hippocampus-specific SynGAP1 knockout. Learn. Mem. 2012;19(7):268–281. doi: 10.1101/lm.026351.112. [DOI] [PubMed] [Google Scholar]
- Mulder E.J., Anderson G.M., Kema I.P., de Bildt A., van Lang N.D.J., den Boer J.A., Minderaa R.B. Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. J. Am. Acad. Child Adolesc. Psychiatr. 2004;43(4):491–499. doi: 10.1097/00004583-200404000-00016. [DOI] [PubMed] [Google Scholar]
- Muller C.L., Anacker A.M.J., Veenstra-VanderWeele J. The serotonin system in autism spectrum disorder: from biomarker to animal models. Neuroscience. 2016;321:24–41. doi: 10.1016/j.neuroscience.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munizza C., Olivieri L., Di Loreto G., Dionisio P. A comparative, randomized, double-blind study of trazodone prolonged-release and sertraline in the treatment of major depressive disorder. Curr. Med. Res. Opin. 2006;22(9):1703–1713. doi: 10.1185/030079906X121039. [DOI] [PubMed] [Google Scholar]
- Musumeci S.A., Colognola R.M., Ferri R., Gigli G.L., Petrella M.A., Sanfilippo S., Bergonzi P., Tassinari C.A. Fragile-X syndrome: a particular epileptogenic EEG pattern. Epilepsia. 1988;29(1):41–47. doi: 10.1111/j.1528-1157.1988.tb05096.x. [DOI] [PubMed] [Google Scholar]
- Musumeci S.A., Ferri R., Elia M., Dal Gracco S., Scuderi C., Stefanini M.C., Castano A., Azan G. Sleep neurophysiology in fragile X patients. Dev. Brain Dysfunct. 1995;8(4–6):218–222. (Scopus) [Google Scholar]
- Musumeci S.A., Hagerman R.J., Ferri R., Bosco P., Dalla Bernardina B., Tassinari C.A., De Sarro G.B., Elia M. Epilepsy and EEG findings in males with fragile X syndrome. Epilepsia. 1999;40(8):1092–1099. doi: 10.1111/j.1528-1157.1999.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Musumeci S.A., Bosco P., Calabrese G., Bakker C., De Sarro G.B., Elia M., Ferri R., Oostra B.A. Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia. 2000;41(1):19–23. doi: 10.1111/j.1528-1157.2000.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Mutluer T., Karakoc Demirkaya S., Abali O. Assessment of sleep problems and related risk factors observed in Turkish children with Autism spectrum disorders. Autism Res.: Official Journal of the International Society for Autism Research. 2016;9(5):536–542. doi: 10.1002/aur.1542. [DOI] [PubMed] [Google Scholar]
- Mylonas D., Machado S., Larson O., Patel R., Cox R., Vangel M., Maski K., Stickgold R., Manoach D.S. Dyscoordination of non-rapid eye movement sleep oscillations in autism spectrum disorder. Sleep. 2022;45(3) doi: 10.1093/sleep/zsac010. zsac010. [DOI] [PubMed] [Google Scholar]
- Naguy A., Alrashidi F., AlShalabi S.R. Mirtazapine for inappropriate sexual behaviors in autism. Am. J. Therapeut. 2019;26(6):e751–e753. doi: 10.1097/MJT.0000000000000908. [DOI] [PubMed] [Google Scholar]
- Naisbitt S., Kim E., Tu J.C., Xiao B., Sala C., Valtschanoff J., Weinberg R.J., Worley P.F., Sheng M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23(3):569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- Nakajima R., Takao K., Hattori S., Shoji H., Komiyama N.H., Grant S.G.N., Miyakawa T. Comprehensive behavioral analysis of heterozygous Syngap1 knockout mice. Neuropsychopharmacology Reports. 2019;39(3):223–237. doi: 10.1002/npr2.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Sekine Y., Ouchi Y., Tsujii M., Yoshikawa E., Futatsubashi M., Tsuchiya K.J., Sugihara G., Iwata Y., Suzuki K., Matsuzaki H., Suda S., Sugiyama T., Takei N., Mori N. Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Arch. Gen. Psychiatr. 2010;67(1):59–68. doi: 10.1001/archgenpsychiatry.2009.137. [DOI] [PubMed] [Google Scholar]
- Nguyen M., Murphy T. Mirtazapine for excessive masturbation in an adolescent with autism. J. Am. Acad. Child Adolesc. Psychiatr. 2001;40(8):868–869. doi: 10.1097/00004583-200108000-00004. [DOI] [PubMed] [Google Scholar]
- Nguyen M., Roth A., Kyzar E.J., Poudel M.K., Wong K., Stewart A.M., Kalueff A.V. Decoding the contribution of dopaminergic genes and pathways to autism spectrum disorder (ASD) Neurochem. Int. 2014;66:15–26. doi: 10.1016/j.neuint.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Nicholson A.N., Pascoe P.A. Presynaptic alpha 2-adrenoceptor function and sleep in man: studies with clonidine and idazoxan. Neuropharmacology. 1991;30(4):367–372. doi: 10.1016/0028-3908(91)90062-g. [DOI] [PubMed] [Google Scholar]
- Nierenberg A.A., Adler L.A., Peselow E., Zornberg G., Rosenthal M. Trazodone for antidepressant-associated insomnia. Am. J. Psychiatr. 1994;151(7):1069–1072. doi: 10.1176/ajp.151.7.1069. [DOI] [PubMed] [Google Scholar]
- Oblak A., Gibbs T.T., Blatt G.J. Reduced serotonin receptor subtypes in a limbic and a neocortical region in autism. Autism Res.: Official Journal of the International Society for Autism Research. 2013;6(6):571–583. doi: 10.1002/aur.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Sanchez R., Comai S., Lacoste B., Bambico F.R., Dominguez-Lopez S., Spadoni G., Rivara S., Bedini A., Angeloni D., Fraschini F., Mor M., Tarzia G., Descarries L., Gobbi G. Promotion of non-rapid eye movement sleep and activation of reticular thalamic neurons by a novel MT2 melatonin receptor ligand. J. Neurosci.: The Official Journal of the Society for Neuroscience. 2011;31(50):18439–18452. doi: 10.1523/JNEUROSCI.2676-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Sanchez R., Comai S., Spadoni G., Bedini A., Tarzia G., Gobbi G. Melatonin, selective and non-selective MT1/MT2 receptors agonists: differential effects on the 24-h vigilance states. Neurosci. Lett. 2014;561:156–161. doi: 10.1016/j.neulet.2013.12.069. [DOI] [PubMed] [Google Scholar]
- Oikonomou G., Altermatt M., Zhang R.-W., Coughlin G.M., Montz C., Gradinaru V., Prober D.A. The serotonergic raphe promote sleep in zebrafish and mice. Neuron. 2019;103(4):686–701.e8. doi: 10.1016/j.neuron.2019.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi Y., Suzuki Y., Takahashi K., Yonezawa T., Kanda T., Takata Y., Cherasse Y., Lazarus M. Activation of ventral tegmental area dopamine neurons produces wakefulness through dopamine D2-like receptors in mice. Brain Struct. Funct. 2017;222(6):2907–2915. doi: 10.1007/s00429-017-1365-7. [DOI] [PubMed] [Google Scholar]
