Abstract
Herein, a regioselective, late-stage two-step arene halogenation method is reported. We propose how unusual Ni(I)/(III) catalysis is enabled by a combination of aryl thianthrenium and Ni redox properties that is hitherto unachieved with other (pseudo)halides. The catalyst is accessed in situ from inexpensive NiCl2·6(H2O) and zinc without the need of supporting ligands.
Direct C–H thianthrenation has emerged as a synthetic tool for functionalization of aromatic rings.1,2 It proceeds under mild conditions and tolerates a wide variety of functional groups and thereby addresses one of the most challenging aspects of C–H functionalization: control of the regio- and chemoselectivity.3 Aryl thianthrenium salts have been shown to be excellent one-electron electrophiles in various photochemical and SET (single electron transfer)-type transformations reported to date.1,2 However, despite the wealth of examples, processes that involve SET reactions of thianthrenium salts using nickel as catalyst are still elusive.4 Here, we report a Ni(I)-catalyzed cross-coupling of arylthianthrenium salts to afford aryl halides (Cl, Br, and I). Because thianthrenation proceeds regio- and chemoselectively at a late stage, halides that would not readily be accessible by late-stage halogenation can be accessed with potential applications in selective radiolabeling, access of pharmaceutical candidates, or organometallic chemistry through halogen/metal exchange.5 The proposed redox cycle fundamentally differs from previously reported halogenation reactions6−8 and may be a consequence of the productive interplay of thianthrenium and nickel featuring distinct redox properties, which enables a smooth and practical reaction at room temperature with a broad substrate scope. The conceptual advance to realize an unusual but straightforward redox cycle is promising for the development of additional thianthrenium chemistry with nickel. Different to the commonly proposed mechansims,9,10 Nocera et al.11 has recently shown that a bipyridine-Ni catalyst system can engage in C–N and C–O bond forming reactions under thermal conditions, invoking a self-sustained Ni(I)/Ni(III) process, thus avoiding the combination with photo-9 or electrocatalysis.10 However, the process is limited to electron-deficient aryl bromides, due to the general reluctance to C–X cleavage of electron-rich substrates using Ni(I) complexes with diamines as ligands.12 Due to the low bond dissociation energy of the C(sp2)–S bond in aryl thianthrenium salts upon reduction, they are excellent candidates to engage in one-electron processes regardless of their substitution pattern.1,2 While halogenation of arylthianthrenium salts has been reported, stoichiometric copper salts and irradiation are required.1a,1e Conventional arene halogenation relies on the use of electrophilic halogenating reagents, such as X2 or element–X-type reagents such as N-bromosuccinimide (NBS) (Figure 1), which commonly results in low regioselectivity, particularly for unbiased substrates.13 Although recent years have witnessed an increasing number of electrophilic halogenating reagents, direct late stage C(sp2)–X bond formation still remains a synthetic challenge.14 Interexchanges of X groups in aromatic Finkelstein-type reactions have been reported,15,16 but they all require the synthesis of the parent aryl halide as starting material. In order to provide a broad and robust method, herein, we report on a thermal Ni(I)-catalyzed halogenation of arylthianthrenium salts with broadly available, nucleophilic halide sources. The catalytic system is simple, featuring one of the least expensive sources of Ni, NiCl2·(H2O)6, and zinc metal for in situ reduction. The protocol is robust, scalable, and broad in scope, as exemplified by the late stage halogenation of densely functionalized molecules, without the need for photocatalysis or electrochemistry.
Figure 1.
Halogenation of arenes: state-of-the-art.
Catalytic nickel chloride hexahydrate combined with zinc in the presence of sodium iodide suffices to convert thianthrenium salt 1 into the corresponding iodinated product 2 at 25 °C (Scheme 1A, entry 1). Notice that this protocol uses common NaI as halogen source and does not require the presence of additional supporting ligands for the metal center.17 During the optimization, it was found that dimethylacetamide (DMA) was a crucial solvent for the reaction to proceed; DMF inhibited the reactivity (entry 2). All the elements in the catalytic system are necessary for the reaction to proceed; omission of either Ni or Zn did not lead to any detectable formation of 2 (entries 3 and 4). In addition to simple Ni(II) salt as catalyst, Ni(1,4-cyclooctadiene (COD))2 or Ni(tBustb)3 can be used as well (entry 5), and both Ni(COD)2 and NiCl2/Zn afforded excellent yields of 3 on 5 mmol