Abstract
Objectives
To investigate factors associated with severe COVID-19 in people with psoriasis (PsO), psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA).
Methods
Demographic data, clinical characteristics and COVID-19 outcome severity of adults with PsO, PsA and axSpA were obtained from two international physician-reported registries. A three-point ordinal COVID-19 severity scale was defined: no hospitalisation, hospitalisation (and no death) and death. ORs were estimated using multivariable ordinal logistic regression.
Results
Of 5045 cases, 18.3% had PsO, 45.5% PsA and 36.3% axSpA. Most (83.6%) were not hospitalised, 14.6% were hospitalised and 1.8% died. Older age was non-linearly associated with COVID-19 severity. Male sex (OR 1.54, 95% CI 1.30 to 1.83), cardiovascular, respiratory, renal, metabolic and cancer comorbidities (ORs 1.25–2.89), moderate/high disease activity and/or glucocorticoid use (ORs 1.39–2.23, vs remission/low disease activity and no glucocorticoids) were associated with increased odds of severe COVID-19. Later pandemic time periods (ORs 0.42–0.52, vs until 15 June 2020), PsO (OR 0.49, 95% CI 0.37 to 0.65, vs PsA) and baseline exposure to TNFi, IL17i and IL-23i/IL-12+23i (OR 0.57, 95% CI 0.44 to 0.73; OR 0.62, 95% CI 0.45 to 0.87; OR 0.67, 95% CI 0.45 to 0.98; respectively; vs no disease-modifying antirheumatic drug) were associated with reduced odds of severe COVID-19.
Conclusion
Older age, male sex, comorbidity burden, higher disease activity and glucocorticoid intake were associated with more severe COVID-19. Later pandemic time periods, PsO and exposure to TNFi, IL17i and IL-23i/IL-12+23i were associated with less severe COVID-19. These findings will enable risk stratification and inform management decisions for patients with PsO, PsA and axSpA during COVID-19 waves or similar future respiratory pandemics.
Keywords: Covid-19; Arthritis, Psoriatic; Arthritis; Autoimmunity; Spondylitis, Ankylosing
WHAT IS ALREADY KNOWN ON THIS TOPIC
Factors associated with severe COVID-19 outcomes have been demonstrated in both registry-based and population-based studies for people with immune-mediated inflammatory diseases (IMIDs) collectively and for specific IMIDs.
However, relevant risk factor data are limited for axial spondyloarthritis (axSpA) and psoriatic disease (including psoriasis without arthritis (PsO) and psoriatic arthritis (PsA)), a group of conditions that shares pathophysiological mechanisms and approved treatments, particularly targeted therapies.
WHAT THIS STUDY ADDS
Older age, male sex, comorbidity burden, higher disease activity and glucocorticoid intake were associated with more severe COVID-19.
Later pandemic time periods, PsO and exposure to TNFi, IL17i and IL-23i/IL-12+23i were associated with less severe COVID-19.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The findings from this study will enable risk stratification for patients with PsO, PsA and axSpA.
These findings will inform the development of tailored management strategies and evidence-based recommendations for patients with PsO, PsA and axSpA.
Introduction
The COVID-19 pandemic has significantly impacted people with immune-mediated inflammatory diseases (IMIDs), particularly those taking immunomodulatory drugs such as biological or targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs).1–4 While risk factors for severe COVID-19 outcomes have been demonstrated in both registry-based and population-based studies, for people with IMIDs collectively and for specific diseases such as rheumatoid arthritis, relevant risk factor data are limited for axial spondyloarthritis (axSpA) and psoriatic disease (including psoriasis without arthritis (PsO) and psoriatic arthritis (PsA)).5–13 The association of specific classes of b/tsDMARDs commonly used in this population, including IL-17 inhibitors (IL17i) and IL-23 or IL-12/23 inhibitors (IL-23i/IL-12+23i), with COVID-19 outcomes has not been well studied. Improved understanding of the risks associated with exposure to these medications in this population will address knowledge gaps as we continue to navigate COVID-19 risks in the postvaccination era.
We used data from the COVID-19 Global Rheumatology Alliance (C19-GRA) and the Psoriasis Patient Registry for Outcomes, Therapy and Epidemiology of COVID-19 Infection (PsoProtect) physician-reported registries to evaluate the associations of baseline characteristics, including different classes of b/tsDMARDs, with COVID-19 severity in people with PsO, PsA and axSpA.
Methods
Data source
The C19-GRA physician-reported observational registry launched on 24 March 2020. Patients are eligible for inclusion if they have both a pre-existing rheumatic disease and SARS-CoV-2 infection. PsoProtect is a physician-reported observational registry launched on 27 March 2020. Patients are eligible for inclusion if they have both pre-existing PsO and SARS-CoV-2 infection. For both registries, data are entered voluntarily into the data entry systems by rheumatologists/dermatologists or under the supervision of rheumatologists/dermatologists. In Argentina, Brazil, France, Germany, Italy, Portugal and Sweden, C19-GRA data are transferred from national registries; in all other countries, data are entered directly into the registries’ data entry systems. Countries were categorised according to the six WHO regions (www.who.int); the ‘Americas’ was further divided into north and south. Further details of the registries have been described elsewhere.13–18 We used data collected on or before 25 October 2021.
COVID-19 reporting and primary outcome of interest
Both confirmed and presumptive cases of COVID-19 were reported. For analysis, patients were subsequently categorised into (1) confirmed or high likelihood of COVID-19 (chest imaging (CT or chest X-ray) showing bilateral infiltrates and/or symptoms after close contact with a known laboratory-confirmed COVID-19 positive patient) or (2) presumptive cases based on symptoms alone.
The primary outcome of interest of this study was COVID-19 outcome, assessed by use of an ordinal COVID-19 severity scale with three mutually exclusive categories: (1) no hospitalisation and no death; (2) hospitalisation, but no death and (3) death. ‘Baseline characteristics’ refer to demographic or clinical characteristics at the time of COVID-19 symptom onset (or diagnosis if asymptomatic).
IMID treatment prior to COVID-19
Medications used to treat the IMID prior to COVID-19 diagnosis were categorised into groups. Immunomodulatory drugs (conventional synthetic (cs)/biological (b)/targeted synthetic (ts) DMARDs) were distinguished from the PsO-specific non-biological systemic agent acitretin as well as from non-steroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids (GC). csDMARDs included antimalarials, cyclosporine, leflunomide, methotrexate and sulfasalazine. bDMARDs included TNFi (eg, adalimumab, certolizumab, etanercept, golimumab, infliximab and TNFi biosimilars), IL-17i (eg, brodalumab, ixekizumab and secukinumab), IL-12/23i (ustekinumab) and IL-23i (eg, guselkumab, risankizumab and tildrakizumab). tsDMARDs included apremilast and JAKi (eg, baricitinib, tofacitinib and upadacitinib). IL-23i and IL-12/23i were combined in the same group for data analysis (IL-23i/IL-12+23 i). Regarding NSAIDs, we asked physicians to report if at the time of COVID-19 symptom onset (or diagnosis if asymptomatic), the patient was taking NSAIDs, without specifying a minimal duration of a continuous treatment with NSAIDs. We chose no current DMARD use as the reference group after considering the groups’ sample size and internal validity to be used as comparator for exposure to the various IMID treatments. For more details regarding the choice of DMARD reference category, refer to online supplemental methods.
ard-2022-223499supp003.pdf (72.1KB, pdf)
Statistical analyses
Descriptive tables were produced for the whole cohort and by diagnostic group (PsO, PsA and axSpA, as defined by the reporting healthcare professional). All patients with confirmed or presumptive COVID-19 were included in the primary analysis.
Independent associations between demographic and disease features and the ordinal COVID-19 outcome were estimated by multivariable ordinal logistic regression using the proportional odds model and were reported as OR and 95% CIs. In ordinal regression analysis, the effect size of a categorical predictor gives the change in log odds of being at least one level higher on the ordinal COVID-19 severity scale compared with the reference category of the predictor variable, while for a continuous predictor, it gives the change in odds of being one level higher on the ordinal COVID-19 severity scale for a unit increase in the continuous predictor. More details about assumptions of the proportional odds model are provided in online supplemental methods.
Factors potentially associated with the COVID-19 outcome considered in the models were age, sex, smoking habits (ever, unknown/missing, never), pandemic calendar period (until 15 June 2020, 16 June 2020 to 31 December 2020, 1 January 2021 and later), key comorbidities (chronic obstructive pulmonary disease (COPD) or asthma, other chronic lung disease, chronic kidney disease (CKD), hypertension, other cardiovascular disease (CVD), obesity, diabetes, cancer), IMID diagnostic category, IMID disease activity as per physician’s global assessment (remission/low vs moderate/high), DMARD treatment prior to COVID-19 diagnosis, GC use and NSAID use.
For patients classified as having more than one IMID or being treated with more than one of the medications of interest, we created a hierarchy based on clinical expertise to categorise patients. This way, non-overlapping (mutually exclusive) categories are obtained, allowing a clear reference group for interpretation of the regression models, and avoiding collinearities. Patients labelled as having both PsA and axSpA were counted as PsA patients. Patients receiving multiple csDMARDs were grouped according to the following hierarchy: cyclosporine>sulfasalazine>leflunomide>methotrexate>antimalarials, where ‘A>B’ means ‘A has priority over B’. Patients receiving a b/tsDMARD and additionally a csDMARD were considered in the model solely in the b/tsDMARD group (ie, b/tsDMARD>csDMARD).
We tested four two-way additive interactions in the models: hypertension and CVD; obesity and diabetes; cancer and smoking habits; and disease activity and prednisolone-equivalent GC use. Online supplemental methods provide more details regarding statistical interactions.
To account for heterogeneity between participating countries regarding healthcare systems and infection dynamics, countries were considered as random effects in the regression analyses. To appropriately estimate the well-established non-linear effect of age on the outcome of SARS-CoV-2 infection, we included restricted cubic splines in the regression models. Four knots were chosen for most analyses, while three knots were chosen for the outcome mortality and the disease-specific analyses due to the limited effective sample size.19
Missing data were handled using multiple imputation; results of the logistic regression analyses for 10 imputed datasets were pooled by Rubin’s rules. As disease activity was missing for all patients entered from France in the C19-GRA registry, country-level life expectancy was used in the imputation model to explain potential structural differences in disease activity between countries not accounted for in the patient-level data (data from 2018, source: http://hdr.undp.org/). For more details regarding excluded patients and handling of missing data, refer to online supplemental methods.
IMIDs differ regarding the DMARDs approved for their treatment. To explore the impact of this heterogeneity on the associations of interest, in addition to the primary analysis with all patients, diagnostic categories were defined, and stratified secondary analyses were undertaken separately for patients with PsO, PsA and axSpA.
The following sensitivity analyses were also performed to examine the robustness of our findings: (1) analysis limited to patients with confirmed or highly likely COVID-19; (2) analysis using the alternative binary outcome ‘hospitalisation’; (3) analysis using the alternative binary outcome ‘death’. In the model using death as dependent variable, comorbidities were analysed as an independent binary variable (3 or more comorbidities vs less than 3), to minimise the risk of overfitting. Data were considered statistically significant for p values<0.05. All analyses were conducted in SAS (V.9.4) and R (V.4.0.4).
