Abstract
Aim:
Laparoscopic surgery is the preferred approach for primary uncomplicated ileocolic resection, however its role for repeat resections is unclear. This study assessed the outcomes of primary and repeated ileocolic resections for Crohn’s disease to examine rates of laparoscopy and patient morbidity.
Methods:
A retrospective review of a prospectively maintained database was conducted at a tertiary centre between 2013–2019. All patients undergoing ileocolic resections for Crohn’s disease were included. The cohort was divided into three groups based on number of resections - primary (1R), secondary (2R) and tertiary or more (>2R) groups. The primary outcome was 30-day postoperative morbidity.
Results:
Over a 6-year period, 474 patients underwent ileocolic resection for Crohn’s disease, including 369 primary (1R, 77.8%) and 105 repeat (≥2R, 22.2%) resections. A laparoscopic approach was less common in the ≥2R versus 1R groups (79.0% vs. 93.8%, p<0.001), but rates of conversion to an open procedure were comparable. Morbidity was higher amongst repeat resections although this was not significant (20.0% vs 14.1%, p=0.18). Amongst cases approached laparoscopically (n=429), rates of conversion and postoperative morbidity did not differ by stage of resection, although operative time was longer for repeat operations. Even in the group undergoing laparoscopy for tertiary or greater resections (>2R, n=29), the rates of conversion (10%) and morbidity (14%) were relatively low.
Conclusion:
In this contemporary series of primary and reoperative ICR for ileal CD, a laparoscopic approach is feasible and safe for the majority of repeat ileocolic resections when performed at a high volume centre.
Keywords: Crohn’s disease, inflammatory bowel disease, ileocolic resection, laparoscopic surgery, morbidity
Introduction
Ileocolic resection (ICR) is the most common operation performed for Crohn’s disease (CD) of the terminal ileum. The benefits of laparoscopy in primary ICR have been well-documented and include decreased time to tolerance of a diet, faster return of bowel function, and shorter hospital length of stay.[1–5] Other studies have suggested fewer short-term postoperative complications, lower incidence of incisional hernia and postoperative small bowel obstruction, and improved cosmesis.[5–8] Laparoscopic and open approaches to resection have similar likelihood of disease recurrence.[6,9,10] Laparoscopic surgery for CD may also be associated with lower overall cost, although operating times are increased.[11] Overall, laparoscopy has become the preferred surgical approach for ileocolic CD, with a recent analysis of the National Surgical Quality Improvement Program (NSQIP) showing an increase in laparoscopic ICR from 40% in 2006 to 61% of all cases in 2015.[7]
In spite of advances in medical therapy including increased use of biologics, many patients will undergo multiple surgical resections for CD during their lifetime. A recent study reported that within 10 years of primary ICR, 55% of patients will have clinical recurrence of ileocolic disease and 19% will undergo repeat resection.[12] The role of laparoscopy in repeat ICR for recurrent ileocolic CD remains unclear. Patients requiring repeat resections often have complex disease including abscesses, strictures, and enteric fistulas.[13] Additional resections portend more severe intra-abdominal adhesions, which can make a laparoscopic approach challenging and may lead to higher rates of conversion. Surgery for recurrent CD has been associated with increased risk of postoperative complications as compared to primary resections, including intra-abdominal sepsis and anastomotic leak.[14–16] While laparoscopy has been shown to be feasible in several small studies for select patients undergoing secondary ICR,[17–26] its role in patients undergoing tertiary or greater resections has not been examined closely.
The current study aims to assess demographic and surgical characteristics amongst patients undergoing primary (1R), secondary (2R), and repeat (>2R) ICR resections at a single tertiary institution including rates of laparoscopic resection, risk of conversion, rates of diverting stoma formation and postoperative morbidity.
Materials and Methods
Database
A consecutive series of patients were identified from a prospectively maintained departmental database of all CD patients who underwent ICR at a tertiary care facility, Mount Sinai Hospital, from January 2013 through September 2019. Data collected include patient demographics, medical history, operative details and postoperative outcomes. Disease behavior was classified according to the Montreal classification system as either inflammatory (non-stricturing/non-penetrating), stricturing or penetrating.[27] Perioperative use of steroids or biologics was defined as the regular use of these medications within 30 days prior to the procedure. Complications within 30-days of surgery were identified through review of the electronic medical record, cross-referenced with our institutional NSQIP database, and graded according to the Clavien-Dindo classification system with final grading confirmed by a board-certified colorectal surgeon.[28] This study was approved by our institutional review board and reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Patient Selection
All patients with CD who underwent ICR were included in the study. Exclusion criteria included surgery for ulcerative colitis or cancer as well as patients undergoing a procedure that did not include ileocolic resection and anastomosis. In cases where a patient underwent multiple resections during the study period, data were included for the most recent resection and any prior operations on that patient were excluded.
Technical Principles
The surgical technique was standardized amongst surgeons at a single institution. Perioperative management of patients was also standardized regardless of operative approach according to our institutional enhanced recovery after surgery program.[29] Preoperative discontinuation of steroids and biologic agents was by surgeon preference, although in general the preferred approach was to wean patients to a dose of ≤ 20 mg of prednisone daily and to discontinue the last dose of biologic agents prior to surgery.
Elective or emergency ICR was performed with removal of all macroscopic disease in a minimally invasive fashion whenever possible, even in patients with previous laparotomies. The decision to forgo a laparoscopic approach and proceed with open ICR was multifactorial and at surgeon discretion; however, all surgeons performing ICR have extensive laparoscopic experience and this is the preferred approach whenever it is felt to be feasible and safe. No patients in the cohort underwent robotic ICR. Additional procedures were performed based on the extent of disease, including takedown of enteric fistula, abdominal washout of abscess or phlegmon, and additional small bowel resection for strictures. A diverting ileostomy was performed when deemed necessary by the surgeon for control of intra-abdominal sepsis. Ileocolonic anastomoses were performed extracorporeally in a side-to-side fashion and were hand-sewn or stapled. Extended ICR was defined as any additional distal bowel margin beyond the caecum or terminal ileum that was necessary due to extent of disease. Conversion to open was defined as an unplanned midline incision or extension of the standard ICR extraction site.
