Abstract
Enteropathogenic Escherichia coli (EPEC) inserts its receptor for intimate adherence (Tir) into host cell membranes by using a type III secretion system. Detergents are frequently used to fractionate infected host cells to investigate bacterial protein delivery into mammalian cells. In this study, we found that the Triton X-100-soluble membrane fraction from EPEC-infected HeLa cells was contaminated with bacterial proteins. We therefore applied a mechanical method of cell lysis and ultracentrifugation to fractionate infected HeLa cells to investigate the biology and biochemistry of Tir delivery and translocation. This method demonstrates that the translocation of Tir into the host cell membrane requires its transmembrane domains, but not tyrosine phosphorylation or binding to Tir's ligand, intimin.
Enteropathogenic Escherichia coli (EPEC) is a human pathogen responsible for outbreaks of diarrhea in both developing and developed countries (23). During infections, EPEC adheres to intestinal epithelial cells through the binding of the outer membrane protein, intimin, to its receptor in the host. Remarkably, EPEC inserts a receptor for intimin, Tir (translocated intimin receptor), into the host cell membrane, where it becomes tyrosine phosphorylated (5, 18). Intimin binding induces the rearrangement of the host cytoskeletal structure to form attaching and effacing (A/E) lesions, which are characterized by the degradation of the brush border microvilli and the formation of actin-rich pedestals upon which the bacteria reside (6). It has been shown recently that Tir tyrosine phosphorylation is required for A/E lesion formation (17). While the mechanism of Tir insertion is not known, it is facilitated by EPEC's type III secretion system and secreted proteins EspA, EspB and EspD (18). Type III secretion systems are specialized protein targeting systems that deliver effectors from the inside of the bacterium directly into the host cell (15). EspA forms a filamentous organelle located on the bacterial surface that is postulated to act as a channel for the type III system to deliver proteins inside the host cell (10, 20). EspB and EspD have been recently shown to be translocated into the host cell membrane, with EspB also found in the cytoplasm, and together potentially form a translocation pore in the host cell membrane (21, 29–31). While Tir is predicted to be a 56-kDa protein, it migrates as a 78-kDa bacterial form and as a 90-kDa tyrosine-, serine-, and/or threonine-phosphorylated host cell form when analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (17, 18). Topological, experimental, and sequence analyses indicate that Tir is an integral membrane protein with a hairpin-like structure, where both the amino and carboxy termini are inside the host cytoplasm, and the extracellular loop between the two transmembrane domains (TMDs) functions as the intimin-binding domain (IBD) (4, 7, 14, 17, 25, 26).
In this report, we investigated the biology and biochemistry of Tir delivery into HeLa cells. Studying the biochemical translocation or transport of a protein from the bacterial cytoplasm into the target host cell requires a reliable method of fractionating infected host cells. Detergent-based separation methods, with Triton X-100 being the favored reagent, have been used extensively to determine the location of bacterial virulence factors inside infected host cells. Examples include Tir, EspB, and EspD in EPEC and enterohemorrhagic E. coli, YopE, YopH, and YopD in Yersinia, exoenzyme S in Pseudomonas, and SipB, SipC, and SptP in Salmonella (3–5, 7, 12, 13, 17, 18, 20, 21, 24, 27, 30, 31). Here, we compared the use of a detergent-based fractionation method with a mechanical separation method to investigate the translocation of Tir and a series of Tir truncations into HeLa cells.
The 78-kDa Tir is detected in the Triton X-100-soluble membrane fraction from HeLa cells infected with a type III mutant.