Results
Study sample and baseline characteristics
The study population included 5045 cases, of which 921 (18.3%) were patients with PsO, 2293 (45.5%) with PsA, and 1831 (36.3%) with axSpA. Overall, the mean age was 50 years (SD 13.5), just over half were male (51.7%) and most were from Europe (77.5%) (table 1). Cases were reported fairly equally across the three pandemic time periods. Most cases had disease (IMIDs) in remission or minimal/low disease activity (82.7%). About half had no key comorbidities reported (52.9%). Of those with comorbidities, the most reported were hypertension (26.5%) and obesity (21.1%). Any csDMARD use was reported in 30.3%, with methotrexate as the most common (23.4%). Only 5.6% reported using sulfasalazine. bDMARD use was reported in 65.7% (TNFi 45.6%, IL17i 12.1%, IL-23i/IL-12+23i 8.1%). Only 1.2% reported JAKi use. Baseline GC use was reported in only 7.3% (4.6%, 0–7.5 mg/day and 1.4%, >7.5 mg/day) and NSAID use in 24%.
Table 1.
Baseline characteristics of the study population (total and stratified by immune-mediated inflammatory disease diagnosis)
| Parameter | Psoriatic arthritis | Axial spondyloarthritis | Psoriasis (without arthritis) | Total |
| N | 2293 | 1831 | 921 | 5045 |
| General | ||||
| Age (years) | 53.2 (12.8) | 46.9 (13.4) | 48.4 (13.6) | 50 (13.5) |
| ≤30 years | 110 (4.8) | 211 (11.5) | 100 (10.9) | 421 (8.3) |
| 31–50 years | 785 (34.2) | 900 (49.2) | 400 (43.4) | 2085 (41.3) |
| 51–65 years | 1032 (45) | 567 (31) | 336 (36.5) | 1935 (38.4) |
| 66–75 years | 280 (12.2) | 109 (6) | 59 (6.4) | 448 (8.9) |
| >75 years | 86 (3.8) | 44 (2.4) | 26 (2.8) | 156 (3.1) |
| Male sex | 1053 (45.9) | 996 (54.4) | 557 (60.5) | 2606 (51.7) |
| Ever-smoker | 566 (33.2) (N=1705) (Missing=588) |
313 (23.9) (N=1311) (Missing=520) |
334 (36.3) (N=921) (Missing=0) |
1213 (30.8) (N=3937) (Missing=1108) |
| Regions | ||||
| African Region | 5 (0.2) | 5 (0.3) | 4 (0.4) | 14 (0.3) |
| Eastern Mediterranean Region | 34 (1.5) | 31 (1.7) | 7 (0.8) | 72 (1.4) |
| European Region | 1707 (74.4) | 1396 (76.2) | 805 (87.4) | 3908 (77.5) |
| North American Region | 454 (19.8) | 221 (12.1) | 53 (5.8) | 728 (14.4) |
| South American Region | 62 (2.7) | 159 (8.7) | 46 (5) | 267 (5.3) |
| South-East Asian Region | 10 (0.4) | 8 (0.4) | 3 (0.3) | 21 (0.4) |
| Western Pacific Region | 21 (0.9) | 11 (0.6) | 3 (0.3) | 35 (0.7) |
| Time period | ||||
| Until 15 June 2020 | 744 (32.4) | 564 (30.8) | 417 (45.3) | 1725 (34.2) |
| From 16 June 2020 to 31 December 2020 | 1043 (45.5) | 880 (48.1) | 340 (36.9) | 2263 (44.9) |
| 1 January 2021 and later | 506 (22.1) | 387 (21.1) | 164 (17.8) | 1057 (21) |
| Ordinal outcome | ||||
| Not hospitalised, no death | 1850 (80.7) | 1576 (86.1) | 794 (86.2) | 4220 (83.6) |
| Hospitalised, no death | 392 (17.1) | 229 (12.5) | 115 (12.5) | 736 (14.6) |
| Death | 51 (2.2) | 26 (1.4) | 12 (1.3) | 89 (1.8) |
| Disease activity | (N=2014) (Missing=279) |
(N=1288) (Missing=532) |
(N=920) (Missing=1) |
N=4233 (Missing=812) |
| Remission/low disease activity | 1670 (82.9) | 1090 (83.9) | 740 (80.4) | 3500 (82.7) |
| Moderate/high disease activity | 344 (17.1) | 209 (16.1) | 180 (19.6) | 733 (17.3) |
| Comorbidities | (N=2266) (Missing=27) |
(N=1809) (Missing=22) |
(N=921) (Missing=0) |
N=4996 (Missing=49) |
| Hypertension | 744 (32.8) | 383 (21.2) | 196 (21.3) | 1323 (26.5) |
| Cardiovascular disease | 179 (7.9) | 88 (4.9) | 71 (7.7) | 338 (6.8) |
| COPD or asthma | 185 (8.2) | 114 (6.3) | 62 (6.7) | 361 (7. |
| Other chronic lung disease | 47 (2.1) | 30 (1.7) | 22 (2.4) | 99 (2) |
| Chronic kidney disease | 60 (2.6) | 24 (1.3) | 17 (1.8) | 101 (2) |
| Diabetes | 316 (13.9) | 126 (7) | 120 (13) | 562 (11.2) |
| Cancer | 65 (2.9) | 26 (1.4) | 24 (2.6) | 115 (2.3) |
| Obesity | 522 (23) | 262 (14.5) | 269 (29.2) | 1053 (21.1) |
| No of comorbidities | 1 (1.2) | 0.6 (1) | 1 (1.2) | 0.8 (1.1) |
| No comorbidity | 1077 (47.5) | 1124 (62.1) | 442 (48) | 2643 (52.9) |
| 1 comorbidity | 573 (25.3) | 418 (23.1) | 248 (26.9) | 1239 (24.8) |
| 2 comorbidities | 350 (15.4) | 181 (10) | 127 (13.8) | 658 (13.2) |
| ≥3 comorbidities | 266 (11.7) | 86 (4.8) | 104 (11.3) | 456 (9.1) |
| DMARDs (monotherapy or combination therapy) |
||||
| csDMARDs | 1068 (46.6) | 353 (19.3) | 107 (11.6) | 1528 (30.3) |
| Antimalarials | 30 (1.3) | 7 (0.4) | 0 | 37 (0.7) |
| Methotrexate | 889 (38.8) | 194 (10.6) | 100 (10.9) | 1183 (23.4) |
| Leflunomide | 94 (4.1) | 13 (0.7) | 0 | 107 (2.1) |
| Sulfasalazine | 119 (5.2) | 164 (9) | 0 | 283 (5.6) |
| Cyclosporine | 11 (0.5) | 0 | 8 (0.9) | 19 (0.4) |
| bDMARDs | 1341 (58.5) | 1347 (73.6) | 627 (68.1) | 3315 (65.7) |
| TNF inhibitors | 895 (39) | 1176 (64.2) | 227 (24.6) | 2298 (45.6) |
| IL-17 inhibitors | 301 (13.1) | 164 (9) | 145 (15.7) | 610 (12.1) |
| IL-23/IL-12+23 inhibitors | 145 (6.3) | 7 (0.4) | 255 (27.7) | 407 (8.1) |
| tsDMARDs | 111 (4.8) | 11 (0.6) | 19 (2.1) | 141 (2.8) |
| JAK inhibitors | 51 (2.2) | 11 (0.6) | 0 | 62 (1.2) |
| Apremilast | 60 (2.6) | 0 | 19 (2.1) | 79 (1.6) |
| No DMARD treatment | 234 (10.2) | 309 (16.9) | 179 (19.4) | 722 (14.3) |
| Other therapies | ||||
| Glucocorticoids (#) | 241 (10.7) (N=2242) (Missing=51) |
110 (6.3) (N=1760) (Missing=71) |
6 (0.7) (N=921) (Missing=0) |
357 (7.3) (N=4923) (Missing=122) |
| 0 mg/day <glucocorticoids ≤7.5 mg/day | 167 (7.5) (N=2215) (Missing=78) |
54 (3.1) (N=1724) (Missing=107) |
3 (0.3) (N=919) (Missing=2) |
224 (4.6) (N=4858) (Missing=187) |
| Glucocorticoids >7.5 mg/day | 46 (2.1) (N=2215) (Missing=78) |
19 (1.1) (N=1724) (Missing=107) |
1 (0.1) (N=919) (Missing=2) |
66 (1.4) (N=4858) (Missing=187) |
| NSAIDs | 512 (24.5) (N=2094) (Missing=199) |
553 (35.5) (N=1559) (Missing=272) |
32 (3.5) (N=921) (Missing=0) |
1097 (24) (N=4574) (Missing=471) |
| Acitretin | 3 (0.1) | 0 | 26 (2.8) | 29 (0.6) |
Data are N (column %) for categorical variables or mean (SD) for continuous variables. Table includes patients diagnosed with psoriasis without arthritis, psoriatic arthritis or axial spondyloarthritis, with a non-missing ordinal outcome and non-missing values for age, sex, time period and DMARDs. Further, patients receiving multiple b/tsDMARDs or DMARDs not typical for the three diagnoses were excluded, as well as patients labelled as having additional inflammatory rheumatic diseases (529 patients excluded in total). Data refer to patients with non-missing values for the respective variable; total N for patients with non-missing values is given in parentheses for variables with missing values; the total number of missing values is also given in parenthesis, for the applicable variables. (#) Includes patients with a missing glucocorticoid dosage.
bDMARD, biological disease-modifying anti-rheumatic drugs; BMI, body mass index; COPD, chronic obstructive pulmonary disease; csDMARD, conventional synthetic DMARD; DMARD, disease-modifying antirheumatic drug; IL, interleukin; JAK, Janus kinase; N, number; NSAID, non-steroidal anti-inflammatory drugs; TNF, tumour necrosis factor; tsDMARD, targeted synthetic DMARD.
When stratified by condition (table 1), the main notable differences were that individuals with PsA were older (mean 53.2 years vs 46.9 years in axSpA and 48.4 years in PsO), a higher proportion of those with PsA had hypertension (32.8% vs 21.2% in axSpA and 21.3% in PsO) and a higher proportion of those with PsO were obese (29.2% vs 23% in PsA and 14.5% in axSpA). csDMARDs were most used among individuals with PsA (46.6% vs 19.3% in axSpA and 11.6% in PsO) while bDMARDs were most used among individuals with axSpA (73.6% vs 58.5% in PsA and 68.1% in PsO). Baseline GC usage was low overall but differed notably between the groups, with almost none in PsO (0.7%) vs 10.7% in PsA and 6.3% in axSpA. There was no difference across disease groups with regard to disease activity.