Statistical Analysis
Data were collected and analyzed using R version 4.4.0.[30] Patients who underwent primary ICR (1R) were compared to those who underwent secondary (2R) and/or tertiary and greater (>2R) ICR. The primary outcome was any complication within 30 days of the operation. Subset analyses were performed on the group of patients undergoing repeat resection (≥2R) as well as the group who were resected using a laparoscopic approach. Categorical variables were compared using the Pearson’s Chi-square test or the Fisher exact test. Continuous data were compared using a Kruskal-Wallis test. Hypothesis testing was performed at 5% level of significance. Missing data, when applicable, are described in the text.
Results
A total of 512 patients were identified in a prospective database; of these, 38 patients found on further review to either have a non-CD diagnosis or a procedure other than ICR were excluded. This left 474 consecutive patients who were included in the analysis. This included 369 (77.8%) patients in the 1R group and 105 (22.2%) patients in the ≥2R group. The ≥2R group was comprised of 65 patients undergoing secondary resections (2R), 31 patients undergoing their third (3R), 6 their fourth (4R), 1 their fifth (5R), and 2 their seventh ileocolic resection (7R). Of the 105 patients in the ≥2R group, 62 (59.0%) had their prior surgery at another institution.
Patients were predominately male (n=331, 69.8%) with a median age of 33 years [IQR 25–47]. Missing data were as follows: Montreal classification (n=1), body mass index (n=4), and ASA class (n=1). All other data were complete. A majority of patients (n=254, 53.6%) underwent at least one additional procedure at the time of ICR, with some patients undergoing multiple additional procedures as follows: abscess drainage (n=114), fistula takedown (n=100), small bowel resection (n=29), stricturoplasty (n=16), low anterior resection (n=22), sigmoidectomy (n=5), left hemicolectomy (n=4) and transverse colectomy (n=1). There were no intraoperative complications.
Overall, 73 patients (15.4%) experienced a postoperative complication. These consisted of minor complications (Clavien-Dindo I/II) in 55 patients (11.6%) including ileus (n=19), minor infections (n=14), and bleeding (n=11). Major complications (Clavien-Dindo III/IV) occurred in 18 patients (3.8%) including intra-abdominal abscess (n=7), anastomotic leak (n=3), and small bowel obstruction (n=3) (Supplemental Table 1). The 30-day reoperation rate was 2.3% (n=11) and 30-day readmission rate was 3.6% (n=17).
In comparison to the 1R group, the ≥2R group was older (median 43 vs. 31 years, p<0.001), but was comparable with regards to gender, BMI, and proportion of patients with ASA ≥3 (Table 1). The ≥2R group had a higher percentage of current and former smokers (26.6% vs. 7.9%, p<0.001). Montreal classification and preoperative indications for ICR were comparable between groups, as were perioperative use of steroids and biologics. Patients undergoing repeat ICR were more likely to have previously undergone other non-ICR abdominal surgery (26.7% vs. 14.9%, p=0.01). Patients in the ≥2R group were less likely to undergo extended resection (2.9% vs. 10%, p=0.02), but rates of emergency surgery and concurrent surgical procedures were comparable between groups.
Table 1:
Comparison of all cases by primary (1R) versus repeat resections (≥2R).
| 1R (N=369) | ≥2R (N=105) | p-value | |
|---|---|---|---|
| Patient and Disease Characteristics | |||
|
| |||
| Male (%) | 264 (71.5) | 67 (63.8) | 0.16 |
|
| |||
| Age (median [IQR]) | 31 [24, 46] | 43 [31, 54] | <0.001 |
|
| |||
| BMI (median [IQR]) | 22.3 [19.7, 25.8] | 22.0 [19.5, 26.0] | 0.68 |
|
| |||
| ASA ≥ 3 (%) | 108 (29.3) | 39 (37.1) | 0.16 |
|
| |||
| Smoker (%) | <0.001 | ||
| Never | 340 (92.1) | 77 (73.3) | |
| Former | 17 (4.6) | 18 (17.1) | |
| Current | 12 (3.3) | 10 (9.5) | |
|
| |||
| Indication for surgery (%) | 0.69 | ||
| Obstruction | 272 (73.7) | 80 (76.2) | |
| Abscess | 68 (18.4) | 17 (16.2) | |
| Fistula | 11 (3.0) | 4 (3.8) | |
| Dysplasia | 8 (2.2) | 0 (0.0) | |
| Failure of medical therapy | 5 (1.4) | 2 (1.9) | |
| Other* | 5 (1.4) | 2 (1.9) | |
|
| |||
| Phenotype (%) | 0.21 | ||
| Inflammatory | 14 (3.8) | 3 (2.9) | |
| Stricturing | 202 (54.7) | 67 (64.4) | |
| Penetrating | 153 (41.5) | 34 (32.4) | |
|
| |||
| History of other abdominal surgery (%) | 55 (14.9) | 28 (26.7) | 0.01 |
|
| |||
| Perioperative steroids (%) | 45 (12.2) | 17 (16.2) | 0.36 |
|
| |||
| Perioperative biologics (%) | 50 (13.6) | 18 (17.1) | 0.44 |
|
| |||
| Operative Characteristics | |||
|
| |||
| Emergency surgery (%) | 9 (2.4) | 3 (2.9) | 0.73 |
|
| |||
| Extended ICR** (%) | 37 (10.0) | 3 (2.9) | 0.02 |
|
| |||
| Concurrent procedures (%) | 195 (52.8) | 59 (56.2) | 0.62 |
|
| |||
| Approach (%) | <0.001 | ||
| Laparoscopic | 314 (85.1) | 75 (71.4) | |
| Converted | 32 (8.7) | 8 (7.6) | |
| Open | 23 (6.2) | 22 (21.0) | |
|
| |||
| Diverting stoma (%) | 16 (4.3) | 5 (4.8) | 0.79 |
|
| |||
| Duration, minutes (median [IQR]) | 120 [94, 157] | 145 [101, 206] | <0.001 |
|
| |||
| Postoperative Outcomes | |||
|
| |||
| Length of stay, days (median [IQR]) | 5 [4, 7] | 6 [5, 9] | 0.02 |
|
| |||
| Any complication (%) | 52 (14.1) | 21 (20.0) | 0.19 |
|
| |||
| Major complication (%) | 14 (3.8) | 4 (3.8) | 0.56 |
|
| |||
| 30-day readmission (%) | 14 (3.8) | 3 (2.9) | 0.77 |
|
| |||
| Reoperation (%) | 11 (3.0) | 0 (0.0) | 0.13 |
Perforation, toxic megacolon, hemorrhage, recurrent appendicitis
Any additional distal bowel margin beyond the cecum
The proportion of patients where laparoscopy was attempted was lower in the ≥2R group (93.8% vs. 79.0%, p<0.001), as was the proportion of total cases completed laparoscopically (85.1% vs. 71.4%, p<0.001) (Table 1). However, the rate of conversion to an open procedure was comparable between groups (32 of 346, 9.2% vs. 8 of 83, 9.6%, p=1.00). Median duration of the procedure was higher in repeated resections (120 [IQR 94–158] vs. 145 [IQR 101–203] minutes, p<0.001). Rates of faecal diversion were comparable between primary (1R) and repeat (≥2R) resection group (Table 1).
