Abstract
Background:
Treatment-resistant schizophrenia (TRS) affects approximately 30% of people with schizophrenia. Clozapine is the gold standard treatment for TRS but is not always suitable, with a proportion of individuals intolerant of side effects or unable to engage in necessary blood monitoring. Given the profound impact TRS can have on those affected, alternative pharmacological approaches to care are needed.
Objectives:
To review the literature on the efficacy and tolerability of high-dose olanzapine (>20 mg daily) in adults with TRS.
Design:
This is a systematic review.
Data Sources and Methods:
We searched for eligible trials published prior to April 2022 in PubMed/MEDLINE, Scopus and Google Scholar. Ten studies met the inclusion criteria [five randomised controlled trials (RCTs), one randomised crossover trial and four open label studies]. Data were extracted for predefined primary outcomes (efficacy, tolerability).
Results:
Compared with standard treatment, high-dose olanzapine was non-inferior in four RCTs, three of which used clozapine as the comparator. Clozapine was superior to high-dose olanzapine in a double-blind crossover trial. Open-label studies demonstrated tentative evidence in support of high-dose olanzapine use. It was better tolerated than clozapine and chlorpromazine in two respective RCTs, and was generally well tolerated in open-label studies.
Conclusion:
This evidence suggests high-dose olanzapine is superior for TRS when compared with other commonly used first- and second-generation antipsychotics, including haloperidol and risperidone. In comparison with clozapine, the data are encouraging for the use of high-dose olanzapine where clozapine use is problematic, but larger, better designed trials are needed to assess the comparative efficacy of both treatments. There is insufficient evidence to consider high-dose olanzapine equivalent to clozapine when clozapine is not contraindicated. Overall, high-dose olanzapine was well tolerated, with no serious side effects.
Registration:
This systematic review was preregistered with PROSPERO [CRD42022312817].
Keywords: clozapine, olanzapine, psychosis, schizophrenia
Introduction
Treatment-resistant schizophrenia (TRS) affects approximately 30% of people with schizophrenia 1 and is associated with significant functional impairment. Clozapine is the gold standard treatment for TRS,2–4 reflected in national clinical guidelines.5–11 For some individuals, alternative treatments are required due to clinical contraindications precluding clozapine use, including clozapine discontinuation due to adverse events 12 or for those who do not wish to start clozapine due to concerns regarding side effects or the monitoring required.
Olanzapine is an effective medication in non refractory schizophrenia. Meta-analyses place it as one of the most efficacious antipsychotics compared with first-generation antipsychotics (FGAs) and other second-generation antipsychotics (SGAs).13,14 In the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study, olanzapine [final mean dose 23.4 mg (SD = 7.9)] was equivalent to clozapine [final mean dose 332.1 mg (SD = 156.9)] in terms of all cause discontinuations [although clozapine showed greater reductions in Positive and Negative Syndrome Scale (PANSS), total scores and a lower number of discontinuations due to lack of efficacy]. 15 A network meta-analysis using both direct and indirect comparisons to create a hierarchy of efficacy identified olanzapine to be as effective as clozapine in adult patients with a TRS, schizophreniform disorder or schizoaffective disorder, 16 notwithstanding limitations with the included studies, with suboptimal mean clozapine dose and inclusion of treatment-intolerant patients. 17 Some of these studies were high-dose olanzapine trials, which may have contributed to olanzapine’s efficacy. Meta-analysis of the dose–response relationships for antipsychotics identified an increasing dose–response efficacy curve for olanzapine, suggestive that higher than licenced doses of olanzapine may be more efficacious. 18
Over the past 20 years, a handful of double-blind trials and open-label studies have indicated that high-dose olanzapine (>20 mg daily) may be an effective therapy in TRS.19,20 However, the use of high-dose olanzapine for this purpose is not well recognised in the wider clinical community. A meta-analysis of randomised controlled trials (RCTs; n = 7) of olanzapine (both standard and high dose) compared with clozapine in TRS identified greater reductions in PANSS positive and negative symptom scores for clozapine compared with olanzapine but with no significant differences in PANSS total symptom scale scores and time to discontinuation between those treated with olanzapine and those treated with clozapine. 21 This review suggested similar effects on total psychotic symptoms in TRS between high-dose olanzapine and clozapine. However, not all of the included RCTs specified cases with TRS defined by inadequate response to at least two antipsychotics and some included treatment-intolerant patients. It is acknowledged that a lack of uniformity in the definition of treatment resistance across studies can make the interpretation of meta-analyses difficult. 22
The aim of this systematic review was to investigate the clinical effects and tolerability of high-dose olanzapine compared with other antipsychotic drugs in the treatment of TRS.
Methods
The protocol of this systematic review is registered in PROSPERO [CRD42022312817] and its details are available at: http://www.crd.york.ac.uk/prospero/
The review was conducted in accordance with the Meta-analyses of Observational Studies in Epidemiology (MOOSE) guidelines 23 and reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement. 24
Search strategy
Literature search
Inclusion criteria:
The search terms are given in Appendix 1.