When stratified by medication group (online supplemental table 1), patients not taking DMARDs were slightly younger (mean 49.9 years) than patients taking DMARDs (range from 50 to 56.2 years, depending on the DMARD group) except for IL-17i/IL-23i/IL12+23i (mean 49.9 years) and TNFi (mean 48.3 years). Moreover, patients not taking DMARDs were slightly less often in remission/low disease activity (71.%) than patients taking DMARDs (range from 80.5% to 86.1%, depending on the DMARD group) except for JAKi (65.3% in remission/low disease activity).
ard-2022-223499supp002.pdf (245.5KB, pdf)
COVID-19 outcomes
Baseline characteristics of the study population stratified by COVID-19 outcome are shown in online supplemental table 2. Most patients (4220, 83.6%) were not hospitalised, 736 (14.6%) were hospitalised and 89 (1.8%) died. The frequency of hospitalisation (without death) and death were slightly higher in PsA (17.1% and 2.2%, respectively), compared with axSpA (12.5% and 1.4%, respectively) and PsO (12.5% and 1.3%, respectively) (table 1).
Associations of baseline characteristics with COVID-19 severity
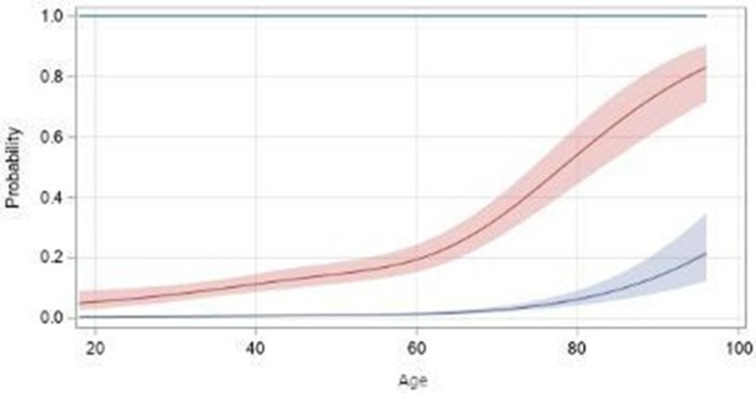
The results of the primary multivariable ordinal logistic regression model are shown in table 2 and the relationship between age and probability of hospitalisation and death is shown in figure 1.
Table 2.
Multivariable ordinal logistic regression analysis of factors associated with COVID-19 severity (primary model, all patients)
| N total | 5045 | |||
| N deaths/hospitalisations without death/neither | 89/736/4220 | |||
| N deaths/hospitalisations without death/neither | OR | 95% CI | ||
| Male sex (vs female) | 57/419/2130 | 1.54 | 1.30 | 1.83 |
| Pandemic time period | ||||
| Until 15 June 2020 | 45/395/1285 | 1 | (Reference) | |
| 16 June 2020–31 December 2020 | 28/217/2018 | 0.42 | 0.34 | 0.51 |
| 1 January 2021 and later | 16/124/917 | 0.52 | 0.41 | 0.67 |
| Comorbidities | ||||
| Hypertension alone (vs no hypertension, no CVD) | 28/242/847 | 1.25 | 1.01 | 1.55 |
| CVD alone (vs no hypertension, no CVD) | 7/38/76 | 1.87 | 1.21 | 2.90 |
| CVD and hypertension (vs no hypertension, no CVD) | 21/63/136 | 1.41 | 0.98 | 2.02 |
| COPD or asthma | 21/87/257 | 1.75 | 1.33 | 2.31 |
| Other lung disease | 11/34/55 | 2.54 | 1.64 | 3.93 |
| Chronic kidney disease | 14/42/46 | 2.32 | 1.50 | 3.58 |
| Obesity alone (vs no obesity, no diabetes) | 11/138/676 | 1.35 | 1.07 | 1.70 |
| Diabetes mellitus alone (vs no obesity, no diabetes) | 15/102/212 | 1.84 | 1.38 | 2.45 |
| Obesity and diabetes mellitus (vs no obesity, no diabetes) | 14/61/162 | 1.89 | 1.34 | 2.68 |
| Cancer and known smoking habits (vs no cancer, never smoked) | 4/29/57 | 1.13 | 0.68 | 1.88 |
| Cancer and unknown smoking habits (vs no cancer, never smoked) | 5/7/13 | 2.89 | 1.19 | 6.97 |
| No cancer and ever smoked or unknown smoking habits (vs no cancer, never smoked) | 38/281/1933 | 0.87 | 0.71 | 1.06 |
| Rheumatic disease | ||||
| Psoriatic arthritis | 51/392/1850 | 1 | (Reference) | |
| Axial spondyloarthritis | 26/229/1576 | 1.07 | 0.86 | 1.33 |
| Psoriasis (without arthritis) | 12/115/794 | 0.49 | 0.37 | 0.65 |
| Medication | ||||
| No DMARD therapy | 21/128/573 | 1 | (Reference) | |
| Antimalarials | 0/4/14 | 1.08 | 0.30 | 3.84 |
| Methotrexate | 13/133/449 | 1.03 | 0.76 | 1.40 |
| Leflunomide | 2/16/42 | 1.08 | 0.55 | 2.11 |
| Sulfasalazine | 12/32/136 | 1.41 | 0.91 | 2.17 |
| Cyclosporine | 0/1/18 | 0.31 | 0.04 | 2.47 |
| TNF inhibitors | 24/248/2026 | 0.57 | 0.44 | 0.73 |
| IL-17 inhibitors | 2/88/520 | 0.62 | 0.45 | 0.87 |
| IL-23/IL-12+23 inhibitors | 5/58/344 | 0.67 | 0.45 | 0.98 |
| JAK inhibitors | 7/10/45 | 1.58 | 0.83 | 3.01 |
| Apremilast | 3/18/58 | 1.04 | 0.57 | 1.91 |
| Disease activity (DA) and glucocorticoids (GCs) | ||||
| Remission/low DA, no GCs | 57/516/3358 | 1 | (Reference) | |
| Remission/low DA, GCs | 13/59/177 | 1.97 | 1.39 | 2.79 |
| Moderate/high DA, no GCs | 15/130/604 | 1.39 | 1.09 | 1.76 |
| Moderate/high DA, GCs | 5/32/82 | 2.23 | 1.39 | 3.58 |
| NSAIDs | 16/151/1072 | 0.77 | 0.60 | 0.98 |
Results for ordinal mixed effects logistic regression analysis in all patients (primary model). Shown are fixed effects, random effects for country are not shown. Missing values are imputed via multiple imputation, patient numbers may thus be rounded. The model was additionally adjusted for age employing four-knot restricted cubic splines. Significant associations highlighted in bold.
bDMARD, biological disease-modifying antirheumatic drug; BMI, body mass index; COPD, chronic obstructive pulmonary disease; csDMARD, conventional synthetic DMARD; CVD, cardiovascular disease; DMARD, disease-modifying antirheumatic drug; IL, interleukin; JAK, Janus kinase; N, number; NSAID, non-steroidal antiinflammatory drug; TNF, tumour necrosis factor; tsDMARD, targeted synthetic DMARD.
Figure 1.
Relationship between age and probability of hospitalisation (red) and death (blue) estimated by four-knot restricted cubic splines, with 95% CIs (primary model, ordinal outcome, all patients).
Age was associated with COVID-19 severity in a non-linear way (stronger association for older age groups). Hypertension without CVD (OR 1.25, 95% CI 1.01 to 1.55), CVD without hypertension (OR 1.87, 95% CI 1.21 to 2.90), COPD or asthma (OR 1.75, 95% CI 1.33 to 2.31), other lung disease (OR 2.54, 95% CI 1.64 to 3.93), CKD (OR 2.32, 95% CI 1.50 to 3.58), cancer in patients with missing data on smoking (OR 2.89, 95% CI 1.19 to 6.97), obesity without diabetes (OR 1.35, 95% CI 1.07 to 1.70), diabetes without obesity (OR 1.84, 95% CI 1.38 to 2.45), and coexistence of obesity and diabetes (OR 1.89, 95% CI 1.34 to 2.68) were associated with greater odds of worse COVID-19 severity compared with referents without each condition. Male sex was associated with 1.54 times greater odds of worse COVID-19 severity compared with female sex (95% CI 1.30 to 1.83). Moderate/high disease activity (with or without GC use) and remission/low disease activity (with GC use) were associated with higher odds of worse COVID-19 outcomes compared with being in remission/low disease activity without GC use (OR ranging from 1.39 to 2.23). Later pandemic time periods were associated with lower odds of worse COVID-19 severity compared with the baseline period of March 2020–15 June 2020 (OR 0.42, 95% CI 0.34 to 0.51 for 16 June 2020–31 December 2020; OR 0.52, 95% CI 0.41 to 0.67 for 1 January 2021 and later). Compared with PsA, PsO was associated with less COVID-19 severity (OR 0.49, 95% CI 0.37 to 0.65). For medication classes, none were associated with higher odds of COVID-19 severity. TNFi, IL17i and IL-23i/IL-12+23i all demonstrated reduced odds of severe COVID-19 outcomes (OR 0.57, 95% CI 0.44 to 0.73; OR 0.62, 95% CI 0.45 to 0.87; OR 0.67, 95% CI 0.45 to 0.98, respectively). Finally, NSAID use compared with no use of NSAIDs was associated with lower odds of severe COVID-19 outcomes (OR 0.77, 95% CI 0.60 to 0.98).
Stratified analyses
When stratified by condition, results were similar to the primary model (online supplemental table 3) and online supplemental figures 1-3) with the following notable exceptions: hypertension alone and CVD alone were only significantly associated with the COVID-19 severity outcome among those with axSpA (OR 1.49, 95% CI 1.01 to 2.19; and OR 2.77, 95% CI 1.25 to 6.13; respectively) whereas COPD and asthma were associated with the COVID-19 severity outcome only among those with PsA (OR 1.95, 95% CI 1.34 to 2.82). The association of IL-23i/IL-12+23i with less severe COVID-19 outcomes was only statistically significant among those with PsO (OR 0.43, 95% CI 0.23 to 0.82); however, IL-23i/IL-12+23i were not used among patients with axSpA (not efficacious/licensed for this indication) and numbers were lower for PsA.
Sensitivity analyses
The results of sensitivity analyses are shown in online supplemental tables 4-6 and online supplemental figures 4-6. When restricting the analysis to confirmed COVID-19 cases (n=4176), multivariable model results were consistent with the primary model. Results were also similar to the primary model for the binary outcome of hospitalisation.
For the binary outcome of death, male sex (OR 2.00, 95% CI 1.22 to 3.26), having three or more comorbidities (OR 3.34, 95% CI 1.98 to 5.63) and baseline GC use (OR 1.91, 95% CI 1.002 to 3.64) remained associated with the outcome of interest. In this model, TNFi and IL17i continued to demonstrate reduced odds of severe COVID-19 outcomes (OR 0.50, 95% CI 0.26 to 0.98 and OR 0.11, 95% CI 0.02 to 0.51; respectively). However, sulfasalazine use (OR 2.64, 95% CI 1.13 to 6.17) and JAKi use (OR 7.49, 95% CI 2.61 to 21.47) were associated with greater odds of severe COVID-19 outcomes in this model.
Discussion
In this registry-based study of individuals with PsO, PsA and axSpA with SARS-CoV-2 infection, we found that known risk factors for the general population (older age, the presence of comorbidities) and for IMIDs overall (higher disease activity, higher baseline GC usage) were associated with more severe COVID-19 outcomes. In addition, a diagnosis of COVID-19 in a later time period during the pandemic was associated with lower disease severity compared with early 2020. Consistent with previous studies, baseline TNFi use was associated with lower odds for severe COVID-19 outcomes; we also found that IL17i and IL-23i/IL-12+23i use had similar associations with lower odds for severe COVID-19 outcomes.