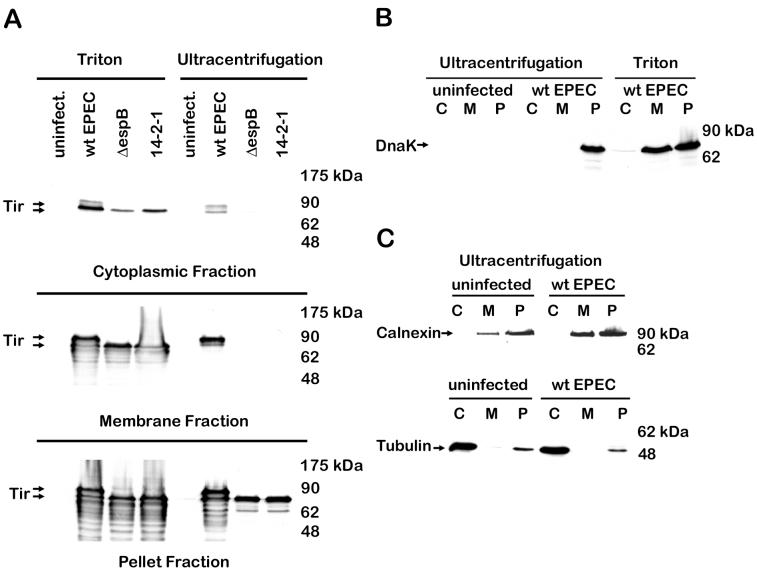
Cellular fractionation was carried out using a detergent-based method as described previously (19). Briefly, cultured HeLa cells were infected with EPEC E2348/69, washed, and treated with 0.2% saponin to release the cytoplasmic fraction in the presence of phosphatase and protease inhibitors (1 mM sodium vanadate, 1 mM sodium fluoride, 100 nM microcystin LR, 1 μM pepstatin). Triton X-100 (1%) was used to solubilize the membrane proteins from the remaining insoluble fraction. As expected, both 90- and 78-kDa Tir were found in the membrane and insoluble fractions of wild-type EPEC-infected HeLa cells (Fig. 1A) (18). Both forms were also detected in the cytoplasmic fraction. Unexpectedly, 78-kDa Tir was detected in the membrane and cytoplasmic fractions of HeLa cells infected with a type III mutant [cfm14-2-1(1)] (8) and with an espB mutant (9), although these mutants are predicted to be unable to translocate Tir. The most likely reason that this was not observed in earlier studies (18) is due to the increased sensitivity and specificity of a newer anti-Tir monoclonal antibody (4). The detection of 78-kDa Tir in cells infected with type III mutants seemed to indicate that phosphorylation of Tir, but not translocation, was dependent on the type III apparatus and secreted proteins. Alternatively, the 78-kDa form of the protein could be present due to bacterial contamination. The potential of bacterial contamination of the fractions was therefore addressed.
FIG. 1.
Triton-soluble membrane fraction of infected HeLa cells is contaminated with bacterial proteins, while the fractions from the mechanical lysis and ultracentrifugation method are free of bacterial contamination. HeLa cells were infected for 3 h with wild-type (wt) EPEC E2348/69, a type III secretion apparatus mutant [cfm14-2-1(1)], or an espB mutant and fractionated into the cytoplasmic (C), membrane (M), and bacterial and host cytoskeletal pellet (P) fractions, using a Triton-based method or the ultracentrifugation method as described in the text. After samples were resolved by SDS-PAGE (10% gel) and transferred to nitrocellulose membranes, immunoblots were probed with anti-Tir monoclonal antibody (A), anti-DnaK monoclonal antibody (B) to monitor bacterial contamination, and anticalnexin polyclonal antibody and antitubulin monoclonal antibody (C) to monitor cross-contamination of the fractions. Arrows indicate 78- and 90-kDa forms of Tir, DnaK, calnexin, and tubulin.
Triton X-100-soluble fraction of infected HeLa cells is contaminated with bacterial proteins.
DnaK is an abundant heat shock protein that functions as a chaperone inside the bacterial cytoplasm (22) and was therefore used to assess the release of cytoplasmic proteins from EPEC. The Triton X-100-soluble membrane fraction of EPEC-infected HeLa cells contained a substantial amount of DnaK (Fig. 1B), indicating that it was indeed contaminated with bacterial cytoplasmic proteins. This was also observed using a variety of other bacterial markers including intimin (inner and outer membrane) and Etk (inner membrane) (16) (data not shown). Low levels of bacterial contamination were detected in the saponin-soluble cytoplasmic fraction (Fig. 1B). Triton X-100 at concentrations as low as 0.1% released Tir, EspB, and other proteins from EPEC in culture (data not shown). Translocation of Tir is especially easy to monitor since the 90-kDa tyrosine-phosphorylated form of Tir is present when host cells are infected but not in bacteria cultured alone (18). Therefore, while Tir is indeed translocated to the host cell, the presence of the 78-kDa form of Tir could be due to bacterial contamination. Thus, to fully understand the process of translocation, a reliable fractionation method, devoid of bacterial contamination, was established.
Mechanical lysis and ultracentrifugation fractions are free of bacterial contamination.
