Abstract
Background
Chest CT displays chest pathology better than chest X-ray (CXR). We evaluated the effects on health outcomes of replacing CXR by ultra-low-dose chest-CT (ULDCT) in the diagnostic work-up of patients suspected of non-traumatic pulmonary disease at the emergency department.
Methods
Pragmatic, multicentre, non-inferiority randomised clinical trial in patients suspected of non-traumatic pulmonary disease at the emergency department. Between 31 January 2017 and 31 May 2018, every month, participating centres were randomly allocated to using ULDCT or CXR. Primary outcome was functional health at 28 days, measured by the Short Form (SF)-12 physical component summary scale score (PCS score), non-inferiority margin was set at 1 point. Secondary outcomes included hospital admission, hospital length of stay (LOS) and patients in follow-up because of incidental findings.
Results
2418 consecutive patients (ULDCT: 1208 and CXR: 1210) were included. Mean SF-12 PCS score at 28 days was 37.0 for ULDCT and 35.9 for CXR (difference 1.1; 95% lower CI: 0.003). After ULDCT, 638/1208 (52.7%) patients were admitted (median LOS of 4.8 days; IQR 2.1–8.8) compared with 659/1210 (54.5%) patients after CXR (median LOS 4.6 days; IQR 2.1–8.8). More ULDCT patients were in follow-up because of incidental findings: 26 (2.2%) versus 4 (0.3%).
Conclusions
Short-term functional health was comparable between ULDCT and CXR, as were hospital admissions and LOS, but more incidental findings were found in the ULDCT group. Our trial does not support routine use of ULDCT in the work-up of patients suspected of non-traumatic pulmonary disease at the emergency department.
Trial registration number
NTR6163.
Keywords: emergency medicine, respiratory infection, pneumonia, imaging/CT MRI etc
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Several studies underscore the higher diagnostic accuracy of chest CT as compared with chest X-ray (CXR), but since no patient outcome measures were collected, the effectiveness of both strategies cannot be compared.
WHAT THIS STUDY ADDS
Our randomised trial is unique in its aim to assess the yield of replacing CXR by ultra-low-dose chest-CT (ULDCT) in the diagnostic work-up of emergency department patients suspected of non-traumatic pulmonary disease in terms of patient outcomes and healthcare efficiency. We showed that ULDCT leads to functional health outcomes at 28 days that are at least similar to those obtained if management is guided by CXR, while resulting in minimal differences in hospital admission rates, length of stay and mortality rates.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
The results of our study enforces the current guidelines that adhere to CXR as first-line imaging technique. Future research should focus on subgroups of patients that might benefit of ULDCT.
Introduction
While chest X-ray (CXR) is a standard diagnostic procedure in patients suspected of non-traumatic pulmonary disease at the emergency department (ED), chest CT highlights chest pathology better than CXR.1 2 Studies in patients with possible community-acquired pneumonia (CAP) and other non-traumatic pulmonary diseases have demonstrated that the diagnostic accuracy of CXR is limited.3–6 Three studies showed CT markedly improved diagnostic accuracy, and subsequently changed diagnoses and clinical management.4 5 7 CT also requires more radiation and increases the risk of radiation-induced cancer.8 9 Ultra-low-dose chest-CT (ULDCT; dose <1 mSv) has overcome this disadvantage, while preserving diagnostic accuracy for many acute pulmonary diseases that present at the ED, like pneumonia and congestive heart failure.10 11
The use of ULDCT reduced false-positive and false-negative CXR findings with consequences for clinical management by 20% in a prospective study in an outpatient setting.7 Yet ULDCT is still more expensive and less accessible than CXR, and incidental findings are more prevalent.12 13 While the superior diagnostic accuracy could lead to faster detection of underlying conditions and timely initiation of effective treatment, incidental findings detected on ULDCT could also complicate healthcare processes, potentially prolonging hospital stay.5
The value of a diagnostic test is not expressed by its accuracy but depends on how it affects patients health.14 New tests should only be introduced into clinical practice when they have demonstrated to impact clinical decision-making, resulting in better patient health outcomes or a simplification of the healthcare process.15 Diagnostic imaging technologies that affect large numbers of patients and hold the potential to substantially increase healthcare costs require more extensive and more robust data on outcomes than those without these attributes.16
At present, there is no direct evidence that patient management in the ED guided by chest-(ULD)CT rather than CXR results in better patient outcomes or a more efficient process of care; for example, with fewer or shorter hospital admissions. We designed a multicentre non-inferiority randomised clinical trial in which we randomly allocated consenting ED patients suspected of non-traumatic pulmonary disease to either ULDCT or CXR.
The link between imaging and health outcomes is an indirect one, and superior accuracy is not guaranteed to lead to improved health outcomes.16 We did not expect ULDCT to lead to better patient outcomes but anticipated that it would result in functional health after 28 days at least as good as obtained with CXR, hence the non-inferiority design. In addition, we hypothesised that improved detection of underlying conditions with ULDCT would lead to a more efficient healthcare process, reflected in fewer hospital admissions and a shorter hospital length of stay, compared with CXR.
