Abstract
Home non-invasive mechanical ventilation (HMV) with home oxygen therapy (HOT) in patients with persistent hypercapnia following an acute exacerbation of chronic obstructive pulmonary disease delays hospital readmission. The economic impact of this treatment is unknown. We evaluated the cost-effectiveness of HMV in the UK healthcare system using data from a previously published efficacy trial. Quality-adjusted life-years (QALYs) were computed from EQ-5D-5L. Accounting for all direct patient costs HOT-HMV was £512 (95%CI £36 to £990) more expensive per patient per year than HOT-alone. This small increase in cost was accompanied by increased quality of life leading to an incremental cost-effectiveness ratio of £10 259 per QALY. HOT-HMV was cost-effective in this clinical population. Trial registration number: NCT00990132.
Keywords: COPD Exacerbations, Non invasive ventilation, Health Economist
Introduction
Chronic obstructive pulmonary disease (COPD) remains a common cause of hospital admission, with patients with persistent hypercapnic respiratory failure having worse outcomes.1 A few studies have evaluated the cost of home non-invasive mechanical ventilation (HMV) with home oxygen therapy (HOT) for patients with COPD with persistent hypercapnia following hospitalisation.2 HOT-HMV has been shown clinically efficacy in a previous UK randomised clinical trial.3 We hypothesised HOT-HMV would be cost-effective in the UK.
Methods
A full description of the trial design can be found with the trial results.3 Patients with persistent hypercapnia (PaCO2≥7 kPa) between 2 weeks and 4 weeks after resolution of acidosis following an admission with an acute exacerbation of COPD were recruited. Patients were randomised to HOT-HMV or HOT. In addition to clinical data, healthcare utilisation, exacerbation frequency and quality-of-life data were collected at each follow-up visit (6 weeks then 3, 6 and 12 months). A detailed description of quality-adjusted life-year (QALY) calculations is provided in online supplemental eMethods. Medical resource utilisation was recorded throughout the trial at routine follow-up, which was reported by patients and verified by electronic health records, where possible. The economic analysis was conducted over 12 months, reflecting the clinical trial. Costs were calculated from 2017 tariff data from a National Health Service perspective (online supplemental eTable 1). Cost-effectiveness was a prespecified outcome of the efficacy trial using an intention-to-treat (ITT) approach. Details of the ITT and per-protocol approach are in online supplemental eMethods. Sensitivity analyses used realistic minimum and maximum costs.
thorax-2022-219653supp001.pdf (394.7KB, pdf)
Results
A total of 116 patients were included in the base-case analysis: 57 in the intervention group (HOT-HMV) and 59 in the control group (HOT) (online supplemental eFigure e1). Baseline patient and retention data are provided in online supplemental eTable 2 and eTable 3. Diary card data were missing for 25 patients (HOT-HMV=8; HOT=17).
thorax-2022-219653supp002.pdf (501.6KB, pdf)
Base-case analyses for UK (ITT)
Average total 1-year device costs per patient for the intervention group were £6679 (95% CI £6447 to £6911) compared with £2684 (95% CI £2007 to £3360) in the control group. For all other cost categories, 1-year costs per patient were lower in the intervention group compared with the control group, including average 1-year total primary and secondary care physician visits (£5947 (95% CI £4394 to £7586) vs £8275 (95% CI £6428 to £10 122)); medication costs (£90 (95% CI £52 to £127) vs £104 (95% CI £61 to £146)) and costs for the treatment of exacerbations (£4679 (95% CI £2866 to £6493) vs £5821 (95% CI £4089 to £7552)). Total average annual direct costs per patient were £17 395 (95% CI £14 309 to £20 482) for the intervention group and £16 883 (95% CI £13 319 to £20 446) for the control group.
The average number of QALYs was 0.36 (95% CI 0.27 to 0.45) and 0.31 (95% CI 0.23 to 0.39) for the intervention group and control group, respectively. The incremental cost-effectiveness ratio (ICER) was £10 259/QALY (95% CI £5438 to £16 449) (table 1).
Table 1.
Cost-effectiveness results for home non-invasive mechanical ventilation (HMV) with home oxygen therapy (HOT) versus HOT alone (intention-to-treat analysis)
| Intervention | Total costs (£) (95% CI) | Total QALYs (95% CI) | ICER (Δcost/ΔQALYs) (95% CI) |
| UK analysis | |||
| HOT alone | £16 883 (£13 319 to £20 446) | 0.31 (0.23 to 0.39) | Ref |
| HOT-HMV | £17 395 (£14 309 to £20 482) | 0.36 (0.27 to 0.45) | £10 259 (£5438 to £16 449) |
ICER, incremental cost-effectiveness ratio; QALYs, quality-adjusted life-years.