As an alternative to a detergent-based method, we investigated a mechanical method to fractionate host cells. Cultured HeLa cells were infected with EPEC, washed, gently scraped, and mechanically disrupted by vigorous passage (six times) through a 22-gauge needle using a 1-ml syringe in a buffer containing 3 mM imidazole (pH 7.4), 250 mM sucrose, 0.5 mM EDTA, and the aforementioned phosphatase and protease inhibitors. A low-speed centrifugation (3,000 × g, 15 min) was used to pellet bacteria, unbroken HeLa cells, host nuclei, and cytoskeleton (bacterial and host cytoskeletal fraction), followed by ultracentrifugation (41,000 × g, 20 min) to separate the membrane (pellet) from the cytoplasmic (supernatant) fractions. Using the ultracentrifugation method, 90-kDaTir and a very small amount of 78-kDa Tir were detected in the membrane fraction of HeLa cells infected with wild-type EPEC (Fig. 1A). Tir was not detected in the cytoplasmic or membrane fractions of cells infected with the type III mutant or espB mutant using this technique, which is in sharp contrast to what was observed with the detergent-based method. No detectable bacterial contamination was observed in either the cytoplasmic or the membrane fractions (Fig. 1B). Host cytoplasmic proteins (e.g., tubulin) were not detected in the ultracentrifugation membrane fraction, and host membrane proteins (e.g., calnexin) were not detected in the cytoplasmic fraction (Fig. 1C), indicating that the ultracentrifugation method produces not only fractions free of bacterial contamination but distinct fractions as well.
Translocation of Tir into the HeLa cell membrane sequentially increases with time.
Using the newly developed fractionation protocol, we examined Tir translocation into HeLa cell membranes over a time course of infection. HeLa cells were infected with wild-type EPEC for 1 to 4 h and fractionated by the ultracentrifugation method. At 1 h postinfection, only a very small amount of the 78-kDa bacterial form of Tir was found in the bacterial and host cytoskeletal fraction, but all subsequent time points showed an increase in both 90- and 78-kDa forms of Tir (Fig. 2); 90-kDa Tir was detected at 1.5 h postinfection and increased throughout the infection in the membrane fraction. A small amount of 78-kDa Tir was detected in the membrane fraction at time points beyond 2.5 h postinfection. At time points after 3 h, small amounts of both forms of Tir were found in the cytoplasmic fraction, while there was no detectable bacterial contamination (data not shown). The 78-kDa form of Tir may be found in the membrane and cytoplasmic fractions because of phosphorylation/dephosphorylation events, or possibly it is the translocated Tir prior to tyrosine, serine, and/or threonine phosphorylation. These results demonstrate that Tir translocation to the HeLa cell membrane increases with the duration of infection.
FIG. 2.
Translocation of Tir into HeLa cell membranes sequentially increases with time. HeLa cells were infected with wild-type (wt) EPEC E2348/69 for 1 to 4 h and fractionated by the ultracentrifugation method as described in the text. After samples were resolved by SDS-PAGE (10% gel) and transferred to nitrocellulose membranes, immunoblots were probed with anti-Tir monoclonal antibody. Arrows indicate 78- and 90-kDa forms of Tir.
Translocation of Tir to the membrane requires its transmembrane domains but not tyrosine phosphorylation or intimin-binding domains.
We deleted tir in a streptomycin-resistant strain of EPEC E2348/69 such that it would be in the same genetic background as the wild-type EPEC and type III mutant [cfm14-2-1(1)] used in this study, using a positive-selection suicide vector as described previously (18). This Δtir strain was transformed with a variety of previously constructed Tir derivatives (1, 4; R. DeVinney and B. B. Finlay, unpublished data). These particular mutants were used to study the role of the amino and carboxy termini, TMDs, IBD, and tyrosine phosphorylation on translocation (Fig. 3).Tir 200-549 and Tir 1-150 were not detected in the bacterial and host cytoskeletal fraction of HeLa cells infected with these strains, indicating that they either were not made in detectable quantities or were degraded by EPEC (Fig. 3B). Small amounts of these two derivatives were found when the bacteria were cultured alone for 6 h, suggesting that these two proteins are poorly expressed (data not shown). This agrees with previous work indicating that Tir's amino terminus is needed for its stability and secretion (1, 4). CesT, Tir's chaperone, is thought to act as an antidegradation factor by specifically binding to Tir's amino terminus, forming a multimeric stabilized complex (1, 11). The other Tir derivatives were produced by EPEC, as evidenced by their presence in the bacterial and host cytoskeletal fraction (Fig. 3B). No bacterial contamination was detected in any of the cytoplasmic and membrane fractions (data not shown).
FIG. 3.