English language studies.
RCTs (including crossover trials) and non-randomised, non-controlled open-label studies evaluating the efficacy and safety of high-dose olanzapine for TRS.
Adult patients with TRS or related disorders.
Dose range of olanzapine used was >20 mg daily or mean dose was >20 mg/day (high-dose olanzapine).
Duration of medication was at least 2 weeks.
High-dose olanzapine was compared with placebo or other antipsychotics or low-dose olanzapine (20 mg or less daily).
At least 60% of the olanzapine-treated study population received high-dose olanzapine or the mean olanzapine dose was >20 mg daily.
Exclusion criteria:
Participants did not meet criteria for TRS.
Participants were labelled with TRS, but had not received adequate previous antipsychotic trials.
Unclear outcome results.
Participants were prescribed a combination of antipsychotic medications.
Meta-analyses and systematic reviews.
Case reports.
Data extraction
Two reviewers (LG and JR) independently screened all articles against the inclusion criteria. Both reviewers then extracted information from relevant articles independently. Any discordance throughout this process was resolved by arbitration with a third reviewer (JL).
Results
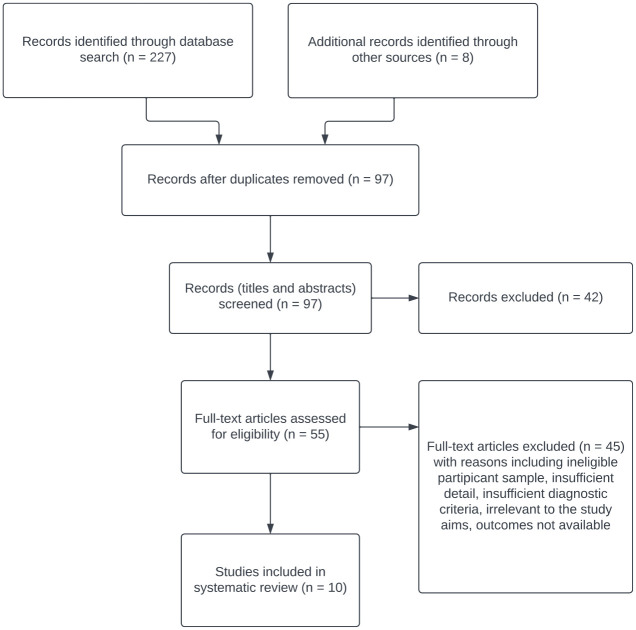
The study selection process, search results and reasons for exclusion are given in Figure 1.
Figure 1.
Flow diagram.
The initial search yielded 227 references. After checking titles and abstracts, 55 full texts were screened. Citations within each article were included as an additional source of references. At the end of the screening process, 10 studies were identified for inclusion. At the full-text review stage, we contacted authors in relation to two studies25,26 but they were not in a position to provide additional information 25 or the data received did not enable the study to be included in the systematic review. 26
Characteristics of included studies
Tables 1 and 2 display an overview of the 10 included studies. Dates of publication ranged from 1998 to 2013. Five studies were conducted in the United States, one each in Canada, Japan, China and Israel, and a further multinational study including participants from 14 countries (Belgium, Denmark, Finland, France, Germany, Italy, Norway, Portugal, South Africa, Spain, Sweden, Switzerland, United Kingdom and Ireland).
Table 1.
RCTs.
| Study | Study type | Subjects recruited | Subjects completed | Study description | Inclusion criteria | Compared drugs (mg/day) | Primary efficacy measures | Definition of clinical response | Response rate | Completion rate | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Conley et al. 27 | Double blind RCT | n = 84 | n = 59 | 8 weeks Fixed dose Inpatients |
TRS | Olanzapine
(25) versus chlorpromazine (1200) with benztropine (4) |
BPRS CGI SANS |
At least 20% reduction in BPRS
score Plus: Endpoint CGI score of 3 or less, or Endpoint BPRS score of 35 or less |
7%/0% | 71%/69% (no significant differences in completion rates) |
Numbers of subjects who showed symptomatic improvement were not significantly different between both groups |
| Tollefson et al. 28 | Double-blind RCT + open-label extension | n = 180 | n = 107 | 18-week double-blind period + 3-year
extension Flexible dose In and outpatients |
TRS | Olanzapine (15 to
25) versus clozapine (200 to 600) |
PANSS BPRS CGI-S |
At least 20% reduction in BPRS
score Plus: Endpoint CGI score of 3 or less, or Endpoint BPRS score of 35 or less |
38.2%/34.5% | 70%/65.6% (rates of discontinuation due to S/E were significantly lower with olanzapine) |
Both groups showed significant within group improvement, with no significant difference between treatments |
| Volavka et al. 29 | Double-blind RCT | n = 157 | n = 91 | 14 weeks Flexible dose Inpatients |
TRS or TR SZA | Olanzapine (10 to
40) versus clozapine (200 to 800) versus risperidone (4 to 16) versus haloperidol (10 to 30) |
PANSS | Compared PANSS scores at endpoint between the four groups | 58% (no significant differences in attrition rates) | Clozapine, olanzapine and risperidone showed significant
