Abstract
Acute graft-versus-host disease (GVHD) is characterized by the production of high levels of T helper 1 (Th1)-type cytokines. Bone marrow transplantation from allogeneic C57BL/6 cells to CBF1 mice produced acute GVHD. Host resistance to Th1-driven Listeria monocytogenes was enhanced, whereas host resistance to Th2-driven Staphylococcus aureus was reduced during acute GVHD. These results suggest that opposite host responses are observed between Th1-driven and Th2-driven bacterial infections in acute GVHD.
Acute graft-versus-host disease (GVHD) is the most common complication of allogeneic bone marrow transplantation (BMT). During acute GVHD, cytokine dysregulation occurs as a consequence of synergistic interactions between cells of both myeloid and lymphoid lineages (14). Antigen-specific CD4+ T helper (Th) cell responses can be divided into two types, Th1 and Th2, based on cytokine production and effector function (15, 28). Differentiation of Th1 cells, which can produce interleukin 2 (IL-2), gamma interferon (IFN-γ), and lymphotoxin, is driven by IL-12 and IFN-γ, while differentiation of Th2 cells, which can produce IL-4, IL-5, IL-10, and IL-13, is driven by IL-4. Acute GVHD is characterized by the production of high levels of Th1-type cytokines, including IFN-γ (1, 23). The perceived role of IFN-γ in acute GVHD has been conflicting. Some studies have demonstrated that IFN-γ plays an important role in the pathogenesis in acute GVHD (4, 32), and other studies have suggested that IFN-γ plays a role in protecting from the disease (16, 33).
Listeria monocytogenes, a facultative intracellular bacterium, induces the Th1 response, including IFN-γ production in the infected host (11), and IFN-γ plays a critical role in antilisterial resistance (8, 12), while Th2-type cytokines, including IL-4 and IL-10, inhibit antilisterial resistance (3, 17, 18, 31). In contrast, our recent study indicated that Th2 response becomes dominant in Staphylococcus aureus infection and that IL-4 and IL-10 might play a protective role (25) but that IFN-γ plays a detrimental role in host resistance against S. aureus infection (19, 25, 34). On the other hand, infections of these pathogens have been reported as the complication of GVHD in humans (6, 26, 35). Therefore, we were interested in investigating the host response to L. monocytogenes or S. aureus infection in a mouse model of acute GVHD which is polarized toward Th1.
In this study, data were expressed as means ± standard deviations (SD), and the Wilcoxon rank-sum test was used to determine the significance of the differences in bacterial counts in the organs and in cytokine titers between the control and experimental groups. The generalized Wilcoxon test was used to determine the significance of differences in the survival rates.
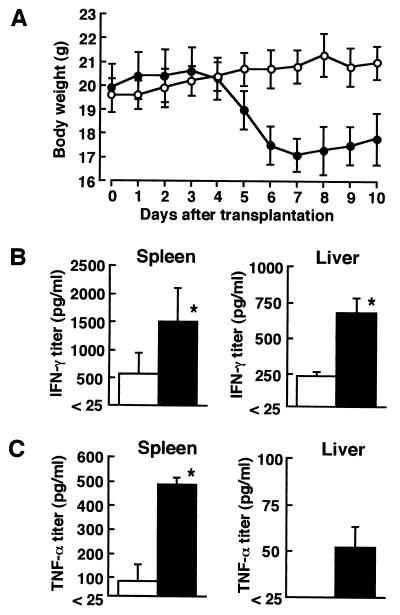
Female C57BL/6 mice (B6 mice; H-2b) and CBF1 mice (BALB/c × B6 H-2b×d), 6 to 8 weeks old, were purchased from SLC Japan, Hamamatsu, Shizuoka, Japan. All animals were maintained in the Institute for Animal Experiment, Hirosaki University School of Medicine. Acute GVHD was induced in irradiated CBF1 mice by intravenous injections of bone marrow cells and spleen cells of B6 mice as described previously (9, 30). Briefly, CBF1 mice received a sublethal 8.0-Gy dose of total body irradiation from an X-ray irradiation apparatus (MBR-1505R2; Hitachi Medico, Ibaraki, Japan). The recipients were injected intravenously with 1 × 107 bone marrow cells and 5 × 107 spleen cells of B6 mice resuspended in RPMI 1640 medium (Nissui Pharmaceutical Co., Tokyo, Japan) 24 h later. As a control, irradiated CBF1 mice were injected with 1 × 107 bone marrow cells and 5 × 107 spleen cells of CBF1 mice. B6 BMT recipients started to lose body weight from day 5 of transplantation as a typical clinical sign of acute GVHD (Fig. 1A). Furthermore, most B6 BMT recipients had diarrhea and lost hair during observation.