One-way sensitivity analyses and bootstrap sensitivity analysis for UK
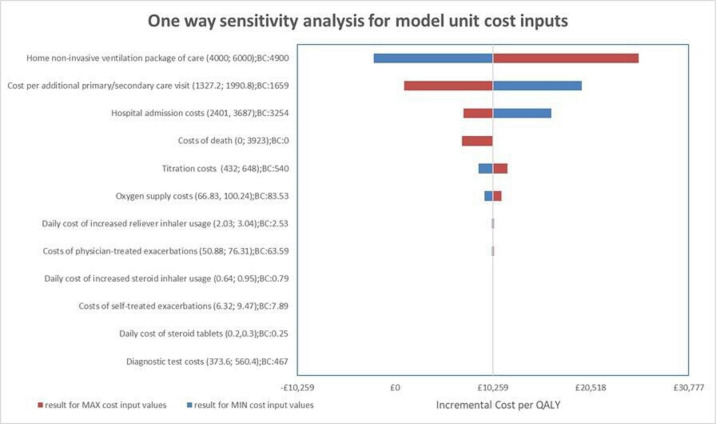
One-way sensitivity analyses demonstrated parameters with the greatest influence on the ICER: HMV package of care costs for 12 months (ICER range −£2244 to £25 542), cost per additional primary and secondary care physician visit (£944 to £19,574) and hospital admission costs (£7152 to £19574) (figure 1). Bootstrap iterations indicated that at £20 000 and £30 000/QALY, the probability that HOT-HMV is cost-effective versus HOT alone is 56% and 61%, respectively (figure 2A). At £30 000/QALY, the probability that HOT-HMV is more costly and more effective than HOT is 45% (figure 2B). The probability that HOT-HMV is less costly and more effective than HOT alone is 34%.
Figure 1.
One-way sensitivity analysis results of home non-invasive ventilation with home oxygen therapy versus home oxygen therapy alone in the UK health systems (intention to treat).
Figure 2.
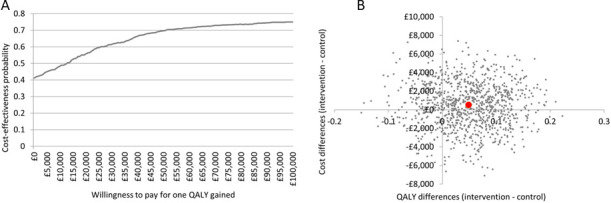
(A) Cost-effectiveness acceptability curve for home non-invasive ventilation with home oxygen therapy versus home oxygen therapy alone in the UK health systems (intention-to-treat analysis); (B) Cost-effectiveness plane for home non-invasive ventilation with home oxygen therapy versus home oxygen therapy alone in the UK health systems (intention-to-treat analysis).
Discussion
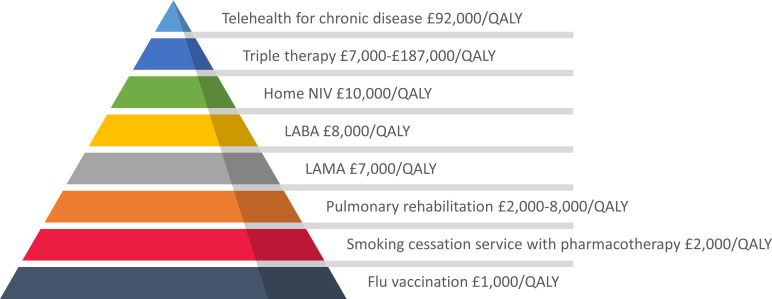
HMV is increasingly used to treat chronic hypercapnic respiratory failure.4 A few publications have examined the cost-effectiveness of HMV, with existing economic evaluation largely confined to different HMV modes or setup strategies.5–7 HOT-HMV has previously demonstrated clinical effectiveness, increasing admission-free survival (time to hospital readmission or death) in patients with COPD following a life-threatening exacerbation requiring acute non-invasive ventilation.3 Our study demonstrates that HOT-HMV is cost-effective, with the upper limit of cost per QALY falling below £20 000. This result is consistent with previously conducted analyses using Markov models, which suggest a cost per QALY of £11 318 with a 99% chance of being cost-effective at the £20 000 threshold.8 9 The cost per QALY of HOT-HMV falls below that considered acceptable for interventions in the UK10 and compares favourably with other interventions commonly used in COPD (figure 3).