improvement, haloperidol did
not; Clozapine + olanzapine showed similar efficacy, superior to risperidone |
|
| Conley et al. 30 | Double-blind crossover | n = 13 | n = 7 | 8 weeks Fixed dose Inpatients |
TRS | Olanzapine
(50) versus clozapine (450) |
BPRS CGI-S |
At least 20% reduction in BPRS score Plus: At least one-point improvement on CGI-S score, or Endpoint BPRS score of 35 or less |
0%/30% | 54%/100% (all subjects who discontinued treatment did so when receiving olanzapine) |
Clozapine demonstrated robust effect size changes compared with olanzapine, in all domains except negative symptoms, where neither improved |
| Meltzer et al. 31 | Double-blind RCT | n = 40 | n = 24 | 6 months Flexible dose Outpatients |
TRS or TR SZA | Olanzapine (25 to
45) versus clozapine (300 to 900) |
PANSS | 20% or greater reduction in PANSS total score | 50%/60% | 60% overall (with higher completion rate favouring olanzapine) |
Significant within group improvement, with no significant difference between groups, except GAF, which favoured clozapine |
| Ermilov et al. 32 | Double-blind RCT | n = 18 | n = 8 | 10 weeks Fixed dose Inpatients |
TRS | Olanzapine (30) versus DSR (3000) |
PANSS | 37.5%/50% (lower completion rate with olanzapine) |
DSR resulted in significantly less improvement than olanzapine |
BPRS, Brief Psychiatric Rating Scale; CGI, Clinical Global Impression; CGI-S, CGI-severity; DSR, D serine; GAF, global assessment of functioning; PANSS, Positive and Negative Syndrome Scale; RCT, randomised control trial; SANS, Scale for the Assessment of Negative Symptoms; SE, standard error; TRS, treatment-resistant schizophrenia; TR SZA, treatment-resistant schizoaffective disorder.
Shaded sections represent unavailable data.
Table 2.
Open-label studies.
| Study | Study type | Subjects recruited | Subjects completed | Study description | Inclusion criteria | Compared drugs (mg/day) | Primary efficacy measures | Definition of clinical response | Response rate | Completion rate | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dursun et al. 33 | Open label | n = 16 | n = 16 | 16 weeks Outpatients |
TRS | Olanzapine (up to 40) | BPRS | At least 20% reduction in BPRS score | 50% | 100% | Significant overall improvement, but difference between high + standard dose not statistically significant |
| Lindenmayer et al. 34 | Open label | n = 43 | n = 27 | 14 weeks Inpatients |
TRS or TR SZA | Olanzapine (up to 40) | PANSS BPRS CGI |
At least 20% reduction in BPRS
score Plus: Endpoint CGI score of 3 or less, or Endpoint BPRS score of 35 or less |
16.7% | 62.8% | Olanzapine was associated with a modest, non-significant improvement |
| Chiu et al. 35 | Open label | n = 51 | n = 43 | 13 weeks Outpatients |
TRS | Olanzapine (up to 25) | PANSS BPRS CGI |
At least 20% reduction in BPRS score Plus: A CGI S score of 3 or less |
39.2% | 84.3% | Significant improvement in positive and negative
symptoms HDO was generally well tolerated |
| Kishi et al. 36 | Open label | n = 13 | n = 4 | 24 weeks Inpatients |
TRS | Olanzapine (up to 40) | PANSS | 10% or greater reduction in PANSS score | 30.8% | 30.8% | Improvements on PANSS scores not statistically significant |
BPRS, Brief Psychiatric Rating Scale; CGI, Clinical Global Impression; HDO, high-dose olanzapine; PANSS, Positive and Negative Syndrome Scale; TRS, treatment-resistant schizophrenia; TR SZA, treatment-resistant schizoaffective disorder.
The sample size in the 10 studies ranged from 13 to 180 subjects. All studies investigated the use of high-dose olanzapine in patients with TRS. Three studies also included patients with treatment-resistant schizoaffective disorder.29,31,34
The objective of 9 of the 10 studies was to assess the efficacy and tolerability of high-dose olanzapine in TRS,27–31,33–36 with the adverse events of the last study 30 reported elsewhere. 37 The tenth study, while producing data pertaining to the same outcomes, had a different primary objective, 32 preliminarily examining the antipsychotic effects of D Serine in TRS, using high-dose olanzapine as a comparator.