FIG. 1.
(A) Effect of allogeneic BMT on body weight and endogenous cytokine production. Body weights of B6 BMT recipients (solid circles) and CBF1 BMT recipients (open circles) were expressed the means ± SD (error bars) for a group of six mice. The data are a representative result of five experiments. (B and C) The titers of endogenous IFN-γ (B) and TNF-α (C) in the spleens and livers of B6 BMT (solid bars) and CBF1 BMT (open bars) recipients were assayed. Each result represents the mean ± SD for a group of four mice. These results were reproduced in three repeated experiments. A single asterisk indicates a significant difference from the CBF1 BMT recipients at a P value of <0.01.
Endogenous cytokines in the spleen and liver homogenates and cytokine responses in the spleen cell cultures of B6 BMT recipients and CBF1 BMT control recipients were investigated on day 7 of transplantation. Preparation of spleen extracts for cytokine assays and preparation of splenocytes for in vitro cultures were performed as described previously (20, 24). Assays for IFN-γ, IL-4, and tumor necrosis factor (TNF-α) were carried out by a double sandwich enzyme-linked immunosorbent assay, as described previously (18, 19). The endogenous IFN-γ titers were significantly higher in the spleens and livers of B6 BMT recipients, compared with those of CBF1 BMT control recipients (Fig. 1B). The higher titers of TNF-α, which is involved in the pathogenesis in acute GVHD (29), were also detected in B6 BMT recipients (Fig. 1C). Next, splenocytes (106/ml), which were isolated from B6 BMT recipients or CBF1 BMT control recipients on day 7 after transplantation, were inoculated into a 24-well tissue culture plate (Greiner, Frickenhausen, Germany), and the cells were stimulated with 2.5 × 106 cells of irradiated splenocytes obtained from B6 mice or from CBF1 mice or stimulated with 145-2C11-derived anti-mouse CD3ɛ monoclonal antibody at a final concentration of 2 μg/ml at 37°C in a humidified 5% CO2 incubator for 48 h. Culture supernatant fluids obtained were assayed for IFN-γ and IL-4 (Table 1). Splenocytes obtained from B6 BMT recipients produced IFN-γ but not IL-4 in response to irradiated CBF1 splenocytes, and both cytokines were produced in response to stimulation with anti-CD3 monoclonal antibody. They did not respond to syngeneic B6 splenocytes. In contrast, splenocytes obtained from CBF1 BMT recipients failed to produce these cytokines in response to any stimuli used herein. These results suggest that the production of the Th1-type cytokine IFN-γ is augmented in B6 BMT recipients.
TABLE 1.
Cytokine production in spleen cell cultures obtained from mice which received allogeneic or syngeneic BMTa
| Mice | Stimulation | Titer (pg/ml) of:
|
|
|---|---|---|---|
| IFN-γ | IL-4 | ||
| CBF1→CBF1 | B6 splenocytes | <25 | <25 |
| CBF1 splenocytes | <25 | <25 | |
| Anti-CD3 | <25 | <25 | |
| B6→CBF1 | B6 splenocytes | <25 | <25 |
| CBF1 splenocytes | 1,108 ± 188 | <25 | |
| Anti-CD3 | 4,030 ± 1493 | 60 ± 5 | |
Spleen cells obtained from CBF1 mice, which had been transplanted with bone marrow cells and spleen cells obtained from B6 mice (B6→CBF1) or CBF1 mice (CBF1→CBF1) 7 days before were stimulated with irradiated B6 or CBF1 splenocytes or with anti-CD3 monoclonal antibody for 48 h, and then the titers of IFN-γ and IL-4 in the culture supernatants were determined. Each result represents the mean ± SD for a group of five mice. The data are reproduced in three experiments.
