Abstract
Purpose of review
We review the cardiovascular toxicities associated with cancer immune therapies and discuss the cardiac manifestations, potential mechanisms, and management strategies.
Recent findings
The recent advances in cancer immune therapy with immune checkpoint inhibitors and adoptive cell transfer have improved clinical outcomes in numerous cancers. The rising use of cancer immune therapy will lead to a higher incidence in immune-related adverse events. Recent studies have highlighted several reports of severe cases of acute cardiotoxic events with immune therapy including fulminant myocarditis. We believe that immune-mediated myocarditis is a driving mechanism behind these cardiovascular toxicities and requires vigilant screening and prompt management with corticosteroids and immune-modulating drugs, especially with combination immune therapies.
Summary
While the incidence of serious cardiovascular toxicities with immune therapy appears low, these can be life-threatening especially when manifesting as acute immune-mediated myocarditis. Further collaborative studies are needed to effectively identify, characterize, and manage these events.
Keywords: Immune therapy, Checkpoint inhibitors, PD-1, CTLA-4, Cardiovascular toxicities, Myocarditis
Introduction
Recent advances in immune therapy have revolutionized cancer treatment paradigms leading to substantially improved clinical outcomes for cancers with traditionally poor prognosis. Immune therapies have been approved as first-line treatments for metastatic melanoma and non-small cell lung cancer, and as second-line therapy for Hodgkin’s lymphoma, head and neck, bladder, and renal cell cancers (see Table 1). In general, these novel therapies modulate and overcome cancer cell specific immune evasion. Distinct therapeutic classes include immune checkpoint inhibitors, chimeric antigen receptor (CAR) T cell therapy, and cancer vaccines. Activated T cell responses, however, may be non-specific to cancer cells and can target normal tissue leading to immune-related adverse events (irAEs). These toxicities most often affect the colon, lung, endocrine glands, skin, and liver, and are usually rapidly reversible with high-dose corticosteroids. However, limited recent reports of a spectrum of myocardial toxicities ranging from mild to fulminant among patients on immune checkpoint inhibitors suggest that immune-related cardiac effects may also be clinically relevant [10••]. With an expected increase in the usage of immune therapies along with prolonged survival, we expect a concomitant rise in the incidence of these cardiac side effects. Therefore, both oncologists and cardiologists will need to be vigilant for immune-mediated cardiotoxicity, and early detection strategies should be explored. In this review, we explore cancer immune therapy in the context of their cardiac-related adverse events and highlight potential mechanism and management.
Table 1.
Summary of case reports and series of cardiovascular toxicities associated with cancer immune therapies
| Ref | # of pts | Age/sex | Tumor type | Stage | Prior Tx | Cancer treatment | Onseta | Clinical presentation |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| [1] | 8 | 22–67 (mean 41 )/both | Melanoma/RCC | IV | N/A | IL-2 | <= 4 days | Various |
| [2] | 44 | N/A/both | Various | IV | N/A | N/A | N/A | |
| [3] | 1 | 61/F | Melanoma | IV | MAGE-A3 vaccination, EFN-alpha, PRAME vaccination | Ipilimumab | 16 | N/A |
| [4] | 1 | 60/M | Melanoma | IV | Adjuvant ipilimumab | Ipilimumab | 18 | Palpitations |
| [5] | 1 | 59/M | Melanoma | IV | None | Ipilimumab | 21 | Chest pain and dyspnea |
| [6] | 1 | 83/F | Melanoma (vaginal) | IV | None | Ipilimumab | 12 | Chest pain and dyspnea |
| [7] | 1 | 69/F | Melanoma (choroidal) | IV | N/A | Nivolumab | 8 | General malaise and palpitations |
| [8] | 1 | 73/F | Melanoma (uveal) | IV | Dacarbazine, ipilimumab | Pembrolizumab | 15 | Dyspnea |
| [9] | 5 | 49–87/both | Melanoma | IV | Various | Nivolumab, pembrolizumab | 2–17 | N/A |
| [10••] | 2 | 65/F | Melanoma | IV | N/A | Ipilimumab and nivolumab | 2 | atypical chest pain, dyspnea, and fatigue |
| 63/M | Melanoma | IV | N/A | Ipilimumab and nivolumab | 2 | Fatigue, myalgias | ||
| [11] | 1 | 72/M | Melanoma | IV | N/A | Ipilimumab and nivolumab | 6 | Dyspnea, peripheral edema and anasarca |
| [12] | 2 | 63/M | Melanoma | IV | Adjuvant EFN, dendritic cell vaccine, ipilimumab | ACT with MAGE-A3 TCR | <1 | Chills, nausea, abdominal pain then cardiopulmonary arrest |
| 57/M | Multiple myeloma | N/A | Lenalidomide/Dex, Bortezomib/ Dex, D-PACE (Dex, Cisplatin, Doxorubicin, Cyclophosphamide, etoposide) | ACT with MAGE-A3 TCR | <1 | Hypotension, hypoxia, AMS | ||
|
| ||||||||
| Ref | Cardiovascular side effects | Labs | Imaging | Histology | Intervention | Tumor response | Outcome of side effect | Notes |
|
| ||||||||
| [1] | Hypotension (5), ischemia (2), atrial/ventricular arrhythmias (4), CKMB myocarditis (6) | Normal to elevated | Non-obstructive and obstructive CAD, | Transmural myocardial necrosis, non-infectious myocarditis | N/A | N/A | Fatal | |
| [2] | Atrial/ventricular arrhythmia (25), ischemia/MI (9), congestive heart failure (1), 1st degree AVB (2), cardiomyopathy (5), sudden death (2) | N/A | N/A | N/A | N/A | N/A | ||