Regarding study design, five were RCTs,27–29,31,32 one was a double-blind crossover trial, 30 and four were prospective open-label studies.33–36 The follow-up period ranged from 8 weeks27,30 to 3 years. 28 Eight studies defined TRS by lack of response to at least two prior antipsychotic periods of adequate dose and duration,27,28,30–32,34–36 one study defined TRS by lack of response to at least one typical antipsychotic treatment period of adequate dose and duration 29 and one study defined TRS by lack of response to at least three antipsychotic treatment periods of adequate dose and duration. 33
Efficacy
High-dose olanzapine for TRS – randomised controlled trials
An 8 week double-blind RCT 27 included 84 inpatients with TRS in Maryland, USA. TRS was defined by two or more trials of antipsychotics at daily doses of at least 1000 mg chlorpromazine equivalent and of at least 6 weeks duration with inadequate response. The subjects received either olanzapine at a fixed dose of 25 mg (n = 42) or chlorpromazine at a fixed dose of 1200 mg (n = 42). Fifty-nine patients completed the trial [71% of olanzapine-treated patients (n = 30); 69% of chlorpromazine-treated patients (n = 29)]. There was no significant difference in efficacy between the two groups (7% met response criteria in the olanzapine group and 0% in the chlorpromazine group), with both treatments displaying modest within-group symptomatic improvement. The most common side effects were drowsiness, dry mouth and orthostatic changes, the latter two more frequently observed with chlorpromazine. Overall, high-dose olanzapine was significantly better tolerated than chlorpromazine, with the olanzapine group specifically displaying less motor and cardiovascular side effects.
A double-blind multicentre RCT 28 evaluated the non-inferiority of olanzapine compared with clozapine in TRS. Participants were recruited from 14 countries. TRS was defined by lack of response to at least two antipsychotic trials of 6 weeks duration at a daily dose of at least 500 mg chlorpromazine equivalent. Patients received olanzapine at doses between 15 and 25 mg/day (n = 90; mean daily dose 20.5 mg) or clozapine at doses between 200 and 600 mg/day (n = 90; mean daily dose 303.6 mg). One hundred and seven participants completed the 18-week double-blind period. Both groups showed within-group improvement with similar treatment responses between the groups. Olanzapine proved non-inferior to clozapine with no statistically significant difference in efficacy found between the two treatments. Regarding clozapine dosing, plasma concentrations were not included, and separate data pertaining to sex or smoking status, potential influencers of antipsychotic metabolism, were not reported. Furthermore, a mean clozapine dose of 304 mg/day is unlikely to reach therapeutic concentration range. Fewer olanzapine-treated individuals discontinued treatment due to adverse events. Dry mouth was the only side effect that was reported statistically significantly more often among olanzapine-treated patients.
A double-blind RCT compared high-dose olanzapine with clozapine in patients with TRS or schizoaffective disorder. 31 TRS was conservatively defined by having failed to respond to at least three 6-week trials of antipsychotics at doses over 1000 mg chlorpromazine equivalent daily. Forty outpatients were recruited, 24 of whom completed the study (olanzapine n = 14; clozapine n = 10). Dosing was flexible, between 25 and 45 mg of olanzapine (mean daily dose 33.6 mg) or between 300 and 900 mg of clozapine (mean daily dose 564 mg). At 8 weeks, 18% of the olanzapine group and 7% of the clozapine group met criteria for treatment response, increasing to 50% of the olanzapine group and 60% of the clozapine group at 6 months, by which point a robust improvement in psychopathology was seen in both groups. There was no statistically significant difference in efficacy between the two treatments, with the exception of the global assessment of function scale score, which favoured clozapine. There was no statistically significant difference in trial completion between the groups (clozapine = 48%; olanzapine = 74%). While this study had a small sample size, the sample size was of sufficient power as determined by power analysis conducted by the study authors. Both groups displayed low rates of extrapyramidal side effects (EPSEs).Treatment with high-dose olanzapine was associated with a significantly higher mean weight gain of 15.9 lb, compared with a mean increase of 3.5 lb on clozapine.
In a double-blind RCT, the efficacy and safety of three SGAs (clozapine, olanzapine and risperidone) were compared with one another and with the FGA haloperidol. 29 Patients had a diagnosis of schizophrenia or schizoaffective disorder and were classified as treatment resistant through the following criteria: (1) persistent positive symptoms after at least six contiguous weeks of treatment, presently or documented in the past, with one or more typical antipsychotics at doses ⩾ 600 mg daily in chlorpromazine equivalents; and (2) a poor level of functioning over the past 2 years. This 14-week trial involved 157 inpatients who were randomly assigned to treatment with clozapine, olanzapine, risperidone or haloperidol. Olanzapine was given at doses up to 40 mg daily. In the first 8 weeks of the trial, mean doses achieved were 7.9 mg daily for olanzapine and 401.6 mg for clozapine. In the last 6 weeks, mean doses achieved were 30.4 mg daily for olanzapine and 526.6 mg for clozapine. The results suggested similar efficacy between clozapine and olanzapine, with risperidone and haloperidol less effective. Furthermore, increased doses of olanzapine were associated with additional significant symptomatic improvement; an effect not seen with the other antipsychotic medications, including clozapine. However, the recruited sample size may not have ensured sufficient power to demonstrate differences between four treatment arms. Treatments were generally well tolerated, although clozapine and olanzapine were associated with higher weight gain. The average weight increase with clozapine was 4.2 kg, and with olanzapine was 5.4 kg.
