Polymyalgia rheumatica (PMR) is an inflammatory disorder characterised by severe pain and stiffness involving the shoulders and proximal aspects of the arms bilaterally. Untreated PMR leads to a significant reduction in quality of life. 1
Glucocorticoids (GCs) are the mainstay for PMR but induce adverse events in long-term therapy. The clinical need for GC-sparing agents remains unmet. From recent reports, tocilizumab shows efficacy, but the assessment of disease activity might be impaired due to its effect on C reactive protein. 2 3 For conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), there is no convincing evidence of efficacy. Tofacitinib, a JAK inhibitor, suppresses interferon-γ-related downstream pathway, thereby alleviating the activity of PMR. To explore its efficacy and safety in PMR, we conducted the Simon’s two-stage minimax designed study (NCT04799262).4
The key inclusion criteria were patients with highly active PMR (PMR Activity Scale (PMR-AS) >17) with positive inflammatory parameters within 2 weeks prior to screening.5 6 The main exclusions included any prior or concurrent use of immunosuppressive therapies within the defined period specified in the protocol, a history of herpes zoster, etc. Tofacitinib of 10 mg/day was administrated concomitantly with prednisone of 15 mg/day at baseline tapered to 2.5 mg/day or less within 20 weeks. GC was tapered following the predefined regimen depending on the response to treatment judged by PMR-AS. During the study, other DMARDs, non-steroidal anti-inflammatory drugs or pregabalin was not allowed. The primary endpoint was the remission response defined as the achievement of sustained low disease activity (LDA) (PMR-AS <7) with GC independence (prednisone ≤2.5 mg/day) for 4 weeks from week 20. Plasma levels of inflammatory cytokines involved in PMR were measured at baseline and endpoint of 24 weeks. The null hypothesis that the true response rate was 0.54 would be tested against a one-sided alternative.7 In the first stage, eight patients would be accrued. If there were more than five responses, six additional patients would be recruited for a total of 14. The null hypothesis would be rejected if 11 or more responses were observed in 14 patients. This design yielded a type I error rate of 0.05 and power of 0.8 when the true response rate was 0.85.2 Two-sided 95% CIs were calculated to estimate the proportion of responses by the Clopper-Pearson approach. Patients were followed up until 48-week extension. The detailed study procedures are available online (online supplemental file 1).
ard-2022-223562supp001.pdf (6.8MB, pdf)
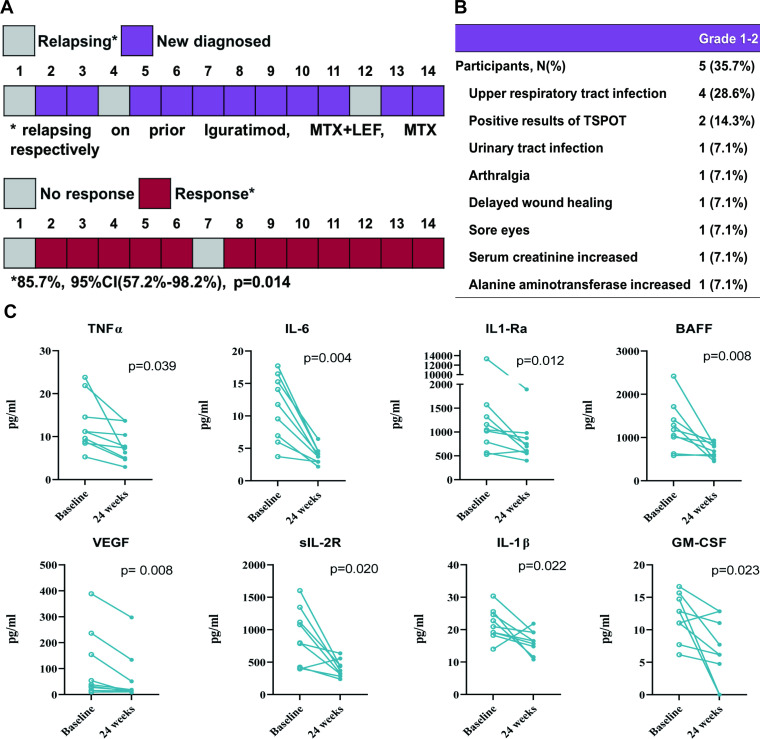
From March to December 2021, 14 participants with highly active PMR were enrolled. Eleven patients were newly diagnosed and three were relapsing on prior csDMARDs with an average disease duration of 9.7 (3.9) months. The baseline PMR-AS was 50.9 (25) with a mean Visual Analogue Scale (VAS)-pain of 71.8 (16). At the endpoint, 12 of 14 (85.7%; 95% CI 57.2% to 98.2%) patients reached the remission response (p=0.014) (figure 1A). A significant reduction in PMR-AS and its components was observed at week 2 from baseline and maintained throughout the study; all achieved LDA at week 24 with a median VAS-pain of 5 (0–17.5) at a prednisone dose of 2.2 (1.1) mg/day (table 1). Additionally, the quality of life assessed by Modified Health Assessment Questionnaire and EQ-5D-3L was both significantly improved (p<0.001). A significant decrease was observed in interleukin (IL)-6, tumour necrosis factor-α, BAFF and IL-1Ra (p<0.05) (figure 1C). During the extension, GC-independent LDA persisted without relapse, and GCs and tofacitinib were further tapered according to PMR-AS. At week 48, the average prednisone dose was 1.3 (1.2) mg with six (42.9%) cases of discontinuation; tofacitinib was halved in six subjects while three participants discontinued it. There were 12 adverse events reported by five (35.7%) participants, while no additional cardiovascular disorders, malignancy or herpes zoster infection were recorded (figure 1B).