- Osorio-Forero A., Cardis R., Vantomme G., Guillaume-Gentil A., Katsioudi G., Devenoges C., Fernandez L.M.J., Lüthi A. Noradrenergic circuit control of non-REM sleep substates. Curr. Biol. 2021;31(22):5009–5023.e7. doi: 10.1016/j.cub.2021.09.041. [DOI] [PubMed] [Google Scholar]
- Ottaviano S., Giannotti F., Cortesi F. The effect of niaprazine on some common sleep disorders in children. A double-blind clinical trial by means of continuous home-videorecorded sleep. Child’s Nerv. Syst.: ChNS: Official Journal of the International Society for Pediatric Neurosurgery. 1991;7(6):332–335. doi: 10.1007/BF00304832. [DOI] [PubMed] [Google Scholar]
- Owens J.A., Rosen C.L., Mindell J.A. Medication use in the treatment of pediatric insomnia: results of a survey of community-based pediatricians. Pediatrics. 2003;111(5 Pt 1):e628–e635. doi: 10.1542/peds.111.5.e628. [DOI] [PubMed] [Google Scholar]
- Ozkan E.D., Creson T.K., Kramár E.A., Rojas C., Seese R.R., Babyan A.H., Shi Y., Lucero R., Xu X., Noebels J.L., Miller C.A., Lynch G., Rumbaugh G. Reduced cognition in Syngap1 mutants is caused by isolated damage within developing forebrain excitatory neurons. Neuron. 2014;82(6):1317–1333. doi: 10.1016/j.neuron.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell W.T., Warren S.T. A decade of molecular studies of fragile X syndrome. Annu. Rev. Neurosci. 2002;25:315–338. doi: 10.1146/annurev.neuro.25.112701.142909. [DOI] [PubMed] [Google Scholar]
- O’hare J.P., O’brien I.a.D., Arendt J., Astley P., Ratcliffe W., Andrews H., Walters R., Corrall R.J.M. Does melatonin deficiency cause the enlarged genitalia of the fragile-X syndrome? Clin. Endocrinol. 1986;24(3):327–333. doi: 10.1111/j.1365-2265.1986.tb03274.x. [DOI] [PubMed] [Google Scholar]
- Pandi-Perumal S.R., Smits M., Spence W., Srinivasan V., Cardinali D.P., Lowe A.D., Kayumov L. Dim light melatonin onset (DLMO): a tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2007;31(1):1–11. doi: 10.1016/j.pnpbp.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Panula P., Yang H.Y., Costa E. Histamine-containing neurons in the rat hypothalamus. Proc. Natl. Acad. Sci. U. S. A. 1984;81(8):2572–2576. doi: 10.1073/pnas.81.8.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzini C.M., Ehlinger D.G., Alchahin A.M., Guo Y., Commons K.G. 16p11.2 deletion syndrome mice perseverate with active coping response to acute stress—rescue by blocking 5-HT2A receptors. J. Neurochem. 2017;143(6):708–721. doi: 10.1111/jnc.14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R.A., Hartman E.E. An 8-year-old boy with autism, 1 year later. JAMA. 2002;287(4):504. doi: 10.1001/jama.287.4.504. [DOI] [PubMed] [Google Scholar]
- Parker M.J., Fryer A.E., Shears D.J., Lachlan K.L., McKee S.A., Magee A.C., Mohammed S., Vasudevan P.C., Park S.-M., Benoit V., Lederer D., Maystadt I., Study D., FitzPatrick D.R. De novo, heterozygous, loss-of-function mutations in SYNGAP1 cause a syndromic form of intellectual disability. Am. J. Med. Genet. 2015;167A(10):2231–2237. doi: 10.1002/ajmg.a.37189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier R., Zhao Y., Perier M., Akaoka H., Lintunen M., Hou Y., Panula P., Watanabe T., Franco P., Lin J.-S. Role of histamine H1-receptor on behavioral states and wake maintenance during deficiency of a brain activating system: a study using a knockout mouse model. Neuropharmacology. 2016;106:20–34. doi: 10.1016/j.neuropharm.2015.12.014. [DOI] [PubMed] [Google Scholar]
- Patzold L.M., Richdale A.L., Tonge B.J. An investigation into sleep characteristics of children with autism and Asperger's Disorder. J. Paediatr. Child Health. 1998;34(6):528–533. doi: 10.1046/j.1440-1754.1998.00291.x. [DOI] [PubMed] [Google Scholar]
- Paul I.M., Yoder K.E., Crowell K.R., Shaffer M.L., McMillan H.S., Carlson L.C., Dilworth D.A., Berlin C.M. Effect of dextromethorphan, diphenhydramine, and placebo on nocturnal cough and sleep quality for coughing children and their parents. Pediatrics. 2004;114(1):e85–e90. doi: 10.1542/peds.114.1.e85. [DOI] [PubMed] [Google Scholar]
- Pavăl D. A dopamine hypothesis of autism spectrum disorder. Dev. Neurosci. 2017;39(5):355–360. doi: 10.1159/000478725. [DOI] [PubMed] [Google Scholar]
- Peça J., Feliciano C., Ting J.T., Wang W., Wells M.F., Venkatraman T.N., Lascola C.D., Fu Z., Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472(7344):437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelayo R., Dubik M. Pediatric sleep pharmacology. Semin. Pediatr. Neurol. 2008;15(2):79–90. doi: 10.1016/j.spen.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Pereira J.A., Ravichandran C.T., Mullett J., McDougle C.J., Keary C.J. Characterization of sleep habits and medication outcomes for sleep disturbance in children and adults with Angelman syndrome. Am. J. Med. Genet. 2020;182(8):1913–1922. doi: 10.1002/ajmg.a.61642. [DOI] [PubMed] [Google Scholar]
- Pfeiffer B.E., Huber K.M. Fragile X mental retardation protein induces synapse loss through acute postsynaptic translational regulation. J. Neurosci.: The Official Journal of the Society for Neuroscience. 2007;27(12):3120–3130. doi: 10.1523/JNEUROSCI.0054-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D., Pagnamenta A.T., Klei L., Anney R., Merico D., Regan R., Conroy J., Magalhaes T.R., Correia C., Abrahams B.S., Almeida J., Bacchelli E., Bader G.D., Bailey A.J., Baird G., Battaglia A., Berney T., Bolshakova N., Bölte S., et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466(7304):368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer N.W., Chandler D.J., Powell J.M., Scappini E.L., Waterhouse B.D., Jensen P. An intersectional viral-genetic method for fluorescent tracing of axon collaterals reveals details of noradrenergic locus coeruleus structure. ENeuro. 2020;7(3) doi: 10.1523/ENEURO.0010-20.2020. ENEURO.0010-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe G.R., Foote S., Eschenko O., Johansen J.P., Bouret S., Aston-Jones G., Harley C.W., Manahan-Vaughan D., Weinshenker D., Valentino R., Berridge C., Chandler D.J., Waterhouse B., Sara S.J. Locus coeruleus: a new look at the blue spot. Nat. Rev. Neurosci. 2020;21(11) doi: 10.1038/s41583-020-0360-9. Article 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politte L.C., Scahill L., Figueroa J., McCracken J.T., King B., McDougle C.J. A randomized, placebo-controlled trial of extended-release guanfacine in children with autism spectrum disorder and ADHD symptoms: an analysis of secondary outcome measures. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2018;43(8):1772–1778. doi: 10.1038/s41386-018-0039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard A.J., Prendergast M. Depressive pseudodementia in a child with autism. Dev. Med. Child Neurol. 2004;46(7):485–489. doi: 10.1017/s0012162204000805. [DOI] [PubMed] [Google Scholar]