Overall 30-day morbidity was higher in the ≥2R group as compared to the 1R group but this difference was not significant (20.0% vs. 14.1% experiencing ≥1 complication, p=0.19). Furthermore, the rate of major complications was comparable between groups (3.8% vs. 3.8%, p=1.00). Postoperative mortality was nil. Length of stay was longer in the ≥2R group (median 6 vs. 5 days, p=0.02). Rates of 30-day readmissions and reoperations were not different between groups.
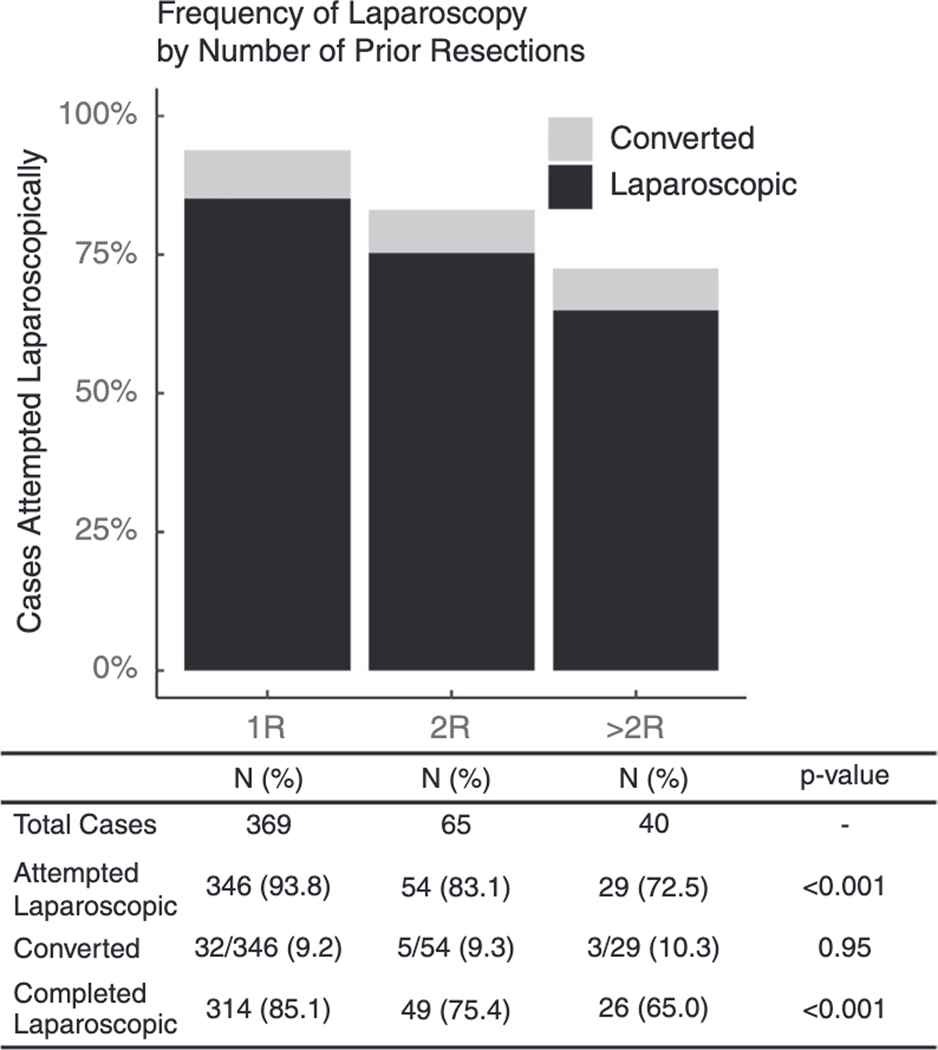
To further explore laparoscopy rates and outcomes, patients in the repeat resection group were separated into those who underwent a secondary resection (2R) and those who underwent a tertiary+ resection (>2R). There were no significant differences between 2R and >2R groups with regards to patient or disease characteristics with the exception of age (40 vs. 44 years, p=0.02). (Supplemental Table 2). Median duration of the procedure increased with repeated resections (1R: 120 [94–158] vs. 2R: 140 [100–190] vs. >2R 167 [122–224] minutes, p<0.001). Laparoscopy rates declined by stage of resection; however, a large portion of cases in the >2R group were still attempted and completed laparoscopically and conversion rates were comparable between all three groups. (Figure 1).
Figure 1:
Proportion of cases that were attempted laparoscopically by stage of resection, demonstrating rates of conversion to open surgery as well as overall proportion of cases completed laparoscopically by stage.