Translocation of Tir to the host cell membrane requires its TMDs but not tyrosine phosphorylation or IBD. (A) Schematic representation of the Tir derivatives used. The CesT chaperone-binding domain (residues 1 to 100) is indicated by a hatched box, the two putative TMDs are shown as black boxes, and the IBD is between residues 277 and 332 (1, 4, 14, 17). (B) HeLa cells were infected for 3 h with wild-type (wt) EPEC E2348/69 or EPEC Δtir transformed with Tir derivatives and fractionated into the cytoplasmic, membrane, and bacterial and host cytoskeletal fractions, using the ultracentrifugation method as described in the text. after samples were resolved by sds-page (10% gel) and transferred to nitrocellulose membranes, immunoblots were probed with anti-tir monoclonal antibody. dots indicate predicted tir polypeptides.
Previously, a Tir truncation missing the two putative TMDs was reported to be inserted into host cell membranes by the detergent-based fractionation method (17). In the present study, Tir 1-200 was not detected in the membrane fraction, although it was present in a very small quantity in the cytoplasmic fraction (Fig. 3B). Tir 1-200 does not have any of the putative TMDs and therefore would not be predicted to be inserted into the membrane. In light of our results with the detergent-based fractionation method, Kenny's result (17) is likely due to bacterial contamination. Tir 1-362 contains only the first putative TMD, and while it was detected in the membrane fraction, a larger portion of it was found in the cytoplasmic fraction, indicating that a more stable membrane association is achieved when Tir has both putative TMDs. Taken together, these results demonstrate that the TMDs, until now only predicted, are required for membrane localization.
Tir lacking the IBD (residues 267 to 350) was still translocated to the membrane and cytoplasm at above wild-type levels (Fig. 3B). This demonstrates that the IBD is not important for insertion into the membrane and deletion of the IBD seems to facilitate delivery. Phosphorylation of tyrosine 474 of Tir has been shown to be essential for actin rearrangement but not for the increase in apparent molecular weight (17). A mutant in which this tyrosine was replaced with a phenylalanine (Tir Y474F) was also found in the membrane fraction, indicating that tyrosine phosphorylation is not needed for Tir insertion into the host cell membrane.
In this study, we found that the Triton X-100-soluble membrane fraction from HeLa cells infected with EPEC was contaminated with bacterial proteins. Another group has detected bacterial contamination of the Triton X-100-soluble membrane fraction (30). While many investigators have used Triton and other detergent-based fractionation procedures (3–5, 7, 12, 13, 17, 18, 20, 21, 24, 27, 30, 31), a few researchers have explored other fractionation methods (28). It is important to recognize that the fractions from the extensively used Triton X-100 method are contaminated with bacterial proteins and that the results obtained using this method should be reevaluated with appropriate controls. The mechanical disruption and ultracentrifugation method described here reliably fractionates HeLa cells infected with EPEC and Yersinia enterocolitica (data not shown) and should be applicable to other gram-negative pathogens.
Whether Tir translocation involves a cytosolic intermediate remains an unresolved issue. There are two possible methods for Tir's insertion into the host membrane: (i) Tir is translocated into the host cytoplasm then sequentially inserted into the membrane; (ii) Tir is translocated directly into the host cell membrane, possibly through an EspB/D pore. In this study we detected Tir only in the cytoplasmic fraction at very late time points, yet it was detected in the membrane fraction as early as 1.5 h postinfection, suggesting that Tir may be translocated directly into the membrane. The cytoplasmic forms of Tir seen at later time points could be due to overloading of the translocation system. However, our results cannot rule out a very rapid and short-lived cytoplasmic intermediate. Recently, Tir was detected in the saponin-soluble cytoplasmic fraction of HeLa cells infected with a Δtir strain complemented with Tir (17), although the cells were infected for a long time (5 h) and contamination of the fractions was not monitored. It was proposed that Tir is translocated into the host cytoplasm, where it is serine and/or threonine phosphorylated, after which it is inserted into the membrane, where it becomes tyrosine phosphorylated (17). In the mitochondria, some nucleus-encoded inner membrane proteins are translocated directly into the inner membrane, while others are transported via a matrix intermediate (2). Therefore, it is also plausible that Tir is translocated directly into the host cell membrane, where it becomes phosphorylated.