The findings of our study reiterate known risk factors in both the general population and among people with IMIDs: older age, male sex and presence of comorbidities, specifically cardiometabolic and pulmonary conditions, were associated with more severe COVID-19 outcomes.5 6 Our findings that disease activity and GC usage at baseline have an additive interaction are consistent with prior findings in the C19-GRA registry.20
In this study, baseline use of TNFi was associated with lower odds of severe COVID-19 outcomes. This was previously shown in the C19-GRA registry,7 in a combined rheumatic disease, inflammatory bowel disease (IBD) and PsO analysis,8 and in a US-based administrative claims database study among individuals with RA.21 Mechanistic plausibility for trialling TNFi therapies for COVID-19 treatment has been discussed in the literature.22 23 These therapies neutralise TNF, a major cytokine in the excess inflammatory phase of COVID-19, and several trials are ongoing. A recent preprint announced results of a large randomised, placebo-controlled clinical trial led by the National Institutes of Health showing that treating adults hospitalised with COVID-19 with infliximab (a TNFi) did not significantly shorten time to recovery but did improve 14-day clinical status and substantially reduced 28-day mortality compared with standard of care24—the peer-reviewed publication is awaited.
We also demonstrated that using IL17i and IL-23i/IL-12+23i was also associated with lower odds of severe COVID-19 outcomes. Prior population-level data from Israel and the UK have shown that the use of IL-17i was not associated with worse COVID-19 outcomes.25 26 At the same time, case reports and case series have also suggested that IL-17 and IL-23 inhibition may not have a negative effect on the course of COVID-19,27–29 though further inference on whether exposure to these medications might be associated with better COVID-19 outcomes is limited. IL-17 may play a pathogenic role in acute respiratory distress syndrome and lung inflammation associated with severe COVID-19. Patients with COVID-19 who experience pulmonary complications have increased and activated Th17 cell populations, and lung damage and hyperinflammation are linked to these patients’ increased Th17 cell responses.30 31 The anti-IL-17 monoclonal antibody netakimab improved survival in a small clinical trial in patients with COVID-19; it decreased lung lesion volume and the need for oxygen support.32 However, in another study, netakimab therapy improved some clinical parameters and decreased C reactive protein levels, but it had no effect on the need for mechanical ventilation or patient survival in COVID-19 patients.33 Suppressing inflammation via a variety of mechanisms has been shown to improve COVID-19 outcomes in people with severe disease (ie, GC, IL-6i, JAKi, maybe TNFi). Whether IL-17 will also have a role remains to be determined and requires further study.34 35 Importantly, in our study, we report associations and therefore we caution against interpreting our estimates causally, as the possibility of selection bias and unmeasured confounding cannot be excluded.
Apremilast was not associated with the severity of COVID-19 in patients with PsO/PsA. Although the number of patients taking apremilast was low, these data are important because they add to limited previous evidence of a favourable safety profile of apremilast on COVID-19 severity in patients with these conditions.36–38
The finding that baseline NSAID use was associated with less COVID-19 severity is interesting but should be interpreted with caution. NSAID use is particularly prone to reporting bias, and inconsistencies in reporting might have resulted from the fact that we did not specify a minimal duration of a continuous treatment with NSAID and did not use a standardised questionnaire to collect NSAID data (eg, type of NSAID, dose and duration of treatment). General population studies in the UK and Denmark have not found associations between NSAID use and COVID-19-related hospitalisation or death.39–41 In our study, this association was seen particularly in individuals with axSpA and may be related to milder disease and/or well controlled of disease activity; confounding by indication cannot be excluded.
Finally, the results of one sensitivity analysis indicated that use of sulfasalazine and JAKi were associated with higher odds of death (binary outcome) due to COVID-19, though there were no associations with the ordinal COVID-19 severity outcome or with hospitalisation (binary outcome). In the C-19 GRA registry, we previously found an association of sulfasalazine use with worse COVID-19 outcome,7 a finding which was also seen in initial analyses of the Surveillance Epidemiology of Coronavirus Under Research Exclusion (IBD) database42 though later analyses were null.43 While there are biologically plausible effects of sulfasalazine on SARS-CoV-2 viral entry,44 our results may be due to residual confounding. The association of JAKi usage with COVID-19 outcomes is consistent with findings from some studies focused on people with RA.9 45 However, results from this sensitivity analysis should be interpreted with caution as the proportion of patients on JAKi was low (and no patients with PsO were taking this medication) and the respective 95% CI was wide.
Our study has several strengths, including the international nature of the combined registries, the large sample size and the granularity of information regarding IMID medications and disease activity. Our study also has limitations. First, the C19-GRA and PsoProtect registries were dependent on voluntary provider entry of cases, and there may be bias towards cases with more severe COVID-19 and those on DMARD therapy, as mostly secondary care clinicians were submitting cases. As such, proportions of events in our study sample should not be interpreted as incidence rates. Second, while we tried to mitigate the impacts of selection bias and confounding by indication, it is possible that our results may still be biased. However, we performed a series of sensitivity analyses to confirm the robustness of our findings, including restricting to a sample of confirmed cases of COVID-19, and our results were consistent across these additional analyses. Third, although we were able to adjust for several potential confounders in our models, there may still be residual unmeasured confounding. We did not have data available on disease duration or prior medication use, apart from what was reported at the time of COVID-19 diagnosis. Finally, vaccination status was not available for the patients in this dataset; however, the model adjustment for pandemic calendar period used in this study may act as a surrogate for vaccination status.
In conclusion, more severe COVID-19 outcomes in PsO, PsA and axSpA are largely associated with age, comorbidities, active disease and GC use. None of the bDMARDs typically used in PsO, PsA and axSpA, including TNFi, IL-17i and IL-23i/IL-12+23i, were associated with severe COVID-19 outcomes, and no biologics-specific differences were found. Our findings will help clinicians, scientific societies and policy makers worldwide develop tailored management strategies for patients with PsO, PsA and axSpA during COVID-19 waves or similar future respiratory pandemics.
ard-2022-223499supp001.pdf (171.1KB, pdf)
Acknowledgments
We wish to thank all healthcare providers who entered data into the registry.
Footnotes
Handling editor: Josef S Smolen
Twitter: @pedrommcmachado, @rheum_cat, @CorinneMiceli, @deshire_alpizar, @emilysirotich, @fredericorajao, @hausmannmd, @carmona_loreto, @yatesmark1, @drbeckyg, @saskiaamber, @wabautistam, @zach_wallace_md, @philipcrobinson
Correction notice: This article has been corrected since it published Online First. The first authorship statement has been added.
Collaborators: COVID-19 Global Rheumatology Alliance (C19-GRA) Consortium: Shafiq Fatima (Akbar 124 Street Medical Group), Adriana de Oliveira Marinho (Acre State Hospital Foundation), Noreen Nasir (Aga Khan University Hospital, Karachi Pakistan), Hesham Hamoud (Al Azhar University Hospitals) Shraddha Jatwani (Albert Einstein Medical Center, PA), Shanmuganandan Krishnan (APOLLO CLINIC), Alba Paula, Alvaro Andres Reyes Torres, Ana Bertoli, Andrea Baños, Boris Kisluk, Carla Gobbi, Carla Maldini, Carla Matellan, Carlevaris Leandro, Carolina Aeschlimann, Cecilia Goizueta, Cecilia Pisoni, Cecilia Romeo, Debora Guaglianone, Eugenia Picco, Fabian Risueño, Federico Nicolas Maldonado, Gelsomina Alle, Gimena Gómez, Gisela Subils, Gustavo Fabián Rodriguez Gil, Hernán Maldonado Ficco, Ivana Romina Rojas Tessel, Jonathan Eliseo Rebak, José Luis Velasco Zamora, Josefina Gallino Yanzi, Juan Alejandro Albiero, Julia Scafati, Julieta Silvana Morbiducci, Karen Roberts, Karina Cogo, Lorena Takashima, Luciana Casalla, Luciana Gonzalez Lucero, Ma. Alicia Lazaro, María Alejandra Cusa, María Alejandra Medina, Maria de la Vega, María Elena Calvo, Maria Isabel Quaglia, María J. Haye Salinas, Maria Julieta Gamba, Maria Marcela Schmid, María Severina, Maria Sol Castaños Menescardi, Maria Soledad Gálvez Elkin, María Victoria Martire, Mariana Pera, Marina Laura Werner, Mercedes García, Micaela Cosatti, Natalia Herscovich, Natalia Lili Cuchiaro, Noelia German, Pablo Maid, Roberto Miguel Baez, Rodolfo Perez Alamino, Romina Nieto, Romina Tanten, Rosana Gallo, Rosana Quintana, Sabrina Porta, Sabrina Solange de la Vega Fernandez, Sandra Petruzzelli, Sebastián Moyano, Silvana Conti, Sofía Ornella, Susana Isabel Pineda, Tatiana Barbich, Vanessa Castro Coello, Veronica Bellomio, Veronica Savio, Yohana Tissera (Argentine Society of Rheumatology), Angela Dahle, Anne Wolff, Archibald Skemp, Emily Pfeifer, Hammad Bajwa, Jeffrey Wilson, Jennifer Morgan, Jody Hargrove, Maren Hilton, Nicholas Lebedoff, Sara Baig, Susan Leonard, Vernon Berglund, Walter Dorman (Arthritis and Rheumatology Consultants, PA), Christopher Morris (Arthritis Associates of Kingsport), Michael Cannon (Arthritis Consultants of Tidewater), Marcela Posada (Artmedica), Antonio Carlos Ximenes, Felipe Omura, Flora