| [3] | Myocarditis | N/A | N/A | Myocardial fibrosis | N/A | PD | Fatal | Presented with concurrent immune-related hepatitis |
| [4] | Paroxysmal atrial fibrillation, left BBB, left ventricular dysfunction, ischemia | N/A | LVEF reduction from 55–60% to 40–45% with abnormal septal motion on echocardiogram, mildly decreased uptake in the anterior segment after stress on pharmacological stress test | N/A | Initiated on lisinopril and carvedilol | N/A | Resolved to normal LVEF after 5 months | Second ipilimumab treatment |
| [5] | Pericarditis, pericardial effusion | Normal troponin | Pericardial thickening and moderate pericardial effusion on CT | Acute fibrinous pericarditis without malignancy or micooorganism | Pericardiocentesis and window, indomethacin, IV steroids to oral taper | N/A | Resolved | Concurrent adrenal insufficiency, hypothyroidism, colitis |
| [6] | Subacute “Takotsubo-like” cardiomyopathy, transient supraventricular/ventricular tachycardia | Elevated troponin (0.98 ng/ml) and ESR (65 mm/hr) | Akinetic apex on echocardiogram with preserved EF, non-obstructive CAD, focal FDG uptake in ballooned LVapex on PET-CT | N/A | Beta-blocker | N/A | Resolved | |
| [7] | Myocarditis | Positive rapid troponin, elevated CK and CKMB | Diffuse hypokinesis of the left ventricle, LVEF 30% on echocardiogram, normal coronary arteries on angiography | Infiltration of CD8+ T cells in myocardium | Oral steroid taper | SD | Return to normal EF | |
| [8] | Acute heart failure, myocarditis | Elevated BNP and troponin | LVEF of 30% with marked ventricular dyssynchrony on echocardiogram, no findings consistent with myocarditis or ischemia on cardiac MRI | Infiltration of CD8+ T cells in myocardium | Oral steroid taper, candesartan, bisoprolol, spironolactone, torsemide | SD | Resolved, EF returned to 52% | |
| [9] | Stable angina, sinus tachycardia, ventricular arrhythmia, asystolia, hypertension, atrial flutter, myocarditis, cardiomyopathy, LV systolic dysfunction | N/A | N/A | N/A | V/PO steroids (1/1), metoprolol (1), antihypertensives (1) | PD (3), PR (1) N/A (1) | Resolved except for one death | |
| [10••] | Myocarditis, myositis, intraventricular conduction delay, complete heart block, refractory ventricular tachycardia Myocarditis, myositis, intraventricular conduction delay, complete heart block |
Elevated CK, CKMB, and troponin Elevated CK, CKMB, and troponin |
Preserved EF (73%) Low normal EF (50%) |
Infiltration of T cells in myocardium, cardiac sinus, AV nodes, and skeletal muscle | IV steroids IV steroids, infliximab |
N/A N/A |
Fatal Fatal |
Evidence of selective clonal T cell populations in the those in tumor and Myocarditis, myositis, intraventricular skeletal muscle |
| [11] | Myocarditis and cardiomyopathy | N/A | LVEF reduction from 50% to 15% on echocardiogram, no evidence of ischemia on cardiac MRI or angiography | Infiltration of lymphocytes with fibrosis in myocardium | Oral steroid taper, rampiril, metoprolol, spironolactone, and furosemide | PR | EF improved to 40% | |
| [12] | Cardiopulmonary arrest due to acute myocardial infarction | Elevated troponin, CKMB, and cytokines | N/A | Autopsy revealed severe coronary disease and myocardial infarction, T cell infiltration of the myocardium with necrosis | None | N/A | Fatal | Concurrent neutronpenic fever, anemia, and cytokine release syndrome |
| Cardiogenic shock | Elevated troponin and cytokines | N/A | Cardiac myonecrosis with T cell infiltrate in myocardium | IV steroids, vasopressors | N/A | Fatal | Concurrent neutronpenic fever, and C. difficile infection | |
Ref reference, Tx treatment
Weeks after initiation of therapy
Historical Perspective
While the field of cancer immune therapy has flourished in the last decade, it actually began over a century ago with observations by Dr. William B. Coley of spontaneous tumor regression in patients after infections, suggesting a connection with the immune system and cancer development. These findings culminated in the first immune therapy known as “Coley’s toxins,” a mixture of heat-killed cultures of Streptocoocus pyogenes and Serratia maracescens, that was used as an injectable treatment for sarcoma patients [13]. While the use of this treatment was met with much skepticism during Dr. Coley’s time and never gained widespread acceptance, our further understanding of cancer immunology has propelled the contemporary field of immune therapy into the forefront of modern cancer treatments.
Early Cytokine Immune Therapy
The earliest effective cancer immune therapies were cytokines in the form of high-dose interleukin-2 (IL-2) and interferon-alpha (IFN-α). IL-2 was identified as a T cell growth factor in 1976 and recombinant forms of IL-2 showed pre-clinical anti-tumor activity in melanoma murine models [14].Clinical trials led to high-dose IL-2 becoming the first FDA-approved immune therapy for renal cell carcinoma in 1992 and metastatic melanoma in 1998 [15]. Similarly, IFN-α therapy has exhibited anti-tumor effects in melanoma mouse models, presumably mediated through immune activation. IFN-α is currently approved for use in the adjuvant setting for high-risk, resected melanoma, and in combination with bevacizumab for advanced renal cell carcinoma.