Methods
Study design
In this pragmatic, multicentre, non-inferiority randomised clinical trial we compared patient outcomes and short-term health process parameters after ULDCT to those after CXR in ED patients suspected of non-traumatic pulmonary disease. The protocol and statistical analysis plan for this trial on the OPTimal IMAging strategy in patients suspected of non-traumatic pulmonary disease at the ED: chest X-ray or CT (OPTIMACT) have been published earlier.17 18 In short, during randomly assigned periods of one calendar month between 31 January 2017 and 31 May 2018, either ULDCT or conventional CXR was used in two participating Dutch hospitals: one university hospital (Amsterdam UMC) and one large teaching hospital (Spaarne Gasthuis).
The trial was performed according to General Data Protection Regulation and Good Clinical Practice standards. Written informed consent was provided by all study participants. This study report was prepared following the CONsolidated Standards Of Reporting Trials (CONSORT) reporting guidelines, using the extension for non-inferiority and equivalence randomised trials.19
Setting and participants
Eligible for inclusion were ED patients aged 18 years and older, suspected of non-traumatic pulmonary disease and requiring CXR according to the attending physician. Patients could be either self-referred or referred by a general practitioner or their treating physician at the hospital. Excluded were patients unable to undergo ULDCT or CXR, incapacitated patients, pregnant women and patients with a life expectancy less than 1 month or with other anticipated barriers to 28-days follow-up data collection; subjects could only participate once.
Study procedures
History taking, physical examination and laboratory tests were initiated by the attending physician. After setting the indication for chest imaging and acquiring informed consent, the attending physician provided a working diagnosis on the structured and standardised radiology request form. This was followed by either ULDCT or CXR, according to the imaging method allocated to the month of presentation. If the clinical question was not adequately answered after obtaining the CXR or ULDCT, standard additional imaging (eg, chest CT with intravenous contrast medium, CT pulmonary angiography) was performed. If there was a high suspicion of pulmonary emboli at ED admission, patients directly underwent a CT pulmonary angiography, in accordance with regular clinical practice. The technical aspects of the imaging methods can be found in the study protocol paper and online supplemental text S1.17
thoraxjnl-2021-218337supp001.pdf (401.4KB, pdf)
Radiologists used a structured standardised report to optimise and standardise reading. Reading and reporting was performed or supervised by the radiologist on call at the time of clinical management, also outside office hours. The ULDCT and CXR were read with prior imaging if available. To increase inter-reader consistency, the residents and radiologist less experienced in the field of chest imaging were supervised by a group of seven radiologists with a subspecialty in chest imaging. The attending physician subsequently formulated an ED discharge diagnosis. Decisions on additional imaging, treatment, hospital admission and discharge were at the discretion of the attending physician, according to national guidelines, if applicable.
Data collection
Baseline ED data included medical history and physical examination, laboratory, microbiological and radiological test results, diagnosis at ED discharge, prescription of antibiotics or diuretics and hospital admission. Follow-up data after ED discharge included disease course, treatment outcome, additional imaging, hospital length of stay, mortality up to day 28 and patients in follow-up after day 28 because of incidental findings. All data were obtained from electronic patient records.
Whenever necessary, additional information was obtained from general practitioners, nursing wards, outpatient clinics or hospitals where patients had been transferred or referred to. Twenty-eight days after ED presentation study participants received the Short Form (SF)-12 questionnaire; the questionnaires were available in Dutch and English and in electronic and paper form. We prompted with frequent reminders to ensure maximum response.
We assigned one or more final diagnoses after 28 days of follow-up, based on a review of all clinical, radiological and microbiological data available. For this purpose, a diagnostic handbook was developed enabling standardised and reproducible categorisation for 32 diagnoses. More details on the methodology of the handbook, its evaluation and validation are available elsewhere.20 Patients in whom the day 28 diagnosis could be assigned were also assigned an ED discharge diagnosis.
All baseline and follow-up data and questionnaires responses were coded and saved in electronic Case Report Forms (Castor EDC, Amsterdam, The Netherlands).
Outcomes
Considering the wide range of conditions and underlying diagnoses, we selected a generic health-related outcome measure with minimal burden for study participants. The primary outcome was functional health at day 28 after ED presentation, as measured by the physical component summary scale (PCS) score of the SF-12 questionnaire V.1 (scale 0–100, higher score corresponds to better functional health).21 Secondary outcomes included hospital admission, hospital length of stay, mortality within 28 days, number of patients requiring follow-up because of incidental findings on ULDCT or CXR after 28 days and mental health measured by the mental component summary scale (MCS) score of the SF-12 (scale 0–100).21 An economic evaluation will be reported elsewhere.
Statistical analysis
We evaluated all effects in those who underwent the allocated imaging method. We express the effect of imaging as an absolute mean difference in the SF-12 PCS score, with a 95% lower CI. A one-point difference in the mean SF-12 PCS score was defined as the non-inferiority margin.
The primary analyses were done using all available data, with sensitivity analyses using multiple imputation of missing questionnaire responses (using age and sex as predictors) and to adjust for confounding. Since we anticipated non-response of the SF-12 questionnaires, we analysed differences in baseline characteristics between responders and non-responders.
We express all effects on secondary outcomes as differences between groups with two-sided 95% CIs. We hypothesised that replacing CXR by ULDCT would lead to a more efficient healthcare process, reflected in fewer admissions and a shorter hospital length of stay. We calculated the median hospital length of stay and the difference between groups in those admitted using the Hodges-Lehmann estimator. The effect on mortality within 28 days was expressed as an absolute risk difference. The effect on incidental findings is reported as the absolute difference in proportions of patients in follow-up. All analyses were performed in SPSS V.26.