Patients who successfully underwent ICR via laparoscopy (n=389) were compared to those who were converted to an open approach (n=40) or underwent open surgery (n=45) (Table 2). Patients who were successfully treated laparoscopically were more likely to be younger, had fewer comorbidities, and had lower incidence of penetrating disease. Laparoscopy was infrequent in patients undergoing emergency surgery and was less common in patients undergoing concurrent surgical procedures in addition to ICR.
Table 2:
Preoperative and intraoperative factors associated with successful laparoscopic completion versus conversion or open approach.
| Laparoscopic (N=389) | Converted or open (N=85) | p-value | |
|---|---|---|---|
| Male (%) | 268 (68.9) | 63 (74.1) | 0.41 |
|
| |||
| Age (median [IQR]) | 32 [25, 46] | 41 [30, 54] | 0.002 |
|
| |||
| BMI (median [IQR]) | 22.4 [19.9, 25.9] | 21.9 [18.1, 25.5] | 0.04 |
|
| |||
| ASA ≥ 3 (%) | 103 (26.5) | 44 (51.8) | <0.001 |
|
| |||
| Smoker (%) | 0.47 | ||
| Never | 345 (88.7) | 72 (84.7) | |
| Former | 28 (7.2) | 7 (8.2) | |
| Current | 16 (4.1) | 6 (7.1) | |
|
| |||
| Indication (%) | 0.04 | ||
| Obstruction | 295 (75.8) | 57 (67.1) | |
| Abscess | 66 (17.0) | 19 (22.4) | |
| Fistula | 12 (3.1) | 3 (3.5) | |
| Dysplasia | 8 (2.1) | 0 (0.0) | |
| Failure of medical therapy | 3 (0.8) | 4 (4.7) | |
| Other* | 5 (1.3) | 2 (2.4) | |
|
| |||
| Phenotype (%) | <0.001 | ||
| Inflammatory | 15 (3.9) | 2 (2.4) | |
| Stricturing | 234 (60.3) | 35 (41.2) | |
| Penetrating | 139 (35.7) | 48 (56.5) | |
|
| |||
| History of non-ICR abdominal surgery (%) | 63 (16.2) | 21 (24.7) | 0.09 |
| Perioperative steroids (%) | 52 (13.4) | 10 (11.8) | 0.83 |
|
| |||
| Perioperative biologics (%) | 51 (13.1) | 17 (20.0) | 0.14 |
|
| |||
| Emergency surgery (%) | 4 (1.0) | 8 (9.4) | <0.001 |
|
| |||
| Extended ICR (%) | 28 (7.2) | 12 (14.1) | 0.06 |
|
| |||
| Concurrent procedures (%) | 185 (47.6) | 69 (81.2) | <0.001 |
Perforation, toxic megacolon, hemorrhage, recurrent appendicitis
Any additional distal bowel margin beyond the cecum
When laparoscopic cases were considered specifically, patients undergoing attempted repeat laparoscopic resections (2R or >2R, n=54 and n=29, respectively) were more commonly male, older, and current or former smokers compared to primary laparoscopic resection (1R, n=346) (Table 3). There were no significant differences with regard to disease phenotype or indications for surgery, or in the use of perioperative steroids or biologics by number of prior resections. Operating time was longer for repeated laparoscopic resections, but rates of faecal diversion, hospital length of stay, complications, readmissions and reoperations did not differ between groups. For patients in the >2R group who underwent attempted laparoscopic repair (n=29), the conversion rate was 10%, with an overall morbidity of 14%.
Table 3:
Characteristics of cases that were approached laparoscopically by stage of disease.
| 1R (N=346) | 2R (N=54) | >2R (N=29) | p-value | |
|---|---|---|---|---|
| Patient and Disease Characteristics | ||||
|
| ||||
| Male (%) | 252 (72.8) | 34 (63.0) | 15 (51.7) | 0.03 |
|
| ||||
| Age (median [IQR]) | 31 [24, 46] | 40 [30, 52] | 43 [35, 53] | <0.001 |
|
| ||||
| BMI (median [IQR]) | 22.5 [19.7, 26.0] | 23.4 [20.1, 26.5] | 20.8 [18.7, 23.1] | 0.03 |
|
| ||||
| ASA ≥ 3 (%) | 98 (28.4) | 14 (25.9) | 12 (41.4) | 0.29 |
|
| ||||
| Smoker (%) | <0.001 | |||
| Never | 319 (92.2) | 43 (79.6) | 18 (62.1) | |
| Former | 16 (4.6) | 7 (13.0) | 9 (31.0) | |
| Current | 11 (3.2) | 4 (7.4) | 2 (6.9) | |
|
| ||||
| Indication (%) | 0.75 | |||
| Obstruction | 259 (74.9) | 43 (79.6) | 22 (75.9) | |
| Abscess | 64 (18.5) | 7 (13.0) | 5 (17.2) | |
| Fistula | 9 (2.6) | 3 (5.6) | 1 (3.4) | |
| Dysplasia | 8 (2.3) | 0 (0.0) | 0 (0.0) | |
| Failure of medical therapy | 3 (0.9) | 0 (0.0) | 0 (0.0) | |
| Other* | 3 (0.9) | 1 (1.9) | 1 (3.4) | |
|
| ||||
| Phenotype (%) | 0.19 | |||
| Inflammatory | 13 (3.8) | 1 (1.9) | 1 (3.4) | |
| Stricture | 193 (55.8) | 39 (73.6) | 18 (62.1) | |
| Penetrating | 140 (40.5) | 13 (24.1) | 10 (34.5) | |
|
| ||||
| History of other abdominal surgery (%) | 50 (14.5) | 16 (29.6) | 8 (27.6) | 0.007 |
|
| ||||
| Perioperative steroids (%) | 43 (12.4) | 12 (22.2) | 4 (13.8) | 0.15 |
|
| ||||
| Perioperative biologics (%) | 46 (13.3) | 9 (16.7) | 5 (17.2) | 0.70 |
|
| ||||
| Operative Characteristics | ||||
|
| ||||
| Emergency surgery (%) | 4 (1.2) | 2 (3.7) | 0 (0.0) | 0.22 |
|
| ||||
| Extended ICR** (%) | 31 (9.0) | 2 (3.7) | 1 (3.4) | 0.27 |
|
| ||||
| Concurrent procedures (%) | 176 (50.9) | 27 (50.0) | 14 (48.3) | 0.96 |
|
| ||||
| Conversion (%) | 32 (9.2) | 5 (9.3) | 3 (10.3) | 0.98 |
|
| ||||
| Diverting stoma (%) | 12 (3.5) | 2 (3.7) | 1 (3.4) | 1.00 |
|
| ||||
| Duration, minutes (median [IQR]) | 120 [94, 155] | 139 [100, 171] | 137 [98, 195] | 0.02 |
|
| ||||
| Postoperative Outcomes | ||||
|
| ||||
| Length of stay, days (median [IQR]) | 5 [4, 7] | 5 [4, 6] | 6 [4, 9] | 0.38 |
|
| ||||
| Any complication (%) | 45 (13.0) | 10 (18.5) | 4 (13.8) | 0.55 |
|
| ||||
| Major complication (%) | 11 (3.2) | 3 (5.6) | 0 (0.0) | 0.55 |
|
| ||||
| 30-day readmission (%) | 11 (3.2) | 1 (1.9) | 1 (3.4) | 1.00 |
|
| ||||
| Reoperation (%) | 9 (2.6) | 0 (0.0) | 0 (0.0) | 0.80 |
Perforation, toxic megacolon, hemorrhage, recurrent appendicitis
Any additional distal bowel margin beyond the cecum