Maria D Andrea Marcolino, Gecilmara Pileggi, Jose Roberto Silva Miranda (Artrocenter), Josephine Dhar (Ascension St. John Hospital), Ellison Smith, Julie Levengood, Kristin Gowin (Asheville Arthritis and Osteoporosis Center), Brahim Dahou (Association Rhumatologues Algériens Privés (ARAP)), Nicola Dalbeth (Auckland District Health Board), Eduardo Cepeda (Austin Diagnostic Clinic), Yves Piette (AZ Sint-Jan Brugge), Bea Maeyaert (AZ Sint-Lucas Brugge), Mieke Devinck (AZ Sint-Lucas Brugge), Karen Joyce Cortez (Baguio General Hospital and Medical Center), Selda ÇELİK (BAKIRKOY DR SADI KONUK EDUCATIONAL AND RESEARCH HOSPITAL, RHEUMATOLOGY DEPARTMENT, Istanbul), Ozan Cemal Icacan (Bakırköy Dr. Sadi Konuk Research And Training Hospital, Istanbul), Frances Stafford (Blackrock Clinic), Aarat Patel (Bon Secours Rheumatology Center), Lucy Thornton (Bradford Royal Infirmary), Derrick Todd, Jeffrey A. Sparks, Kristin D'Silva, Naomi Serling-Boyd, Tiffany Y-T Hsu, Zachary Wallace, (Brigham and Women's Hospital), Kendra Zuckerman (Bryn Mawr Medical Specialists), Suzanne Chapnick (Cambridge Health Alliance), Denise Hare, Tina Linehan (Capital Health Rheumatology), Laurie Bergstrom (Catalina Pointe Arthritis), Alain Sanchez Rodriguez (Centro Medico ABC), Beatriz Elena Zazueta-Montiel (Centro Medico del Angel), Angel Alejandro Castillo Ortiz (Centro Medico Las Americas), Jaime Hadid (Centro Medico Nacional 20 de Niviembre), Lilia Andrade Ortega (Centro Medico Nacional 20 de Noviembre ISSSTE), Xóchitl Jiménez (Centro Medico Naval), Erick Zamora Tehozol (Centro Medico Pensiones), Cummins Lue (CHI Little Rock Diagnostic Clinic), Ho So (Chinese University of Hong Kong), Mari Yamamoto (Chubu Rosai Hospital), Cassandra Calabrese (Cleveland Clinic) Sebastián Ibáñez (Clínica Alemana de Santiago), Juan Carlos Arana Ruiz (Clinica de Excelencia en Reumatología), Sergio Durán-Barragán (Clínica de Investigación en Reumatología y Obesidad), Lui Cajas (Clinica Universitaria Colombia - Centro Medico Providencia Sanitas), Tea Ahel Pavelić (Clinical Hospital Center Rijeka), Samuel Katsuyuki Shinjo (Clinical Hospital of Faculty of Medicine of University of Sao Paulo), Ricardo Xavier (Clinical Hospital of Porto Alegre), Michel Alexandre Yazbek (Clinical Hospital of University of Campinas), Montserrat Corteguera Coro (Complejo Asistencial Avila), Enrique Giraldo (Complejo Hospitalario), Anne-Marie Chassin-Trubert (Complejo Hospitalario San José), Sasha Dunt (Countess of Chester NHS Foundation Trust), Laura Muntean (County Emergency Hospital), Ileana Filipescu, Ioana Felea, Maria Magdelena Tamas, Simona Rednic (County Emergency Hospital, Cluj Napoca), Celia Fernandez (Cullman Internal Medicine), Barbara Goldstein (Denver Arthritis Clinic), Maria Greenwald (Desert Medical Advances), Branimir Anić (Div Clin Immunol Rheumatol; Dept Int Med, School of Med Zagreb, University Hospital Center Zagreb), Christopher Adams (East Alabama Medical Center), Jimmy Gene Villo, Rizza Navarro (East Avenue Medical Center), Arezou Khosroshahi (Emory University), Eva Strakova (Faculty Hospital Prešov), Nilzio Antonio da Silva (Faculty of Medicine of Goias Federal University), Ricardo Acayaba de Toledo (Faculty of Medicine of Sao Jose do Rio Preto), Babur Salim (Fauji Foundation Hospital), Sandra Lucia Euzebio Ribeiro (Federal University of Amazonas), Viviane Angelina de Souza (Federal University of Juiz de Fora), Adriana Maria Kakehasi (Federal University of Minas Gerais), Ana Karla Guedes de Melo (Federal University of Paraiba), Haim Cesar, Sueli Coelho da Silva Carneiro (Federal University of Rio de Janeiro), Edgard Torres dos Reis Neto (Federal University of Sao Paulo), Roberto Ranza (Federal University of Uberlandia), Martina Skamlova (FNSPFDR, Banská Bystrica), Brian Oppermann MD (Geisinger Health System), Samia Araujo de Sousa Studart (General Hospital of Fortaleza), Adam Kilian (George Washington University), Douglas White, Melanie Winter (Gundersen Health System), Gozde Kübra Yardımcı, Umut Kalyoncu (Hacettepe University Faculty of Medicine), Gozd Kubra Yardımcı (Hacettepe University Faculty of Medicine, Ankara), Samar Al-Emadi (Hamad Medical Corporation), Christine Graver (Hampshire Hospitals NHS Trust), Eva Rath (Hanusch Krankenhaus, Vienna), Dimitrios Vassilopoulos (Hippokration General Hospital, Athens), Luis Francisco Valdes Corona (Hospital Angeles Lomas), Mercedes Pinto Ortiz (Hospital Ángeles Mocel), Elisa Palalane (Hospital Central de Maputo), Monica Vazquez del Mercado (Hospital Civil de Guadalajara "Dr. Juan I. Menchaca"), Jose A Gomez Puerta (Hospital Clinic Barcelona), CLAUDIA MARQUES (HOSPITAL DAS CLÍNICAS – UFPE), Maria del Pilar Cruz Domínguez (Hospital de Especialidades La Raza (asociación de escleroderma)), Gabriela Maria Guzman Melgar (Hospital del Valle, Honduras), Roberto Muñoz Louis (Hospital Docente Padre Billini), Juan Carlos Cobeta Garcia (Hospital Ernest Lluch, Calatayud), Ariel Salinas (Hospital Essalud Alberto Sabogal Sologuren), Caroline Siegel (Hospital for Special Surgery), Theodore Fields (Hospital for Special Surgery), Mathia Cecilia Aguiar (Hospital General Agustin O'horan), Everardo Alvarez Hernandez (Hospital General de México "Dr. Eduardo Liceaga"), Jose Eduardo Navarro Zarza (Hospital General de Texco "Adolfo Prieto"), David Vega (Hospital General de Zona #17), LUCIA MAYA (Hospital General de Zona #48 IMSS), Salvador Loredo Alanis (Hospital General de Zona No. 3), Fernando Cobos Villanueva (Hospital General de Zona No. 30), Mónica Nancy Fuentes-Hernandez (Hospital General Issste Toluca), Aline Ranzolin (Hospital Getulio Vargas), Glaucio Ricardo Werner de Castro (Hospital Governador Celso Ramos), Jossiell Then Báez (Hospital Metropolitano de Santiago (HOMS)), Vladimir Aroja Santos (Hospital Municipal La Portada (Hospital Centinela de COVID-19)), Maria Carmen Torres Martin (Hospital Nuestra Senora Sonsoles, Avila), Blanca Mota (Hospital Privado), Shakira Selvananda (Hospital Pulau Pinang), JOSE ANTONIO VELOZ ARANDA (HOSPITAL REGIONAL ISSSTE LEON), BERNARDO CUNHA (HOSPITAL SARAH-BRASÍLIA), GUO RUEY LING (Hospital Sibu), Juan José Alegre Sancho (Hospital Universitari Dr Peset, Valencia), Cassandra Michel Skinner Taylor (Hospital Universitario "Dr. José Eleuterio González"), Dionicio Angel Galarza Delgado (Hospital Universitario "Dr. José Eleuterio González"), Lorena Pérez-Barbosa (Hospital Universitario "Dr. José Eleuterio González"), Francinne Machado Ribeiro (Hospital Universitário Pedro Ernesto Universidade do Estado do Rio de Janeiro), Jose Campos, Natalia de la Torre-Rubio (Hospital Universitario Puerta de Hierro), Iris Jazmin Colunga Pedraza (Hospital Universitario, Universidad Autonoma de Nuevo Leon), Rebecca Grainger (Hutt Hospital), Laurindo Ferreira da Rocha Junio (IMIP), David Alejandro Herrera-van Oostdam (IMSS), Juárez Mora Ingrid Maribel (IMSS), LILIANA PABLO-OLIVARES (IMSS), Oscar Marquez-Miranda (IMSS), Rina Dalva Neubarth (Institute of Medical Assistance to State Civil Servants of Sao Paulo), Concetta Lamore, Elliot Rosenstein, Melissa Harvey, Neil Kramer, Nicole Daver (Institute of Rheumatic and Autoimmune Diseases), Jiri Vencovsky, Maria Filkova (Institute of Rheumatology, Prague), Andrés Zúñiga-Vera (Instituto de Reumatología, Hematología y Dermatología (IRHED)), Luis H. Silveira (Instituto Nacional de Cardiologia), Diana Cervántes Rosete, Eduardo Martín Nares, Marina Rull Gabayet, Tatiana Sofia Rodriguez-Reyna (Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán), Suneya Hogarty (Integrative Arthritis and Pain Consultants), Laurie Hughell, Lindsey Clark (Iowa Arthritis & Osteoporosis Center), Mahdi Vojdanian (Iran Rheumatology Center), Monique Hoekstra, Theo Zijlstra (Isala Hospital, Zwolle), Servet Akar (Izmir Katip Celebi University Atatürk Training and Research Hospital, Izmir), Hirofumi Amano (Juntendo University), Karen Yeter (Kaiser Permanente), Elena Nikiphorou, Lucia Fusi, Rosaria Salerno (King's College Hospital), Loreta Bukauskiene (Klaipeda University Hospital), Fatemah Abutiban (Kuwait Rheumatology Association), Elizabeth Macphie (Lancashire and South Cumbria NHS Foundation Trust), Claire Vandevelde (Leeds Teaching Hospitals NHS Trust), Elizabeth Warner (Lister Hospital), Caroline Mulvaney Jones (Llandudno Hospital), VANEET SANDHU (Long Island Regional Arthritis Osteoporosis Care, PC), Leanna Wise (Los Angeles County + USC Medical Center), Daniela Spisakova, Marieta Senčarová (Louis Pasteur University Hospital, Košice), Faizah Siddique (Loyola University Medical Center), Jennifer Magno (Makati Medical Center), Ivy Rivera-Go (Manila Med), Rachel Wallwork (Massachusetts General Hospital), Kristin M. D’Silva, MD, Naomi J. Patel, MD (Massachusetts General Hospital, Harvard Medical School), Geraldine McCarthy (Mater Misericordiae University Hospital), Alí Duarte-García, Emily Gilbert, Maria Valenzuela Almada (Mayo Clinic Health System), Marta Píchová (Medipont plus s.ro., České Budějovice), Gabriela Belakova (Medman s.r.o., Martin), Byung Ban (Medstar Georgetown University Hospital), Elaine Zobrist, Lynn Ludmer (Mercy Hospital), Deshiré Alpízar-Rodríguez (Mexican College of Rheumatology), Jonathon Brooks, Mark Sapsford (Middlemore Hospital), Sarah Horton (Minerva Health Centre), Farida Al Balushi (Moh), Tamar Tanner (Montefiore Medical Center), Viktoriia Vasylets (Multifield Medical Centre, Odessa), Kyoko Motomura (National Center for Global health and Medicine), Shiro Ohshima (National Hospital Organization Osaka Minami Medical Center), Dagmar Mičeková, Helena Raffayova, Martin Zlnay, Olga Lukacova, Vanda Mlynarikova (National Institute of Rheumatic Diseases, Piešťany), Marina Hamaguchi (Nihon University Itabashi Hospital), Jane Leeder (Norfolk & Norwich University Hospital), Eric Ruderman (Northwestern Memorial), Evangeline Scopelitis, Jerald Zakem, Karen Toribio Toribio, Robert Quinet, Tameka Webb-Detiege, William Davis (Ochsner Medical Center Rheumatology Department), Laura Sampson (Orthopaedics and Rheumatology of the North Shore (ORNS)), NORIHIRO NISHIMOTO (Osaka Rheumatology Clinic), Kristen Young (Parkland Hospital), Inita Bulina, Julija Zepa (Pauls Stradins