Early clinical data revealed a high incidence of cardiovascular effects from high-dose IL-2 and IFN-α therapy ranging from severe hypotension (up to 65%), arrhythmias (up to 57%), and ischemia (up to 20%) [1, 2, 16, 17]. With high-dose IL-2 therapy, vascular leak syndrome and a myriad of cardiovascular complications resulting in hypotension and tachycardia can occur [16, 17]. Up to 10% of patients may have arrhythmias including atrial fibrillation, which can contribute to the hypotension. Rare cases of myocardial infarction or myocarditis have also been reported [1]. These findings highlight the substantial toxicities of IL-2, which is reserved for patients with excellent performance status and preserved organ function. While most of these changes result from cardiac stress and hemodynamic changes, pre-clinical studies suggest that IL-2 activated lymphocytes may also directly damage endothelial cells and cardiac myocytes [18].
In IFN-α therapy, cardiac adverse reports have been reported but are less frequent as compared to high-dose IL-2. Moreover, in eight phase 1 trials involving IFN-α, no significant cardiotoxic adverse events were reported. In one small case series of 44 cancer patients treated with interferon, cardiotoxic effects ranged from arrhythmias (25/44), dilated cardiomyopathy (5/44), and ischemic heart disease manifested in MI (9/44) and sudden death (2/44) [2]. No relationship between cardiotoxic effects and dosage could be found. Most of these effects were ultimately reversible with cessation of treatment.
Immune Checkpoint Inhibitors
Ipilimumab (Anti-CTLA-4 Therapy)
The concept of immune checkpoints as potential targets for cancer treatment was introduced in the 1990s. Initial understanding of T cell biology revolved around the concept of a two-signal model which required T cell receptor (TCR) recognition and engagement by major histocompatibility complex (MHC) bound antigens (“signal 1”) as well as engagement by co-stimulatory molecules, CD28 and B7 (“signal 2”), for an effective immune response (Fig. 1) [19]. However, the discovery that CTLA-4, the first immune checkpoint identified, could directly antagonize T cell responses by opposing this CD28 co-stimulation shifted the focus from enhancing anti-tumor immune responses to removing inhibitors of T cell function [20–22]. Pre-clinical studies revealed that inhibition of CTLA-4 could enhance anti-tumor responses leading to the development and approval of ipilimumab, a monoclonal antibody to CTLA-4 [23, 24]. With its landmark approval, ipilimumab represented the first drug to demonstrate a survival benefit in metastatic melanoma, a uniformly deadly cancer with a historical long-term survival of less than 10% [25]. Since the first clinical trials, 5–10-year follow-up data with ipilimumab has shown a doubling of that long-term survival to approximately 21% [26]. The clinical trial experience with ipilimumab revealed a distinct class of toxicities, termed immune-related adverse events (irAEs), including colitis, hepatitis, endocrinopathies, and dermatitis. While these irAEs were frequent and clinically significant leading to reversible morbidity with courses of high-dose corticosteroids, they rarely led to mortality (~1%) [27•].
Fig. 1.

Timeline of breakthroughs in cancer immune therapy
Cardiac adverse events were uncommon in the initial studies of ipilimumab. One patient treated with single-agent high-dose ipilimumab (10 mg/kg) developed fatal myocarditis [28•]. There has been a range of notable cardiac-related side effects reported post-marketing. A multicenter retrospective study (n=752) revealed one patient with fatal concurrent myocardial fibrosis and hepatitis who received ipilimumab for metastatic melanoma[3].Other case reports revealed a case of reversible left ventricular dysfunction, one case of late onset pericardial effusion, constrictive pericarditis with biopsy-proven pericarditis, a case of cardiac tamponade which responded to high-dose corticosteroids, and one case of Takotsubo cardiomyopathy with apical ballooning on echocardiography (Table 1) [4–6].
Anti-PD-1 Therapy
After the clinical success of anti-CTLA-4 therapy with ipilimumab in melanoma, intensive research efforts identified other immune checkpoints. Two such molecules were programmed death 1 (PD-1) and its ligand, PD-L1, which were initially discovered to inhibit T cell proliferation in murine models [29]. Similar to the CTLA-4/B7 interaction, binding of PD-1, expressed on activated T cells, to PD-L1, expressed on cancer or stromal cells, leads to T cell exhaustion. However, unlike CTLA-4 and B7, which interact at the level of the lymph nodes between T cell and antigen presenting cells (APCs), PD-1 and PD-L1 interact at the level of the tumor microenvironment (Fig. 2). Blockade of PD-1 was shown to reverse the “exhausted” T cell phenotype and produce anti-tumor responses in murine models [30]. Subsequent clinical trials with nivolumab and pembrolizumab, both anti-PD-1 monoclonal antibodies, successfully demonstrated a clinical benefit and improved overall survival compared with conventional therapies in various tumor types including melanoma, renal cell, and non-small cell lung cancer [31–36].
Fig. 2.

Mechanism of checkpoint inhibition in a lymphatic tissue and b peripheral tissue
From these clinical trials of anti-PD-1, there have been several reports of clinically significant cardiotoxic effects, including cases of pericarditis, hypertension, atrial and ventricular arrhythmias as well as a case of fatal case of a myocardial infarction [32, 37–39]. Case reports and case series have confirmed these findings outside the clinical trial setting [7, 8] (Table 1). One case series of 496 melanoma patients treated with anti-PD-1 therapy revealed a 1% incidence of cardiac disorders which ranged from a case of fatal ventricular arrhythmia due to myocarditis, various arrhythmias (atrial flutter, ventricular arrhythmia), asystole due to cardiomyopathy, hypertension, myocarditis, and left ventricular dysfunction [9]. These events occurred at various times ranging from 2 to 17 weeks after treatment; the non-fatal cases improved or resolved with treatment with corticosteroids or supportive medications. Other case reports include autoimmune myocarditis with varying degrees of severity, from steroid-reversible disease to fulminant myocarditis [40, 41]. At this time, there does not appear to be any known association with the particular anti-PD-1 drug (pembrolizumab or nivolumab), tumor response, tumor type, or specific clinical features that predispose these patients to these serious cardiac adverse events. Ultimately, clinically significant cardiac events are infrequent with single-agent immune checkpoint inhibition.