- Portas C.M., Grønli J. In: Serotonin and Sleep: Molecular, Functional and Clinical Aspects. Monti J.M., Pandi-Perumal S.R., Jacobs B.L., Nutt D.J., editors. 2008. Involvement of the 5-HT1A and the 5-HT1B receptor in the regulation of sleep and waking; pp. 325–369. Birkhäuser. [DOI] [Google Scholar]
- Portas C.M., McCarley R.W. Behavioral state-related changes of extracellular serotonin concentration in the dorsal raphe nucleus: a microdialysis study in the freely moving cat. Brain Res. 1994;648(2):306–312. doi: 10.1016/0006-8993(94)91132-0. [DOI] [PubMed] [Google Scholar]
- Portas C.M., Thakkar M., Rainnie D., McCarley R.W. Microdialysis perfusion of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) in the dorsal raphe nucleus decreases serotonin release and increases rapid eye movement sleep in the freely moving cat. J. Neurosci.: The Official Journal of the Society for Neuroscience. 1996;16(8):2820–2828. doi: 10.1523/JNEUROSCI.16-08-02820.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portas C.M., Bjorvatn B., Fagerland S., Grønli J., Mundal V., Sørensen E., Ursin R. On-line detection of extracellular levels of serotonin in dorsal raphe nucleus and frontal cortex over the sleep/wake cycle in the freely moving rat. Neuroscience. 1998;83(3):807–814. doi: 10.1016/s0306-4522(97)00438-7. [DOI] [PubMed] [Google Scholar]
- Portmann T., Yang M., Mao R., Panagiotakos G., Ellegood J., Dolen G., Bader P.L., Grueter B.A., Goold C., Fisher E., Clifford K., Rengarajan P., Kalikhman D., Loureiro D., Saw N.L., Zhengqui Z., Miller M.A., Lerch J.P., Henkelman M., et al. Behavioral abnormalities and circuit defects in the basal ganglia of a mouse model of 16p11.2 deletion syndrome. Cell Rep. 2014;7(4):1077–1092. doi: 10.1016/j.celrep.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey D.I., Litwiller M., Koburn A., McDougle C.J. Paroxetine in autism. J. Am. Acad. Child Adolesc. Psychiatr. 1999;38(2):111–112. doi: 10.1097/00004583-199902000-00004. [DOI] [PubMed] [Google Scholar]
- Posey D.J., Guenin K.D., Kohn A.E., Swiezy N.B., McDougle C.J. A naturalistic open-label study of mirtazapine in autistic and other pervasive developmental disorders. J. Child Adolesc. Psychopharmacol. 2001;11(3):267–277. doi: 10.1089/10445460152595586. [DOI] [PubMed] [Google Scholar]
- Posey D.J., Puntney J.I., Sasher T.M., Kem D.L., McDougle C.J. Guanfacine treatment of hyperactivity and inattention in pervasive developmental disorders: a retrospective analysis of 80 cases. J. Child Adolesc. Psychopharmacol. 2004;14(2):233–241. doi: 10.1089/1044546041649084. [DOI] [PubMed] [Google Scholar]
- Posey D.J., Erickson C.A., Stigler K.A., McDougle C.J. The use of selective serotonin reuptake inhibitors in autism and related disorders. J. Child Adolesc. Psychopharmacol. 2006;16(1–2):181–186. doi: 10.1089/cap.2006.16.181. [DOI] [PubMed] [Google Scholar]
- Posey D.J., Stigler K.A., Erickson C.A., McDougle C.J. Antipsychotics in the treatment of autism. J. Clin. Investig. 2008;118(1):6–14. doi: 10.1172/JCI32483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prchalova D., Havlovicova M., Sterbova K., Stranecky V., Hancarova M., Sedlacek Z. Analysis of 31-year-old patient with SYNGAP1 gene defect points to importance of variants in broader splice regions and reveals developmental trajectory of SYNGAP1-associated phenotype: case report. BMC Med. Genet. 2017;18(1):62. doi: 10.1186/s12881-017-0425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propper L. Managing disruptive behaviour in autism-spectrum disorder with guanfacine. J. Psychiatry Neurosci.: J. Psychiatr. Neurosci. 2018;43(5):359–360. doi: 10.1503/jpn.180039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucilowska J., Vithayathil J., Pagani M., Kelly C., Karlo J.C., Robol C., Morella I., Gozzi A., Brambilla R., Landreth G.E. Pharmacological inhibition of ERK signaling rescues pathophysiology and behavioral phenotype associated with 16p11.2 chromosomal deletion in mice. J. Neurosci.: The Official Journal of the Society for Neuroscience. 2018;38(30):6640–6652. doi: 10.1523/JNEUROSCI.0515-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puizillout J.J., Gaudin-Chazal G., Daszuta A., Seyfritz N., Ternaux J.P. Release of endogenous serotonin from “encéphale isolé” cats. II - correlations with raphe neuronal activity and sleep and wakefulness. J. Physiol. (Paris) 1979;75(5):531–537. [PubMed] [Google Scholar]
- Qu W.-M., Xu X.-H., Yan M.-M., Wang Y.-Q., Urade Y., Huang Z.-L. Essential role of dopamine D2 receptor in the maintenance of wakefulness, but not in homeostatic regulation of sleep, in mice. J. Neurosci.: The Official Journal of the Society for Neuroscience. 2010;30(12):4382–4389. doi: 10.1523/JNEUROSCI.4936-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowski J., Kubiak P., Aston-Jones G. Locus coeruleus activity in monkey: phasic and tonic changes are associated with altered vigilance. Brain Res. Bull. 1994;35(5–6):607–616. doi: 10.1016/0361-9230(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Rapin I. An 8-year-old boy with autism. JAMA. 2001;285(13):1749–1757. doi: 10.1001/jama.285.13.1749. [DOI] [PubMed] [Google Scholar]
- Rashaid A.H.B., Alqhazo M.T., Nusair S.D., Adams J.B., Bashtawi M.A., Al-Fawares O. Profiling plasma levels of thiamine and histamine in Jordanian children with autism spectrum disorder (ASD): potential biomarkers for evaluation of ASD therapies and diet. Nutr. Neurosci. 2022;1–8 doi: 10.1080/1028415X.2022.2101976. [DOI] [PubMed] [Google Scholar]