Finally, we considered the cohort of patients who underwent a repeat resection (≥2R, n=105) and examined whether the prior resection was done open or laparoscopically; of these, 31 patients (30%) had prior laparoscopic resections only, 63 had previously had at least one open resection (60%), and for 11 patients the type of prior resection was unknown. Of patients with a prior laparoscopic resection (n=31), the redo resection was attempted laparoscopically in 27 patients (87%) with a conversion rate of 7% (n=2) and overall morbidity of 19% (n=5). Of patients with a prior open resection (n=63), the redo resection was attempted laparoscopically in 46 patients (73%) with a conversion rate of 9% (n=4) and overall morbidity of 17% (n=8). Thus, while patients with a history of open ICR were less likely than patients with a history of laparoscopic ICR be approached laparoscopically at the time of repeat resection (73% vs. 87%, p<0.001), rates of conversion and overall complications were comparable regardless of prior operative approach.
Discussion
This study aimed to review the role of laparoscopy in management of recurrent Crohn’s terminal ileal disease at a tertiary care institution specializing in IBD. From 2013–2019, 474 patients underwent ICR, with 82.1% of cases performed laparoscopically and an overall morbidity rate of 15.4%. Our results suggest that at experienced centers, laparoscopy for secondary and even tertiary+ ICR is safe and feasible in a majority of patients with a relatively low rate of conversion. Although the rate of attempted laparoscopy decreased with increasing stage of resection, a majority of patients in this tertiary or greater resection group (>2R) were attempted and completed laparoscopically (73% and 65%, respectively). In carefully selected patients, laparoscopic ICR was feasible, with acceptable rates of conversion (10%) and overall morbidity rates (14%) that are comparable to patients undergoing primary or secondary ICR. Furthermore, appropriately selected patients who underwent laparoscopic ICR following a prior open ICR (n=63) also had low rates of conversion (9%) and morbidity (17%).
In this contemporary ICR cohort, 22.2% of patients underwent repeat resection (≥2R), with some patients having undergone as many as six prior resections, highlighting the risk of repeat surgery in CD despite advances in medical therapies. Our findings are consistent with a recent longitudinal study demonstrating that approximately 20% of patients will undergo a repeat ICR for recurrent disease within 10 years of their initial operation.[12] Given this high proportion of young patients undergoing multiple resections over their lifetimes, determining the feasibility and safety of a laparoscopic approach to repeated resections is crucial.
In this study, overall morbidity of patients undergoing repeat resections was higher than in the primary resection group, although this difference was not significant. Higher morbidity for repeat resections has been documented in several larger studies of ICR for CD.[16,31] Additionally, the median operating time increased significantly in repeat resections, both overall and in the laparoscopic cohort. These findings are consistent with the existing literature that suggests that repeat resections are at higher risk for more intra-abdominal adhesions and inflammation.[32–34] Indications for repeat ileocolic resections include severe inflammation, strictures, and abscesses; these findings can make identification of anatomical landmarks and control of inflamed Crohn’s mesentery technically difficult during laparoscopy.[35] The current study is likely underpowered to detect a small difference in increased overall morbidity for repeat resections. However, when broken down by Clavien-Dindo score, morbidity was driven by minor complications (Supplemental Table 1).
A laparoscopic approach is clearly preferred in primary ICR for Crohn’s disease, with fewer complications than open resection and improved early postoperative outcomes.[1,2,11,35,36] The most recent meta-analysis of laparoscopic surgery for CD also found a decrease in total perioperative complications in the laparoscopic group compared to the open group (RR 0.71).[8] However, this analysis looked at all bowel resections and did not account for patients specifically undergoing repeat resections.
The safety of laparoscopic approach for recurrent CD has been addressed in numerous small observational studies (Table 4).[17–26] Rates of conversion in earlier studies were quite high, ranging from 13–62%. A meta-analysis of these earlier studies found that the conversion rate was higher in recurrent CD as compared to primary resection (OR 2.53, 95% CI 1.22–5.25, p=0.01).[37] However, more recent series have reported acceptable conversion rates between 5–32%. This likely reflects growing comfort with advanced laparoscopic technique. Brouquet et al. recently reported on 20 patients who underwent laparoscopic ICR for tertiary+ resection (>2R) and also reported a low rate of conversion (15%); however, only 34% of tertiary+ cases in this series were attempted laparoscopically.[15] Similarly, a recent national study in Italy found that only 31% of repeat resections were attempted laparoscopically, with no increase in laparoscopy rates for repeat resections at high-volume centers.[16] In our study, 73% of tertiary+ cases were approached laparoscopically with only 10% conversion.