Figure 3.
Value pyramid for interventions in the management of COPD. COPD, chronic obstructive pulmonary disease; LABA, long-acting inhaled beta agonist; LAMA, long-acting inhaled muscarinic antagonist; NIV, non-invasive ventilation; QALY, quality-adjusted life-year.
Limitations
The efficacy trial included a small population with a completion rate (64/116) limited principally by patient death (35/116), which was numerically but not statistically larger in the HOT-alone group. The cost-effectiveness analysis accounts for the lower completion rates as death significantly impacts QALY. Of the patients who withdrew, most (>70%) were in the HOT-alone group. The the most common withdrawal reason was disease progression, which is associated with worse quality of life and so would favour the control arm rather than the intervention.
All centres contributing data to the efficacy trial had established HMV services; therefore, it is possible that less established centres would take longer to set up HMV and would thus increase costs with HOT-HMV. Recent data have demonstrated outpatient setup of HMV in COPD not only is feasible but may be more cost-effective than inpatient titration,5 although this was not the case on a recent study of patients with obesity hypoventilation syndrome.11
The trial design allowed patients initially allocated to HOT alone to have HMV if they breached safety criteria after reaching the primary outcome. As expected, the high number of cross-over patients diluted the impact on quality of life between intervention and control arm as these control arm patients were in poorer health than the HOT patients who did not cross-over. Importantly, an additional and modified per-protocol analysis showed increased ICERs compared with ITT (online supplemental eResults).
Finally, the health economic analysis required simplifications. The use of average costs of medical resources does not necessarily reflect actual individual healthcare expenditures but provides typical costs for the patient population. Furthermore, the use of QALYs as an effectiveness measure necessitates breaking the multidimensional construct of quality of life into one value. However, this approach is consistent with other research.12
Conclusion
HMV with HOT in patients with persistent hypercapnia following an acute life-threatening exacerbation of COPD is likely to be cost-effective in the UK.
thorax-2022-219653supp003.pdf (69.7KB, pdf)
thorax-2022-219653supp004.pdf (183.3KB, pdf)
Footnotes
Twitter: @NickHartGSTT
Presented at: ATS 20 May 2018, San Diego, A2517: Cost-Effectiveness of Home Oxygen Therapy-Home Mechanical Ventilation (HOT-HMV) for the Treatment of Chronic Obstructive Pulmonary Disease (COPD) with Chronic Hypercapnic Respiratory Failure Following an Acute Exacerbation of COPD in the United Kingdom (UK). A2518: Cost-Effectiveness of Home Oxygen Therapy-Home Mechanical Ventilation (HOT-HMV) for Treatment of Chronic Obstructive Pulmonary Disease (COPD) with Chronic Hypercapnic Respiratory Failure Following an Acute Exacerbation of COPD in the USA.
Contributors: Conception and design: NH, PBM, TR, BB and TG. Data collection: PBM, BB and QG. Model development and data interpretation: TR, BB, QG and TG. Manuscript drafting: NH, PBM, LF, QG, TR, BB and TG. Manuscript review, critical appraisal and final approval: NH, PBM, GC, LF, QG, TR, BB and TG.
Funding: The original randomised controlled trial was supported by unrestricted educational grants from Philips-Respironics (Pennsylvania, USA), ResMed (California, USA), ResMed Foundation and Guy’s & St Thomas’ Charity. Philips-Respironics provided the Harmony 2 ventilators and Actiwatch spectrum devices used in the study. ResMed provided VPAP III ST-A devices used in the study. The study was supported by Guy’s and St Thomas’ NHS Foundation Trust and King’s College London, National Institute of Health Research Comprehensive Biomedical Research Centre, London, UK and the NIHR Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London. The health economic analysis was funded equally by Philips-Respironics and ResMed. TG reports grants from ResMed and grants from Philips Respironics during the conduct of the study.