Combination Immune Therapy
As the mechanisms of anti-CTLA and anti-PD-1 therapy are distinct, pre-clinical models supported the combination to synergistically improve anti-tumor responses [42]. The first combination immune therapies involved ipilimumab and nivolumab in melanoma. These trials demonstrated a significant improvement in clinical responses as compared to single-agent therapy (58% in the combination, 44% in nivolumab alone, and 19% in ipilimumab alone) [28•]. However, this efficacy was juxtaposed against higher frequency of serious immune-related side effects (55% in the combination, 16% in nivolumab alone, and 27% in ipilimumab alone). There was at least one case of fatal ventricular arrhythmia in the combination arm of an earlier phase II study of ipilimumab and nivolumab [43]. In general, these side effects were generally reversible with cessation of treatment and corticosteroids.
Emerging data suggest that fulminant myocarditis can result from combination of ipilimumab and nivolumab treatment. Two patients with metastatic melanoma developed myositis with rhabdomyolysis, early progressive and refractory cardiac electrical instability, and myocarditis with a robust presence of T cell and macrophage infiltrates [10••]. Both patients died despite prompt high-dose corticosteroids use and, in one case, treatment with infliximab. T cell infiltrates were only seen in striated muscle (both skeletal muscle and myocardium) and in the tumor metastases, but not in other tissue types. Characterization of the TCR revealed indications of shared high-frequency TCRs targeting tumor, heart and skeletal muscle suggesting possible T cell cross-reactivity targeting a common antigen.
Another case report identified LV dysfunction in a patient treated with combination nivolumab and ipilimumab [11]. The patient presented with heart failure symptoms with a significant reduction in LVEF from 50 to 15% after three infusions of the combination immune therapy. Biopsy confirmed the diagnosis of immune-mediated myocarditis. However, his LVEF improved to 40% after 2 months of high-dose corticosteroids and medical treatment of his heart failure.
A review from a large safety database from the manufacturer of ipilimumab and nivolumab, Bristol-Myers Squibb, revealed that myocarditis occurred more frequently (0.27 vs. 0.06%) and severely (0.17 vs <0.01% fatal cases) with combination therapy with ipilimumab and nivolumab than with nivolumab monotherapy [10••]. Of note, these data were collected retrospectively from a single manufacturer where no prospective standardized screening of cardiac issues was performed, so this likely represents an underrepresentation of the true incidence. Further efforts on pharmacovigiliance should be promoted with screening methods detailed below. In addition, as numerous companies have developed checkpoint inhibitor, these data need to be assessed with other drugs.
Pathophysiology of Immune Checkpoint Inhibitor-Induced Cardiovascular Events
The underlying pathophysiology for these immune-mediated cardiovascular events is unknown, but the development of myocarditis is now an established side effect related to checkpoint inhibition. Pre-clinical data suggest that both CTLA-4 and PD-1 play critical roles regulating immune homeostasis in the myocardium. CTLA-4−/− mice develop severe autoimmune myocarditis mediated by CD8+ T cells, which is rapidly fatal after birth [22, 44]. PD-1-deficient mice are also predisposed to spontaneous myocarditis, likely due to cytotoxic activity of effector T cells against cardiac myocytes [45–49]. Interestingly, in BALB/c genetic background, PD-1−/− mice develop spontaneous dilated cardiomyopathy due to autoantibodies to cardiac troponin, which suggests a possible antibody-mediated etiology in some genetic backgrounds [48, 49]. PD-1 deficiency in the autoimmune MRL-Faslpr mice, a model for lupus, led to the development of fatal myocarditis with T cell and macrophage infiltration into the myocardium with evidence of heart-specific autoantibodies [46, 47]. In our own cases, we did not observe any evidence of antibody deposition, although these diverse mechanisms suggest that distinct immune processes may be triggered by immune checkpoint inhibitors. Ultimately, further studies are needed to clarify the pathophysiology of immune checkpoint inhibitors, as single agents or in combination therapy, in relation to myocarditis.
Adoptive Cell Transfer
Another novel approach to leverage immunity for cancer treatment involves adoptive cell transfer (ACT), where a patient’s own T cells are engineered to specifically target tumor cells. The principles of ACT are based on initial studies where pre-existing tumor infiltrating lymphocytes (TILs) were collected, expanded, and then re-introduced into a patient immune microenvironment concurrently with systemic IL-2. Through these methods, ACT with autologous TILs has been shown to achieve a response rate of 53% with complete responses in 24% in 101 patients with melanoma [50]. However, the success with autologous TILs has been largely limited to melanoma as obtaining tumor-reactive TILs is difficult in other cancer types; furthermore, responses in other solid tumors have rarely been observed [51]. Further advances in ACT with genetic engineering of modified TCRs or CAR into T cells have expanded the use of ACT into several other cancer types. Today, intensive research efforts are underway to expand this technology.