scale (entries 1 and 5). When using undistilled “wet” DMA, the reaction can still occur, albeit the yield halts at 73% (entry 6). However, the inert atmosphere was crucial for the reaction, and only a trace amount of 2 was detected if the reaction is run open-air (entry 7). Based on precedents in ligandless Ni catalysis,18 the need for a reducing agent when using a simple Ni(II) salt points to the possible generation of Ni(I) as the active species. Along these lines, when using Wilke-Morandi’s well-defined Ni(I) complex Ni(COD)(OAr*) without Zn,192 was also obtained in high yields (entry 8). Catalytic amounts of Ni(0) precursors are able to catalyze the reaction, presumably via the in situ formation of Ni(I) species through initial oxidation by the arylthianthrenium salt (entry 6 and vide infra). Larger amounts of Ni(0) led to a linear decrease of yield, ultimately reaching only trace amounts of 2 with 1.0 equiv of Ni(COD)2 (Scheme 1B, top). However, when the reaction with 100 mol % Ni(COD)2 is diluted to 0.02 M in DMA, a 41% yield of 2 is obtained, thus suggesting that aggregations of Ni(0) deactivate the Ni catalyst at high concentrations (see the Supporting Information). When one-electron oxidant FeCp2BArF was added to the reaction with 1.0 equiv of Ni(COD)2 prior to the addition of the thianthrenium salt, reactivity was restored and product 2 was obtained in 75% yield, supporting that one full equivalent of Ni(I) is able to sustain the reactivity (Scheme 1B, bottom). Electron paramagnetic resonance (EPR) analysis of an equimolar mixture of Ni(COD)2 and FeCp2BArF in toluene:THF revealed the formation of a Ni(I) species consistent with cationic [Ni(COD)2]+ (Scheme 1C).20 Addition of 1 and NaI to this mixture led to a rapid color change from light yellow to red, and 75% of 2 was obtained. Moreover, in situ EPR analysis during the reaction at different reaction times clearly indicates the presence of one paramagnetic Ni species distinct from the cationic Ni(I) made from Ni(COD)2 and FeCp2BArF. This species appears at high concentration and slowly fades away as the reaction progresses. The signal is analogous to the one obtained by Reisman while monitoring the Ni-catalyzed triflate-iodide exchange reaction of enol ethers (see the Supporting Information for details).21 Although insights on the exact structure of the active Ni(I) catalyst are still elusive given that it evaded isolation, the in situ EPR experiments further support the Ni(I) hypothesis (see the Supporting Information for details). Based on the above experiments, we speculate that the reaction mechanism proceeds through the initial formation of the L–Ni(I)–X species A (Scheme 1D), where L is COD or DMA, with appropriate redox potential for oxidative addition to the aryl-TT salt. After initial SET from Ni(I) to the cationic thianthrenium salt, mesolytic cleavage of the C–S bond would lead to the formation of an Ar radical, which prior to escaping the solvent cage in B, undergoes oxidative ligation to the Ni(II) species, to afford Ni(III) intermediate C. Reductive elimination from C leads to the formation of the C–halogen bond and presumably D, which after rapid anion metathesis regenerates the active species A. It should be pointed out that the mechanism in Scheme 1, although plausible, remains purely speculative at present. The ability to undergo oxidative addition to a “naked” Ni(I) compound, even for electron-rich arenes, distinguishes the arylthianthrenium salts from aryl-halides and other pseudohalides and underscores the utility of arylthianthreniums beyond conventional cross-coupling precursors.11,12
Scheme 1. (A) Discovery and Optimization; (B) Influence of the Catalyst Loading and Oxidants; (C) Evidence for the Formation of Ni(I) Intermediates; (D) Proposed Mechanism of the Ni-Catalyzed Halogenation of Arylthianthrenium Salts.
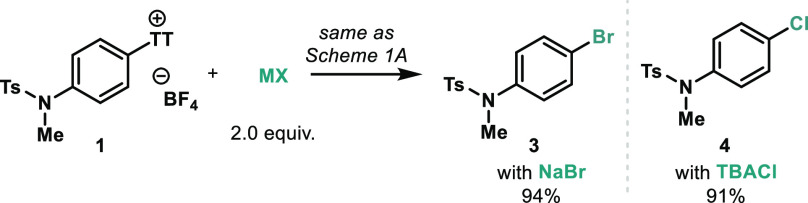
Sodium iodide could successfully be swapped for NaBr or tetrabutylammonium chloride leading to the corresponding aryl bromide (3) and chloride (4), respectively, in excellent yields (Scheme 2). Attempts to extend the scope to fluorination in this manner were thus far unsuccessful. Other MX salts have also been tested employing the optimized conditions to probe the extent of additional C–X coupling reactions: salts such as tetrabutylammonium difluorotriphenylsilicate (TBAT), tris(dimethylamino)sulfonium difluorotrimethylsilicate (TASF), KF, and NaF (for C–F) or KOAc and PhCO2Na (for C–O bonds) did not afford the corresponding products, and the starting material remained unreacted. It is important to note that one single, simple catalytic system allows for the synthesis of a triad of halogenated arenes by simply selecting the desired halide salt.
Scheme 2. Expansion of the Protocol to the Corresponding Bromination and Chlorination.