An SD of 10 on the SF-12 was anticipated.22 A power analysis showed that using a 0.05 significance level, 2400 participants were needed to achieve 80% power in excluding a 1 point lower mean SF-12 PCS score for ULDCT, using the two-sample t-test statistic, assuming no actual difference in the mean scores. This 1-point non-inferiority margin comes down to a 0.1 effect size.
Results
Participants
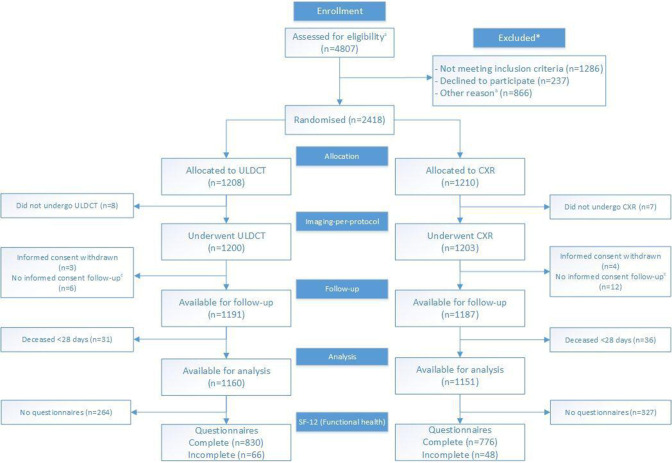
4807 patients presented at the ED with suspected non-traumatic pulmonary disease (figure 1). Of these, 2418 were included: 1208 were allocated to ULDCT and 1210 to CXR (online supplemental table S1 for information on the total eligible group and online supplemental figure S1 for location and month of inclusion). Baseline characteristics of included patients were comparable between groups (table 1). Presenting symptoms and clinical indication on the radiology request form were largely similar, although slightly more ULDCT patients presented with cough and fever and had bronchitis as possible diagnosis (table 1).
Figure 1.
Trial profile. CXR, chest X-ray; SF-12, Short Form 12; ULDCT, ultra-low-dose chest-CT. a Due to privacy regulations the total number of patients assessed for eligibility, and the total number of patients excluded for randomisation are incomplete. These numbers are composed of complete data (Amsterdam UMC, location AMC) and data from a random sample of non-included patients (Spaarne Gasthuis). Specified in online supplemental table 1. b Specified in online supplemental table 1. c Short informed consent form signed at the emergency department as patient was too ill for full consent, giving permission to use imaging information for study purposes. No full informed consent form, giving permission for collection of follow-up information was signed.
Table 1.
Baseline characteristics of study participants*†
| ULDCT (n=1208) | CXR (n=1210) | |
| Mean age (±SD), years | 59.0 (18.1) | 59.0 (18.6) |
| Female sex | 613 (50.7) | 587 (48.5) |
| Comorbidity | ||
| Charlson Comorbidity Index (IQR)‡ | 3.0 (1.0–5.0) | 3.0 (1.0–5.0) |
| Immunocompromised | 285 (23.6) | 246 (20.3) |
| Malignancy§ | 229 (19.0) | 222 (18.3) |
| Diabetes§ | 230 (19.0) | 245 (20.2) |
| Pulmonary disease | ||
| Chronic obstructive pulmonary disease§ | 175 (14.5) | 179 (14.8) |
| Asthma | 141 (11.7) | 109 (9.0) |
| Interstitial lung disease | 29 (2.4) | 28 (2.3) |
| Cystic fibrosis | 14 (1.2) | 14 (1.2) |
| Cardiac disease | ||
| Myocardial infarction§ | 159 (13.2) | 169 (14.0) |
| Chronic cardiac failure§ | 98 (8.1) | 98 (8.1) |
| Neurological disease§ | 140 (11.6) | 149 (12.3) |
| Kidney disease§ | 104 (8.6) | 119 (9.8) |
| Thromboembolic disease | 92 (7.6) | 107 (8.8) |
| Presenting symptoms | ||
| Dyspnoea | 663 (54.9) | 695 (57.4) |
| Cough | 679 (56.2) | 631 (52.1) |
| Fever | 514 (42.5) | 468 (38.7) |
| Thoracic pain | 442 (36.6) | 460 (38.0) |
| Sputum production | 378 (31.3) | 354 (29.3) |
| Haemoptysis | 54 (4.5) | 41 (3.4) |
| Confusion | 50 (4.1) | 41 (3.4) |
| Clinical question on radiology request form | ||
| Pneumonia | 837 (69.3) | 822 (67.9) |
| Pulmonary congestion | 76 (6.3) | 114 (9.4) |
| Bronchitis | 108 (8.9) | 57 (4.7) |
| Pneumothorax | 36 (3.0) | 69 (5.7) |
| Pleural effusion | 35 (2.9) | 46 (3.8) |
| Pulmonary tumour | 10 (0.8) | 10 (0.8) |
| Atelectasis | 4 (0.3) | 3 (0.3) |
| Pulmonary metastases | 4 (0.3) | 3 (0.2) |
| Other | 90 (7.5) | (6.5) |
*See online supplemental table S5 for a detailed composition of this table including specification of variables.
†Values are numbers (percentages) unless otherwise specified.
‡Charlson Comorbidity Index, excluding AIDS. Predicts 10-year survival in patients with multiple comorbidities.32
§Variables included in the Charlson Comorbidity Index.32
CXR, chest X-ray; ULDCT, ultra-low-dose chest CT.