- Rasmussen K., Morilak D.A., Jacobs B.L. Single unit activity of locus coeruleus neurons in the freely moving cat. I. During naturalistic behaviors and in response to simple and complex stimuli. Brain Res. 1986;371(2):324–334. doi: 10.1016/0006-8993(86)90370-7. [DOI] [PubMed] [Google Scholar]
- Relia S., Ekambaram V. Pharmacological approach to sleep disturbances in autism spectrum disorders with psychiatric comorbidities: a literature review. Med. Sci. 2018;6(4):95. doi: 10.3390/medsci6040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richdale A.L. A descriptive analysis of sleep behaviour in children with Fragile X. J. Intellect. Dev. Disabil. 2003;28(2):135–144. doi: 10.1080/1366825031000147076. [DOI] [Google Scholar]
- Richdale A.L., Prior M.R. The sleep/wake rhythm in children with autism. Eur. Child Adolesc. Psychiatr. 1995;4(3):175–186. doi: 10.1007/BF01980456. [DOI] [PubMed] [Google Scholar]
- Richdale A.L., Schreck K.A. Sleep problems in autism spectrum disorders: prevalence, nature, & possible biopsychosocial aetiologies. Sleep Med. Rev. 2009;13(6):403–411. doi: 10.1016/j.smrv.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Richdale A.L., Baker E., Short M., Gradisar M. The role of insomnia, pre-sleep arousal and psychopathology symptoms in daytime impairment in adolescents with high-functioning autism spectrum disorder. Sleep Med. 2014;15(9):1082–1088. doi: 10.1016/j.sleep.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Richelson E., Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000;68(1):29–39. doi: 10.1016/s0024-3205(00)00911-5. [DOI] [PubMed] [Google Scholar]
- Rossi P.G., Posar A., Parmeggiani A., Pipitone E., D'Agata M. Niaprazine in the treatment of autistic disorder. J. Child Neurol. 1999;14(8):547–550. doi: 10.1177/088307389901400814. [DOI] [PubMed] [Google Scholar]
- Rossignol D.A., Frye R.E. Melatonin in autism spectrum disorders: a systematic review and meta-analysis. Dev. Med. Child Neurol. 2011;53(9):783–792. doi: 10.1111/j.1469-8749.2011.03980.x. [DOI] [PubMed] [Google Scholar]
- Russo R.M., Gururaj V.J., Allen J.E. The effectiveness of diphenhydramine HCI in pediatric sleep disorders. J. Clin. Pharmacol. 1976;16(5–6):284–288. doi: 10.1002/j.1552-4604.1976.tb02406.x. [DOI] [PubMed] [Google Scholar]
- Sala C., Vicidomini C., Bigi I., Mossa A., Verpelli C. Shank synaptic scaffold proteins: keys to understanding the pathogenesis of autism and other synaptic disorders. J. Neurochem. 2015;135(5):849–858. doi: 10.1111/jnc.13232. [DOI] [PubMed] [Google Scholar]
- Sallee F.R., McGough J., Wigal T., Donahue J., Lyne A., Biederman J., SPD503 STUDY GROUP. Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: a placebo-controlled trial. J. Am. Acad. Child Adolesc. Psychiatr. 2009;48(2):155–165. doi: 10.1097/CHI.0b013e318191769e. [DOI] [PubMed] [Google Scholar]
- Sanders S.J., Ercan-Sencicek A.G., Hus V., Luo R., Murtha M.T., Moreno-De-Luca D., Chu S.H., Moreau M.P., Gupta A.R., Thomson S.A., Mason C.E., Bilguvar K., Celestino-Soper P.B.S., Choi M., Crawford E.L., Davis L., Wright N.R.D., Dhodapkar R.M., DiCola M., et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70(5):863–885. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper C.B., Fuller P.M. Wake-sleep circuitry: an overview. Curr. Opin. Neurobiol. 2017;44:186–192. doi: 10.1016/j.conb.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara S.J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009;10(3):211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Saré R.M., Harkless L., Levine M., Torossian A., Sheeler C.A., Smith C.B. Deficient sleep in mouse models of fragile X syndrome. Front. Mol. Neurosci. 2017;10:280. doi: 10.3389/fnmol.2017.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterstrom F.K., Kosmicki J.A., Wang J., Breen M.S., De Rubeis S., An J.-Y., Peng M., Collins R., Grove J., Klei L., Stevens C., Reichert J., Mulhern M.S., Artomov M., Gerges S., Sheppard B., Xu X., Bhaduri A., Norman U., et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell. 2020;180(3):568–584.e23. doi: 10.1016/j.cell.2019.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell T.E., Arrigoni E., Lipton J.O. Neural circuitry of wakefulness and sleep. Neuron. 2017;93(4):747–765. doi: 10.1016/j.neuron.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schain R.J., Freedman D.X. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J. Pediatr. 1961;58:315–320. doi: 10.1016/s0022-3476(61)80261-8. [DOI] [PubMed] [Google Scholar]
- Schatzberg A., DeBattista C. Schatzberg's Manual of Clinical Psychopharmacology. American Psychiatric Association Publishing; 2019. Antidepressants. [DOI] [Google Scholar]
- Schatzberg A., DeBattista C. Schatzberg's Manual of Clinical Psychopharmacology. American Psychiatric Association Publishing; 2019. Antipsychotic drugs.https://psychiatryonline-org.proxy.library.upenn.edu/doi/full/10.1176/appi.books.9781615372997.AS04 [Google Scholar]
- Scherman D., Hamon M., Gozlan H., Henry J.P., Lesage A., Masson M., Rumigny J.F. Molecular pharmacology of niaprazine. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 1988;12(6):989–1001. doi: 10.1016/0278-5846(88)90093-0. [DOI] [PubMed] [Google Scholar]
- Schnoes C.J., Kuhn B.R., Workman E.F., Ellis C.R. Pediatric prescribing practices for clonidine and other pharmacologic agents for children with sleep disturbance. Clin. Pediatr. 2006;45(3):229–238. doi: 10.1177/000992280604500304. [DOI] [PubMed] [Google Scholar]
- Schotte A., Janssen P.F., Gommeren W., Luyten W.H., Van Gompel P., Lesage A.S., De Loore K., Leysen J.E. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology. 1996;124(1–2):57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- Schreck K.A., Mulick J.A., Smith A.F. Sleep problems as possible predictors of intensified symptoms of autism. Res. Dev. Disabil. 2004;25(1):57–66. doi: 10.1016/j.ridd.2003.04.007. [DOI] [PubMed] [Google Scholar]