As shown in Scheme 3, both simple (2–37) and complex functionalized small molecules (38–50) can successfully be halogenated. A main feature of this protocol is the mild conditions, which permit tolerance of a wide range of functionalities, namely, sulfonamides, amides, imides, ethers, esters, halogens, nitriles, carbamates, hydroxyl, trifluoromethyl, ketones, biaryl, aldehydes, alkyl- and arylhalides, unsaturated heterocycles, amines, or encumbered olefin among others. It is also important to mention that halogenation occurs smoothly regardless of the substitution pattern: ortho- (37, 39, 40, 49–50), meta- (31, 47), and para-substituted arenes (2–25, 29, 30, 32, 34–36, 41–43) are all smoothly converted into the corresponding aryl halides. Arenes with free amines react with the thianthrene-S-oxide and lead to the iminothianthrenes, which have been used in allylic amination.22 It is worth pointing out that, due to the electrophilic nature of the thianthrenation step, substrates bearing electron-releasing groups are more suitable for this process. As thianthrenation is not only restricted to aromatic C–H bonds, we have attempted the halogenation of alkenyl thianthrenium salts derived from simple olefins.23 Although terminal alkenylthianthrenium salts remained unreactive (see the Supporting Information), internal alkenylthianthrenium salts were smoothly converted into iodinated (51, 53–55) or brominated (52, 56) products successfully. It is worth pointing out that the stereochemistry of the starting alkenylthianthrenium salts were preserved during the course of the reaction (51, 52, and 54). Chlorination falls beyond the scope of the protocol, leaving the starting material unreacted. In order to benchmark our protocol with other C–H halogenation strategies, the synthesis of compounds 36 and 46 was attempted with state-of-the-art electrophilic iodination methods and resulted in either regioselectivity issues or low yields (see the Supporting Information). It is noteworthy that this Ni-catalyzed platform levels the functional group tolerance of the thianthrenation step, thus accommodating a broad range of functional groups.
Scheme 3. Scope of Halogenation of Aryl Thianthrenium Salts.

Yields of isolated products are indicated in each case. Standard reaction conditions: thianthrenium salt (1.0 equiv), NiCl2·6H2O (10 mol %), Zn (325 mesh) (20 mol %), halogenation source (NaI, NaBr, or TBACl) (2.0 equiv), DMA (0.2 M), 25 °C, 1–16 h. a1H NMR yield by using 1,3,5-trimethoxybenzene as the internal standard.
In order to highlight the valuable formation of aryl halides, we provide two examples where the formation of the aryl halide can be telescoped in a two-step procedure: a reductive coupling with N2O to produce phenols (58, 51% yield) (Scheme 4A)24 and C–C bond formation with redox-active esters (60, 58% yield) (Scheme 4B).25
Scheme 4. Two-Step One-Pot Transformation.
The halogenation protocol presented here highlights that thermal Ni redox catalysis without the need of supporting ligands is within reach with suitably activated electrophilic aryl counterparts. Our laboratories are currently exploring these possibilities further.
Acknowledgments
Financial support for this work was provided by Max-Planck-Gesellschaft, Max-Planck-Institut für Kohlenforschung, Max Planck Institute for Energy Conversion and the Fonds der Chemischen Industrie (VCI-FCI). We thank the analytical departments (NMR spectroscopy, Mass spectrometry, and HPLC) at the MPI für Kohlenforschung for support in the characterization of compounds.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c02611.
Experimental procedures and analytical data (1H, 13C, and 19F NMR, HRMS) for new compounds (PDF)
Open access funded by Max Planck Society.
The authors declare no competing financial interest.
Supplementary Material
References