The effect of acute GVHD on host resistance against L. monocytogenes infection was investigated. L. monocytogenes 1b-1684 cells were prepared as described previously (18). B6 BMT recipients and CBF1 BMT control recipients were infected intravenously with 104 CFU of L. monocytogenes on day 7 post-BMT, and the number of bacterial cells in the spleens and livers was determined on days 2 and 4 of infection by culturing on tryptic soy agar (Difco Laboratories, Detroit, Mich.) (18) (Table 2). Bacteria in the organs were not detectable on days 2 and 4 of infection in B6 BMT recipients, while significant numbers of bacteria in the organs were observed on day 2 and then the numbers were decreased on day 4 of infection in CBF1 BMT control recipients. It is recognized that antilisterial resistance depends on innate immunity on day 2 and is performed by cytokine-induced activated phagocytes on day 4 of systemic L. monocytogenes infection (13). Therefore, the results suggest that innate immunity to L. monocytogenes infection is augmented in acute GVHD.
TABLE 2.
Effect of allogeneic BMT on host resistance against L. monocytogenes infection and S. aureus infection
| Infectiona | Days postinfectionb | Mouse groupc | Log bacteria (CFU/organ)d in:
|
||
|---|---|---|---|---|---|
| Spleen | Liver | Kidney | |||
| L. monocytogenes | 2 | CBF1→CBF1 | 4.81 ± 0.18 | 4.13 ± 0.26 | NDe |
| B6→CBF1 | <2 | <2 | ND | ||
| 4 | CBF1→CBF1 | 4.14 ± 0.17 | 2.60 ± 0.44 | ND | |
| B6→CBF1 | <2 | <2 | ND | ||
| S. aureus | 2 | CBF1→CBF1 | 2.08 ± 0.36 | 4.26 ± 0.06 | 6.35 ± 0.67 |
| B6→CBF1 | 3.51 ± 0.46f | 4.26 ± 0.13 | 6.63 ± 0.45 | ||
| 7 | CBF1→CBF1 | 2.25 ± 0.19 | 3.29 ± 0.26 | 7.26 ± 0.65 | |
| B6→CBF1 | 3.95 ± 0.37f | 5.79 ± 0.26f | 7.68 ± 0.34 | ||
BMT recipients were infected intravenously with 104 CFU of L. monocytogenes or 107 CFU of S. aureus on day 7 post-BMT.
The number of bacterial cells in the organs was determined at the indicated times.
Irradiated CBF1 mice were transplanted with bone marrow cells and spleen cells obtained from B6 mice (B6→CBF1) or CBF1 mice (CBF1→CBF1).
Each result represents the mean ± SD for a group of four mice. These results were reproduced in three repeated experiments.
ND, not determined.
Significantly different from value for CBF1→CBF1 group (P < 0.05).