- Schroder C.M., Malow B.A., Maras A., Melmed R.D., Findling R.L., Breddy J., Nir T., Shahmoon S., Zisapel N., Gringras P. Pediatric prolonged-release melatonin for sleep in children with autism spectrum disorder: impact on child behavior and caregiver's quality of life. J. Autism Dev. Disord. 2019;49(8):3218–3230. doi: 10.1007/s10803-019-04046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweimer J.V., Ungless M.A. Phasic responses in dorsal raphe serotonin neurons to noxious stimuli. Neuroscience. 2010;171(4):1209–1215. doi: 10.1016/j.neuroscience.2010.09.058. [DOI] [PubMed] [Google Scholar]
- Sebat J., Lakshmi B., Malhotra D., Troge J., Lese-Martin C., Walsh T., Yamrom B., Yoon S., Krasnitz A., Kendall J., Leotta A., Pai D., Zhang R., Lee Y.-H., Hicks J., Spence S.J., Lee A.T., Puura K., Lehtimäki T., et al. Strong association of de novo copy number mutations with autism. Science (New York, N.Y.) 2007;316(5823):445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seese R.R., Wang K., Yao Y.Q., Lynch G., Gall C.M. Spaced training rescues memory and ERK1/2 signaling in fragile X syndrome model mice. Proc. Natl. Acad. Sci. U. S. A. 2014;111(47):16907–16912. doi: 10.1073/pnas.1413335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifritz E., Moore P., Trachsel L., Bhatti T., Stahl S.M., Gillin J.C. The 5-HT1A agonist ipsapirone enhances EEG slow wave activity in human sleep and produces a power spectrum similar to 5-HT2 blockade. Neurosci. Lett. 1996;209(1):41–44. doi: 10.1016/0304-3940(96)12607-0. [DOI] [PubMed] [Google Scholar]
- Sherin J.E., Shiromani P.J., McCarley R.W., Saper C.B. Activation of ventrolateral preoptic neurons during sleep. Science (New York, N.Y.) 1996;271(5246):216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Silverman J.L., Tolu S.S., Barkan C.L., Crawley J.N. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2010;35(4):976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivertsen B., Posserud M.-B., Gillberg C., Lundervold A.J., Hysing M. Sleep problems in children with autism spectrum problems: a longitudinal population-based study. Autism: The International Journal of Research and Practice. 2012;16(2):139–150. doi: 10.1177/1362361311404255. [DOI] [PubMed] [Google Scholar]
- Slater J.W., Zechnich A.D., Haxby D.G. Second-generation antihistamines: a comparative review. Drugs. 1999;57(1):31–47. doi: 10.2165/00003495-199957010-00004. [DOI] [PubMed] [Google Scholar]
- Smith-Hicks C., Wright D., Kenny A., Stowe R.C., McCormack M., Stanfield A.C., Holder J.L. Sleep abnormalities in the synaptopathies-SYNGAP1-related intellectual disability and phelan-McDermid syndrome. Brain Sci. 2021;11(9):1229. doi: 10.3390/brainsci11091229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfelt L., Ursin R. The 5-HT2 antagonist ritanserin decreases sleep in cats. Sleep. 1993;16(1):15–22. doi: 10.1093/sleep/16.1.15. [DOI] [PubMed] [Google Scholar]
- Soorya L., Kolevzon A., Zweifach J., Lim T., Dobry Y., Schwartz L., Frank Y., Wang A.T., Cai G., Parkhomenko E., Halpern D., Grodberg D., Angarita B., Willner J.P., Yang A., Canitano R., Chaplin W., Betancur C., Buxbaum J.D. Prospective investigation of autism and genotype-phenotype correlations in 22q13 deletion syndrome and SHANK3 deficiency. Mol. Autism. 2013;4(1):18. doi: 10.1186/2040-2392-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souders M.C., Mason T.B.A., Valladares O., Bucan M., Levy S.E., Mandell D.S., Weaver T.E., Pinto-Martin J. Sleep behaviors and sleep quality in children with autism spectrum disorders. Sleep. 2009;32(12):1566–1578. doi: 10.1093/sleep/32.12.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel R., DeVos J.E. Central effects of guanfacine and clonidine during wakefulness and sleep in healthy subjects. Br. J. Clin. Pharmacol. 1980;10(Suppl. 1):165S–168S. doi: 10.1111/j.1365-2125.1980.tb04925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfels G.F., Heym J., Strecker R.E., Jacobs B.L. Behavioral correlates of dopaminergic unit activity in freely moving cats. Brain Res. 1983;258(2):217–228. doi: 10.1016/0006-8993(83)91145-9. [DOI] [PubMed] [Google Scholar]
- Stessman H.A.F., Xiong B., Coe B.P., Wang T., Hoekzema K., Fenckova M., Kvarnung M., Gerdts J., Trinh S., Cosemans N., Vives L., Lin J., Turner T.N., Santen G., Ruivenkamp C., Kriek M., van Haeringen A., Aten E., Friend K., et al. Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases. Nat. Genet. 2017;49(4):515–526. doi: 10.1038/ng.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigler K.A., Posey D.J., McDougle C.J. Aripiprazole for maladaptive behavior in pervasive developmental disorders. J. Child Adolesc. Psychopharmacol. 2004;14(3):455–463. doi: 10.1089/cap.2004.14.455. [DOI] [PubMed] [Google Scholar]
- Stigler K.A., Posey D.J., McDougle C.J. Ramelteon for insomnia in two youths with autistic disorder. J. Child Adolesc. Psychopharmacol. 2006;16(5):631–636. doi: 10.1089/cap.2006.16.631. [DOI] [PubMed] [Google Scholar]
- Sullivan B.J., Ammanuel S., Kipnis P.A., Araki Y., Huganir R.L., Kadam S.D. Low-dose perampanel rescues cortical gamma dysregulation associated with parvalbumin interneuron GluA2 upregulation in epileptic Syngap1+/- mice. Biol. Psychiatr. 2020;87(9):829–842. doi: 10.1016/j.biopsych.2019.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.-X., Wang D.-R., Ye C.-B., Hu Z.-Z., Wang C.-Y., Huang Z.-L., Yang S.-R. Activation of the ventral tegmental area increased wakefulness in mice. Sleep Biol. Rhythm. 2017;15(2):107–115. doi: 10.1007/s41105-017-0094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntsova N., Szymusiak R., Alam Md N., Guzman-Marin R., McGinty D. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J. Physiol. 2002;543(Pt 2):665–677. doi: 10.1113/jphysiol.2002.023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift K.M., Gross B.A., Frazer M.A., Bauer D.S., Clark K.J.D., Vazey E.M., Aston-Jones G., Li Y., Pickering A.E., Sara S.J., Poe G.R. Abnormal locus coeruleus sleep activity alters sleep signatures of memory consolidation and impairs place cell stability and spatial memory. Curr. Biol.: Cailiao Baohu. 2018;28(22):3599–3609.e4. doi: 10.1016/j.cub.2018.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Y.Y. Pitolisant: first global approval. Drugs. 2016;76(13):1313–1318. doi: 10.1007/s40265-016-0620-1. [DOI] [PubMed] [Google Scholar]