- For selected examples of aryl thianthrenium salts, see:; a Berger F.; Plutschack M. B.; Riegger J.; Yu W.; Speicher S.; Ho M.; Frank N.; Ritter T. Site-selective and versatile aromatic C–H functionalization by thianthrenation. Nature 2019, 567, 223–228. 10.1038/s41586-019-0982-0. [DOI] [PubMed] [Google Scholar]; b Engl P. S.; Häring A. P.; Berger F.; Berger G.; Pérez-Bitrián A.; Ritter T. C–N Cross-Couplings for Site-Selective Late-Stage Diversification via Aryl Sulfonium Salts. J. Am. Chem. Soc. 2019, 141, 13346–13351. 10.1021/jacs.9b07323. [DOI] [PubMed] [Google Scholar]; c Sang R.; Korkis S. E.; Su W.; Ye F.; Engl P. S.; Berger F.; Ritter T. Site-Selective C–H Oxygenation via Aryl Sulfonium Salts. Angew. Chem., Int. Ed. 2019, 58, 16161–16166. 10.1002/anie.201908718. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ye F.; Berger F.; Jia H.; Ford J.; Wortman A.; Börgel J.; Genicot C.; Ritter T. Aryl Sulfonium Salts for Site-Selective Late-Stage Trifluoromethylation. Angew. Chem., Int. Ed. 2019, 58, 14615–14619. 10.1002/anie.201906672. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Li J.; Chen J.; Sang R.; Ham W.-S.; Plutschack M. B.; Berger F.; Chabbra S.; Schnegg A.; Genicot C.; Ritter T. Photoredox catalysis with aryl sulfonium salts enables site-selective late-stage fluorination. Nat. Chem. 2020, 12, 56–62. 10.1038/s41557-019-0353-3. [DOI] [PubMed] [Google Scholar]; f Aukland M. H.; Šiaučiulis M.; West A.; Perry G. J. P.; Procter D. J. Metal-free photoredox-catalysed formal C–H/C–H coupling of arenes enabled by interrupted Pummerer activation. Nat. Catal. 2020, 3, 163–169. 10.1038/s41929-019-0415-3. [DOI] [Google Scholar]; g Péter Á.; Perry G. J. P.; Procter D. J. Radical C–C Bond Formation using Sulfonium Salts and Light. Adv. Synth. Catal. 2020, 362, 2135–2142. 10.1002/adsc.202000220. [DOI] [Google Scholar]; h Lansbergen B.; Granatino P.; Ritter T. Site-Selective C–H alkylation of Complex Arenes by a Two-Step Aryl Thianthrenation-Reductive Alkylation Sequence. J. Am. Chem. Soc. 2021, 143, 7909–7914. 10.1021/jacs.1c03459. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Cabrera-Afonso M. J.; Granados A.; Molander G. A. Sustainable Thioetherification via Electron Donor–Acceptor Photoactivation Using Thianthrenium Salts. Angew. Chem., Int. Ed. 2022, 61, e202202706. 10.1002/anie.202202706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected reviews of aryl thianthrenium salts, see:; a Lou J.; Wang Q.; Wu P.; Wang H.; Zhou Y.-G.; Yu Z. J. C. S. R. Transition-metal mediated carbon–sulfur bond activation and transformations: an update. Chem. Soc. Rev. 2020, 49, 4307–4359. 10.1039/C9CS00837C. [DOI] [PubMed] [Google Scholar]; b Kozhushkov S. I.; Alcarazo M. Synthetic Applications of Sulfonium Salts. Eur. J. Inorg. Chem. 2020, 2020, 2486–2500. 10.1002/ejic.202000249. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Yorimitsu H. Catalytic Transformations of Sulfonium Salts via C-S Bond Activation. Chem. Rec. 2021, 21, 3356–3369. 10.1002/tcr.202000172. [DOI] [PubMed] [Google Scholar]; d Meng H.; Liu M.-S.; Shu W. Organothianthrenium salts: synthesis and utilization. Chem. Sci. 2022, 13, 13690–13707. 10.1039/D2SC04507A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected reviews of C–H functionalization, see:; a Lyons T. W.; Sanford M. S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 2010, 110, 1147–1169. 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ackermann L. Carboxylate-assisted transition-metal-catalyzed C–H bond functionalizations: mechanism and scope. Chem. Rev. 2011, 111, 1315–1345. 10.1021/cr100412j. [DOI] [PubMed] [Google Scholar]; c Neufeldt S. R.; Sanford M. S. Controlling Site Selectivity in Palladium-Catalyzed C–H Bond Functionalization. Acc. Chem. Res. 2012, 45, 936–946. 10.1021/ar300014f. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Brückl T.; Baxter R. D.; Ishihara Y.; Baran P. S. Innate and Guided C–H Functionalization Logic. Acc. Chem. Res. 2012, 45, 826–839. 10.1021/ar200194b. [DOI] [PMC free article] [PubMed] [Google Scholar]; e He J.; Wasa M.; Chan K. S. L.; Shao Q.; Yu J.-Q. Palladium-catalyzed transformations of alkyl C–H bonds. Chem. Rev. 2017, 117, 8754–8786. 