Eight ULDCT and seven CXR patients did not undergo the allocated imaging method. Another 92 patients (40 ULDCT and 52 CXR) only signed a short informed consent, retracted their informed consent during follow-up or died within 28 days, leaving 1160 ULDCT patients and 1151 CXR patients available for analysis of the primary outcome (figure 1).
The results of ULDCT and CXR were directly communicated to the attending physician by phone. Clinical management was based on the initial report. In only 1.0% of ULDCTs and 0.6% of CXRs the initial report was adjusted by the supervisor, this was directly communicated with the attending physician. Information on the availability of prior imaging can be found in online supplemental text S2.
The median ULDCT radiation dose was 0.2 mSv (IQR 0.2–0.3 mSv). The median CXR dose was for portable anterior posterior (AP) CXR 0.02 mSv (IQR 0.02–0.03 mSv) and bucky CXR posterior anterior (PA) and lateral 0.05 mSv (IQR 0.03–0.07 mSv).
Management at the emergency department
More patients in the ULDCT group had a clinical diagnosis of CAP at ED discharge: 255/1161 (22.0%) versus 189/1151 (16.4%), a difference of 5.5% (95% CI: 2.3% to 8.8%). In the ULDCT group, more patients had a diagnosis at ED discharge of (possible) influenza A/B (table 2).
Table 2.
Diagnoses at ED discharge and at Day 28*†
| Diagnosis | ULDCT (n=1161) | CXR (n=1151) | Difference (95% CI) | |||
| Diagnosis at ED discharge | Diagnosis at Day 28 | Diagnosis at ED discharge | Diagnosis at Day 28 | Diagnosis at ED discharge |
Diagnosis at Day 28 |
|
| Community-acquired pneumonia | 255 (22.0) | 225 (19.4) | 189 (16.4) | 169 (14.7) | 5.5 (2.3 to 8.8) | 4.7 (1.6 to 7.8) |
| Lower respiratory tract infection other than pneumonia | 101 (8.7) | 121 (10.4) | 100 (8.7) | 116 (10.1) | 0 (–2.3 to 2.3) | 0.3 (–2.1 to 2.8) |
| COPD exacerbation | 83 (7.1) | 116 (10.0) | 72 (6.3) | 127 (11.0) | 0.8 (–1.1 to 2.9) | −1.0 (–3.5 to 1.5) |
| (Possible) influenza A/B‡ | 74 (6.4) | 96 (8.3) | 37 (3.2) | 73 (6.3) | 3.2 (1.4 to 4.9) | 1.9 (–0.2 to 4.0) |
| Congestive heart failure | 66 (5.7) | 66 (5.7) | 87 (7.6) | 110 (9.6) | −1.9 (–3.9 to 1.5) | −3.9 (–6.0 to –1.7) |
| Asthma exacerbation | 51 (4.4) | 75 (6.5) | 34 (3.0) | 49 (4.3) | 1.4 (–0.1 to 3.0) | 2.2 (0.4 to 4.0) |
| Upper respiratory tract infection | 49 (4.2) | 52 (4.5) | 48 (4.2) | 64 (5.6) | 0 (–1.6 to 1.7) | −1.1 (–2.9 to 0.7) |
| Healthcare-associated pneumonia | 4 (0.3) | 50 (4.3) | 8 (0.7) | 37 (3.2) | −0.4 (–0.9 to 0.2) | 1.1 (–0.5 to 2.6) |
| Extra-thoracic pathology | 259 (22.3) | 299 (25.8) | 246 (21.4) | 338 (29.4) | 0.9 (–2.4 to 4.3) | −3.6 (–7.3 to 0.03) |
| Thoracic pain of unknown origin | 110 (9.5) | 122 (10.5) | 112 (9.7) | 135 (11.7) | −0.3 (–2.7 to 2.2) | −1.2 (–3.8 to 1.3) |
| Fever of unknown origin | 111 (9.6) | 53 (4.6) | 85 (7.4) | 46 (4.0) | 2.2 (–0.1 to 4.4) | 0.6 (–1.1 to 2.2) |
| Other thoracic pathology | 57 (4.9) | 60 (5.2) | 66 (5.7) | 63 (5.5) | −0.8 (–2.7 to 1.0) | −0.3 (–2.1 to 1.5) |
| No definite diagnosis yet | 78 (6.7) | – | 108 (9.4) | – | −2.7 (–4.9 to 0.5) | – |
*Values are numbers (percentages) unless otherwise noted.
†Only diagnoses that occurred in more than 50 patients either at ED discharge or at Day 28 are reported in this table. See online supplemental table S6 for the results of all 32 diagnostic categories. Patients could have more than one diagnosis.
‡(Possible) influenza A/ B: at ED discharge a diagnosis of possible influenza was assigned if a patient was treated for influenza A/B awaiting the results of the PCR test. At day 28 diagnosis of influenza A/B was assigned to PCR positive patients accordingly.
COPD, chronic obstructive pulmonary disease; CXR, chest X-ray; ED, emergency department; ULDCT, ultra-low-dose chest-CT.
The number of clinically relevant incidental findings was 100 (8.3%; 95% CI: 7.0% to 10.0%) in the ULDCT group versus 14 (1.2%; 95% CI: 1.0% to 2.0%) in the CXR group (absolute difference 7.2; 95% CI: 5.5 to 8.9). The most frequently reported incidental findings were pulmonary nodules (ULDCT 54 and CXR 7; online supplemental table S2).