- Szakacs Z., Dauvilliers Y., Mikhaylov V., Poverennova I., Krylov S., Jankovic S., Sonka K., Lehert P., Lecomte I., Lecomte J.-M., Schwartz J.-C., HARMONY-CTP study group Safety and efficacy of pitolisant on cataplexy in patients with narcolepsy: a randomised, double-blind, placebo-controlled trial. The Lancet. Neurology. 2017;16(3):200–207. doi: 10.1016/S1474-4422(16)30333-7. [DOI] [PubMed] [Google Scholar]
- Szymusiak R., Alam N., Steininger T.L., McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803(1–2):178–188. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- Tabet A.-C., Pilorge M., Delorme R., Amsellem F., Pinard J.-M., Leboyer M., Verloes A., Benzacken B., Betancur C. Autism multiplex family with 16p11.2p12.2 microduplication syndrome in monozygotic twins and distal 16p11.2 deletion in their brother. Eur. J. Hum. Genet. 2012;20(5):540–546. doi: 10.1038/ejhg.2011.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri F., Esmaeilpour K., Sepehri G., Sheibani V., Ur Rehman N., Maneshian M. Histamine H3 receptor antagonist, ciproxifan, alleviates cognition and synaptic plasticity alterations in a valproic acid-induced animal model of autism. Psychopharmacology. 2022;239(8):2673–2693. doi: 10.1007/s00213-022-06155-z. [DOI] [PubMed] [Google Scholar]
- Taira M., Takase M., Sasaki H. Sleep disorder in children with autism. Psychiatr. Clin. Neurosci. 1998;52(2):182–183. doi: 10.1111/j.1440-1819.1998.tb01018.x. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Lin J.-S., Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J. Neurosci.: The Official Journal of the Society for Neuroscience. 2006;26(40):10292–10298. doi: 10.1523/JNEUROSCI.2341-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Lin J.-S., Sakai K. Characterization and mapping of sleep-waking specific neurons in the basal forebrain and preoptic hypothalamus in mice. Neuroscience. 2009;161(1):269–292. doi: 10.1016/j.neuroscience.2009.02.075. [DOI] [PubMed] [Google Scholar]
- Takase M., Taira M., Sasaki H. Sleep-wake rhythm of autistic children. Psychiatr. Clin. Neurosci. 1998;52(2):181–182. doi: 10.1111/j.1440-1819.1998.tb01017.x. [DOI] [PubMed] [Google Scholar]
- Testa-Silva G., Loebel A., Giugliano M., de Kock C.P.J., Mansvelder H.D., Meredith R.M. Hyperconnectivity and slow synapses during early development of medial prefrontal cortex in a mouse model for mental retardation and autism. Cerebr. Cortex. 2012;22(6):1333–1342. doi: 10.1093/cercor/bhr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D., Stoppel L.J., Heynen A.J., Lindemann L., Jaeschke G., Mills A.A., Bear M.F. Contribution of mGluR5 to pathophysiology in a mouse model of human chromosome 16p11.2 microdeletion. Nat. Neurosci. 2015;18(2):182–184. doi: 10.1038/nn.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissier M.H., Lainey E., Fattaccini C.M., Hamon M., Adrien J. Effects of ipsapirone, a 5-HT1A agonist, on sleep/wakefulness cycles: probable post-synaptic action. J. Sleep Res. 1993;2(2):103–109. doi: 10.1111/j.1365-2869.1993.tb00070.x. [DOI] [PubMed] [Google Scholar]
- Tononi G., Pompeiano M., Cirelli C. Suppression of desynchronized sleep through microinjection of the alpha 2-adrenergic agonist clonidine in the dorsal pontine tegmentum of the cat. Pflueg. Arch. Eur. J. Physiol. 1991;418(5):512–518. doi: 10.1007/BF00497780. [DOI] [PubMed] [Google Scholar]
- Tordjman S., Anderson G.M., Pichard N., Charbuy H., Touitou Y. Nocturnal excretion of 6-sulphatoxymelatonin in children and adolescents with autistic disorder. Biol. Psychiatr. 2005;57(2):134–138. doi: 10.1016/j.biopsych.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Tordjman S., Anderson G.M., Bellissant E., Botbol M., Charbuy H., Camus F., Graignic R., Kermarrec S., Fougerou C., Cohen D., Touitou Y. Day and nighttime excretion of 6-sulphatoxymelatonin in adolescents and young adults with autistic disorder. Psychoneuroendocrinology. 2012;37(12):1990–1997. doi: 10.1016/j.psyneuen.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Trulson M.E., Jacobs B.L. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163(1):135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- Trulson M.E., Preussler D.W. Dopamine-containing ventral tegmental area neurons in freely moving cats: activity during the sleep-waking cycle and effects of stress. Exp. Neurol. 1984;83(2):367–377. doi: 10.1016/S0014-4886(84)90105-5. [DOI] [PubMed] [Google Scholar]
- Trulson M.E., Preussler D.W., Howell G.A. Activity of substantia nigra units across the sleep-waking cycle in freely moving cats. Neurosci. Lett. 1981;26(2):183–188. doi: 10.1016/0304-3940(81)90346-3. [DOI] [PubMed] [Google Scholar]
- Tsujino N., Nakatani Y., Seki Y., Nakasato A., Nakamura M., Sugawara M., Arita H. Abnormality of circadian rhythm accompanied by an increase in frontal cortex serotonin in animal model of autism. Neurosci. Res. 2007;57(2):289–295. doi: 10.1016/j.neures.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Tudor M.E., Walsh C.E., Mulder E.C., Lerner M.D. Pain as a predictor of sleep problems in youth with autism spectrum disorders. Autism: The International Journal of Research and Practice. 2015;19(3):292–300. doi: 10.1177/1362361313518994. [DOI] [PubMed] [Google Scholar]
- Tufan A.E., Kutlu H. Adjunctive quetiapine may help depression comorbid with pervasive developmental disorders. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2009;33(8):1570–1571. doi: 10.1016/j.pnpbp.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Tural Hesapcioglu S., Ceylan M.F., Kasak M., Sen C.P. Olanzapine, risperidone, and aripiprazole use in children and adolescents with Autism Spectrum Disorders. Research in Autism Spectrum Disorders. 2020;72 doi: 10.1016/j.rasd.2020.101520. [DOI] [Google Scholar]
- Valentino R.J., Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur. J. Pharmacol. 2008;583(2–3):194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valicenti-McDermott M.R., Demb H. Clinical effects and adverse reactions of off-label use of aripiprazole in children and adolescents with developmental disabilities. J. Child Adolesc. Psychopharmacol. 2006;16(5):549–560. doi: 10.1089/cap.2006.16.549. [DOI] [PubMed] [Google Scholar]