10.1021/acs.chemrev.6b00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples of cross-coupling reactions using sulfonium salts through oxidative addition by a low-valent Ni(0) complex, see:; a Srogl J.; Allred G. D.; Liebeskind L. S. Sulfonium Salts. Participants par Excellence in Metal-Catalyzed Carbon–Carbon Bond-Forming Reactions. J. Am. Chem. Soc. 1997, 119, 12376–12377. 10.1021/ja9726926. [DOI] [Google Scholar]; b Aukland M. H.; Talbot F. J. T.; Fernández-Salas J. A.; Ball M.; Pulis A. P.; Procter D. J. An Interrupted Pummerer/Nickel-Catalysed Cross-Coupling Sequence. Angew. Chem., Int. Ed. 2018, 57, 9785–9789. 10.1002/anie.201805396. [DOI] [PubMed] [Google Scholar]; c Yamada K.; Yanagi T.; Yorimitsu H. Generation of Organozinc Reagents from Arylsulfonium Salts Using a Nickel Catalyst and Zinc Dust. Org. Lett. 2020, 22, 9712–9718. 10.1021/acs.orglett.0c03782. [DOI] [PubMed] [Google Scholar]; d Yanagi T.; Somerville R. J.; Nogi K.; Martin R.; Yorimitsu H. Ni-Catalyzed Carboxylation of C(sp2)–S Bonds with CO2: Evidence for the Multifaceted Role of Zn. ACS Catal. 2020, 10, 2117–2123. 10.1021/acscatal.9b05141. [DOI] [Google Scholar]
- For selected reviews of halogen/metal exchange reactions, see:; a Kuzmina O. M.; Steib A. K.; Moyeux A.; Cahiez G.; Knochel P. Recent Advances in Iron-Catalyzed Csp2–Csp2 Cross-Couplings. Synthesis 2015, 47, 1696–1705. 10.1055/s-0034-1380195. [DOI] [Google Scholar]; b Hammann J. M.; Hofmayer M. S.; Lutter F. H.; Thomas L.; Knochel P. Recent Advances in Cobalt-Catalyzed Csp2 and Csp3 Cross-Couplings. Synthesis 2017, 49, 3887–3894. 10.1055/s-0036-1588430. [DOI] [Google Scholar]; c Balkenhohl M.; Knochel P. Recent Advances of the Halogen–Zinc Exchange Reaction. Chem.—Eur. J. 2020, 26, 3688–3697. 10.1002/chem.201904794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples of Pd-catalyzed halogenation reactions, see:; a Dick A. R.; Hull K. L.; Sanford M. S. A Highly Selective Catalytic Method for the Oxidative Functionaliztion of C–H Bonds. J. Am. Chem. Soc. 2004, 126, 2300–2301. 10.1021/ja031543m. [DOI] [PubMed] [Google Scholar]; b Wan X.; Ma Z.; Li B.; Zhang K.; Cao S.; Zhang S.; Shi Z. Highly Selective C–H Functionalization/Halogenation of Acetanilide. J. Am. Chem. Soc. 2006, 128, 7416–7417. 10.1021/ja060232j. [DOI] [PubMed] [Google Scholar]; c Zhao X.; Dimitrijevic E.; Dong V. M. Palladium-Catalyzed C–H Bond Functionalization with Arylsulfonyl Chlorides. J. Am. Chem. Soc. 2009, 131, 3466–3467. 10.1021/ja900200g. [DOI] [PubMed] [Google Scholar]; d Powers D. C.; Ritter T. Bimetallic Pd(III) Complexes in Palladium-Catalyzed Carbon–Heteroatom Bond Formation. Nat. Chem. 2009, 1, 302–309. 10.1038/nchem.246. [DOI] [PubMed] [Google Scholar]; e Dudnik A. S.; Chernyak N.; Huang C.; Gevorgyan V. A General Stratrgy Toward Aromatic 1,2-Ambiphilic Synthons: Palladium-Catalyzed ortho-Halogenation of PyDipSi-Arenes. Angew. Chem., Int. Ed. 2010, 49, 8729–8732. 10.1002/anie.201004426. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Noel T.; Maimone T. J.; Buchwald S. L. Accelerating Palladium-Catalyzed C–F Bond Formation: Use of a Microflow Packed-Bed Reactor. Angew. Chem., Int. Ed. 2011, 50, 8900–8903. 10.1002/anie.201104652. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Sun X.; Shan G.; Sun Y.; Rao Y. Regio- and Chemoselective C–H Chlorination/Bromination of Electron-Deficient Arenes by Weak Coordination and Study of Relative Directing-Group Abilities. Angew. Chem., Int. Ed. 2013, 52, 4440–4444. 10.1002/anie.201300176. [DOI] [PubMed] [Google Scholar]
- For selected examples of Cu-catalyzed halogenation reactions, see:; a Chen X.; Hao X.-S.; Goodhue C. E.; Yu J.-Q. Cu(II)-Catalyzed Functionalizations of Aryl C–H Bonds using O2 as an Oxidant. J. Am. Chem. Soc. 2006, 128, 6790–6791. 10.1021/ja061715q. [DOI] [PubMed] [Google Scholar]; b Casitas A.; King A. E.; Parella T.; Costas M.; Stahl S. S.; Ribas X. Direct Observation of Cu(I)/Cu(III) Redox Steps Relevant to Ullman-Type Coupling Reactions. Chem. Sci. 2010, 1, 326–330. 10.1039/c0sc00245c. [DOI] [Google Scholar]; c Urones B.; Martínez Á. M.; Rodríguez N.; Arrayás R. G.; Carretero J. C. Copper-catalyzed ortho-halogenation of protected anilines. Chem. Commun. 2013, 49, 11044–11046. 10.1039/c3cc47174h. [DOI] [PubMed] [Google Scholar]