Another RCT in TRS investigated the efficacy of D Serine, which works through N methyl D aspartate (NMDA) receptor function enhancement, with that of high-dose olanzapine. 32 18 inpatients who met criteria for treatment resistance 38 were included in the study. These criteria were (1) persistent positive symptoms, that is, item score ⩾ 4 on at least two of four positive symptom scales on the Brief Psychiatric Rating Scale (BPRS) (2) total BPRS score ⩾ 45 on the 18-item scale and a score of ⩾ 4 on Clinical Global Impressions (CGIs) (3) no period of good social or occupational functioning within the last 5 years (4) at least three periods in the preceding 5 years of treatment with conventional antipsychotics from at least two chemical classes at doses ⩾ 1000 mg/day of chlorpromazine equivalent for 6 weeks, each without significant symptom relief, and failure to improve by at least 20% in total BPRS score. Of note, patients were excluded from the study if they were treated with clozapine. Participants received a fixed dose of either D Serine (monotherapy) 3000 mg (n = 10) or olanzapine 30 mg (n = 8) for 10 weeks. Eight patients completed the trial (D Serine n = 5; olanzapine n = 3) with less symptomatic improvement in the D Serine-treated group compared with high-dose olanzapine, as measured by PANSS total scores. No side effects were noted with D Serine, while two patients withdrew from olanzapine treatment due to EPSEs and drowsiness.
A double-blind crossover trial compared high-dose olanzapine with clozapine in 13 in-patients with TRS. 30 The criteria for treatment resistance were as follows: (1) at least two periods of treatment in the preceding 5 years with an antipsychotic drug (from at least two different chemical classes, excluding haloperidol), at dosages ⩾ 1000 mg/day of chlorpromazine equivalents, for 6 weeks without significant symptomatic relief; (2) no period of good functioning within the past 5 years; and (3) severity of psychopathology indicated by a total score of 45 or more on the BPRS, a CGI severity score of 4 or more, and a score of 4 or more on at least two of the BPRS psychosis items. Of note, patients were not included if they had previously failed clozapine treatment. For the first 8-week period, participants were assigned to olanzapine at a fixed dose of 50 mg (n = 8), or clozapine at a fixed dose of 450 mg (n = 5). For the second 8-week period, treatments were switched and patients were prescribed the alternative treatment at the same fixed doses. Patients were titrated to the fixed dose over the initial 2 weeks. Around 30% of clozapine-treated patients had a 20% or more reduction in BPRS scores compared with 0% of olanzapine-treated patients. It was found that participants initially treated with clozapine had worsening of symptoms when subsequently treated with olanzapine. Similarly, improvements were observed in the second 8 weeks when patients initially treated with olanzapine were switched to clozapine, with nearly half of these responding to clozapine. Regarding attrition rate, the six patients who discontinued treatment did so during the olanzapine phases (three during the first 8 weeks and three during the second 8 weeks), mostly due to clinical deterioration rather than adverse events, while no patients dropped out of the study when receiving clozapine. Clozapine withdrawal symptoms in the study population were not specifically reported. In relation to this study, adverse events were documented elsewhere 37 reporting a mean weight gain of 3.4 kg for the olanzapine group versus 1.2 kg for the clozapine group. The olanzapine group had less pronounced mean changes in glucose, serum triglyceride and total cholesterol levels compared with the clozapine group (olanzapine group = 5% mean increase in triglyceride levels [mean increase of 6.6 mg/dL (SD = 33.1)], compared with clozapine group = 92% [mean increase of 162.8 mg/dL (SD = 258.1)]; olanzapine group = 2% increase in mean total cholesterol levels [mean increase of 4.3 mg/dL (SD = 35.6)], compared with clozapine group = mg/dL 18% [mean increase of 37.6 mg/dL (SD = 41.2)].
High-dose olanzapine for TRS – open-label studies (non-randomised, non-controlled)
An open-label study in Canada assessed high-dose olanzapine in patients with TRS, using flexible dosing up to a maximum of 40 mg daily. 33 A conservative definition of TRS was used: no significant symptomatic improvement after at least three antipsychotic treatments, on daily doses of 1000 mg chlorpromazine equivalents for at least 6 weeks within the preceding 5 years. Sixteen outpatients were recruited via consecutive referrals and commenced on olanzapine, which was increased based on clinical need. All patients completed the trial and at the end (mean daily olanzapine dose 28.1 mg), 50% met criteria for treatment response, as defined by a reduction of 20% or more in BPRS score. Subjects who received doses above 20 mg (n = 11) demonstrated slightly greater improvement. None of the participants discontinued treatment, suggesting that even at increased doses, olanzapine was tolerated reasonably well.