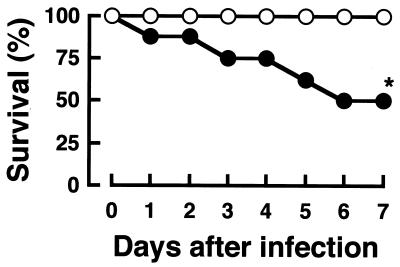
Next, the effect of acute GVHD on host resistance to S. aureus infection was investigated. S. aureus 834 cells were prepared as described previously (24). B6 BMT recipients and CBF1 BMT control recipients were infected intravenously with 107 CFU of S. aureus on day 7 post-BMT. Half of the infected B6 BMT recipients succumbed until day 6 of infection, whereas all CBF1 BMT control recipients survived (Fig. 2). The number of bacterial cells in the spleens, livers, and kidneys of the infected mice was determined on days 2 and 7 of infection (Table 2). It is presumed that host resistance depends on innate immunity on day 2, while the reduction of bacterial numbers is observed by day 7 in the spleens and livers during systemic S. aureus infection (19, 25). Bacterial numbers in the spleens on day 2 and in the spleens and livers on day 7 but not in the kidneys were markedly increased in B6 BMT recipients, compared with those in preferentially CBF1 BMT control recipients. Our previous study indicated that S. aureus colonizes and proliferates along with abscess formation in kidneys, but large numbers of bacterial cells were detected in spleen and liver when they were shifted to a lethal systemic infection (19). Therefore, it is possible that B6 BMT recipients might succumb to septicemia. The present results suggest that B6 BMT recipients are more susceptible to S. aureus infection during acute GVHD.
FIG. 2.
Effect of allogeneic BMT on survival rates of mice infected with S. aureus. Eight B6 BMT recipients (solid circles) and eight CBF1 BMT control recipients (open circles) were infected intravenously with 107 CFU of S. aureus on day 7 post-BMT. An asterisk indicates a significant difference from the CBF1 BMT recipients at a P value of <0.01. These results were reproduced in three repeated experiments.
In the present study, opposite susceptibilities between Th1-driven and Th2-driven bacterial infections were expressed in acute GVHD. A Th1-driven acute GVH reaction augmented host resistance to Th1-driven L. monocytogenes infection in the early stage (Table 2). It is known that Th1 or Th1-related cytokines, especially IFN-γ and TNF-α, are involved in antilisterial resistance (8, 10, 12, 17, 21, 22). Therefore, in the present study, upregulation of innate immunity to L. monocytogenes infection might be due to activation of effector cells, including macrophages (2, 13), by cytokines, including IFN-γ and TNF-α produced during acute GVH reaction (Fig. 1 and Table 1). Recently, some studies showed that prior or concurrent immunization with bacteria such as Mycobacterium bovis BCG and Brucella abortus, which induce Th1-type responses, abrogates antigen-specific, immunoglobulin E-mediated allergic recall response to ovalbumin (5, 27). Similarly, our present results suggested that host resistance to S. aureus infection, in which IL-4 and IL-10 might play a beneficial role and IFN-γ might play a detrimental role (19, 25), was suppressed during Th1-type acute GVHD (Fig. 2 and Table 2). In humans, staphylococci are involved in opportunistic infections in patients with GVHD (6, 26). Given our present results, it may be significant that S. aureus, which causes nosocomial infections, is noticed in acute GVHD.
In conclusion, opposite susceptibilities to infectious diseases may be observed in BMT recipients suffering from acute GVHD, depending on the inducibility of Th cell responses by pathogens.
Acknowledgments
This work was supported in part by the Aomori Bank Research Grants for the Organ Transplantation Study Group at the Hirosaki University School of Medicine and grants-in-aid for general scientific research (08670297 and 10670247) provided by the Japanese Ministry of Education, Science, Sports, and Culture.