In the ULDCT group 475/1208 patients (39.3%) had one or more additional imaging procedures within 28 days after the initial ULDCT, compared with 652/1210 (53.9%) in the CXR group after the initial CXR, an absolute difference of −14.6% (95% CI: −18.5% to −10.6%).
In the ULDCT group median ED length of stay was 4:47 hours (IQR 3:39–6:21). For CXR median ED length of stay was 4:36 hours (IQR 3:22–6:14). In the ULDCT group 638/1208 (52.7%) patients were admitted to hospital, with a median hospital length of stay of 4.8 days (IQR 2.1–8.8). After CXR 659/1210 (54.5%) patients were admitted to hospital; their median hospital length of stay was 4.6 days (IQR 2.1–8.8). There was a significant difference in the median ED length of stay (0:14 hours, IQR 0:04–0:23) in favour of ULDCT, there was no significant difference in the proportion admitted (1.7%; 95% CI: −0.06 to 0.02) or in the median hospital length of stay between both groups (0.04 days; 95% CI: −0.3 to 0.5). After ULDCT 50/1208 patients (4.1%) were admitted to the intensive care unit versus 44/1210 (3.6%) patients in the CXR group.
Outcomes
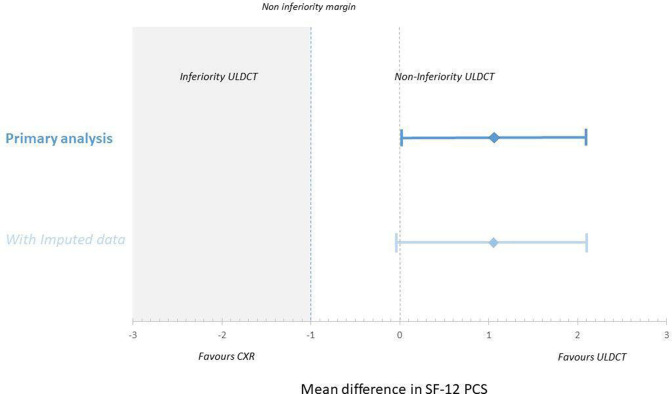
In the ULDCT group 896/1160 (77.2%) of the SF-12 questionnaires were returned (830 complete and 66 incomplete) versus 824/1151 (71.6%) for CXR (776 complete and 48 incomplete). There were only minor differences in baseline characteristics between responders and non-responders (online supplemental tables S3 and S4). Functional health measured by the mean PCS score was 37.0 (SD: 11.1, 95% CI: 36.2 to 37.8) in the ULDCT group compared with 35.9 (SD: 10.6, 95% CI: 35.2 to 36.7) in the CXR group, a difference of 1.1 points (95% lower CI: 0.003) and demonstrating non-inferiority of ULDCT (figure 2, online supplemental figure S2). A sensitivity analysis including 109 imputed PCS scores of partially incomplete questionnaires showed results similar to the primary analysis, with a mean PCS score 36.9 in the ULDCT group (n=892) versus 35.9 in the CXR group (n=823), a difference of 1.0 (one-sided 95% lower CI: −0.06). Additional sensitivity analyses did not produce substantially different findings (online supplemental text S3, figure 2).
Figure 2.
Equivalence plot of the physical component summary scale (PCS) scores of the Short Form (SF)-12 questionnaires. CI primary analysis: 0.003 to 2.13 (difference is 1.1) and CI imputed data: −0.06 to 2.10 (difference is 1.0). CXR, chest X-ray; ULDCT, ultra-low-dose chest-CT.
In 66/2378 patients who gave permission for follow-up (2.8%; 30 ULDCT and 36 CXR) insufficient follow-up data were available to assign the day 28 diagnosis, leaving 1161 ULDCT and 1151 CXR patients. With ULDCT, more patients had a day 28 diagnosis of CAP and asthma exacerbation, while patients in the CXR group more often had a day 28 diagnosis of congestive heart failure (table 2).
At day 28, 26 ULDCT patients (2.2%; 95% CI: 1.0% to 3.0%) and four CXR patients (0.3%; 95% CI: 0.0% to 1.0%) were in follow-up because of clinically relevant incidental findings (absolute difference 1.8; 95% CI: 1.0 to 2.7). Of these, three ULDCT patients and two CXR patients were lost to follow-up.
Mental health measured by the mean MCS score for ULDCT was 46.2 (95% CI: 44.5 to 46.0) versus 45.2 (95% CI: 45.5 to 46.9) for CXR, a mean difference of 1.0 (95% CI: −0.1 to 2.0). Mortality rates within 28 days were 2.6% for ULDCT and 3.0% for CXR, resulting in an absolute risk difference of 0.4% (95% CI: −0.9% to 1.7%).
Discussion
In this trial, we showed that using ULDCT in the diagnostic work-up of patients suspected of non-traumatic pulmonary disease at the ED resulted in functional health outcomes at 28 days that are at least similar to those obtained with management guided by CXR, with minimal differences in ED length of stay, hospital admissions, hospital length of stay and mortality rates, less additional imaging but more incidental findings.