Clinical University Hospital, Riga), Sow Lai Kan (Penang General Hospital), Márta Király (Petz Aladár University Teaching Hospital, Győr), Soňa Žlnayová (Poliklinika MarMedico, s.r.o., Nové Mesto nad Váhom), Mauro Keiserman (Pontifical Catholic University of Rio Grande do Sul), A. Patrice Pollock, Abeer Eid, Ashley Maier, Atzintli Martínez, Cara Bailey, Caroline Arroyo, David Snow, Eduardo Martin-Nares, Elvia Moreta, Ericsson Trieu, Ernst Markus Klaus, Fedra Irazoque, Genevieve Katigbak, Gilbert Kepecs, Greta Reyes-Cordero, Jaimie Russell, Jessica Chapman, Joseph Huffstutter, Kara Long, Khurram Abbass, Lauren Kaufman, Marco Martinez Martinez, MONICA MACIAS-PALACIOS, NAGA PRABU, Paloma De Abreu Trigueros, Raheem Kherani, Randall Beyl, Sarah Middleton, Sharath Kumar, Susan Barrett, Viktoria Pavlova, Yanira Yinde (Private Practice), Samir Patel (Queen Elizabeth Hospital Woolwich), Alexandra Balbir-Gurman (Rambam Rheumatology Institute, Haifa), Bruno Ferreira (Rede Sarah de Hospitais de Rabilitação), Kathleen Anthony (Rheumatology and Osteoporosis Specialists), Richard Stern (Rheumatology Associastes), Lilliam Miranda (Rheumatology Center INC), Shabbir Chikani (Rheumatology centre, HCG hospital), Ann Clarke (Richmond Road Diagnostic And Treatment Center), Ammar Haikal, Michael Guma, Sushama Mody (Riverside Medical Group), Diana O'Kane (RNHRD at Royal United Hospital Bath), Sheila O'Reilly (Royal Derby Hospital) HUMAID AL WAHSHI, Nasra Al-Adhoubi (Royal Hospital), Jenny Tyler (Royal United hospital), Jennifer Tyler (Royal United Hospital, Bath), Vivien Hsu (Rutgers Robert Wood Johnson), Mária Oetterová (Safarik University hospital, Kosice), Audrey Low, Beverley Harrison (Salford Royal NHS FT), Guillermo Pons-Estel (Sanatorio Parque), Mariana Peixoto Guimaraes Ubirajara e Silva de Souza (Santa Casa of Belo Horizonte), Carolina Zorzanelli Costa (Santa Casa of Vitoria), Veronika Sharp (Santa Clara Valley Medical Center), Laura Groseanu (Sf Maria Clinical Hospital, Bucharest), Daric Mueller (Shores Rheumatology PC), Lillian Barra, Tom Appleton (SJHC London), South African Rheumatism and Arthritis Association (South Africa), Laura Chadwick (St Helens & Knowsley NHS Foundation Trust), Richard Conway (St James' Hospital, Dublin), Janet Pope (St. Joseph's Health Care), Maria del Carmen Hernandez (Star Medica Centro), Cecília Resende Brunow Bazzo (State University of Londrina), Steven Feldman (Steven Feldman MD PA), Anna Sabová (Súkromná Reumatologická Ambulancia, Vranov nad Topľou), Talal Ali Al Lawati (Sultan Qaboos University Hospital), Rachael Flood (Tallaght University Hospital), MOOIKHIN HNG (Tareum) Arundathi Jayatilleke (Temple University Hospital) Simeon Grazio (The teaching hospital sisters of charity), Keiko Koshiba, Masashi Funada (Toho University Omori Medical Center), Midori Sato (Tokyo Medical Center), Fukumi NAKAMURA (Tokyo Metropolitan Bokutoh General Hospital), Kazuhisa Yokota (Tokyo Metropolitan Health and Hospitals Corporation, Ebara Hosipital), Lingli Dong (Tongji Hospital), A. Silvia Ross (Triangle Arthritis & Rheumatology), John FitzGerald, Tanaz Kermani (UCLA), Boris Karanovic (UHC Zagreb), Alojzija Hocevar (UMC Ljubljana), Martina Bakosova (UNB Nemocnica Stare Mesto, Bratislava), Manuel Ugarte-Gil (Universidad Científica del Sur-Hospital Guillermo Almenara Irigoyen), Marcelo Pinheiro (Universidade Federal De São Paulo Escola Paulista de Medicina e Escola Paulista de Enfermagem), Su-Ann Yeoh (University College London Hospital), Maria Oetterova, Nicola Gullick (University Hospital), Emőke Šteňová (University Hospital Bratislava), Marko Barešić, Ivan Padjen (University Hospital Center Zagreb), Melanie-Ivana Čulo (University Hospital Dubrava, Zagreb), Wilson Bautista Molano (University Hospital Fundación Santa Fe de Bogotá), James Pilcher (University Hospital Lewisham), Kristina Kovačević Stranski (University Hospital Osijek), Lubica Capova (University Hospital, Bratislava), Zelmira Macejova (University Hospital, Košice), Jarosław Nowakowski (University Hospital, Krakow), Takashi Kida (University Hospital, Kyoto Prefectural University of Medicine), SAUDATU ISSAKA (University Hospitals), Licia Maria Henrique da Mota (University of Bras), JoAnn Zell (University of Colorado), Christine Peschken (University of Manitoba), Angelito Flora, Evelyn Salido, Geraldine Zamora (University of the Philippines, Philippine General Hospital), Alison Bays (University of Washington, Seattle), David Karp, Ezzati Fatemeh, Guillermo Quiceno, Kathryn Dao (UT Southwestern Medical Center), APARNA DAS (UTMB), Lauro Quintanilla (Virginia Medica), Vicki Quincey (Waikato Hospital), Deborah Parks (Washington University Div of Rheumatology), Kelly Weselman (Wellstar Kennestone Hospital), Fabiane Shizue Sakai (Windmills Hospital), Katie Williams (York District Hospital), Kirsty Devine (York/Scarborough Hospitals) Lenny Geurts-van Bon (Ziekenhuisgroep Twente), Sarah Goglin (Zuckerberg San Francisco General Hospital). Psoriasis Patient Registry for Outcomes, Therapy and Epidemiology of COVID-19 Infection (PsoProtect) Consortium: A van geest, Aadarsh Shah, AC de Waal, Alba Catala, Alberto Barea, Alberto Romero Maté, Alekya Singapore, Alexandra Paolino, Alexandra Vincent, Alice Mwale, Alison Sears, Amy de la Breteque, Amy Foulkes, Ana Brasileiro, Ana Maria Morales Callaghan, Ana Martinez, Andrea Carugno, Andrea Chiricozzi, Andrea Conti, Andrew DeCrescenzo, Andrew Pink, Angela Braeger, Anke Piekar, Ann Jones, Ann Sergeant, Anna Baran, Anne Groeneveld, Annette Essex, Anthony Bewley, Antoine Fauconneau, Antony Raharja, Aparna Sinha, Areti Makrygeorgou, Arias Martín Nadia, Astrid van Huizen, Beata Fabos, Beatriz Pérez Suárez, Benhadou Farida, Birgitta Wilson Claréus, Bola Coker, Canelle Mazaud, Caoimhe Fahy, Caoimhe M. R. Fahy, Carla Tubau Prims, Carmen Bugarin Diz, Caroline Campbell, Carolyn Martin, Carrie Davis, Catherine Holden, Catherine Motosko, Catherine Quinlan, Catriona Maybury, Cesar Gonzalez, Charlotte Barclay, Chifari Angelo, Choon Siew Eng, Cid Yazigi Sabbag, Claudia de la Cruz, Claudia Guebenlian Bakerdjian, Claudio Greco, Cristina Echeverria, Cristina Mariela Echeverría, Dagmara Samselska, Danang Tri Wahyudi, Daniela Armijo, Danielle Brassard, Daryl Teo, David Fairhurst, Deanna Cummings, Deepti Kolli, Denis Jullien, Denise Peeters, Descamps Vincent, Diana Patricia Ruiz Genao, Diana Ruiz Genao, Eileen Parry, Elaine Agius, Eleanor Henderson, Elena Hawryluk, Eliseo Martínez-García, Elizabeth Molina, Elizabeth Stewart, Ellie Henderson, Elzbieta Klujszo, Emily Brown, Emily Dwyer, Emmanuel Mahe, Emmanuel Toni, Emmerson Gale Vista, Emmylou Casanova, Enikö Sonkoly, Enrique Loayza, Erin Kamp, Esteban Daudén, Esther Balogh, F Aubin, Felicity Edwards, Ferial Ismail, Fernando Valenzuela, Fikki Orekoya, Florentina-Silvia Delli, Francesca Capon, Freya Meynell, Gabriel Magariños, Gabrielle Becher, Gabrielle Key, Gaelle Quereux, Gaurav Dhawan, Gemma Keogh, Georgi Popov, Georgie King, Girard Celine, Gloria Aparicio, Gordana Krnjevic Pezic, Graham Johnston, Gustavo Anibal Cardozo, H. El Khattabi, Haleema Alfailakawi, Hazel Oon, Hazel Rooney, Helen McAteer, Helene Aubert, Hervé Bachelez, Hoseah Waweru, Ian Pearson, Ignacio Yanguas, Iman Kotb, Ines Barbosa, Isabella Tosi, J Charles, Jack French, James John Murphy, Jamie Weisman, Jennifer Elias, Jennifer Soung, Jenny Carolan, Jenny Hughes, Jill Ramsay, Jing Husaini, JM. Ortiz, Jo Lambert, Joanne Topliffe, Joel M Gelfand, John Newsham, Jonathan Ng, Jose-Manuel Carrascosa, Joseph J Schwartz, Julia Bowman, Julie Charles, Juul van de Reek, Karina Jackson, Karolina Vorčáková, Katarzyna Grys, Katherine Poirier, Kathryn Kerisit, MD, Kayleigh J Mason, Keith Wu, Kimberly Anne G. Ednalino, Kirsty Wynes, Kyle Eash, Lars Iversen, Laura Speck, Laure Mery-Bossard, Lauren Booker, Leah Hoggan, leandro perrotat, Leila Asfour, Leila Kattach, Leontien de Graaf, Lesort Cécile, Lian van der Gang, Lieve Meuleman, Lim Jin Huang, Linda Lawson, Linda McMahon, Line Kibsgaard, Lisa Kirby, Lisa van der Rijst, Liv Eidsmo, Lluís Rusiñol Batlle, Lone Skov, Lorraine Gribben, Lucy Moorhead, Luigi Naldi, Luis Puig, Lyndsey Florence, M.M. Bakker, Mahira Hamdy El Sayed, Mahmood Abubakar, Malcolm Rustin, Manel Velasco, Mangiarotti Germán, Manisha Panchal, Manja Bloem, Manpreet Lakhan, Manuel Dario Franco, Mara Maccarone, Margot Common, Maria Fernanda Lui, Marie Dedroog, Marie-Eve Fortier, Marie-Louise Svensson, Mariusz Sikora, Mark Vandaele, Maruska Marovt, Masanori Okuse, Matthias Schmuth, Melanie Bruton, Melanie Claridge, Melanie westmoreland, Melissa Sweeney, Michela Magnano, Mireille van Baar, Miriam Saposnik, Mohamed EL-Komy, Musumeci Maria Letizia, N Beneton, Nick J Reynolds, Nick Reynolds, Nora Noemí Kogan, Omid Zargari, Pablo De Caso, Pamela Campbell, Paola Di Meglio, Paolo Gisondi, Paula Bourren, Paula luna, Paulo Varela, Penny Nash, Peter Foley, Peter Holló, Peter Jenkin, Phan Céline, Philip Hampton, Phyllis Spuls, Pia Tookey, Piergiacomo Calzavara-Pinton, Portia Goldsmith, Pter Holló, Rachel Bak, Radhika Patel, Raquel Rivera Diaz, Rebecca Rose, Reinhart Speeckaert, Ricardo Romiti, Richard Warren, Richard Woolf, Rogelio Mercado, Rohima Khatun, Rolland Gyulai, Romana Ceovic, Romana Machackova, Ronald Vender, Rosa Andres Ejarque, Rosa Taberner, Rosalie Juch, Russell cohen md faad, Sandy McBride, Sara Cacciapuoti, Sarah Drummond, Sarah Kirk, Sarah McCusker, Saskia Reeken, Selva Anahí Gutiérrez Yañez, Shanti Ayob, Shrita Shinton, Silvia Pérez Barrio, Simina Stefanescu, Sinan Doğan, Sinéad M Langan, Sophia Mohme, Sophia Strong-Sheldrake, Sophie Wanten, Stefano Piaserico, Stephanie Ball, Stephanie Ogden, Susan Ann Lloyd, Susan Hall, Susan Woollett, Susana Armesto Alonso, Susannah Hoey, Taku Suzuki, Tatiana del Río, Tatiana Pecova, Teena Mackenzie, Telgdy Enikő, Teresa Tsakok, Thomas Beaulieu, Tiago Torres, Ting Seng Tang, Tom Hillary, Tomas Kampe, Toomas Talme, Tracy Brown, Tracy Smith, Tran Hong Truong, Trupti Desai, Victoria Brown, Victoria King, Vito Di Lernia, Wisam Alwan, Ya-Hsin Wang, Yena Kim, Zafeiriou Efterpi, zahira koreja, Zaida Troyano, Zeeshaan Hasan, Zeljko Mijuskovic, Zenas Yiu, Zeynep Topkarci.