An open-label study in Japan investigated the efficacy and tolerability of high-dose olanzapine among inpatients with TRS. 36 Thirteen patients were administered standard-dose olanzapine for the 2-week pretrial phase. No patients achieved a significant response in this phase and three dropped out, leaving 10 to enter the 24-week trial of high-dose olanzapine. Doses were increased to a maximum of 40 mg based on individual response. Four subjects completed the study with only one meeting response criteria (greater than 40% reduction in PANSS total score). Regarding tolerability, no serious side effects were noted. There were no significant changes in weight or laboratory test results, though mean levels were not reported.
Another open-label study enrolled 51 subjects with TRS from outpatient clinics in Taiwan. 35 Treatment resistance was defined by previous lack of response to at least two different antipsychotics (including clozapine) at daily doses equivalent to 750 mg chlorpromazine during a minimum trial of 4 weeks each, as well as evidence of impaired social functioning for 2 years. All patients were treated with olanzapine at daily doses between 10 and 25 mg for 13 weeks. At the end, significant improvements were seen in both positive and negative symptoms, with 20 of 51 patients meeting criteria for treatment response. Of note, five patients dropped out of the study due to worsening psychotic symptoms, and all five of these had previously not responded to clozapine. One other patient who was a clozapine non-responder completed the study, but did not respond to olanzapine treatment. The most common adverse events were nervousness, insomnia and weight gain. The increase in weight was statistically (though not clinically) significant, with mean bodyweight increasing from 63.9 kg to 66 kg (3.2%). In general, the medication was well tolerated.
A fourth open-label study investigated high-dose olanzapine in 43 in-patients with TRS or schizoaffective disorder. 34 Treatment resistance was defined by (1) at least 6 weeks of continuous treatment with one or more antipsychotics (risperidone or clozapine) at dosages of at least 600 mg of chlorpromazine equivalents; (2) poor level of functioning over the last 2 years; and (3) treatment resistance to risperidone at ⥸ 8 mg daily for at least 6 weeks or clozapine at ⥸ 400 mg daily for at least 14 weeks (and meeting criteria for ultra-treatment resistance). During the study, patients were treated with olanzapine at doses up to 40 mg daily for 14 weeks. In this study, 16.7% of participants met response criteria. In those receiving olanzapine above 20 mg daily, there were significant increases in the PANSS Negative factor change and the PANSS Positive factor change compared with those treated with olanzapine 20 mg or less (PANSS Negative factor mean change on >20 mg olanzapine = 2.0, and on ⩽20 mg olanzapine = 0.0, and PANSS Positive factor mean change on >20 mg olanzapine = 2.0, and on ⩽20 mg olanzapine = 0.1). There was no significant difference in symptom improvement between people who previously did not respond to risperidone and people who previously did not respond to clozapine. Regarding side effects, a significant yet modest increase in weight gain was observed.
Discussion
Therapeutic efficacy of high-dose olanzapine
To our knowledge, this is the first systematic review examining the use of high-dose olanzapine in adequately defined TRS. Our review included 10 studies comprising 615 participants in total. Our review findings, although insufficient to firmly support the use of high-dose olanzapine for TRS, suggest that it may be an efficacious treatment alternative when clozapine cannot be used, and that olanzapine at higher than recommended doses is generally well tolerated.
In the systematic review, four trials compared high-dose olanzapine with clozapine.28–31 Three of these, conducted as double-blind RCTs, found that high-dose olanzapine was equivalent to clozapine.28,29,31 The other, a double-blind crossover trial, found that clozapine was superior. 30 When interpreting these findings, potential flaws in the clozapine comparator procedure in some trials should be considered. Plasma medication concentrations were not reported in these studies, the omission of which may impact on making direct comparisons between the two treatments. Using mean dose as an indicator, it is unlikely that a mean clozapine daily dose of 303.6 mg would reach the therapeutic range. 28 Furthermore, in the crossover trial, 2 patients out of 10 who were set to have a fixed clozapine daily dose of 450 mg had this reduced to 300 mg for clinical reasons. 30 Each phase of this trial was 8 weeks long, and with clozapine known to have an increased effect over time, this may not be comparing like with like. It is possible that the participants who switched from clozapine to high-dose olanzapine could have experienced withdrawal symptoms, which may have had an impact on the perceived efficacy of high-dose olanzapine in this group. 30 However, in a population of clozapine responders, Littrell et al. 39 previously showed that 90% of those who converted from clozapine to olanzapine to avoid side effects showed a successful response to olanzapine, favouring conversion as a treatment strategy. In the four arm RCT, 29 it was reported that for clinical reasons, clozapine needed to be titrated slower than the other antipsychotics, but that this was taken into account in analyses as a potential confounding factor. None of these studies focused on early-episode schizophrenia, with an open-label trial in first-episode schizophrenia showing that 75% of non-responders to two consecutive trials of standard doses of olanzapine and risperidone responded to a trial of clozapine as a third-line treatment. 40