- van der Heijden K.B., Stoffelsen R.J., Popma A., Swaab H. Sleep, chronotype, and sleep hygiene in children with attention-deficit/hyperactivity disorder, autism spectrum disorder, and controls. Eur. Child Adolesc. Psychiatr. 2018;27(1):99–111. doi: 10.1007/s00787-017-1025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankov A., Hervé-Minvielle A., Sara S.J. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur. J. Neurosci. 1995;7(6):1180–1187. doi: 10.1111/j.1460-9568.1995.tb01108.x. [DOI] [PubMed] [Google Scholar]
- Veasey S.C., Fornal C.A., Metzler C.W., Jacobs B.L. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J. Neurosci.: The Official Journal of the Society for Neuroscience. 1995;15(7 Pt 2):5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch O.J., Maxwell-Horn A.C., Malow B.A. Sleep in autism spectrum disorders. Current Sleep Medicine Reports. 2015;1(2):131–140. doi: 10.1007/s40675-015-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch O.J., Pendergast J.S., Allen M.J., Leu R.M., Johnson C.H., Elsea S.H., Malow B.A. Genetic variation in melatonin pathway enzymes in children with autism spectrum disorder and comorbid sleep onset delay. J. Autism Dev. Disord. 2015;45(1):100–110. doi: 10.1007/s10803-014-2197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch O.J., Reynolds A., Katz T., Weiss S.K., Loh A., Wang L., Malow B.A. Sleep in children with autism spectrum disorders: how are measures of parent report and actigraphy related and affected by sleep education? Behav. Sleep Med. 2016;14(6):665–676. doi: 10.1080/15402002.2015.1065408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J., Muller C.L., Iwamoto H., Sauer J.E., Owens W.A., Shah C.R., Cohen J., Mannangatti P., Jessen T., Thompson B.J., Ye R., Kerr T.M., Carneiro A.M., Crawley J.N., Sanders-Bush E., McMahon D.G., Ramamoorthy S., Daws L.C., Sutcliffe J.S., Blakely R.D. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc. Natl. Acad. Sci. U. S. A. 2012;109(14):5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellino F., Zanotto E., Ravera G., Veneselli E. Open-label risperidone treatment of 6 children and adolescents with autism. Canadian Journal of Psychiatry. Revue Canadienne De Psychiatrie. 2001;46(6):559–560. doi: 10.1177/070674370104600614. [DOI] [PubMed] [Google Scholar]
- Verhoeff M.E., Blanken L.M.E., Kocevska D., Mileva-Seitz V.R., Jaddoe V.W.V., White T., Verhulst F., Luijk M.P.C.M., Tiemeier H. The bidirectional association between sleep problems and autism spectrum disorder: a population-based cohort study. Mol. Autism. 2018;9:8. doi: 10.1186/s13229-018-0194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk A.J., Pieretti M., Sutcliffe J.S., Fu Y.H., Kuhl D.P., Pizzuti A., Reiner O., Richards S., Victoria M.F., Zhang F.P. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Vijapura S., Schofield M., Maneta E., Coffey B.J. Mania in an adolescent with autism and premenstrual mood variation: a diagnostic and treatment dilemma. J. Child Adolesc. Psychopharmacol. 2014;24(3):161–164. doi: 10.1089/cap.2014.2432. [DOI] [PubMed] [Google Scholar]
- Vlaskamp D.R.M., Shaw B.J., Burgess R., Mei D., Montomoli M., Xie H., Myers C.T., Bennett M.F., XiangWei W., Williams D., Maas S.M., Brooks A.S., Mancini G.M.S., van de Laar I.M.B.H., van Hagen J.M., Ware T.L., Webster R.I., Malone S., Berkovic S.F., et al. SYNGAP1 encephalopathy. Neurology. 2019;92(2):e96–e107. doi: 10.1212/WNL.0000000000006729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stülpnagel C., Funke C., Haberl C., Hörtnagel K., Jüngling J., Weber Y.G., Staudt M., Kluger G. SYNGAP1 mutation in focal and generalized epilepsy: a literature overview and A case report with special aspects of the EEG. Neuropediatrics. 2015;46(4):287–291. doi: 10.1055/s-0035-1554098. [DOI] [PubMed] [Google Scholar]
- Wang Y.-Q., Takata Y., Li R., Zhang Z., Zhang M.-Q., Urade Y., Qu W.-M., Huang Z.-L. Doxepin and diphenhydramine increased non-rapid eye movement sleep through blockade of histamine H1 receptors. Pharmacol. Biochem. Behav. 2015;129:56–64. doi: 10.1016/j.pbb.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Wang G., Liu Z., Xu G., Jiang F., Lu N., Baylor A., Owens J. Sleep disturbances and associated factors in Chinese children with autism spectrum disorder: a retrospective and cross-sectional study. Child Psychiatr. Hum. Dev. 2016;47(2):248–258. doi: 10.1007/s10578-015-0561-z. [DOI] [PubMed] [Google Scholar]
- Wasdell M.B., Jan J.E., Bomben M.M., Freeman R.D., Rietveld W.J., Tai J., Hamilton D., Weiss M.D. A randomized, placebo-controlled trial of controlled release melatonin treatment of delayed sleep phase syndrome and impaired sleep maintenance in children with neurodevelopmental disabilities. J. Pineal Res. 2008;44(1):57–64. doi: 10.1111/j.1600-079X.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Taguchi Y., Hayashi H., Tanaka J., Shiosaka S., Tohyama M., Kubota H., Terano Y., Wada H. Evidence for the presence of a histaminergic neuron system in the rat brain: an immunohistochemical analysis. Neurosci. Lett. 1983;39(3):249–254. doi: 10.1016/0304-3940(83)90308-7. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Taguchi Y., Shiosaka S., Tanaka J., Kubota H., Terano Y., Tohyama M., Wada H. Distribution of the histaminergic neuron system in the central nervous system of rats; a fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res. 1984;295(1):13–25. doi: 10.1016/0006-8993(84)90811-4. [DOI] [PubMed] [Google Scholar]
- Waterhouse B.D., Predale H.K., Plummer N.W., Jensen P., Chandler D.J. Probing the structure and function of locus coeruleus projections to CNS motor centers. Front. Neural Circ. 2022;16 doi: 10.3389/fncir.2022.895481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F., Dan Y. Circuit-based interrogation of sleep control. Nature. 2016;538(7623):51–59. doi: 10.1038/nature19773. [DOI] [PubMed] [Google Scholar]