Contributors: PMM and MS had access to the study data and vouch for the data and analyses. MS performed the statistical analyses. PMM, MS and JL drafted the first version of the manuscript. PMM, MS, SKM, JL, LG, ND, AP, AS, ACR, BF, CGA, CGSS, CEMG, CL, CM-R, DW, DAR, DW, EFM, ES, ERS, FMR, FO, FRM, HS, JD, JNB, JH, KLH, LG, LS, LJ, LC, MMP, MDZ, MdlAS, MY, MD, MG-M, NR, NH, PS, RG, RH, RH, SL-T, SB, TP, TO, WB-M, ZSW, ZZNY, JY, PCR and CHS contributed to data collection, data quality control, data analysis and interpretation of the data. PMM is the guarantor and accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. All authors contributed intellectual content during the drafting and revision of the work and approved the final version to be published.
Funding: The study received support from the American College of Rheumatology (ACR) and European Alliance of Associations for Rheumatology (EULAR).
Competing interests: PMM has received honoraria from Abbvie, BMS, Celgene, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Orphazyme, Pfizer, Roche and UCB, all unrelated to this manuscript, and is supported by the National Institute for Health Research (NIHR), University College London Hospitals (UCLH), Biomedical Research Centre (BRC). MS reports a joint, unconditional grant from a consortium of 14 companies supporting the German RABBIT register (AbbVie, Amgen, BMS, Fresenius Kabi, Galapagos, Hexal, Lilly, MSD, Pfizer, Roche, Samsung, Sanofi, Viatris and UCB). SKM reports departmental income from AbbVie, Almirall, Eli Lilly, Janssen-Cilag, Novartis, Sanofi, UCB, outside the submitted work. JL has nothing to disclose. LG reports personal consultant fees/honoraria/travelling support from AbbVie, Amgen, Bristol-Myers Squibb, Biogen, Celgene, Celltrion, Galapagos, Janssen, Lilly, MSD, Novartis, Pfizer, and UCB, and grants from Sandoz and UC, all unrelated to this manuscript. ND has nothing to disclose. AP has nothing to disclose. AS reports a joint, unconditional grant from a consortium of 14 companies supporting the German RABBIT register (AbbVie, Amgen, BMS, Fresenius Kabi, Galapagos, Hexal, Lilly, MSD, Pfizer, Roche, Samsung, Sanofi, Viatris and UCB) and personal fees from lectures for AbbVie, Amgen, Celltrion, MSD, Janssen, Lilly, Roche, BMS and Pfizer, all unrelated to this work. ACR reports a joint, unconditional grant from a consortium of 12 companies supporting the German RABBIT-SpA register (AbbVie, Amgen, Biogen, Celltrion, Hexal, Janssen-Cilag, Lilly, MSD, Novartis, Pfizer, Viatris and UCB) and personal fees from lectures for Novartis, Roche, and Pfizer, all unrelated to this work. BF has nothing to disclose. CGA has nothing to disclose. CGSS has nothing to disclose. CEMG reports grants and/or personal fees from AbbVie, Almirall, Amgen, Anaptysbio, BMS, Boehringer-Ingelheim, Evelo Bioscience, Inmagene, GSK, Janssen, Kyowa Kirin, LEO, Lilly, Novartis, ONO Pharmaceutical, Pfizer and UCB Pharma, outside the submitted work. CL has nothing to disclose. CM-R has nothing to disclose. DW has received personal speaker/consultant fees from AbbVie, BMS, MSD, Pfizer, Roche Chugai, Amgen, Nordic Pharma, UCB, Novartis, Janssen, Lilly, Sandoz, Grunenthal, Galapagos. DAR is Scientific Advisor for GSK, unrelated to this work. DW has nothing to disclose. EFM has received personal consultant fees from Boehringer Ingelheim Portugal, Lda; LPCDR received support for specific activities: grants from Abbvie, Novartis, Lilly Portugal, Amgen Biofarmacêutica, Grünenthal S.A., MSD, Medac and from A. Menarini Portugal-Farmacêutica, S.A.; grants and non-financial support from Pfizer, and non-financial support from Grünenthal GmbH, outside the submitted work. ES has nothing to disclose. ESR reports grants/contracts from Novartis, Pfizer, Amgen and Elea, honoraria from Amgen, Abbvie, BMS, Eli Lilly, Janssen, Novartis, Pfizer, Sandoz, and UCB, support for attending meetings from Abbvie, Pfizer, UCB, and Janssen, and participation on data safety monitoring board or advisory board from Abbvie, Janssen, Pfizer, Amgen, Sandoz and Novartis. FMR has nothing to disclose. FO has nothing to disclose. FRM has nothing to disclose. HS has nothing to disclose. JD has nothing to disclose. JNB reports grants and/or personal fees from AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol-Meyers-Squibb, J&J, LEO Pharma, Lilly, Novartis, Pfizer, Samsung, Sun Pharma, and UCB, outside of the submitted work. JH has nothing to disclose. KLH has received non-personal speaker’s fees from Abbvie and grant income from BMS, UCB, and Pfizer, all unrelated to this manuscript, and is supported by the NIHR Manchester Biomedical Research Centre. LG has received consulting fees and/or research support from Abbvie, Acelyrin, Eli Lilly, Fresenius Kabi, Janssen, Novartis, Pfizer and UCB, all unrelated to this manuscript, and research support from UCB. LS has nothing to disclose. LJ has nothing to disclose. LC has not received fees or personal grants from any laboratory, but her institute works by contract for laboratories among other institutions, such as Abbvie Spain, Eisai, Gebro Pharma, Merck Sharp & Dohme España, S.A., Novartis Farmaceutica, Pfizer, Roche Farma, Sanofi Aventis, Astellas Pharma, Actelion Pharmaceuticals España, Grünenthal GmbH and UCB Pharma. MMP has nothing to disclose. MDZ has nothing to disclose. MdlAS has nothing to disclose. MY has nothing to disclose. MD has nothing to disclose. NG-S has nothing to disclose. NR has nothing to disclose. NH has received consulting fees from Abbvie, Eli Lilly, Janssen, Novartis and UCB. PS has nothing to disclose. RG has received consulting/speaker’s fees from Abbvie, Janssen, Novartis, Pfizer, and Cornerstones, all unrelated to this manuscript, and travel grants from Pfizer and Janssen. RH reports consulting fees from AbbVie, GSK, Novartis, Pfizer, Amgen, Biogen, BMS, Galapagos, Lilly, Mylan, Gilead, Janssen, TAKEDA/Shire, Roche/Chugai, research grant from Pfizer, all unrelated to this manuscript. SL-T has nothing to disclose. SB reports consulting fees from AbbVie, Horizon, Novartis, and Pfizer, and is an employee of Pfizer, Inc. TP reports personal consultant fees from Abbvie, Amgen, Biogen, BMS, Celgene, Celltrion, Fresenius-Kabi, Galapagos, Gilead, Janssen, Lilly, MSD, Nordic, Novartis, Pfizer, Roche-Chugai, Sandoz, Sanofi and UCB, all unrelated to this manuscript. TO reports consultancy fees from Merck Sharp & Dohme, unrelated to the present work. WBM has received personal speaker/consultant fees from AbbVie, Pfizer, Amgen, Novartis, Janssen, Lilly, Biopas. ZSW has nothing to disclose. ZZNY has nothing to disclose. JY is supported by NIH/NIAMS K24 AR07534 and AHRQ R01HS028024. She has received research grants from Gilead, Aurinia, BMS Foundation and Astra Zeneca and performed consultation for Astra Zeneca, Pfizer, and Aurinia. PCR has nothing to disclose. CHS reports grants from AbbVie, Sanofi, Novartis, and Pfizer and through consortia with multiple academic partners (psort.org.uk, BIOMAP-IMI.eu), outside the submitted work; she is a member of the British Association of Dermatologists Guideline committee on biologics in psoriasis.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Reasonable data requests will be considered, wherever legally and ethically possible. Applications to access the data should be made to the C19-GRA Steering Committee.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Given that the registries collect deidentified data, the UK Health Research Authority, the University of California San Francisco Institutional Review Board and the Leeds Research Ethics Committee (UK) considered them exempt from patient consent. Local regulatory guidelines were followed by international sites.