- For selected examples of Rh-, Co-, Ru-, Ag-, and Au-catalyzed halogenation reactions, see:; a Schröder N.; Wencel-Delord J.; Glorius F. High Yielding, Versatile and Practical [Rh(III)Cp*]-Catalyzed ortho-Bromination and Iodination of Arenes. J. Am. Chem. Soc. 2012, 134, 8298–8301. 10.1021/ja302631j. [DOI] [PubMed] [Google Scholar]; b Yu D.-G.; Gensch T.; de Azambuja F.; Vásquez-Céspedes S.; Glorius F. Co(III)-Catalyzed C–H Activation/Formal SN-Type Reactions: Selective and Efficient Cyanation, Halogenation, and Allylation. J. Am. Chem. Soc. 2014, 136, 17722–17725. 10.1021/ja511011m. [DOI] [PubMed] [Google Scholar]; c Hwang H.; Kim J.; Jeong J.; Chang S. Regioselective Introduction of Heteroatoms at the C-8 Position of Quinoline NOxides: Remote C–H Activation Using N-Oxide as a Stepping Stone. J. Am. Chem. Soc. 2014, 136, 10770–10776. 10.1021/ja5053768. [DOI] [PubMed] [Google Scholar]; d Wang L.; Ackermann L. Ruthenium-Catalyzed ortho-C–H Halogenations of Benzamides. Chem. Commun. 2014, 50, 1083–1085. 10.1039/C3CC47852A. [DOI] [PubMed] [Google Scholar]; e Luo Y.; Pan X.; Wu J. Silver-Catalyzed Decarboxylative Halogenation of Carboxylic Acids. Tetrahedron Lett. 2010, 51, 6646–6648. 10.1016/j.tetlet.2010.10.054. [DOI] [Google Scholar]; f Winston M. S.; Wolf W. J.; Toste F. D. Halide-Dependent Mechanisms of Reductive Elimination from Gold(III). J. Am. Chem. Soc. 2015, 137, 7921–7928. 10.1021/jacs.5b04613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Corcoran E. B.; Pirnot M. T.; Lin S.; Dreher S. D.; DiRocco D. A.; Davies I. W.; Buchwald S. L.; MacMillan D. W. C. Aryl amination using ligand-free Ni(II) salts and photoredox catalysis. Science 2016, 353, 279–283. 10.1126/science.aag0209. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Till N. A.; Tian L.; Dong Z.; Scholes G. D.; MacMillan D. W. C. Mechanistic Analysis of Metallaphotoredox C–N Coupling: Photocatalysis Initiates and Perpetuates Ni(I)/Ni(III) Coupling Activity. J. Am. Chem. Soc. 2020, 142, 15830–15841. 10.1021/jacs.0c05901. [DOI] [PubMed] [Google Scholar]
- a Li C.; Kawamata Y.; Nakamura H.; Vantourout J. C.; Liu Z.; Hou Q.; Bao D.; Starr J. T.; Chen J.; Yan M.; Baran P. S. Electrochemically Enabled, Nickel-Catalyzed Amination. Angew. Chem., Int. Ed. 2017, 56, 13088–13093. 10.1002/anie.201707906. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Kawamata Y.; Vantourout J. C.; Hickey D. P.; Bai P.; Chen L.; Hou Q.; Qiao W.; Bamacrman K.; Edwards M. A.; Garrido-Castro A. F.; deGruyter J. N.; Nakamura H.; Knouse K.; Qin C.; Clay K. J.; Bao D.; Li C.; Starr J. T.; Garcia-Irizarry C.; Sach N.; White H. S.; Neurock M.; Minteer S. D.; Baran P. S. Electrochemically Driven, Ni-Catalyzed Aryl Amination: Scope, Mechanism, and Applications. J. Am. Chem. Soc. 2019, 141, 6392–6402. 10.1021/jacs.9b01886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R.; Qin Y.; Nocera D. G. General Paradigm in Photoredox Nickel-Catalyzed Cross-Coupling Allows for Light-Free Access to Reactivity. Angew. Chem., Int. Ed. 2020, 59, 9527–9533. 10.1002/anie.201916398. [DOI] [PubMed] [Google Scholar]
- Gisbertz S.; Reischauer S.; Pieber B. Overcoming limitations in dual photoredox/nickel-catalysed C–N cross-couplings due to catalyst deactivation. Nat. Catal. 2020, 3, 611–620. 10.1038/s41929-020-0473-6. [DOI] [Google Scholar]
- For selected examples of arene halogenation, see:; a Djerassi C. Brominations with N-Bromosuccinimide and Related Compounds. The Wohl-Ziegler Reaction. Chem. Rev. 1948, 43, 271–317. 10.1021/cr60135a004. [DOI] [PubMed] [Google Scholar]; b Rozen S.; Brand M.; Lidor R. Aromatic bromination using bromine fluoride with no Friedel-Crafts catalyst. J. Org. Chem. 1988, 53, 5545–5547. 10.1021/jo00258a030. [DOI] [Google Scholar]; c Eguchi H.; Kawaguchi H.; Yoshinaga S.; Nishida A.; Nishiguchi T.; Fujisaki S. Halogenation Using N-Halogenocompounds. II. Acid Catalyzed Bromination of Aromatic Compounds with 1,3-Dibromo-5,5-dimethylhydantoin. Bull. Chem. Soc. Jpn. 1994, 67, 1918–1921. 