CAP was more often diagnosed at ED discharge, with subsequent confirmation of diagnosis at day 28, in the ULDCT group, reflecting the higher accuracy of ULDCT for CAP.1 2 5 6 However, this hardly affected clinical management. These results are in line with a previous prospective study comparing clinical management and outcomes in adults hospitalised with CAP who had radiological evidence of pneumonia on chest-CT but not on CXR, as compared with patients with radiological evidence on CXR. The 66/2251 (2.9%) patients with only evidence of pneumonia on chest-CT had management and clinical outcomes comparable to CXR patients.23
Similarly, congestive heart failure was more often diagnosed with CXR at day 28. The presumed reason for underdiagnosing congestive heart failure with ULDCT was unfamiliarity of radiologists to detect congestive heart failure on ULDCT in the ED setting.
The similarity in health outcomes with CXR and ULDCT, despite the well-documented lower diagnostic accuracy of CXR,1 2 5–7 is likely explained by CXR’s ability to detect the most relevant diagnoses in ED patients.24 In case a presumed clinical diagnosis is not confirmed by CXR, the attending physician may decide to prompt treatment nonetheless, or perform additional imaging. Indeed, in our study CXR patients underwent significantly more additional imaging procedures within 28 days than in ULDCT patients.
As expected, more ULDCT patients had incidental findings and more were in follow-up because of these findings at 28 days, mostly due to pulmonary nodules. The number of clinically relevant incidental pulmonary nodules and patients in follow-up at day 28 is lower than in previous studies. In a retrospective study of 1000 CT pulmonary angiographies ordered at the ED in a group of patients with an age distribution comparable to our ULDCT cohort, 9.9% of the patients with incidental pulmonary nodules required follow-up, compared with 4.1% in our study (online supplemental table S2).25 This difference is very likely due to differences in comorbidities between both cohorts.
The use of computer-aided diagnosis for lung nodule detection can aid in the detection and follow-up of clinical relevant incidental pulmonary nodules, especially in the hectic work environment of the ED, but the software was not yet available in the radiology departments during our trial. Further studies should evaluate the added value of artificial intelligence in this setting. The impact of incidental findings on long-term functional health could not be assessed in our trial because of the limited follow-up period.
As our study included consecutive ED patients, study limitations are mostly inherent to the demanding workflow at the ED, which sometimes interfered with obtaining informed consent and the logistics to perform ULDCT. Due to the pragmatic nature of this trial, concealment of allocation was not possible. This could have potentially led to information and selection biases. It may explain the higher number of patients with a clinical suspicion of bronchitis for ULDCT and the higher number of patients with possible pulmonary congestion and pneumothorax for CXR. The higher number of patients with a baseline comorbidity of asthma in the ULDCT group, and the resulting higher number of patients with a day 28 diagnosis of asthma exacerbation in the ULDCT group, are probably due to a seasonal increase of asthma exacerbations in the months February, March and April, when ULDCT was more often the allocated method.26 However, the similarity in baseline characteristics and presenting symptoms in both study groups indicates that this was unlikely to result in a systematic bias in our study. The SF-12 questionnaire response (ULDCT 77.2% and CXR 71.6%) was, despite many efforts, lower than anticipated but higher than in earlier studies at the ED.27 28 However, there were only minor differences in baseline characteristics between responders and non-responders (online supplemental tables 2 and 3).
We designed this study as a non-inferiority trial, since we anticipated that the superior accuracy of ULDCT would lead to health outcomes at least as good as after CXR, with a more efficient healthcare process. The point estimate of the mean difference between the two groups shows a 1.1 point difference in mean PCS score in favour of ULDCT. With more returned questionnaires, the precision in this estimate would have been larger and power increased, potentially demonstrating statistically significant superiority of the ULDCT strategy. However, we believe that as difference of 1.1 in mean the PCS-score found is close to the prespecified non-inferiority margin of 1 point and unlikely to be of clinical relevance.
Multiple radiologists with different levels of experience in chest imaging were involved in reading the ULDCT and CXR examinations for this study. This has caused inter-reader variability, although the low proportion of initial reports adjusted by the supervisor (1.0% ULDCTs and 0.6% CXRs) shows that reporting was largely consistent.
This study was performed before the outbreak of the corona virus infectious disease-19 (COVID-19) pandemic. The pandemic changed the incidence, presentation and management of patients suspected of CAP compared with the usual situation, which is not accounted for in our study.29
To our knowledge, this large-scale pragmatic randomised trial is unique in its aim to assess the yield of replacing CXR by ULDCT in the diagnostic work-up of ED patients suspected of non-traumatic pulmonary disease, not in terms of diagnostic accuracy, but in terms of short-term patient outcomes and healthcare efficiency. Participants were included in an urban university hospital and a suburban teaching hospital ensuring an unselected, mixed study population, making results widely applicable. The development and use of the diagnostic handbook adds to the consistency of disease classification and can also be considered one of the strengths of our approach.20
Improved diagnostic accuracy on itself does not automatically translate to improved patient outcomes, as the impact of imaging depends on the outcome of clinical interventions that follow.16 It is therefore argued that significant changes in treatment planning and a meaningful change in patient outcome should be documented for new radiological applications to be accepted.30 Our study showed that ULDCT leads to no marked effect on healthcare efficiency, in terms of number of admissions and hospital length of stay or on short-term health outcomes, in the broad population of ED patients suspected of non-traumatic pulmonary disease. Furthermore, ULDCT has higher immediate imaging costs than CXR while both the examination and reading require more time, which might be a disadvantage at a busy ED. The findings in this trial enforce the current guidelines which adhere to CXR as first-line imaging technique in ED patients suspected of non-traumatic pulmonary disease.31
Conclusion
Considering the availability and costs of ULDCT, the results of our trial do not support the routine use of ULDCT in the work-up of patients presenting with non-traumatic pulmonary disease at the ED.