Genetically modified TCRs allow for higher affinity towards tumor antigens that are normally not well-engaged by wild-type TCRs. These tumor-associated antigens are often produced from cancer-germline (or cancer/testis) genes that are restrictively expressed in immunologically protected germline cells of the testes and trophoblasts, but become abnormally expressed in various cancers. Common cancer-germline antigens include NYESO-1 and MAGE-A3, which are highly expressed on various cancers [52,53].In early trials, there was a response rate of 45 and 67% in melanoma and synovial cell sarcoma patients, respectively. The majority of adverse events with treatment are related to preparative therapy or IL-2, but there has been occasional evidence of cross-reactivity towards other normal cells with fatal consequences. In one case series, the use of genetically modified TCRagainstMAGE-A3, another cancer-germline antigen, led to development of fatal cardiogenic shock in two patients [12]. Mechanistic studies revealed no evidence of MAGE-A3 expression on cardiac tissue, but significant myocardial damage with T cell infiltration which targeted titin, an unrelated myocardial protein [54]. A similar case was reported with modified MART-1 TCR in a metastatic melanoma patient who experienced irreversible neurologic damage and cardiac arrest 6 days after T cell infusion [55]. While infused T cells were found in cardiac tissue, no cross-reactivity was seen with cardiomyocytes suggesting an alternative mechanism likely related to a cytokine release syndrome.
CAR T cell therapy has shown the greatest benefit in hematopoietic malignancies such as B cell acute lymphoblastic leukemia (B-ALL) where CD19, a ubiquitously expressed antigen on malignant cells and differentiated B cells, has been targeted. In heavily pre-treated B-ALL patients, 70–90% of patients experience complete responses to CAR T cell therapy, where traditionally <10% of patients survive beyond 5 years [56, 57]. Like other novel immune therapies, CAR T cell therapy produces its own unique toxicity profile. In particular, cytokine release syndrome (CRS), a systemic inflammatory response that correlates with the in vivo activation and proliferation of CAR T cells, has been a hallmark adverse event of this therapy. Clinically, cardiovascular manifestations of CRS include hypotension, arrhythmias (including tachycardia), decreased left ventricular systolic ejection fraction, troponinemia, and QT prolongation [58–60]. The pathophysiology for cardiac dysfunction is unclear, but resembles cardiomyopathy seen in stress and sepsis. In one dramatic case of cardiac dysfunction, a patient treated with CAR T cell therapy experienced cardiac arrest 7 days after infusion with subsequent reduction in LVEF to <25% from a normal baseline [59]. While the onset of these cardiac side effects can be rapid and severe, they are typically reversible.
Review of Myocarditis
As immune-mediated myocarditis has been a defining feature of cardiotoxicity in immune therapy, it will be critical to fully characterize the nature of myocarditis to determine preventive and treatment strategies. In general, myocarditis is an inflammatory disease of the heart muscle or myocardium. Myocarditis can have many etiologies and has a highly variable presentation that ranges from the incidental findings of an abnormal cardiac biomarker to clinical manifestations that include fatigue, chest pain, heart failure, arrhythmias, heart block, cardiogenic shock, and even sudden death. The range of presentations likely reflects the variability of disease involvement (focal or diffuse) and severity (mild to severe).
Because of the variability of clinical presentation, there are no specific findings for myocarditis and myocarditis is often a diagnosis of exclusion. When myocarditis is suspected, a range of tests from electrocardiogram (EKG), biomarkers, chest radiology, cardiac imaging, and cardiac sampling may be suggested. An EKG is cheap and widely available but the EKG findings are non-specific and can be normal even in significant myocarditis. Cardiac troponin I and troponin T are cardiac-specific makers of cardiomyocyte damage (including in cases of ongoing myocardial inflammation as in myocarditis). Both EKG and troponin measurements are recommended as initial diagnostic tests in cases of suspected myocarditis [61, 62]. Other cardiac biomarkers including BNP or NT-proBNP are markers of myocardial stretch but may be normal in milder forms of myocarditis.
Definitive diagnosis is generally based upon an invasive endomyocardial biopsy (EMB) in which histology reveals inflammatory infiltrates in the myocardium not typical of ischemic damage from coronary artery disease [63].Cardiovascular magnetic resonance (CMR) imaging can serve as the imaging test of choice and may be used for diagnosis. Specifically, CMR can be used to provide standard measures of cardiac size and function; however, it is the unique tissue characterization provided by CMR especially after the administration of gadolinium-based contrast agents that facilitates the noninvasive diagnosis of myocarditis. Specifically, myocarditis is associated with increased capillary permeability, increased myocardial water content, and cellular necrosis. These pathognomic features are detected on CMR by measuring T1, T2 and detecting late gadolinium enhancement in the cardiac muscle. A combination of these CMR criteria has a sensitivity of 76% and specificity of 96% for myocarditis [64, 65]. Therefore, using current consensus criteria a diagnosis is based heavily on an integrated assessment of clinical, imaging, and laboratory findings and is generally a diagnosis of exclusion.
Management of Immune-Mediated Myocarditis for Oncologist and Cardiologists
With our understanding of myocarditis, the monitoring and screening for immune-mediated myocarditis and cardiovascular toxicities will prove to be challenging. However, given the recent reports of fatal, fulminant myocarditis in combination immune therapies, ongoing clinical trials involving combination immune therapies have begun to include further cardiovascular screening such as baseline EKGs to address screening for these cardiovascular side effects. Additionally, it is the practice at some institutions to obtain not only baseline EKGs, but also baseline and weekly cardiac biomarkers such as troponin I in the first few weeks of patients treated with combination immune therapies such as ipilimumab and nivolumab. At this time, there are no specific guidelines regarding exclusion criteria related to prior cardiovascular health.