10.1246/bcsj.67.1918. [DOI] [Google Scholar]; d Groweiss A. Use of Sodium Bromate for Aromatic Bromination: Research and Development. Org. Process Res. Dev. 2000, 4, 30–33. 10.1021/op9901947. [DOI] [Google Scholar]; e Smeaton E. S.; Smith M. H.; White M. J.. Science of Synthesis, Reagents: Halogenation; Georg Thieme Verlag KG: Stuttgart, NY, 2013. [Google Scholar]; f de Almeida L. S.; de Mattos M. C. S.; Esteves P. M. Tribromoisocyanuric Acid in Trifluoroacetic Acid: An Efficient System for Smooth Brominating of Moderately Deactivated Arenes. Synlett 2013, 24, 603–606. 10.1055/s-0032-1317795. [DOI] [Google Scholar]
- a Payne J. T.; Poor C. B.; Lewis J. C. Directed evolution of RebH for site-selective halogenation of large biologically active molecules. Angew. Chem., Int. Ed. 2015, 54, 4226–4230. 10.1002/anie.201411901. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Fosu S. C.; Hambira C. M.; Chen A. D.; Fuchs J. R.; Nagib D. A. Site-Selective C–H Functionalization of (Hetero)Arenes via Transient, Non-symmetric Iodanes. Chem. 2019, 5, 417–428. 10.1016/j.chempr.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Xie W.; Wang M.; Yang S.; Chen Y.; Feng J.; Huang Y. C–H chlorination of (hetero)anilines via photo/organo co-catalysis. Org. Bio. Chem. 2022, 20, 5319–5324. 10.1039/D2OB00834C. [DOI] [PubMed] [Google Scholar]; d Wang W.; Yang X.; Dai R.; Yan Z.; Wei J.; Dou X.; Qiu X.; Zhang H.; Wang C.; Liu Y.; Song S.; Jiao N. Catalytic Electrophilic Halogenation of Arenes with Electron-Withdrawing Substituents. J. Am. Chem. Soc. 2022, 144, 13415–13425. 10.1021/jacs.2c06440. [DOI] [PubMed] [Google Scholar]
- For selected examples of Ni-catalyzed aromatic Finkelstein-type reactions, see:; a Cramer R.; Coulson D. R. Nickel-Catalyzed Displacement Reactions of Aryl Halides. J. Org. Chem. 1975, 40, 2267–2273. 10.1021/jo00904a001. [DOI] [Google Scholar]; b Takagi K.; Hayama N.; Okamoto T. Synthesis of Aryl Iodides from Aryl Halides and Potassium Iodide by Means of Nickel Catalyst. Chem. Lett. 1978, 7, 191–192. 10.1246/cl.1978.191. [DOI] [Google Scholar]; c Takagi K.; Hayama N.; Inokawa S. The in Situ-generated Nickel(0)-catalyzed Reaction of Aryl Halides with Potassium Iodide and Zinc Powder. Bull. Chem. Soc. Jpn. 1980, 53, 3691–3695. 10.1246/bcsj.53.3691. [DOI] [Google Scholar]; d Tsou T. T.; Kochi J. K. Nickel Catalysis in Halogen Exchange with Aryl and Vinylic Halides. J. Org. Chem. 1980, 45, 1930–1937. 10.1021/jo01298a035. [DOI] [Google Scholar]; e Yang S. H.; Li C. S.; Cheng C. H. Halide Exchange Reactions between Aryl Halides and Alkali Halides Catalyzed by Nickel Metal. J. Org. Chem. 1987, 52, 691–694. 10.1021/jo00380a041. [DOI] [Google Scholar]; f O’Connor K. J.; Burrows C. J. Catalysis of Aryl-Halogen Exchange by Nickel(II) Complexes using Sodium Hypochlorite. J. Org. Chem. 1991, 56, 1344–1346. 10.1021/jo00003a088. [DOI] [Google Scholar]; g Cant A. A.; Bhalla R.; Pimlott S. L.; Sutherland A. Nickel-Catalyzed Aromatic Finkelstein Reaction of Aryl and Heteroaryl Bromides. Chem. Commun. 2012, 48, 3993–3995. 10.1039/c2cc30956d. [DOI] [PubMed] [Google Scholar]; h Cant A. A.; Champion S.; Bhalla R.; Pimlott S. L.; Sutherland A. Nickel-Mediated Radioiodination of Aryl and Heteroaryl Bromides: Rapid Synthesis of Tracers for SPECT Imaging. Angew. Chem., Int. Ed. 2013, 52, 7829–7832. 10.1002/anie.201302800. [DOI] [PubMed] [Google Scholar]; i Feng Y.; Luo H.; Zheng W.; Matsunaga S.; Lin L. Light-Promoted Nickel-Catalyzed Aromatic Halogen Exchange. ACS Catal. 2022, 12, 11089–11096. 10.1021/acscatal.2c03354. [DOI] [Google Scholar]