References
- 1. Landewé RBM, Kroon FPB, Alunno A, et al. EULAR recommendations for the management and vaccination of people with rheumatic and musculoskeletal diseases in the context of SARS-cov-2: the november 2021 update. Ann Rheum Dis 2022;81:1628–39. 10.1136/annrheumdis-2021-222006 [DOI] [PubMed] [Google Scholar]
- 2. Mikuls TR, Johnson SR, Fraenkel L, et al. American College of rheumatology guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic: version 3. Arthritis Rheumatol 2021;73:e1–12. 10.1002/art.41596 [DOI] [PubMed] [Google Scholar]
- 3. Curtis JR, Johnson SR, Anthony DD, et al. American College of rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 4. Arthritis Rheumatol 2022;74:e21–36. 10.1002/art.42109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grainger R, Kim AHJ, Conway R, et al. COVID-19 in people with rheumatic diseases: risks, outcomes, treatment considerations. Nat Rev Rheumatol 2022;18:191–204. 10.1038/s41584-022-00755-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kroon FPB, Najm A, Alunno A, et al. Risk and prognosis of SARS-cov-2 infection and vaccination against SARS-cov-2 in rheumatic and musculoskeletal diseases: a systematic literature review to inform EULAR recommendations. Ann Rheum Dis 2022;81:422–32. 10.1136/annrheumdis-2021-221575 [DOI] [PubMed] [Google Scholar]
- 6. Conway R, Grimshaw AA, Konig MF, et al. SARS-cov-2 infection and COVID-19 outcomes in rheumatic diseases: a systematic literature review and meta-analysis. Arthritis Rheumatol 2022;74:766–75. 10.1002/art.42030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis 2021;80:930–42. 10.1136/annrheumdis-2020-219498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Izadi Z, Brenner EJ, Mahil SK, et al. Association between tumor necrosis factor inhibitors and the risk of hospitalization or death among patients with immune-mediated inflammatory disease and COVID-19. JAMA Netw Open 2021;4:e2129639. 10.1001/jamanetworkopen.2021.29639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sparks JA, Wallace ZS, Seet AM, et al. Associations of baseline use of biologic or targeted synthetic dmards with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 global rheumatology alliance physician registry. Ann Rheum Dis 2021;80:1137–46. 10.1136/annrheumdis-2021-220418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sattui SE, Conway R, Putman MS, et al. Outcomes of COVID-19 in patients with primary systemic vasculitis or polymyalgia rheumatica from the COVID-19 global rheumatology alliance physician registry: a retrospective cohort study. Lancet Rheumatol 2021;3:e855–64. 10.1016/S2665-9913(21)00316-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ugarte-Gil MF, Alarcón GS, Izadi Z, et al. Characteristics associated with poor COVID-19 outcomes in individuals with systemic lupus erythematosus: data from the COVID-19 global rheumatology alliance. Ann Rheum Dis 2022;81:970–8. 10.1136/annrheumdis-2021-221636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. MacKenna B, Kennedy NA, Mehrkar A, et al. Risk of severe COVID-19 outcomes associated with immune-mediated inflammatory diseases and immune-modifying therapies: a nationwide cohort study in the opensafely platform. Lancet Rheumatol 2022;4:e490–506. 10.1016/S2665-9913(22)00098-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahil SK, Dand N, Mason KJ, et al. Factors associated with adverse COVID-19 outcomes in patients with psoriasis-insights from a global registry-based study. J Allergy Clin Immunol 2021;147:60–71.:S0091-6749(20)31413-5. 10.1016/j.jaci.2020.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis 2020;79:859–66. 10.1136/annrheumdis-2020-217871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liew JW, Bhana S, Costello W, et al. The COVID-19 global rheumatology alliance: evaluating the rapid design and implementation of an international registry against best practice. Rheumatology (Oxford) 2021;60:353–8.:keaa483. 10.1093/rheumatology/keaa483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mahil SK, Yates M, Yiu ZZN, et al. Describing the burden of the COVID-19 pandemic in people with psoriasis: findings from a global cross-sectional study. J Eur Acad Dermatol Venereol 2021;35:e636–40. 10.1111/jdv.17450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lawson-Tovey S, Strangfeld A, Hyrich KL, et al. EULAR COVID-19 registry: lessons learnt and future considerations. Ann Rheum Dis 2021;80:1110–5. 10.1136/annrheumdis-2021-220319 [DOI] [PubMed] [Google Scholar]
- 18. Yeoh S-A, Gianfrancesco M, Lawson-Tovey S, et al. Factors associated with severe COVID-19 in people with idiopathic inflammatory myopathy: results from the COVID-19 global rheumatology alliance physician-reported registry. RMD Open 2022;8:e002508. 10.1136/rmdopen-2022-002508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harrell F. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. Chapter 5: Resampling, Validating, and Simplifying the Model 2001;3:88–103. [Google Scholar]
- 20. Schäfer M, Strangfeld A, Hyrich KL, et al. Response to: “ correspondence on ‘factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 global rheumatology alliance physician reported registry’” by mulhearn et al. Ann Rheum Dis 2023;82:e116. 10.1136/annrheumdis-2021-220134 [DOI] [PubMed] [Google Scholar]
- 21. Curtis JR, Zhou X, Rubin DT, et al. Characteristics, comorbidities, and outcomes of SARS-cov-2 infection in patients with autoimmune conditions treated with systemic therapies: a population-based study. J Rheumatol 2022;49:320–9. 10.3899/jrheum.210888 [DOI] [PubMed] [Google Scholar]
- 22. Feldmann M, Maini RN, Woody JN, et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet 2020;395:1407–9.:S0140-6736(20)30858-8. 10.1016/S0140-6736(20)30858-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robinson PC, Richards D, Tanner HL, et al. Accumulating evidence suggests anti-TNF therapy needs to be given trial priority in COVID-19 treatment. Lancet Rheumatol 2020;2:e653–5. 10.1016/S2665-9913(20)30309-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O’Halloran J, Kedar E, Anstrom KJ, et al. Infliximab for treatment of adults hospitalized with moderate or severe covid-19. MedRxiv 26, 2022.:2022.09.22.22280245. 10.1101/2022.09.22.22280245 [DOI] [Google Scholar]
- 25. Kridin K, Schonmann Y, Damiani G, et al. Tumor necrosis factor inhibitors are associated with a decreased risk of COVID-19-associated hospitalization in patients with psoriasis-A population-based cohort study. Dermatol Ther 2021;34:e15003. 10.1111/dth.15003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kridin K, Schonmann Y, Solomon A, et al. Risk of COVID-19 infection, hospitalization, and mortality in patients with psoriasis treated by interleukin-17 inhibitors. J Dermatolog Treat 2022;33:2014–20. 10.1080/09546634.2021.1905766 [DOI] [PubMed] [Google Scholar]
- 27. Balestri R, Rech G, Girardelli CR. SARS-cov-2 infection in a psoriatic patient treated with IL-17 inhibitor. J Eur Acad Dermatol Venereol 2020;34:e357–8. 10.1111/jdv.16571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Foti R, Amato G, Visalli E. SARS-cov-2 infection in a psoriatic arthritis patient treated with IL-17 inhibitor. Med Hypotheses 2020;144:110040.:S0306-9877(20)31385-2. 10.1016/j.mehy.2020.110040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Messina F, Piaserico S. SARS-cov-2 infection in a psoriatic patient treated with IL-23 inhibitor. J Eur Acad Dermatol Venereol 2020;34:e254–5. 10.1111/jdv.16468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu J, Tang L, Ma Y, et al. Immunological profiling of COVID-19 patients with pulmonary sequelae. MBio 2021;12:e0159921. 10.1128/mBio.01599-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sadeghi A, Tahmasebi S, Mahmood A, et al. Th17 and Treg cells function in SARS-cov2 patients compared with healthy controls. J Cell Physiol 2021;236:2829–39. 10.1002/jcp.30047 [DOI] [PubMed] [Google Scholar]
- 32. Maslennikov R, Ivashkin V, Vasilieva E, et al. Interleukin 17 antagonist netakimab is effective and safe in the new coronavirus infection (COVID-19). Eur Cytokine Netw 2021;32:8–14. 10.1684/ecn.2021.0463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Avdeev SN, Trushenko NV, Tsareva NA, et al. Anti-IL-17 monoclonal antibodies in hospitalized patients with severe COVID-19: a pilot study. Cytokine 2021;146:.:S1043-4666(21)00213-1. 10.1016/j.cyto.2021.155627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mills KHG. Il-17 and IL-17-producing cells in protection versus pathology. Nat Rev Immunol 2023;23:38–54. 10.1038/s41577-022-00746-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Najm A, Alunno A, Mariette X, et al. Pathophysiology of acute respiratory syndrome coronavirus 2 infection: a systematic literature review to inform EULAR points to consider. RMD Open 2021;7:e001549. 10.1136/rmdopen-2020-001549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Queiro Silva R, Armesto S, González Vela C, et al. COVID-19 patients with psoriasis and psoriatic arthritis on biologic immunosuppressant therapy vs apremilast in North Spain. Dermatol Ther 2020;33:e13961. 10.1111/dth.13961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lytvyn Y, Georgakopoulos JR, Mufti A, et al. Incidence and prognosis of covid-19 in patients with psoriasis on apremilast: a multicentre retrospective cohort study. J Eur Acad Dermatol Venereol 2022;36:e94–5. 10.1111/jdv.17749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mugheddu C, Pizzatti L, Sanna S, et al. COVID-19 pulmonary infection in erythrodermic psoriatic patient with oligodendroglioma: safety and compatibility of apremilast with critical intensive care management. J Eur Acad Dermatol Venereol 2020;34:e376–8. 10.1111/jdv.16625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Drake TM, Fairfield CJ, Pius R, et al. Non-Steroidal anti-inflammatory drug use and outcomes of COVID-19 in the ISARIC clinical characterisation protocol UK cohort: a matched, prospective cohort study. Lancet Rheumatol 2021;3:e498–506. 10.1016/S2665-9913(21)00104-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lund LC, Kristensen KB, Reilev M, et al. Adverse outcomes and mortality in users of non-steroidal anti-inflammatory drugs who tested positive for SARS-cov-2: a Danish nationwide cohort study. PLoS Med 2020;17:e1003308. 10.1371/journal.pmed.1003308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wong AY, MacKenna B, Morton CE, et al. Use of non-steroidal anti-inflammatory drugs and risk of death from COVID-19: an opensafely cohort analysis based on two cohorts. Ann Rheum Dis 2021;80:943–51. 10.1136/annrheumdis-2020-219517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology 2020;159:481–491.S0016-5085(20)30655-7. 10.1053/j.gastro.2020.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ungaro RC, Brenner EJ, Gearry RB, et al. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut 2021;70:725–32. 10.1136/gutjnl-2020-322539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Konig MF, Grzes KM, Robinson PC, et al. Sulfasalazine: a risk factor for severe COVID-19? Lancet Rheumatol 2022;4:e388–9. 10.1016/S2665-9913(22)00067-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bower H, Frisell T, Di Giuseppe D, et al. Impact of the COVID-19 pandemic on morbidity and mortality in patients with inflammatory joint diseases and in the general population: a nationwide Swedish cohort study. Ann Rheum Dis 2021;80:1086–93. 10.1136/annrheumdis-2021-219845 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ard-2022-223499supp003.pdf (72.1KB, pdf)
ard-2022-223499supp002.pdf (245.5KB, pdf)
ard-2022-223499supp001.pdf (171.1KB, pdf)
Data Availability Statement
Reasonable data requests will be considered, wherever legally and ethically possible. Applications to access the data should be made to the C19-GRA Steering Committee.