In conclusion, we have developed a new protocol to fractionate infected host cells into distinct fractions which are free of bacterial contamination. This technique can be used to study the targeting of other type III effectors into host cells and other processes involved in host-pathogen interactions. Using this mechanical disruption and ultracentrifugation method, all Tir derivatives containing at least one putative TMD were found in the host cell membrane fraction, while a construct lacking the TMDs (Tir 1-200) was detected only in the cytoplasmic fraction. These results indicate that Tir's TMDs are indeed required for membrane insertion and anchoring. A Tir mutant lacking the IBD between the TMDs and a derivative that is not tyrosine phosphorylated were found in the same location as wild-type Tir. Taken together, these findings demonstrate that Tir's membrane association is dependent on having its TMDs but not on its ability to bind intimin or its state of tyrosine phosphorylation.
Acknowledgments
We thank Olivia Steele-Mortimer for suggesting a mechanical disruption method for fractionating infected host cells, and we thank Linda Matsuuchi, Rebekah DeVinney, and members of the Finlay lab for helpful discussions and critical reading of the manuscript. We also thank Ilan Rosenshine for providing anti-Etk and anti-intimin antisera.
This work was supported by a doctoral research award to A.G. and an operating grant to B.B.F. from the Medical Research Council of Canada (MRC) and by a Basque Government postdoctoral fellowship to M.G. B.B.F. is an MRC scientist and a Howard Hughes International Research Scholar.
REFERENCES
- 1.Abe A, de Grado M, Pfuetzner R A, Sanchez-Sanmartin C, Devinney R, Puente J L, Strynadka N C, Finlay B B. Enteropathogenic Escherichia coli translocated intimin receptor, Tir, requires a specific chaperone for stable secretion. Mol Microbiol. 1999;33:1162–1175. doi: 10.1046/j.1365-2958.1999.01558.x. [DOI] [PubMed] [Google Scholar]
- 2.Bauer M F, Hofmann S, Neupert W, Brunner M. Protein translocation into mitochondria: the role of TIM complexes. Trends Cell Biol. 2000;10:25–31. doi: 10.1016/s0962-8924(99)01684-0. [DOI] [PubMed] [Google Scholar]
- 3.Collazo C M, Galan J E. The invasion-associated type III system of Salmonella typhimurium directs the translocation of Sip proteins into the host cell. Mol Microbiol. 1997;24:747–756. doi: 10.1046/j.1365-2958.1997.3781740.x. [DOI] [PubMed] [Google Scholar]
- 4.de Grado M, Abe A, Gauthier A, Steele-Mortimer O, DeVinney R, Finlay B B. Identification of the intimin-binding domain of Tir of enteropathogenic Escherichia coli. Cell Microbiol. 1999;1:7–17. doi: 10.1046/j.1462-5822.1999.00001.x. [DOI] [PubMed] [Google Scholar]
- 5.Deibel C, Kramer S, Chakraborty T, Ebel F. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells, where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol Microbiol. 1998;28:463–474. doi: 10.1046/j.1365-2958.1998.00798.x. [DOI] [PubMed] [Google Scholar]
- 6.DeVinney R, Gauthier A, Abe A, Finlay B B. Enteropathogenic Escherichia coli: a pathogen that inserts its own receptor into host cells. Cell Mol Life Sci. 1999;55:961–976. doi: 10.1007/PL00013202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeVinney R, Stein M, Reinscheid D, Abe A, Ruschkowski S, Finlay B B. Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated. Infect Immun. 1999;67:2389–2398. doi: 10.1128/iai.67.5.2389-2398.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnenberg M S, Calderwood S B, Donohue-Rolfe A, Keusch G T, Kaper J B. Construction and analysis of TnphoA mutants of enteropathogenic Escherichia coli unable to invade HEp-2 cells. Infect Immun. 1990;58:1565–1571. doi: 10.1128/iai.58.6.1565-1571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnenberg M S, Yu J, Kaper J B. A second chromosomal gene necessary for intimate attachment of enteropathogenic Escherichia coli to epithelial cells. J Bacteriol. 1993;175:4670–4680. doi: 10.1128/jb.175.15.4670-4680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebel F, Podzadel T, Rohde M, Kresse A U, Kramer S, Deibel C, Guzman C A, Chakraborty T. Initial binding of Shiga toxin-producing Escherichia coli to host cells and subsequent induction of actin rearrangements depend on filamentous EspA-containing surface appendages. Mol Microbiol. 1998;30:147–161. doi: 10.1046/j.1365-2958.1998.01046.x. [DOI] [PubMed] [Google Scholar]