- For selected examples of Cu-, Pd-, and Fe-catalyzed or photoinduced aromatic Finkelstein-type reactions, see:; a Klapars A.; Buchwald S. L. Copper-Catalyzed Halogen Exchange in Aryl Halides: An Aromatic Finkelstein Reaction. J. Am. Chem. Soc. 2002, 124, 14844–14845. 10.1021/ja028865v. [DOI] [PubMed] [Google Scholar]; b Casitas A.; Canta M.; Solà M.; Costas M.; Ribas X. Nucleophilic Aryl Fluorination and Aryl Halide Exchange Mediated by a CuI/CuIII Catalytic Cycle. J. Am. Chem. Soc. 2011, 133, 19386–19392. 10.1021/ja2058567. [DOI] [PubMed] [Google Scholar]; c Feng X.; Qu Y.; Han Y.; Yu X.; Bao M.; Yamamoto Y. Copper-catalyzed Conversion of Aryl and Heteroaryl Bromides into the Corresponding Chlorides. Chem. Commun. 2012, 48, 9468–9470. 10.1039/c2cc34944b. [DOI] [PubMed] [Google Scholar]; d Bonney K. J.; Proutiere F.; Schoenebeck F. Dinuclear Pd(I) Complexes-solely Precatalysts? Demonstration of Direct Reactivity of A Pd(I) Dimer with an Aryl Iodide. Chem. Sci. 2013, 4, 4434–4439. 10.1039/c3sc52054d. [DOI] [Google Scholar]; e Wang Y.; Li L.; Ji H.; Ma W.; Chen C.; Zhao J. Iron(III)- Mediated Photocatalytic Selective Substitution of Aryl Bromine by Chlorine with High Chloride Utilization Efficiency. Chem. Commun. 2014, 50, 2344–2346. 10.1039/c3cc48246d. [DOI] [PubMed] [Google Scholar]; f Li L.; Liu W.; Zeng H.; Mu X.; Cosa G.; Mi Z.; Li C. Photo-induced Metal-Catalyst-Free Aromatic Finkelstein Reaction. J. Am. Chem. Soc. 2015, 137, 8328–8331. 10.1021/jacs.5b03220. [DOI] [PubMed] [Google Scholar]; g Chen M.; Ichikawa S.; Buchwald S. L. Rapid and Efficient Copper–Catalyzed Finkelstein Reaction of (Hetero)Aromatics under Continuous– Flow Conditions. Angew. Chem., Int. Ed. 2015, 54, 263–266. 10.1002/anie.201409595. [DOI] [PubMed] [Google Scholar]; i Petrone D. A.; Ye J.; Lautens M. Modern Transition-Metal-Catalyzed Carbon–Halogen Bond Formation. Chem. Rev. 2016, 116, 8003–8104. 10.1021/acs.chemrev.6b00089. [DOI] [PubMed] [Google Scholar]
- Chu C. K.; Liang Y.; Fu G. C. Silicon–Carbon Bond Formation via Nickel-Catalyzed Cross-Coupling of Silicon Nucleophiles with Unactivated Secondary and Tertiary Alkyl Electrophiles. J. Am. Chem. Soc. 2016, 138, 6404–6407. 10.1021/jacs.6b03465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. A.; Vásquez-Céspedes S.; Shenvi R. A. Iron–Nickel Dual-Catalysis: A New Engine for Olefin Functionalization and the Formation of Quaternary Centers. J. Am. Chem. Soc. 2018, 140, 11317–11324. 10.1021/jacs.8b05868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Jolly P. W.; Wilke G.. The Organic Chemistry of Nickel. Vol. I: Organonickel Complexes; Academic Press: 1974. [Google Scholar]; b Bismuto A.; Müller P.; Finkelstein P.; Trapp N.; Jeschke G.; Morandi B. One to Find Them All: A General Route to Ni(I)–Phenolate Species. J. Am. Chem. Soc. 2021, 143, 10642–10648. 10.1021/jacs.1c03763. [DOI] [PubMed] [Google Scholar]
- Schwab M. M.; Himmel D.; Kacprzak S.; Kratzert D.; Radtke V.; Weis P.; Ray K.; Scheidt E.-W.; Scherer W.; de Bruin B.; Weber S.; Krossing I. [Ni(cod)2][Al(ORF)4], a Source for Naked Nickel(I) Chemistry. Angew. Chem., Int. Ed. 2015, 54, 14706–14709. 10.1002/anie.201506475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstra J. L.; Poremba K. E.; Shimozono A. M.; Reisman S. E. Nickel-Catalyzed Conversion of Enol Triflates into Alkenyl Halides. Angew. Chem., Int. Ed. 2019, 58, 14901–14905. 10.1002/anie.201906815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q.; Chen J.; Lin S.; Ritter T. Allylic Amination of Alkenes with Iminothianthrenes to Afford Alkyl Allylamines. J. Am. Chem. Soc. 2020, 142, 17287–17293. 10.1021/jacs.0c08248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Li J.; Plutschack M. B.; Berger F.; Ritter T. Regio- and Stereoselective Thianthrenation of Olefins To Access Versatile Alkenyl Electrophiles. Angew. Chem., Int. Ed. 2020, 59, 5616–5620. 10.1002/anie.201914215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Vaillant F.; Mateos Calbet A.; González-Pelayo S.; Reijerse E. J.; Ni S.; Busch J.; Cornella J. Catalytic synthesis of phenols with nitrous oxide. Nature 2022, 604, 677–683. 10.1038/s41586-022-04516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huihui K. M. M.; Caputo J. A.; Melchor Z.; Olivares A. M.; Spiewak A. M.; Johnson K. A.; DiBenedetto T. A.; Kim S.; Ackerman L. K. G.; Weix D. J. Decarboxylative Cross-Electrophile Coupling of N-Hydroxyphthalimide Esters with Aryl Iodides. J. Am. Chem. Soc. 2016, 138, 5016–5019. 10.1021/jacs.6b01533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.