REFERENCES
- 1.Allen R D, Staley T A, Sidman C L. Differential cytokine expression in acute and chronic murine graft-versus-host disease. Eur J Immunol. 1993;23:333–337. doi: 10.1002/eji.1830230205. [DOI] [PubMed] [Google Scholar]
- 2.Bancroft G J, Schreiber R D, Unanue E R. Natural immunity: a T-cell-independent pathway of macrophage activation, defined in the scid mouse. Immunol Rev. 1991;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 3.Dai W, Köhler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 1997;158:2259–2267. [PubMed] [Google Scholar]
- 4.Ellison C A, Fischer J M M, HayGlass K T, Gartner J G. Murine graft-versus-host disease in an F1-hybrid model using IFN-γ gene knockout donors. J Immunol. 1998;161:631–640. [PubMed] [Google Scholar]
- 5.Erb K J, Holloway J W, Sobeck A, Moll H, Le Gros G. Infection of mice with Mycobacterium bovis-Bacillus Calmette-Guérin (BCG) suppressed allergen-induced airway eosinophilia. J Exp Med. 1998;187:561–569. doi: 10.1084/jem.187.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg N S, Ahmed T, Robinson B, Ascensao J, Horowitz H. Staphylococcal scalded skin syndrome mimicking acute graft-vs-host disease in a bone marrow transplant recipient. Arch Dermatol. 1989;125:85–87. [PubMed] [Google Scholar]
- 7.Haak-Frendsho M, Brown J F, Iizawa Y, Wagner R D, Czuprynski C J. Administration of anti-IL-4 monoclonal antibody 11B11 increases the resistance of mice to Listeria monocytogenes infection. J Immunol. 1992;148:3978–3985. [PubMed] [Google Scholar]
- 8.Harty J T, Bevan M. Specific immunity to Listeria monocytogenes in the absence of IFNγ. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 9.Hattori K, Hirano T, Ushiyama C, Miyajima H, Yamakawa N, Ebata T, Wada Y, Ikeda S, Yoshino K, Tateno M, Oshimi K, Kayagaki N, Yagita H, Okumura K. A metalloproteinase inhibitor prevents lethal acute graft-versus-host disease in mice. Blood. 1997;90:542–548. [PubMed] [Google Scholar]
- 10.Havell E A. Production of tumor necrosis factor during murine listeriosis. J Immunol. 1987;139:4225–4231. [PubMed] [Google Scholar]
- 11.Hsieh C-S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K H. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 12.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-γ receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 14.Krenger W, Hill G R, Farrara J L M. Cytokine cascades in acute graft-versus-host disease. Transplantation. 1997;64:553–558. doi: 10.1097/00007890-199708270-00001. [DOI] [PubMed] [Google Scholar]
- 15.Mosmann T R, Cherwinski H, Bond M, Giedlin M A, Coffman R L. Two types of murine helper T-cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;36:2348–2357. [PubMed] [Google Scholar]
- 16.Murphy W J, Welniak L A, Taub D D, Wiltrout R H, Taylor P A, Vallera D A, Kopf M, Young H, Longo D L, Blazar B R. Differential effects of the absence of interferon-γ and IL-4 in acute graft-versus-host disease after allogeneic bone marrow transplantation in mice. J Clin Investig. 1998;102:1742–1748. doi: 10.1172/JCI3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakane A, Minagawa T, Kohanawa M, Chen Y, Sato H, Moriyama M, Tsuruoka T. Interactions between endogenous gamma interferon and tumor necrosis factor in host resistance against primary and secondary Listeria monocytogenes infections. Infect Immun. 1989;57:3331–3337. doi: 10.1128/iai.57.11.3331-3337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakane A, Nishikawa S, Sasaki S, Miura T, Asano M, Kohanawa M, Ishiwata K, Minagawa T. Endogenous interleukin-4, but not interleukin-10, is involved in suppression of host resistance against Listeria monocytogenes infection in gamma interferon-depleted mice. Infect Immun. 1996;64:1252–1258. doi: 10.1128/iai.64.4.1252-1258.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakane A, Okamoto M, Asano M, Kohanawa M, Minagawa T. Endogenous gamma interferon, tumor necrosis factor, and interleukin-6 in Staphylococcus aureus infection in mice. Infect Immun. 1995;63:1165–1172. doi: 10.1128/iai.63.4.1165-1172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishikawa S, Nakane A. Host resistance against Listeria monocytogenes is reciprocal during the course of infection in alymphoplastic aly mutant mice. Cell Immunol. 1998;187:88–94. doi: 10.1006/cimm.1998.1329. [DOI] [PubMed] [Google Scholar]