Disclaimer: The funders were not involved in design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests: PBM reports grants and personal fees from Philips, grants and personal fees from ResMed, grants and personal fees from F&P, grants and personal fees from B&D Electromedical, personal fees from Santhera outside the submitted work. BB was consultant to Boston Healthcare and received consultancy payment from ResMed and Phillips. TR was consultant to Boston Healthcare and received consultancy payment from Resmed and Phillips. QG reports grants from ResMed and grants from Philips Respironics during the conduct of the study. LF reports grants from ResMed and grants from Philips Respironics during the conduct of the study. GC reports grants from Boehringer- Ingelheim, grants from Novartis, grants from Astra Zeneca, grants from Respironics, grants from MedImmune, grants from Actelion, grants from Forest, grants from Pearl grants from Ikaria, grants from Aeris, grants from PneumRx, grants from Pulmonx other from HGE Health Care Solutions, other from Amirall, other from Boehringer-Ingelheim, other from Holaira, outside the submitted work. NH is on the Pulmonary Research Advisory Board for Philips and the funds for this are given to Guy's & St Thomas' NHS Foundation Trust. NH’s research group has received unrestricted grants (managed by Guy's & St Thomas' Foundation Trust) from Philips-Respironics, Philips, Resmed, Fisher-Paykel and B&D Electromedical. Philips and Philips-Respironics are contributing to the development of the MYOTRACE technology.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by St Thomas’ Hospital Research Ethics committee (09/H0802/2). Participants gave informed consent to participate in the study before taking part.
References
- 1. Murray I, Paterson E, Thain G, et al. Outcomes following non-invasive ventilation for hypercapnic exacerbations of chronic obstructive pulmonary disease. Thorax 2011;66:825–6. 10.1136/thx.2010.152264 [DOI] [PubMed] [Google Scholar]
- 2. Tuggey JM, Plant PK, Elliott MW. Domiciliary non-invasive ventilation for recurrent acidotic exacerbations of COPD: an economic analysis. Thorax 2003;58:867–71. 10.1136/thorax.58.10.867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murphy PB, Rehal S, Arbane G, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. JAMA 2017;317:2177–86. 10.1001/jama.2017.4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patout M, Ramsay M, Mackie M, et al. Home mechanical ventilation (HMV): setup and outcome in Europe. Eur Resp J 2015;46:OA4780. 10.1183/13993003.congress-2015.OA4780 [DOI] [Google Scholar]
- 5. Duiverman ML, Vonk JM, Bladder G, et al. Home initiation of chronic non-invasive ventilation in COPD patients with chronic hypercapnic respiratory failure: a randomised controlled trial. Thorax 2020;75:244–52. 10.1136/thoraxjnl-2019-213303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mandal S, Arbane G, Murphy P, et al. Medium-Term cost-effectiveness of an automated non-invasive ventilation outpatient set-up versus a standard fixed level non-invasive ventilation inpatient set-up in obese patients with chronic respiratory failure: a protocol description. BMJ Open 2015;5:e007082. 10.1136/bmjopen-2014-007082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Masa JF, Mokhlesi B, Benítez I, et al. Cost-Effectiveness of positive airway pressure modalities in obesity hypoventilation syndrome with severe obstructive sleep apnoea. Thorax 2020;75:459–67. 10.1136/thoraxjnl-2019-213622 [DOI] [PubMed] [Google Scholar]
- 8. Dretzke J, Blissett D, Dave C, et al. The cost-effectiveness of domiciliary non-invasive ventilation in patients with end-stage chronic obstructive pulmonary disease: a systematic review and economic evaluation. Health Technol Assess 2015;19:1–246. 10.3310/hta19810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hall J, Turner AM, Dretzke J, et al. Cost-Effectiveness of domiciliary non-invasive ventilation in patients with chronic obstructive pulmonary disease. Thorax 2021;77:976-86. 10.1136/thoraxjnl-2021-217463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Towse A. Should NICE's threshold range for cost per QALY be raised? Yes. BMJ 2009;338:b181. 10.1136/bmj.b181 [DOI] [PubMed] [Google Scholar]
- 11. Murphy PB, Patout M, Arbane G, et al. Cost-Effectiveness of outpatient versus inpatient non-invasive ventilation setup in obesity hypoventilation syndrome: the OPIP trial. Thorax 2023;78:24-31. 10.1136/thorax-2021-218497 [DOI] [PubMed] [Google Scholar]
- 12. National Institute for Health and Clinical Excellence . Continuous positive airway pressure for the treatment of obstructive sleep apnoea/hypopnoea syndrome. technology appraisal guidance. 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thorax-2022-219653supp001.pdf (394.7KB, pdf)
thorax-2022-219653supp002.pdf (501.6KB, pdf)
thorax-2022-219653supp003.pdf (69.7KB, pdf)
thorax-2022-219653supp004.pdf (183.3KB, pdf)