As the use of immune therapies continues to rise and expand in oncology, practicing clinicians must be vigilant for immune-mediated myocarditis. Symptoms such as chest pain, dyspnea, palpitation, and peripheral edema should lead to further cardiac investigation. Prompt evaluation for myocarditis including EKG and cardiac biomarkers should be initiated. If the initial findings are highly suspicious for immune-related myocarditis, a prompt referral to a cardiologist should be made with escalation to noninvasive imaging such as echocardiogram and CMR. An EMB should be considered if indicated as above. Other etiologies of these symptoms such as immune-related pneumonitis and pulmonary embolism or acute coronary syndromes should also be evaluated concomitantly.
While there currently are no consensus guidelines for management of immune-mediated myocarditis in the setting of cancer immune therapy, we advocate the early screening of patients receiving combination immune therapy with cardiac troponins given the variable presentation of this potentially fatal side effect (see Fig. 3). If there is an asymptomatic elevation in cardiac troponins, holding immune therapy and monitoring the cardiac troponin will inform clinicians on the nature of the elevation. Furthermore, our experience has shown that once a diagnosis of immune-mediated myocarditis from immune therapy is highly suspected or made clinically, treatment should be prompt and based on the severity of the presentation. As some patients may present with severe, fulminant disease in acute heart failure or other cardiovascular compromise, immunosuppressive therapy in the form of high-dose glucocorticoids should be rapidly initiated (i.e., methylprednisolone 1000 mg per day for 3 days followed by prednisone 1 mg/kg). Additional immunosuppressive therapy is generally warranted in these cases as has been suggested for other severe irAEs. While infliximab is a familiar choice as an adjunctive immunosuppressant for other immune-related adverse events (pneumonitis, colitis), it has been associated with heart failure and is contraindicated at higher doses than 5 mg/kg in patients with moderate or severe heart failure (New York Heart Association class III/IV) [66]. In the presence of moderate to severe heart failure, we would consider instead using anti-thymocyte globulin or tacrolimus (given their efficacy in cardiac allograft rejection) in addition to high-dose steroids. Concurrently, standard heart failure and anti-arrhythmic management should also be initiated. Ultimately, the optimal treatment algorithm for this entity has yet to be defined.
Fig. 3.
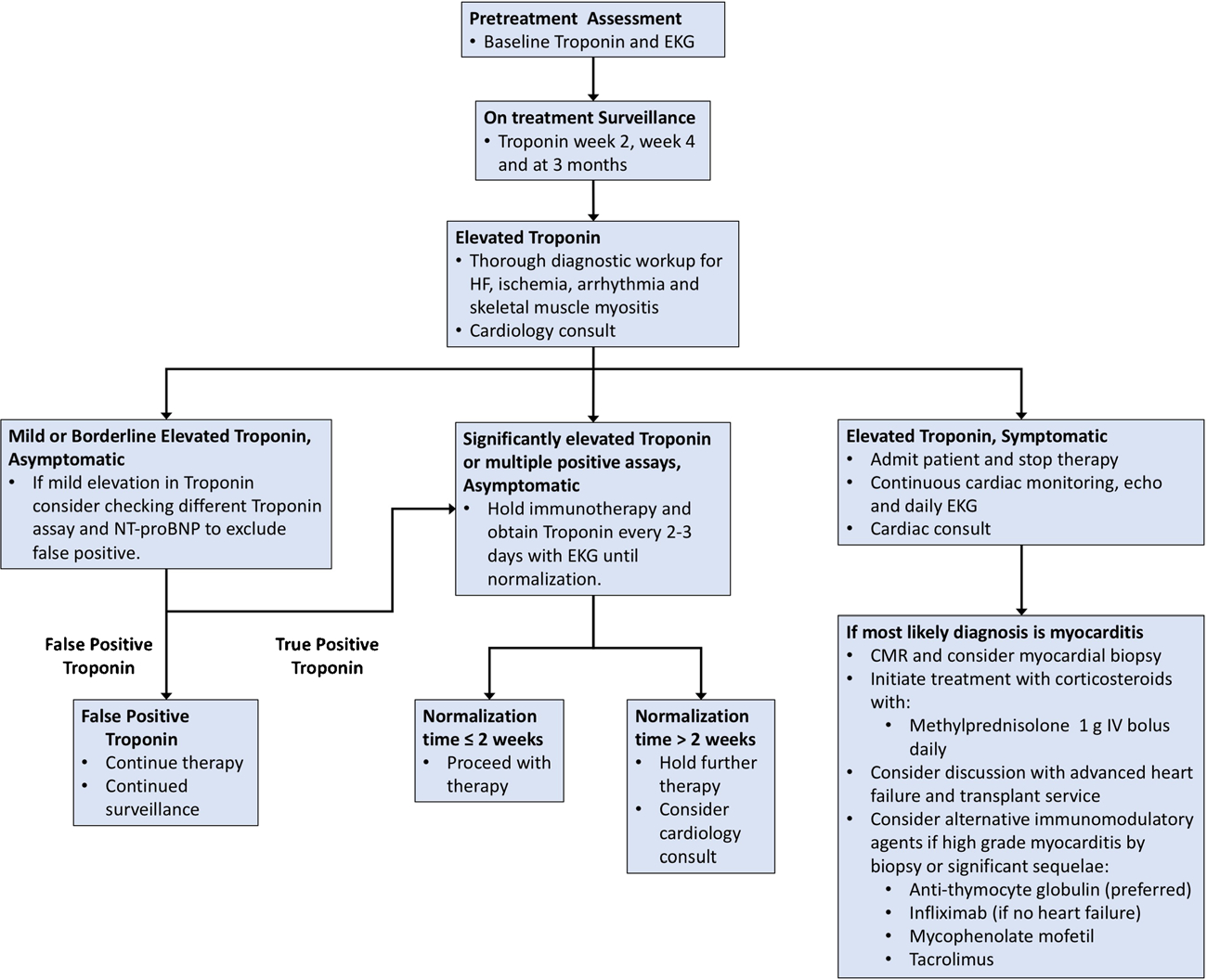
Proposed mechanism for management of immune-mediated myocarditis
For adoptive cell transfer and specifically for CAR T cell therapy, the use of tocilizumab, an anti-interleukin-6 receptor antibody, has significantly reduced the severity and incidence of CRS without affecting excellent clinical outcomes [58–60]. Originally approved for rheumatologic disorders, tocilizumab has not been FDA approved for CRS, but is widely used in managing this toxicity in CAR T cell therapy.