TABLE 4.
Review of prior studies examining laparoscopic ICR for recurrent resection in ileal Crohn's disease.
| Study | Study years | Study type | Type of surgery | Recurrent laparoscopic cases (N) | Comparator group | Conversion rate | Complication rate | Other findings |
|---|---|---|---|---|---|---|---|---|
| Wu et al. (1997) | 1992–1996 | Prospective | ICR | 10 | N=22 primary laparoscopic, N=70 open | 20% (2/10) | 10% (1/10) | Longer LOS and higher morbidity in open group, similar amongst laparoscopic groups |
| Hasegawa et al. (2003) | 1994–2002 | Retrospective | ICR | 16 | N=45 primary laparoscopic | 13% (2/16) | 19% (3/16) | Similar conversion rate, morbidity and LOS compared to primary group |
| Uchikoshi et al. (2004) | 1997 – 2003 | Retrospective | ICR | 23* | N=20 recurrent open | 43% (10/23) | 13% (3/23) | Lower morbidity, shorter LOS compared to open group |
| Moorthy et al. (2004) | 1991–2001 | Retrospective | ICR/other | 26 | N=31 primary laparoscopic | 42% (11/26) | 15% (4/26) | Higher conversion rate and longer time to diet compared to primary group |
| Edden et al. (2008) | 1992–1998 | Prospective | ICR | 34* | N=124 primary laparoscopic | 62% (21/34) | NR | Similar conversion rate overall, converted patients had similar morbidity |
| Chaudhary et al. (2010) | 2002–2010 | Prospective | ICR | 30 | N=29 primary laparoscopic | 7% (2/30) | 30% (9/30) | Longer operative time, similar conversion rates and morbidity compared to primary group |
| Holubar et al. (2010) | 1998–2008 | Retrospective | ICR | 40 | None | 25% (10/40) | 10% (3/30)** | Longer LOS but similar morbidity in patients who were converted to open |
| Pinto et al. (2011) | 2001–2008 | Retrospective | ICR/other | 50 | N=80 primary laparoscopic | 32% (16/50) | 12% (6/50) | LOS and complications similar to primary group |
| Bandyopadhyay et al. (2011) | 2005–2009 | Prospective | ICR | 27 | None | 7% (2/27) | 7% (2/27) | No comparator group |
| Huang et al. (2012) | 2005–2010 | Retrospective | ICR/other | 48*** | N=82 primary laparoscopic | 21% (10/48) | 27% (13/48) | Similar operative time, conversion rate, and morbidity to primary group |
| Aytac et al (2012) | 1997 – 2011 | Retrospective case-match | ICR/other | 26 | N=26 open recurrent | 12% (3/26) | 39% (9/26) | Lower wound infection rate compared to open group, similar operative time, LOS, and overall morbidity |
| Panteleimonitis (2017) | 2006 – 2016 | Retrospective | ICR/other | 19 | N=87 primary laparoscopic | 5% (1/19) | NR | Shorter LOS in primary group, similar conversions, 30-day readmissions and reoperations |
| Current study | 2013 – 2019 | Retrospective | ICR | 83 | N=346 primary laparoscopic | 10% (8/83) | 17% (14/83) | Similar conversion and morbidity to primary group, longer operative time |
ICR (ileocolic resection), NR (not reported), LOS (length of hospital stay)
Including patients undergoing hand-assisted laparoscopic surgery (HALS)
For patients who were not converted only
Any prior surgery type
Morbidity following a laparoscopic approach for recurrent CD in other small series has been reported between 10–39%.[17–20,22–25] Several studies comparing laparoscopic resection for recurrent versus primary disease, generally report similar complications, length of stay, reoperations and readmissions between these groups.[17,18,23,25] A meta-analysis found comparable complication rates between recurrent and open groups approached laparoscopically (OR 1.41, 95% CI 0.86–2.34).[37] Similar to our findings, Chaudhary et al. noted longer operating time in the repeat resections approached laparoscopically.[38] In our study, overall complications did not differ significantly between primary and repeat laparoscopic resection, even though the repeat resection cohort was older and had higher rates of smoking compared to the primary resection cohorts.
Taken together, these studies suggest that laparoscopic ICR can be performed in appropriately selected cases of recurrent CD, with comparable outcomes to primary laparoscopic resection, although conversion rates may be higher and operating times may be longer. Our study furthers this growing body of literature by demonstrating that select cases of tertiary or greater CD recurrences can also be managed laparoscopically with acceptable outcomes. Recurrent disease or prior open ICR should not be considered a contraindication to a laparoscopic approach. Indeed, a majority of recurrent cases, including those done for tertiary+ resections can be approached using laparoscopy in the absence of other contraindications. Our data suggest that other factors, such as penetrating disease type, complexity of disease, patient comorbidities, and surgeon experience may be more important considerations when making the decision to attempt laparoscopy in patients with CD.
As a retrospective review of a prospectively maintained database, there are inherent limitations in the study design. Patients undergoing laparoscopic resection for recurrent CD are clearly a selected cohort, and while patients undergoing repeat laparoscopic resections in this study appeared to be equivalent or higher operative risk, there are additional factors that were not measured and examined. The retrospective nature of the approach makes it difficult to determine the exact reasons for conversion. Patients selected for an “upfront” open approach had higher ASA class, higher rates of penetrating disease, and were much more likely to be undergoing emergency surgery – all of which can contribute to higher complication rates. It is clear that patients need to be appropriately selected for a laparoscopic approach in order to achieve good outcomes. Additionally, some of the groups analyzed were small and underpowered to detect small differences in morbidity. Finally, this study was performed at a single institution that specializes in laparoscopic surgery, with a majority of operations performed by two high-volume surgeons. Thus, findings may not be generalizable to centers and surgeons with less experience. Our patient cohort consists of patients who are relatively young with normal BMI and lower ASA scores. It is unclear whether older and sicker patients would have the same outcomes. In the future, the severity of the disease can be quantified with metrics such as the Crohn’s Disease Activity Index (CDAI).[39]
Conclusion:
In this contemporary series of primary and repeat ICR for ileal CD, when performed at a high-volume tertiary centre, a laparoscopic approach was found to be feasible and safe for repeat ICR, including cases performed for tertiary or greater resections. Separate from other factors, recurrent disease with history of prior ICR, whether performed open or laparoscopically, should not be considered a contraindication to a laparoscopic approach.