Acknowledgments
We kindly acknowledge the contribution of Professor Johannes A. Romijn, internist, CEO and Dean of Amsterdam University Medical Centres, for his contribution to the concept of this study.
Footnotes
Twitter: @MiddeldorpS, @MRidderikhof, @pmmbossuyt
IAHvdB and MMNPK contributed equally.
Contributors: JS and JMP were the principal investigators of the trial and contributed equally. JS and JMP were involved in design, planning and execution of the trial, data interpretation and drafting of the manuscript. IAHvdB and MMNPK were involved in design, planning and execution of the trial, data acquisition, data analysis, data verification, data interpretation and drafting of the manuscript. IAHvdB and MMNPK contributed equally. TSRvE was involved in design, planning and execution of the trial, data acquisition, data analysis and data interpretation and revised the manuscript critically. PMB, MGWD and SB were involved in design, planning, data analysis and drafting of the manuscript. JTA, LFMB, MG, SM, ADMvS and MLR were involved in planning of the trial, data interpretation and revised the manuscript critically. JA, BB, MKB, PB, EE, MG, FH, JK, RBvL, MK, PL, LjJM, LS, RSp and MW were involved in data acquisition, and revised the manuscript critically. SH, MK, IvdL, PL, RSo and WdM were involved in planning and execution of the trial, and revised the manuscript critically. All authors approved the final version of the manuscript and agree to be accountable for all aspects of this trial. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. IAHvdB and MMNPK act as guarantors for the work and accept full responsibility for the work and the conduct of the study, had access to the data and controlled the decision to publish. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. JMP and JS shared last authorship.
Funding: The research was funded by an innovation grant of the Amsterdam University Medical Centres (Amsterdam UMC), location Academic Medical Centre (AMC), University of Amsterdam and a Health Care Efficiency Program grant of the Netherlands Organization for Health Research and Development (ZonMW: 843001806).
Competing interests: All authors have completed and submitted the ICMJE form for Disclosure of Potential Conflicts of Interest. JS reported receipt of an innovation grant of the Amsterdam UMC, location AMC, University of Amsterdam and a grant of Health Care Efficiency Program of the Netherlands Organization for Health Research and Development (ZonMW:843001806). MMNPK reports to have received personal fees from the before-mentioned grants. SM reports to have received grants and personal fees from Bayer, BMS Pfizer, Boehringer Ingelheim, Daiichi Sankyo, Portola, GSK and Aspen. TSRvE reports to have received a personal fee, PhD Scholarship grant, from Amsterdam UMC.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. The data that support the findings of this study are available from the corresponding author, IAHvdB, upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Ethical approval by the medical ethical commission of the Amsterdam UMC, location AMC, is obtained on 24 November 2016 (NL57923.018.16). Participants gave informed consent to participate in the study before taking part.
References
- 1. Hayden GE, Wrenn KW. Chest radiograph vs. computed tomography scan in the evaluation for pneumonia. J Emerg Med 2009;36:266–70. 10.1016/j.jemermed.2007.11.042 [DOI] [PubMed] [Google Scholar]
- 2. Self WH, Courtney DM, McNaughton CD, et al. High discordance of chest X-ray and computed tomography for detection of pulmonary opacities in ED patients: implications for diagnosing pneumonia. Am J Emerg Med 2013;31:401–5. 10.1016/j.ajem.2012.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mueller-Lenke N, Rudez J, Staub D, et al. Use of chest radiography in the emergency diagnosis of acute congestive heart failure. Heart 2006;92:695–6. 10.1136/hrt.2005.074583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gezer NS, Balcı P, Tuna Kemal Çağlar, et al. Utility of chest CT after a chest X-ray in patients presenting to the ED with non-traumatic thoracic emergencies. Am J Emerg Med 2017;35:623–7. 10.1016/j.ajem.2016.12.058 [DOI] [PubMed] [Google Scholar]
- 5. Claessens Y-E, Debray M-P, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med 2015;192:974–82. 10.1164/rccm.201501-0017OC [DOI] [PubMed] [Google Scholar]
- 6. Syrjälä H, Broas M, Suramo I, et al. High-Resolution computed tomography for the diagnosis of community-acquired pneumonia. Clin Infect Dis 1998;27:358–63. 10.1086/514675 [DOI] [PubMed] [Google Scholar]
- 7. Kroft LJM, van der Velden L, Girón IH, et al. Added value of Ultra-low-dose computed tomography, dose equivalent to chest X-ray radiography, for diagnosing chest pathology. J Thorac Imaging 2019;34:179–86. 10.1097/RTI.0000000000000404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hart D, Wall BF, Hillier MC. Frequency and collective dose for medical and dental X-ray examinations in the UK, 2008: HPA-CRCE. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/340154/HPA-CRCE-012_for_website.pdf [Accessed 31 July 2021].
- 9. Backgrounder on biological effects of radiation: U.S. NRC, 2017. Available: https://www.nrc.gov/reading-rm/doc-collections/fact-sheets/bio-effects-radiation.html [Accessed 31 July 2021].