Future Directions
There are still many unknowns regarding the cardiac-related adverse events in cancer immune therapy. Pre-clinical and early clinical insights have helped to focus next steps in research and clinical care. First, a better estimation of the true incidence of these events are needed in both the clinical trial and post-marketing setting. This involves careful screening and monitoring protocols of patients receiving combination immunotherapy. Second, subclinical cardiac adverse events that occur on treatment may have lasting effects as these patients live longer. Third, further research must be focused on the acute management of these often, rapidly fatal cardiotoxic events. This will require a collaborative effort from cardiologist, oncologists, and immunologists to develop consensus guidelines that can control and reverse the havoc released on the myocardium by the immune system. Lastly, a better understanding of the pathophysiology that trigger these events and the general relationship between the immune system and myocardium is needed. This knowledge will help us more safely move into the new world of combination immune therapies.
Conclusion
As cancer immune therapy continues to improve clinical outcomes in patients, cardiac-related adverse events, especially immune-mediated myocarditis, remain a challenge to predict, diagnose, and treat. These cardiac events, while rare, may lead to significant morbidity and even mortality. Collaborative research efforts to manage these events will be critical moving forward.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Daniel Y. Wang, Gosife Donald Okoye, and Thomas G. Neilan declare that they have no conflict of interest.
Douglas B. Johnson reports being on the advisory board for BMS and Genoptix, and grants from Incyte.
Javid J. Moslehi reports personal fees from Pfizer, Novartis, Bristol-Myers Squibb, Takeda, Ariad, Vertex, Acceleron, Incyte, Verastem, RGenix, StemCentRx, Heat Biologics, and Pharmacyclics
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Kragel AH, Travis WD, Steis RG, Rosenberg SA, Roberts WC. Myocarditis or acute myocardial infarction associated with interleukin-2 therapy for cancer. Cancer. 1990;66(7):1513–6. [DOI] [PubMed] [Google Scholar]
- 2.Sonnenblick M, Rosin A. Cardiotoxicity of interferon. A review of 44 cases. Chest. 1991;99(3):557–61. [DOI] [PubMed] [Google Scholar]
- 3.Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8(1):e53745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth ME, Muluneh B, Jensen BC, Madamanchi C, Lee CB. Left ventricular dysfunction after treatment with ipilimumab for meta-static melanoma. Am J Ther. 2016;23(6):e1925–e1928. [DOI] [PubMed] [Google Scholar]
- 5.Yun S, Vincelette ND, Mansour I, Hariri D, Motamed S. Late onset ipilimumab-induced pericarditis and pericardial effusion: a rare but life threatening complication. Case Rep Oncol Med. 2015;2015: 794842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geisler BP, Raad RA, Esaian D, Sharon E, Schwartz DR. Apical ballooning and cardiomyopathy in a melanoma patient treated with ipilimumab: a case of takotsubo-like syndrome. J Immunother Cancer. 2015;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tadokoro T, Keshino E, Makiyama A, Sasaguri T, Ohshima K, Katano H, et al. Acute lymphocytic myocarditis with anti-PD-1 antibody nivolumab. Circ Heart Fail. 2016;9(10). [DOI] [PubMed] [Google Scholar]
- 8.Laubli H, Balmelli C, Bossard M, Pfister O, Glatz K, Zippelius A. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J Immunother Cancer. 2015;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmer L, Goldinger SM, Hofmann L, Loquai C, Ugurel S, Thomas I, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60: 210–25. [DOI] [PubMed] [Google Scholar]
- 10. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–55. •• Case report documenting fulminant myocarditis with combination ICI with histopathologic evidence of selective clonal T-cell populations within the myocardium, tumor and skeletal muscle.