Supplementary Material
What does this paper add to the literature?
In this study we found that while a laparoscopic approach was less common in repeat versus primary ileocolic resection for Crohn’s disease, rates of conversion to an open procedure were comparable as was overall morbidity. Prior resection is not a contraindication to laparoscopy, which is possible in most patients.
Disclaimers:
VB is currently receiving a grant NIDDK-T32 DK 7017 from NIH. There are no other specific funding sources for this work. PS serves as a consultant for Ethicon, Medtronic, Olympus, Karl Storz, Safeheal, and Boston Scientific. The other authors have no financial or other conflicts of interest to report. This study was approved by the local institutional review board with a waiver of patient consent.
Contributor Information
Heather Carmichael, Department of General Surgery, University of Colorado School of Medicine.
Daniel Peyser, Department of Surgery, Icahn School of Medicine at Mount Sinai Hospital.
Vanessa M Baratta, Department of General Surgery, Yale University School of Medicine.
Deepika Bhasin, Department of Surgery, Icahn School of Medicine at Mount Sinai Hospital.
Adrienne Dean, Icahn School of Medicine at Mount Sinai Hospital.
Sergey Khaitov, Division of Colon and Rectal Surgery, Department of Surgery, Icahn School of Medicine at Mount Sinai Hospital.
Alexander J Greenstein, Division of Colon and Rectal Surgery, Department of Surgery, Icahn School of Medicine at Mount Sinai Hospital.
Patricia Sylla, Division of Colon and Rectal Surgery, Department of Surgery, Icahn School of Medicine at Mount Sinai Hospital.
References
- 1.Maartense S, Dunker MS, Slors JFM, Cuesta MA, Pierik EGJM, Gouma DJ, et al. Laparoscopic-assisted versus open ileocolic resection for Crohn’s disease: A randomized trial. Ann. Surg. 2006; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milsom JW, Hammerhofer KA, Böhm B, Marcello P, Elson P, Fazio VW, et al. Prospective, randomized trial comparing laparoscopic vs. conventional surgery for refractory ileocolic Crohn’s disease. Dis. Colon Rectum 2001; [DOI] [PubMed] [Google Scholar]
- 3.Lee Y, Fleming FJ, Deeb AP, Gunzler D, Messing S, Monson JRT. A laparoscopic approach reduces short-term complications and length of stay following ileocolic resection in Crohn’s disease: An analysis of outcomes from the NSQIP database. Color. Dis. 2012; [DOI] [PubMed] [Google Scholar]
- 4.Polle SW, Wind J, Ubbink DT, Hommes DW, Gouma DJ, Bemelman WA. Short-term outcomes after laparoscopic ileocolic resection for Crohn’s disease: A Systematic review. Dig. Surg.2007; [DOI] [PubMed] [Google Scholar]
- 5.Tan JJY, Tjandra JJ. Laparoscopic surgery for Crohn’s disease: A meta-analysis. Dis. Colon Rectum 2007; [DOI] [PubMed] [Google Scholar]
- 6.Rosman AS, Melis M, Fichera A. Metaanalysis of trials comparing laparoscopic and open surgery for Crohn’s disease. Surg. Endosc. Other Interv. Tech. 2005; [DOI] [PubMed] [Google Scholar]
- 7.Alizadeh RF, Chaudhry HH, Li S, Jafari MD, Mills SD, Carmichael JC, et al. Ileocolic resection for Crohn’s disease: A minimally invasive approach claims its place. Am. Surg. 2018; [PubMed] [Google Scholar]
- 8.Patel SV, Patel SV, Ramagopalan SV., Ott MC. Laparoscopic surgery for Crohn’s disease: A meta-analysis of perioperative complications and long term outcomes compared with open surgery. BMC Surg. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dasari BV, McKay D, Gardiner K. Laparoscopic versus Open surgery for small bowel Crohn’s disease. Cochrane Database Syst. Rev. 2011; [DOI] [PubMed] [Google Scholar]
- 10.Lowney JK, Dietz DW, Birnbaum EH, Kodner IJ, Mutch MG, Fleshman JW. Is there any difference in recurrence rates in laparoscopic ileocolic resection for Crohn’s disease compared with conventional surgery? A long-term, follow-up study. Dis. Colon Rectum 2006; [DOI] [PubMed] [Google Scholar]
- 11.Young-Fadok TM, Hall Long K, McConnell EJ, Gomez Rey G, Cabanela RL. Advantages of laparoscopic resection for ileocolic Crohn’s disease improved outcomes and reduced costs. Surg. Endosc. 2001; [DOI] [PubMed] [Google Scholar]
- 12.de Buck van Overstraeten A, Eshuis EJ, Vermeire S, Van Assche G, Ferrante M, D’Haens GR, et al. Short- and medium-term outcomes following primary ileocaecal resection for Crohn’s disease in two specialist centres. Br. J. Surg. 2017; [DOI] [PubMed] [Google Scholar]
- 13.Sapci I, Gorgun E. Minimally Invasive Surgery in Complex Crohn’s Disease. Clin. Colon Rectal Surg. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang W, Tang Y, Nong L, Sun Y. Risk factors for postoperative intra-abdominal septic complications after surgery in Crohn’s disease: A meta-analysis of observational studies. J. Crohn’s Colitis 2015; [DOI] [PubMed] [Google Scholar]
- 15.Brouquet A, Blanc B, Bretagnol F, Valleur P, Bouhnik Y, Panis Y. Surgery for intestinal Crohn’s disease recurrence. Surgery 2010; [DOI] [PubMed] [Google Scholar]