An RCT comparing high-dose olanzapine with chlorpromazine demonstrated equivalence between the two treatments. 27 Of note, in a prospective open-label follow-up study of this RCT, 27 of the high-dose olanzapine non-responders were treated with clozapine. Over an 8-week period, 41% of those who had not responded to olanzapine responded to the subsequent clozapine trial. 41 This important finding demonstrated that clozapine is effective in cases resistant to high-dose olanzapine and at a similar rate to what is expected in TRS. As would be expected, high-dose olanzapine displayed superior efficacy when compared with D Serine, though the primary aim of this RCT was to provide a preliminary evaluation of D Serine monotherapy for psychosis. 32
Of the four non-randomised, non-controlled open-label studies included in our review, three demonstrated a positive response to high-dose olanzapine,33–35 while one detected no significant improvement in symptomatology. 36 Two studies included patients who may be considered ultra-treatment resistant, as some of them had previously not responded to clozapine.34,35 In one of these studies, none of the clozapine non-responders improved with high-dose olanzapine treatment, and the majority of them dropped out of the trial due to worsening psychotic symptoms. 35 In the other study, there was no significant difference in improvement for those who had not responded to clozapine before. 34 Findings from open-label studies provide tentative support for high-dose olanzapine as a treatment option worthy of further exploration, but little support for effectiveness in clozapine non-responders. In contrast to this, a case series by Launer 42 showed that patients who had not responded to previous antipsychotic trials, as well as clozapine, then responded to a trial of olanzapine at 40–60 mg/day, as noted by improved Global Assessment Scale and CGIs Scale scores. This case series was not included in the systematic review due to insufficient available data.
Dose–response curve
The dose–response curve of olanzapine has been studied across broader groups (i.e. treatment-resistant and non-TRS samples). Recent meta-analysis has identified that the olanzapine dose–response efficacy curve may increase at doses beyond the identified 95% effective dose (i.e. 15.8 mg for olanzapine). 18 In a non-TRS population of patients with severe symptoms (n = 599), a dose–response relationship was found with olanzapine 40 mg daily compared with 20 mg and 10 mg daily. 43
Building on this, and specifically in a TRS population, this systematic review identified four RCTs and three open-label studies demonstrating a therapeutic response to high-dose olanzapine.27–29,31,33–35 indicating potential benefits in increasing olanzapine to higher than recommended doses. Regarding the three RCTs that demonstrated equivalence between high-dose olanzapine and clozapine, the average dose of olanzapine in one trial was 20.5 mg, 28 the average dose in another was 30.4 mg, 29 and the dose range in the third was 25–45 mg. 31 In the RCT where clozapine was found to be superior, 30 olanzapine was administered at a fixed dose of 50 mg; therefore, we have not identified a trend across trials for dose–response beyond the defined cut-off for high-dose olanzapine, which was 20 mg or higher daily.
Cognitive function
A feature of our review that merits discussion is the secondary outcome measure of cognitive function investigated in two studies.31,36 Both reported statistically significant improvements in cognition with high-dose olanzapine, including in working memory and motor speed scores. 36 These results were supported by previous findings 44 reporting a significant improvement on overall neuropsychological test performance and specific cognitive tests compared with baseline scores after 3 months of olanzapine treatment at doses between 20 and 40 mg.
Aggressive behaviour
One patient factor predicting the use of high-dose antipsychotics is a history of violence and aggression. 45 The efficacy of high-dose olanzapine for patients with schizophrenia or related disorders presenting with aggression has been studied, with higher doses proving effective for aggressive behaviour and in the symptomatic treatment of agitation. 19
Adverse events
A limiting factor of high-dose olanzapine use is concern regarding tolerability. In the 10 studies included in this review, olanzapine at higher than standard doses was not associated with any serious or fatal events, and was generally well tolerated. Table 3 displays a cumulative prevalence of side effects across trials.
Table 3.
Side effects.