Figure 1.
Enrolment and primary outcome (N=14) (A), summary of adverse events (N=14) (B) and changes of inflammatory cytokines in the 24-week study (N=9) (C). GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; LEF, leflunomide; MTX, methotrexate; TNF, tumour necrosis factor; VEGF, vascular endothelial growth factor.
Table 1.
Treatment and disease characteristics during the follow-up (N=14)
| Week 0 | Week 2 | Week 4 | Week 8 | Week 12 | Week 16 | Week 20 | Week 24 | Week 48 | |
| PMR-AS | 50.9 (25) | 4.0 (2.6–11.3)* | 4.3 (3.8)* | 4.4 (3.2)* | 2.2 (1.1)* | 1.3 (0.6–2.8)* | 2.2 (1.9)* | 2.1 (1.4)* | 1.9 (1.5)* |
| VAS-pain | 71.8 (16) | 30 (15.6)* | 19.3 (14.9)* | 11.5 (9.1)* | 11.4 (9.9)* | 7.9 (7.7)* | 2.5 (0–10)* | 5 (0–17.5)* | 0 (0–0)* |
| MST (min) | 55.9 (14.9) | 0 (0–0)* | 0 (0–0)* | 0 (0)* | 0 (0)* | 0 (0)* | 0 (0)* | 0 (0)* | 0 (0)* |
| EUL=0, N (%) | 4 (28.6) | 12 (85.7)† | 14 (100)* | 14 (100)* | 14 (100)* | 14 (100)* | 14 (100)* | 14 (100)* | 14 (100)* |
| PtGA | 7.5 (1.9) | 2.5 (1.2)* | 1.8 (1.4)* | 1.0 (0–2)* | 1.1 (1)* | 1 (0–1.8)* | 0.5 (0–1)* | 0.5 (0–1)* | 0 (0–1)* |
| PhGA | 7.1 (1.3) | 2.5 (1.2)* | 1.6 (1.3)* | 1.0 (0.9)* | 1.1 (0.9)* | 1 (0–1)* | 0.5 (0–1)* | 0 (0–1)* | 0 (0–1)* |
| ESR (mm/hour)‡ | 66.0 (26.6) | 26.9 (19.4)* | 10.7 (7)* | 15.4 (11.9)* | 11.4 (5.7)* | 12.3 (10.4)* | 13.2 (10.1)* | 11.9 (7.4)* | 11.4 (7.4)* |
| CRP (mg/L) | 36.5 (26.1) | 2 (0.8–9)† | 0.9 (0.5–5)† | 3.4 (3.2)† | 1.1 (0.8)* | 0.7 (0.5–1)* | 0.7 (0.5–1.5)* | 0.8 (0.5–2.5)* | 0.5 (0.5–1)* |
| LDA, N (%) | 0 | 9 (64.3)† | 9 (64.3)† | 11 (78.6)* | 14 (100)* | 13 (92.9)* | 13 (92.9)* | 14 (100)* | 14 (100)* |
| GC (mg/day) | 15 (0) | 10 (0)* | 11.2 (1.2)* | 7.9 (1.9)* | 5.7 (11.7)* | 3.8 (1.2)* | 2.2 (0.7)* | 2.2 (1.1)* | 1.3 (1.2)* |
| Discontinuation, N (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 (42.9%)† |
| Tofacitinib (mg/day) | 10 (0) | 10 (0) | 10 (0) | 10 (0) | 10 (0) | 10 (0) | 9.6 (1.3) | 9.6 (1.3) | 5.7 (3.7)† |
| Discontinuation, N (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (21.4) |
| MHAQ | 3 (1–3) | 0.4 (0.2–0.8)* | 0.3 (0.1–0.4)* | 0.3 (0–0.5)* | 0.3 (0–0.3)* | 0.1 (0–0.3)* | 0.1 (0–0.3)* | 0 (0–0.3)* | 0 (0–0.2)* |
| EQ-5D | 0.3 (0.2) | 0.7 (0.1)* | 0.7 (0.1)* | 0.8 (0.1)* | 0.8 (0.1)* | 0.8 (0.1)* | 0.9 (0.1)* | 0.9 (0.1)* | 0.9 (0.1)* |
Data are mean (SD) or median (IQR), unless stated otherwise. Significant differences were compared between the visit point and week 0.