- 16.National variations in perioperative assessment and surgical management of Crohn’s disease: a multicentre study. Color. Dis. 2020; [DOI] [PubMed] [Google Scholar]
- 17.Wu JS, Birnbaum EH, Kodner IJ, Fry RD, Read TE, Fleshman JW. Laparoscopic-assisted ileocolic resections in patients with Crohn’s disease: Are abscesses, phlegmons, or recurrent disease contraindications? Surgery 1997; [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa H, Watanabe M, Nishibori H, Okabayashi K, Hibi T, Kitajima M. Laparoscopic surgery for recurrent Crohn’s disease. Br. J. Surg. 2003; [DOI] [PubMed] [Google Scholar]
- 19.Uchikoshi F, Ito T, Nezu R, Tanemura M, Kai Y, Mizushima T, et al. Advantages of laparoscope-assisted surgery for recurrent Crohn’s disease. Surg. Endosc. Other Interv. Tech. 2004; [DOI] [PubMed] [Google Scholar]
- 20.Moorthy K, Shaul T, Foley RJ. Factors that predict conversion in patients undergoing laparoscopic surgery for Crohn’s disease. Am. J. Surg. 2004; [DOI] [PubMed] [Google Scholar]
- 21.Edden Y, Ciardullo J, Sherafgan K, Harris MT, Bub DS, Gorfine SR, et al. Laparoscopic-assisted ileocolic resection for Crohn’s disease. J. Soc. Laparoendosc. Surg. 2008; [PMC free article] [PubMed] [Google Scholar]
- 22.Holubar SD, Dozois EJ, Privitera A, Cima RR, Pemberton JH, Young-Fadok T, et al. Laparoscopic surgery for recurrent ileocolic Crohn’s disease. Inflamm. Bowel Dis. 2010; [DOI] [PubMed] [Google Scholar]
- 23.Pinto RA, Shawki S, Narita K, Weiss EG, Wexner SD. Laparoscopy for recurrent Crohn’s disease: How do the results compare with the results for primary Crohn’s disease? Color. Dis. 2011; [DOI] [PubMed] [Google Scholar]
- 24.Bandyopadhyay D, Sagar PM, Mirnezami A, Lengyel J, Morrison C, Gatt M. Laparoscopic resection for recurrent Crohn’s disease: Safety, feasibility and short-term outcomes. Color. Dis. 2011; [DOI] [PubMed] [Google Scholar]
- 25.Huang R, Valerian BT, Lee EC. Laparoscopic approach in patients with recurrent Crohn’s disease. Am. Surg. 2012; [PubMed] [Google Scholar]
- 26.Panteleimonitis S, Ahmed J, Parker T, Qureshi T, Parvaiz A. Laparoscopic resection for primary and recurrent Crohn’s disease: A case series of over 100 consecutive cases. Int. J. Surg. 2017; [DOI] [PubMed] [Google Scholar]
- 27.Silverberg MS, Satsangi J, Ahmad T, Arnott IDR, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. 2005; [DOI] [PubMed] [Google Scholar]
- 28.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg.2004; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Andrea AP, Khetan P, Miller R, Sylla P, Divino CM. Outcomes After Bowel Resection for Inflammatory Bowel Disease in the Era of Surgical Care Bundles and Enhanced Recovery. J. Gastrointest. Surg. 2020; [DOI] [PubMed] [Google Scholar]
- 30.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2020; [Google Scholar]
- 31.Bouquot M, Maggiori L, Hain E, Prost a la Denise J, Bouhnik Y, Panis Y What is the outcome for patients undergoing more than two ileocolonic resections for recurrent Crohn’s disease? A comparative study of 569 consecutive procedures. Color. Dis. 2019; [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto T, Allan RN, Keighley MRB. Risk factors for intra-abdominal sepsis after surgery in Crohn’s disease. Dis. Colon Rectum 2000; [DOI] [PubMed] [Google Scholar]
- 33.Alves A, Panis Y, Bouhnik Y, Pocard M, Vicaut E, Valleur P. Risk factors for intra-abdominal septic complications after a first ileocecal resection for Crohn’s disease: A multivariate analysis in 161 consecutive patients. Dis. Colon Rectum 2007; [DOI] [PubMed] [Google Scholar]
- 34.Simillis C, Yamamoto T, Reese GE, Umegae S, Matsumoto K, Darzi AW, et al. A meta-analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus nonperforating Crohn’s disease. Am. J. Gastroenterol. 2008; [DOI] [PubMed] [Google Scholar]
- 35.Nguyen SQ, Teitelbaum E, Sabnis AA, Bonaccorso A, Tabrizian P, Salky B. Laparoscopic resection for Crohn’s disease: An experience with 335 cases. Surg. Endosc. 2009; [DOI] [PubMed] [Google Scholar]
- 36.Soop M, Larson DW, Malireddy K, Cima RR, Young-Fadok TM, Dozois EJ. Safety, feasibility, and short-term outcomes of laparoscopically assisted primary ileocolic resection for Crohn’s disease. Surg. Endosc. 2009; [DOI] [PubMed] [Google Scholar]
- 37.Shigeta K, Okabayashi K, Hasegawa H, Tsuruta M, Seishima R, Kitagawa Y. Meta-analysis of laparoscopic surgery for recurrent Crohn’s disease. Surg. Today 2016; [DOI] [PubMed] [Google Scholar]
- 38.Chaudhary B, Glancy D, Dixon AR. Laparoscopic surgery for recurrent ileocolic Crohn’s disease is as safe and effective as primary resection. Color. Dis. 2011; [DOI] [PubMed] [Google Scholar]
- 39.Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn’s Disease Activity Index: National Cooperative Crohn’s Disease Study. Gastroenterology 1976; [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.