- 10. Kim Y, Kim YK, Lee BE, et al. Ultra-low-dose CT of the thorax using iterative reconstruction: evaluation of image quality and radiation dose reduction. AJR Am J Roentgenol 2015;204:1197–202. 10.2214/AJR.14.13629 [DOI] [PubMed] [Google Scholar]
- 11. Tækker M, Kristjánsdóttir B, Graumann O, et al. Diagnostic accuracy of low-dose and ultra-low-dose CT in detection of chest pathology: a systematic review. Clin Imaging 2021;74:139–48. 10.1016/j.clinimag.2020.12.041 [DOI] [PubMed] [Google Scholar]
- 12. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology 2017;284:228–43. 10.1148/radiol.2017161659 [DOI] [PubMed] [Google Scholar]
- 13. Munden RF, Carter BW, Chiles C, et al. Managing incidental findings on thoracic CT: mediastinal and cardiovascular findings. A white paper of the ACR incidental findings Committee. J Am Coll Radiol 2018;15:1087–96. 10.1016/j.jacr.2018.04.029 [DOI] [PubMed] [Google Scholar]
- 14. Ferrante di Ruffano L, Hyde CJ, McCaffery KJ, et al. Assessing the value of diagnostic tests: a framework for designing and evaluating trials. BMJ 2012;344:e686. 10.1136/bmj.e686 [DOI] [PubMed] [Google Scholar]
- 15. Fineberg HV. Evaluation of computed tomography: achievement and challenge. AJR Am J Roentgenol 1978;131:1–4. 10.2214/ajr.131.1.1 [DOI] [PubMed] [Google Scholar]
- 16. Gazelle GS, Kessler L, Lee DW, et al. A framework for assessing the value of diagnostic imaging in the era of comparative effectiveness research. Radiology 2011;261:692–8. 10.1148/radiol.11110155 [DOI] [PubMed] [Google Scholar]
- 17. van den Berk IAH, Kanglie MMNP, van Engelen TSR, et al. OPTimal IMAging strategy in patients suspected of non-traumatic pulmonary disease at the emergency department: chest X-ray or ultra-low-dose CT (OPTIMACT)-a randomised controlled trial chest X-ray or ultra-low-dose CT at the ED: design and rationale. Diagn Progn Res 2018;2:20. 10.1186/s41512-018-0038-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanglie MMNP, Bipat S, van den Berk IAH, et al. Optimal imaging strategy in patients suspected of non-traumatic pulmonary disease at the emergency department: chest X-ray or ultra-low-dose chest CT (OPTIMACT) trial-statistical analysis plan. Trials 2020;21:407. 10.1186/s13063-020-04343-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Piaggio G, Elbourne DR, Pocock SJ, et al. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 2012;308:2594–604. 10.1001/jama.2012.87802 [DOI] [PubMed] [Google Scholar]
- 20. van Engelen TSR, Kanglie MMNP, van den Berk IAH, et al. Classifying the diagnosis of study participants in clinical trials: a structured and efficient approach. Eur Radiol Exp 2020;4:44. 10.1186/s41747-020-00169-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ware J, Kosinski M, Keller SD. A 12-Item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33. 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 22. Jenkinson C, Layte R, Jenkinson D, et al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med 1997;19:179–86. 10.1093/oxfordjournals.pubmed.a024606 [DOI] [PubMed] [Google Scholar]
- 23. Upchurch CP, Grijalva CG, Wunderink RG, et al. Community-Acquired pneumonia visualized on CT scans but not chest radiographs: pathogens, severity, and clinical outcomes. Chest 2018;153:601–10. 10.1016/j.chest.2017.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franquet T. Imaging of pneumonia: trends and algorithms. Eur Respir J 2001;18:196–208. 10.1183/09031936.01.00213501 [DOI] [PubMed] [Google Scholar]
- 25. Blagev DP, Lloyd JF, Conner K, et al. Follow-Up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol 2014;11:378–83. 10.1016/j.jacr.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 26. Osborne NJ, Alcock I, Wheeler BW, et al. Pollen exposure and hospitalization due to asthma exacerbations: daily time series in a European City. Int J Biometeorol 2017;61:1837–48. 10.1007/s00484-017-1369-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oei EHG, Nikken JJ, Ginai AZ, et al. Costs and effectiveness of a brief MRI examination of patients with acute knee injury. Eur Radiol 2009;19:409–18. 10.1007/s00330-008-1162-z [DOI] [PubMed] [Google Scholar]
- 28. Watson MC, Ferguson J, Barton GR, et al. A cohort study of influences, health outcomes and costs of patients' health-seeking behaviour for minor ailments from primary and emergency care settings. BMJ Open 2015;5:e006261. 10.1136/bmjopen-2014-006261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020;296:200642. 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goehler A, Gazelle GS. Examining the use of comparative and cost-effectiveness analyses in radiology. AJR Am J Roentgenol 2014;203:939–44. 10.2214/AJR.14.12887 [DOI] [PubMed] [Google Scholar]
- 31. Expert Panel on Thoracic Imaging:, Jokerst C, Chung JH, et al. ACR Appropriateness Criteria® Acute Respiratory Illness in Immunocompetent Patients. J Am Coll Radiol 2018;15:S240–51. 10.1016/j.jacr.2018.09.012 [DOI] [PubMed] [Google Scholar]
- 32. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2021-218337supp001.pdf (401.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. The data that support the findings of this study are available from the corresponding author, IAHvdB, upon reasonable request.