- 11.Elliott S J, Hutcheson S W, Dubois M S, Mellies J L, Wainwright L A, Batchelor M, Frankel G, Knutton S, Kaper J B. Identification of CesT, a chaperone for the type III secretion of Tir in enteropathogenic Escherichia coli. Mol Microbiol. 1999;33:1176–1189. doi: 10.1046/j.1365-2958.1999.01559.x. [DOI] [PubMed] [Google Scholar]
- 12.Francis M S, Wolf-Watz H. YopD of Yersinia pseudotuberculosis is translocated into the cytosol of HeLa epithelial cells: evidence of a structural domain necessary for translocation. Mol Microbiol. 1998;29:799–813. doi: 10.1046/j.1365-2958.1998.00973.x. [DOI] [PubMed] [Google Scholar]
- 13.Frithz-Lindsten E, Du Y, Rosqvist R, Forsberg A. Intracellular targeting of exoenzyme S of Pseudomonas aeruginosa via type III-dependent translocation induces phagocytosis resistance, cytotoxicity and disruption of actin microfilaments. Mol Microbiol. 1997;25:1125–1139. doi: 10.1046/j.1365-2958.1997.5411905.x. [DOI] [PubMed] [Google Scholar]
- 14.Hartland E L, Batchelor M, Delahay R M, Hale C, Matthews S, Dougan G, Knutton S, Connerton I, Frankel G. Binding of intimin from enteropathogenic Escherichia coli to Tir and to host cells. Mol Microbiol. 1999;32:151–158. doi: 10.1046/j.1365-2958.1999.01338.x. [DOI] [PubMed] [Google Scholar]
- 15.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ilan O, Bloch Y, Frankel G, Ullrich H, Geider K, Rosenshine I. Protein tyrosine kinases in bacterial pathogens are associated with virulence and production of exopolysaccharide. EMBO J. 1999;18:3241–3248. doi: 10.1093/emboj/18.12.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenny B. Phosphorylation of tyrosine 474 of the enteropathogenic Escherichia coli (EPEC) Tir receptor molecule is essential for actin nucleating activity and is preceded by additional host modifications. Mol Microbiol. 1999;31:1229–1241. doi: 10.1046/j.1365-2958.1999.01265.x. [DOI] [PubMed] [Google Scholar]
- 18.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 19.Kenny B, Finlay B. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-γ1. Infect Immun. 1997;65:2528–2536. doi: 10.1128/iai.65.7.2528-2536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knutton S, Rosenshine I, Pallen M J, Nisan I, Neves B C, Bain C, Wolff C, Dougan G, Frankel G. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 1998;17:2166–2176. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kresse A U, Rohde M, Guzman C A. The EspD protein of enterohemorrhagic Escherichia coli is required for the formation of bacterial surface appendages and is incorporated in the cytoplasmic membranes of target cells. Infect Immun. 1999;67:4834–4842. doi: 10.1128/iai.67.9.4834-4842.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liberek K, Georgopoulos C, Zylicz M. Role of the Escherichia coli DnaK and DnaJ heat shock proteins in the initiation of bacteriophage lambda DNA replication. Proc Natl Acad Sci USA. 1988;85:6632–6636. doi: 10.1073/pnas.85.18.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nataro J, Kaper J. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Persson C, Nordfelth R, Holmstrom A, Hakansson S, Rosqvist R, Wolf-Watz H. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol Microbiol. 1995;18:135–150. doi: 10.1111/j.1365-2958.1995.mmi_18010135.x. [DOI] [PubMed] [Google Scholar]
- 25.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenshine I, Ruschkowski S, Stein M, Reinscheid D J, Mills S D, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 27.Rosqvist R, Magnusson K E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skrzypek E, Cowan C, Straley S C. Targeting of the Yersinia pestis YopM protein into HeLa cells and intracellular trafficking to the nucleus. Mol Microbiol. 1998;30:1051–1065. doi: 10.1046/j.1365-2958.1998.01135.x. [DOI] [PubMed] [Google Scholar]
- 29.Taylor K A, O'Connell C B, Luther P W, Donnenberg M S. The EspB protein of enteropathogenic Escherichia coli is targeted to the cytoplasm of infected HeLa cells. Infect Immun. 1998;66:5501–5507. doi: 10.1128/iai.66.11.5501-5507.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wachter C, Beinke C, Mattes M, Schmidt M A. Insertion of EspD into epithelial target cell membranes by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1999;31:1695–1707. doi: 10.1046/j.1365-2958.1999.01303.x. [DOI] [PubMed] [Google Scholar]
- 31.Wolff C, Nisan I, Hanski E, Frankel G, Rosenshine I. Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1998;28:143–155. doi: 10.1046/j.1365-2958.1998.00782.x. [DOI] [PubMed] [Google Scholar]