- Weber F., Chung S., Beier K.T., Xu M., Luo L., Dan Y. Control of REM sleep by ventral medulla GABAergic neurons. Nature. 2015;526(7573):435–438. doi: 10.1038/nature14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L.A., Shen Y., Korn J.M., Arking D.E., Miller D.T., Fossdal R., Saemundsen E., Stefansson H., Ferreira M.A.R., Green T., Platt O.S., Ruderfer D.M., Walsh C.A., Altshuler D., Chakravarti A., Tanzi R.E., Stefansson K., Santangelo S.L., Gusella J.F., Autism Consortium Association between microdeletion and microduplication at 16p11.2 and autism. N. Engl. J. Med. 2008;358(7):667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- Wiggs L., Stores G. Sleep patterns and sleep disorders in children with autistic spectrum disorders: insights using parent report and actigraphy. Dev. Med. Child Neurol. 2004;46(6):372–380. doi: 10.1017/s0012162204000611. [DOI] [PubMed] [Google Scholar]
- Wilens T.E., Bukstein O., Brams M., Cutler A.J., Childress A., Rugino T., Lyne A., Grannis K., Youcha S. A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatr. 2012;51(1):74–85.e2. doi: 10.1016/j.jaac.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Wilson H., Wong A., Shaw S., Tse W., Stapleton G., Phelan M., Hu S., Marshall J., McDermid H. Molecular characterisation of the 22q13 deletion syndrome supports the role of haploinsufficiency of SHANK3/PROSAP2 in the major neurological symptoms. J. Med. Genet. 2003;40(8):575–584. doi: 10.1136/jmg.40.8.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintler T., Schoch H., Frank M.G., Peixoto L. Sleep, brain development, and autism spectrum disorders: insights from animal models. J. Neurosci. Res. 2020;98(6):1137–1149. doi: 10.1002/jnr.24619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirojanan J., Jacquemont S., Diaz R., Bacalman S., Anders T.F., Hagerman R.J., Goodlin-Jones B.L. The efficacy of melatonin for sleep problems in children with autism, fragile X syndrome, or autism and fragile X syndrome. J. Clin. Sleep Med.: JCSM: Official Publication of the American Academy of Sleep Medicine. 2009;5(2):145–150. [PMC free article] [PubMed] [Google Scholar]
- Wisor J.P., Nishino S., Sora I., Uhl G.H., Mignot E., Edgar D.M. Dopaminergic role in stimulant-induced wakefulness. J. Neurosci.: The Official Journal of the Society for Neuroscience. 2001;21(5):1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B., Sims D., Smart S., Alwazeer A., Alderson-Day B., Allgar V., Whitton C., Tomlinson H., Bennett S., Jardine J., McCaffrey N., Leyland C., Jakeman C., Miles J. Melatonin versus placebo in children with autism spectrum conditions and severe sleep problems not amenable to behaviour management strategies: a randomised controlled crossover trial. J. Autism Dev. Disord. 2011;41(2):175–184. doi: 10.1007/s10803-010-1036-5. [DOI] [PubMed] [Google Scholar]
- Wright C., Shin J.H., Rajpurohit A., Deep-Soboslay A., Collado-Torres L., Brandon N.J., Hyde T.M., Kleinman J.E., Jaffe A.E., Cross A.J., Weinberger D.R. Altered expression of histamine signaling genes in autism spectrum disorder. Transl. Psychiatry. 2017;7(5):e1126. doi: 10.1038/tp.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Hopf F.W., Li S.-B., de Lecea L. In vivo cell type-specific CRISPR knockdown of dopamine beta hydroxylase reduces locus coeruleus evoked wakefulness. Nat. Commun. 2018;9(1) doi: 10.1038/s41467-018-07566-3. Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y., Matsuishi T., Murakami Y., Kato H. Sleep disorder in Rett syndrome and melatonin treatment. Brain & Dev. 1999;21(8):570. doi: 10.1016/s0387-7604(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Yang M., Lewis F.C., Sarvi M.S., Foley G.M., Crawley J.N. 16p11.2 Deletion mice display cognitive deficits in touchscreen learning and novelty recognition tasks. Learn. Mem. 2015;22(12):622–632. doi: 10.1101/lm.039602.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Mahrt E.J., Lewis F., Foley G., Portmann T., Dolmetsch R.E., Portfors C.V., Crawley J.N. 16p11.2 deletion syndrome mice display sensory and ultrasonic vocalization deficits during social interactions. Autism Res.: Official Journal of the International Society for Autism Research. 2015;8(5):507–521. doi: 10.1002/aur.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavuz-Kodat E., Reynaud E., Geoffray M.-M., Limousin N., Franco P., Bonnet-Brilhault F., Bourgin P., Schroder C.M. Disturbances of continuous sleep and circadian rhythms account for behavioral difficulties in children with autism spectrum disorder. J. Clin. Med. 2020;9(6):1978. doi: 10.3390/jcm9061978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X., Jones N., Yang J., Asraoui N., Mathieu M.-E., Cai L., Chen S.X. Delayed motor learning in a 16p11.2 deletion mouse model of autism is rescued by locus coeruleus activation. Nat. Neurosci. 2021;24(5):646–657. doi: 10.1038/s41593-021-00815-7. [DOI] [PubMed] [Google Scholar]
- Yu X., Ye Z., Houston C.M., Zecharia A.Y., Ma Y., Zhang Z., Uygun D.S., Parker S., Vyssotski A.L., Yustos R., Franks N.P., Brickley S.G., Wisden W. Wakefulness is governed by GABA and histamine cotransmission. Neuron. 2015;87(1):164–178. doi: 10.1016/j.neuron.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Bonnan A., Bony G., Ferezou I., Pietropaolo S., Ginger M., Sans N., Rossier J., Oostra B., LeMasson G., Frick A. Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1(-/y) mice. Nat. Neurosci. 2014;17(12):1701–1709. doi: 10.1038/nn.3864. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Kaiser T., Monteiro P., Zhang X., Van der Goes M.S., Wang D., Barak B., Zeng M., Li C., Lu C., Wells M., Amaya A., Nguyen S., Lewis M., Sanjana N., Zhou Y., Zhang M., Zhang F., Fu Z., Feng G. Mice with Shank3 mutations associated with ASD and schizophrenia display both shared and distinct defects. Neuron. 2016;89(1):147–162. doi: 10.1016/j.neuron.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitnik G.A. Control of arousal through neuropeptide afferents of the locus coeruleus. Brain Res. 2016;1641(Pt B):338–350. doi: 10.1016/j.brainres.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Zuddas A., Di Martino A., Muglia P., Cianchetti C. Long-term risperidone for pervasive developmental disorder: efficacy, tolerability, and discontinuation. J. Child Adolesc. Psychopharmacol. 2000;10(2):79–90. doi: 10.1089/cap.2000.10.79. [DOI] [PubMed] [Google Scholar]