*P<0.001.
†P<0.05.
‡The upper limit of normal value of ESR was 20 mm/hour and/or 8 mg/L for CRP.
CRP, C reactive protein; EQ-5D, EuroQol five-dimension questionnaire; ESR, erythrocyte sedimentation rate; EUL, elevation of upper limbs; GC, glucocorticoid; LDA, low disease activity; MHAQ, Modified Health Assessment Questionnaire; MST, morning stiffness; PhGA, Physician’s Global Assessment of VAS for disease activity; PMR-AS, Polymyalgia Rheumatica Activity Scale; PtGA, Patient’s Global Assessment of VAS for disease activity; VAS, Visual Analogue Scale.
To our knowledge, this is the first protocolised study on tofacitinib in PMR. It shows a high clinical efficacy with a good safety profile. Expected benefits may include sustained LDA, improved quality of life and reduced need for GCs.
Acknowledgments
The authors would like to thank the investigators, research staff, healthcare providers, patients and caregivers who contributed to this study.
Footnotes
Handling editor: Josef S Smolen
LZ, JL and HY contributed equally.
Correction notice: This article has been corrected since it published Online First. The order of authors has been corrected.
Contributors: All people who contributed to this work are listed as coauthors or collaborators.
Funding: The study was sponsored by the National Natural Science Foundation Youth Project (71804109) and Rising Stars of Medical Talents-Youth Development Program (SHWSRS(2021)_099).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
The data are available after approval from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Obtained.
Ethics approval
This study involves human participants and was approved by Shanghai Jiao Tong University, School of Medicine, Renji Hospital Ethics Committee (no. KY2021-088-A). Patients gave informed consent prior to participation.
References
- 1. González-Gay MA, Matteson EL, Castañeda S. Polymyalgia rheumatica. Lancet 2017;390:1700–12. 10.1016/S0140-6736(17)31825-1 [DOI] [PubMed] [Google Scholar]
- 2. Devauchelle-Pensec V, Berthelot JM, Cornec D, et al. Efficacy of first-line tocilizumab therapy in early polymyalgia rheumatica: a prospective longitudinal study. Ann Rheum Dis 2016;75:1506–10. 10.1136/annrheumdis-2015-208742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonelli M, Radner H, Kerschbaumer A, et al. Tocilizumab in patients with new onset polymyalgia rheumatica (PMR-SPARE): a phase 2/3 randomised controlled trial. Ann Rheum Dis 2022;81:838–44. 10.1136/annrheumdis-2021-221126 [DOI] [PubMed] [Google Scholar]
- 4. Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10:1–10. 10.1016/0197-2456(89)90015-9 [DOI] [PubMed] [Google Scholar]
- 5. Leeb BF, Bird HA. A disease activity score for polymyalgia rheumatica. Ann Rheum Dis 2004;63:1279–83. 10.1136/ard.2003.011379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dasgupta B, Cimmino MA, Maradit-Kremers H. Provisional classification criteria for polymyalgia rheumatica: a European League against Rheumatism/American College of rheumatology collaborative initiative. Ann Rheum Dis 2012;2012:484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salvarani C, Macchioni P, Manzini C, et al. Infliximab plus prednisone or placebo plus prednisone for the initial treatment of polymyalgia rheumatica: a randomized trial. Ann Intern Med 2007;146:631–9. 10.7326/0003-4819-146-9-200705010-00005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ard-2022-223562supp001.pdf (6.8MB, pdf)
Data Availability Statement
The data are available after approval from the corresponding author on reasonable request.