- 11.Heinzerling L, Ott PA,Hodi FS, Husain AN, Tajmir-Riahi A,Tawbi H, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer. 2016;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122(6):863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res. 1991(262):3–11. [PubMed] [Google Scholar]
- 14.Rosenberg SA, Mule JJ, Spiess PJ, Reichert CM, Schwarz SL. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med. 1985;161(5):1169–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AtkinsMB Lotze MT, Dutcher JP Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between1985 and 1993. J Clin Oncol. 1999;17(7):2105–16. [DOI] [PubMed] [Google Scholar]
- 16.White RL Jr, Schwartzentruber DJ, Guleria A, MacFarlane MP, White DE, Tucker E, et al. Cardiopulmonary toxicity of treatment with high dose interleukin-2 in 199 consecutive patients with metastatic melanoma or renal cell carcinoma. Cancer. 1994;74(12): 3212–22. [DOI] [PubMed] [Google Scholar]
- 17.Lee RE, Lotze MT, Skibber JM, Tucker E, Bonow RO, Ognibene FP, et al. Cardiorespiratory effects of immunotherapy with interleukin-2. J Clin Oncol. 1989;7(1):7–20. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Yu ZX, Hilbert SL, Yamaguchi M, Chadwick DP, Herman EH, et al. Cardiotoxicity of human recombinant interleukin-2 in rats. A morphological study. Circulation. 1993;87(4):1340–53. [DOI] [PubMed] [Google Scholar]
- 19.Bretscher PA. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc Natl Acad Sci U S A. 1999;96(1):185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1(5):405–13. [DOI] [PubMed] [Google Scholar]
- 21.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182(2):459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–7. [DOI] [PubMed] [Google Scholar]
- 23.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–6. [DOI] [PubMed] [Google Scholar]
- 25.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. [DOI] [PubMed] [Google Scholar]
- 26.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33(17):1889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845–55. • Phase 3 clinical trial with higher dose of ipilimumab with one reported case of myocarditis.
- 28. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. • Phase 3 clinical trial that led to the approval of the first combination immune therapy in cancer. One patient developed myocarditis in the single ipilimumab arm.
- 29.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65(3): 1089–96. [PubMed] [Google Scholar]
- 31.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. [DOI] [PubMed] [Google Scholar]
- 32.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–32. [DOI] [PubMed] [Google Scholar]
- 33.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1positive non-small-cell lung cancer. N Engl J Med. 2016;375: 1823–33. [DOI] [PubMed] [Google Scholar]
- 35.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced non-squamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315(15):1600–9. [DOI] [PubMed] [Google Scholar]
- 38.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374(26): 2542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PDL1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027): 1540–50. [DOI] [PubMed] [Google Scholar]
- 40.Gibson R, Delaune J, Szady A, Markham M. Suspected autoimmune myocarditis and cardiac conduction abnormalities with nivolumab therapy for non-small cell lung cancer. BMJ Case Rep. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koelzer VH, Rothschild SI, Zihler D, Wicki A, Willi B, Willi N, et al. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors-an autopsy study. J Immunother Cancer. 2016;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107(9):4275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Love VA, Grabie N, Duramad P, Stavrakis G, Sharpe A, Lichtman A. CTLA-4 ablation and interleukin-12 driven differentiation synergistically augment cardiac pathogenicity of cytotoxic T lymphocytes. Circ Res. 2007;101(3):248–57. [DOI] [PubMed] [Google Scholar]
- 45.Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol. 2012;188(10):4876–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lucas JA, Menke J, Rabacal WA, Schoen FJ, Sharpe AH, Kelley VR. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J Immunol. 2008;181(4):2513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, Okazaki IM, Yoshida T, Chikuma S, Kato Y, Nakaki F, et al. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol. 2010;22(6):443–52. [DOI] [PubMed] [Google Scholar]
- 48.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–22. [DOI] [PubMed] [Google Scholar]
- 49.Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9(12):1477–83. [DOI] [PubMed] [Google Scholar]
- 50.Goff SL, Dudley ME, Citrin DE, Somerville RP, Wunderlich JR, Danforth DN, et al. Randomized, prospective evaluation comparing intensity of lymphodepletion before adoptive transfer of tumor infiltrating lymphocytes for patients with metastatic melanoma. J Clin Oncol. 2016;34(20):2389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344(6184):641–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29(7): 917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jungbluth AA, Antonescu CR, Busam KJ, Iversen K, Kolb D, Coplan K, et al. Monophasic and biphasic synovial sarcomas abundantly express cancer/testis antigen NY-ESO-1 but not MAGE-A1 or CT7. Int J Cancer. 2001;94(2):252–6. [DOI] [PubMed] [Google Scholar]
- 54.Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, et al. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med. 2013;5(197):197ra03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van den Berg JH, Gomez-Eerland R, van de Wiel B, Hulshoff L, van den Broek D, Bins A, et al. Case report of a fatal serious adverse event upon administration of T cells transduced with a MART-1-specific T-cell receptor. Mol Ther. 2015;23(9):1541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T-cells forward. Nat Rev Clin Oncol. 2016;13(6):370–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109(3):944–50. [DOI] [PubMed] [Google Scholar]
- 58.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224): 224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation. 1997;95(1):163–8. [PubMed] [Google Scholar]
- 62.Lauer B, Niederau C, Kuhl U, Schannwell M, Pauschinger M, Strauer BE, et al. Cardiac troponin T in patients with clinically suspected myocarditis. J Am Coll Cardiol. 1997;30(5):1354–9. [DOI] [PubMed] [Google Scholar]
- 63.Aretz HT, Billingham ME, Edwards WD, Factor SM, Fallon JT, Fenoglio JJ Jr, et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1(1):3–14. [PubMed] [Google Scholar]
- 64.Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109(10):1250–8. [DOI] [PubMed] [Google Scholar]
- 65.Mahrholdt H, Wagner A, Judd RM, Sechtem U. Assessment of myocardial viability by cardiovascular magnetic resonance imaging. Eur Heart J. 2002;23(8):602–19. [DOI] [PubMed] [Google Scholar]
- 66.Kwon HJ, Cote TR, Cuffe MS, Kramer JM, Braun MM. Case reports of heart failure after therapy with a tumor necrosis factor antagonist. Ann Intern Med. 2003;138(10):807–11. [DOI] [PubMed] [Google Scholar]