- 21.Pfeffer K, Matsuyama T, Kündig T M, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi P S, Krönke M, Mak T W. Mice deficient for the 55kd tumor necrosis factor receptor are resistant to endotoxin shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 22.Rothe J, Lesslauer W, Lötscher H, Lang Y, Koebel P, Köntgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumor necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 23.Rus V, Svetic A, Nguyen P, Gause W C, Via C S. Kinetics of Th1 and Th2 cytokine production during the early course of acute and chronic murine graft-versus-host disease. J Immunol. 1993;155:2396–2406. [PubMed] [Google Scholar]
- 24.Sasaki S, Miura T, Nishikawa S, Yamada K, Hirasue M, Nakane A. Protective role of nitric oxide in Staphylococcus aureus in mice. Infect Immun. 1998;66:1017–1022. doi: 10.1128/iai.66.3.1017-1022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasaki S, Nishikawa S, Miura T, Mizuki M, Yamada K, Madarame H, Tagawa Y-I, Iwakura Y, Nakane A. Interleukin-4 and interleukin-10 are involved in host resistance to Staphylococcus aureus infection through regulation of gamma interferon. Infect Immun. 2000;68:2424–2430. doi: 10.1128/iai.68.5.2424-2430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sayer H G, Longton G, Bowden R, Pepe M, Storb R. Increased risk of infection in marrow transplant patients receiving methylprednisolone for graft-versus-host disease prevention. Blood. 1994;84:1328–1332. [PubMed] [Google Scholar]
- 27.Scott D E, Agranovich I, Gober M, Golding B. Inhibition of primary and recall allergen-specific Th2-mediated responses by a Th1 stimulus. J Immunol. 1997;159:107–116. [PubMed] [Google Scholar]
- 28.Seder R A, Paul W E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 29.Speiser D E, Bachmann M F, Frick T W, McKall-Faienza K, Griffiths E, Pfeffer K, Mak T W, Ohashi P S. TNF receptor p55 controls early acute graft-versus-host disease. J Immunol. 1997;158:5185–5190. [PubMed] [Google Scholar]
- 30.Ushiyama C, Hirano T, Miyajima H, Okumura K, Ovary O, Hashimoto H. Anti-IL-4 antibody prevents graft-versus-host disease in mice after bone marrow transplantation. The IgE allotype is an important marker of graft-versus-host disease. J Immunol. 1995;154:2687–2696. [PubMed] [Google Scholar]
- 31.Wagner R D, Maroushek N M, Brown J F, Czuprynski C J. Treatment with anti-interleukin-10 monoclonal antibody enhances early resistance to but impairs complete clearance of Listeria monocytogenes infection in mice. Infect Immun. 1994;62:2345–2353. doi: 10.1128/iai.62.6.2345-2353.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williamson E, Garside P, Bradley J A, More I A R, Mowat A M. Neutralizing IL-12 during induction of murine acute graft-versus-host disease polarizes the cytokine profile toward a Th2-type alloimmune response and confers long term protection from disease. J Immunol. 1997;159:1208–1215. [PubMed] [Google Scholar]
- 33.Yang Y-G, Dey B R, Sergio J J, Pearson D A, Sykes M. Donor-derived interferon γ is required for inhibition of acute graft-versus-host disease by interleukin 12. J Clin Investig. 1998;102:2126–2135. doi: 10.1172/JCI4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y-X, Tarkowski A. Impact of interferon-γ receptor deficiency on experimental Staphylococcus aureus septicemia and arthritis. J Immunol. 1995;155:5736–5742. [PubMed] [Google Scholar]
- 35.Zomas A, Mehta J, Powles R, Treleaven J, Iveson T, Sihghal S, Jameson B, Paul B, Brincat S, Catovsky D. Unusual infections following allogeneic bone marrow transplantation for chronic lymphocytic leukemia. Bone Marrow Transplant. 1994;14:799–803. [PubMed] [Google Scholar]