| Side effect | Overall prevalence with HDO (%) | Conley et al. 27 | Tollefson et al. 28 | Lindenmayer et al. 34 | Volavka et al. 29 | Conley et al. 30 | Chiu et al. 35 | Ermilov et al. 32 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HDO (25 mg fixed) |
Control | HDO (20.5 mg average) |
Control | HDO (40 mg max) |
HDO (30.4 mg average) | Control (Clozapine) | Control (Risperidone) | Control (Haloperidol) | HDO (50 mg fixed) |
Control | HDO (23.5 mg average) |
Control (N/A) |
HDO (30 mg fixed) |
Control | ||
| Dry mouth | 48 | 16/42 (38.1%) |
31/42 (73.8%) |
24/89 (27%) |
11/86 (12.8%) |
8/10 (80%) |
2/10 (20%) |
|||||||||
| Drowsiness/lethargy | 44 | 15/42 (35.7%) |
22/42 (52.4%) |
23/89 (25.8%) |
41/86 (47.7%) |
10/10 (100%) |
10/10 (100%) |
1/8 (12.5%) |
0/10 (0%) |
|||||||
| Nausea/vomiting | 36 | 5/42 (11.9%) |
5/42 (11.9%) |
5/90 (5.6%) |
15/90 (16.7%) |
9/10 (90%) |
6/10 (60%) |
|||||||||
| Headache | 33 | 12/42 (28.6%) |
8/42 (19%) |
10/90 (11.1%) |
5/90 (5.6%) |
6/10 (60%) |
4/10 (40%) |
|||||||||
| Dizziness | 27 | 6/42 (14.3%) |
7/42 (16.7%) |
6/89 (6.7%) |
26/86 (30.2%) |
6/10 (60%) |
6/10 (60%) |
|||||||||
| Constipation | 26 | 5/42 (11.9%) |
13/42 (31%) |
6/90 (6.7%) |
17/90 (18.9%) |
6/10 (60%) |
6/10 (60%) |
|||||||||
| Blurred vision | 25 | 4/42 (9.5%) |
5/42 (11.9%) |
4/10 (40%) |
0/10 (0%) |
|||||||||||
| Dyspepsia | 20 | 4/42 (9.5%) |
9/42 (21.4%) |
3/10 (30%) |
7/10 (70%) |
|||||||||||
| Orthostatic changes | 20 | 4/42 (9.5%) |
30/42 (71.4%) |
3/10 (30%) |
1/10 (10%) |
|||||||||||
| Increased weight/appetite | 18 | 6/90 (6.7%) |
6/90 (6.7%) |
4/10 (40%) |
5/10 (50%) |
3/51 (5.9%) | ||||||||||
| EPSEs/tardive dyskinesia | 15 | 12/42 (28.6%) |
21/42 (50%) |
5/89 (5.6%) |
0/86 (0%) |
5/39 (12.8%) |
5/40 (12.5%) |
13/41 (31.7%) |
1/8 (12.5%) |
0/10 (0%) |
||||||
| Increased salivation | 12 | 13/89 (14.6%) |
54/86 (62.8%) |
1/10 (10%) |
8/10 (80%) |
|||||||||||
| Insomnia | 11 | 6/42 (14.3%) |
2/42 (4.8%) |
7/90 (7.8%) |
3/90 (3.3%) |
6/51 (11.8%) | ||||||||||
| Agitation | 11 | 10/90 (11.1%) |
4/90 (4.4%) |
|||||||||||||
| Sweating | 10 | 8/89 (9%) |
19/86 (22.1%) |
1/10 (10%) |
5/10 (50%) |
|||||||||||
| Tachycardia | 9 | 1/42 (2.4%) |
7/42 (16.7%) |
5/90 (5.6%) |
5/90 (5.6%) |
2/10 (20%) |
0/10 (0%) |
|||||||||
| Increased blood glucose levels | 7 | 3/42 (7.1%) |
||||||||||||||
| Gait disturbances | 2 | 1/42 (2.4%) |
15/42 (35.7%) |
|||||||||||||
| Hypotonia | 2 | 2/89 (2.2%) |
9/86 (10.5%) |
|||||||||||||
| Fever | 1 | 1/90 (1.1%) |
5/90 (5.6%) |
|||||||||||||
| Reduced WCC | 1 | 1/90 (1.1%) |
5/90 (5.6%) |
3/40 (7.5%) |
2/41 (4.8%) |
1/37 (2.7%) |
EPSE, extrapyramidal side effect; HDO, high-dose olanzapine; WCC, white blood cell count.
The most prevalent side effect with high-dose olanzapine was dry mouth (48%), which was observed more frequently with high-dose olanzapine than with the comparative agent in two of the three trials in which it was measured,28,30 side effects of the latter trial reported elsewhere. 37 The next most prevalent side effect was drowsiness (44%). Despite this, drowsiness was observed less frequently with high-dose olanzapine than with the comparative agent in most trials27,28,30,37 and was generally manageable.
An overall prevalence of 15% for EPSEs was found with high-dose olanzapine. However, in the RCTs, there was no statistically significant increase in EPSEs associated with high-dose olanzapine use.28,30,31,37 An observational case series (n = 91) found that EPSEs were identified in 27% of inpatients treated with high-dose olanzapine (range 45–160 mg daily), though these were determined to be mild. 46
Given its potential impact on morbidity and mortality, weight gain in response to higher than recommended doses of olanzapine must be prioritised as an indicator of tolerability. In this review, an overall prevalence of 18% of patients experienced weight gain, although the proportion of patients who gained weight was only reported in three included studies,28,30,35,37 each demonstrating a significant association between high-dose olanzapine and increased weight. When compared with clozapine in one controlled trial, there was a significantly greater mean weight increase with high-dose olanzapine compared with clozapine. 31 A significant dose–response relationship between weight gain and high-dose olanzapine was observed in a non-TRS population. 47 An open-label study supported this association in a TRS sample. 48 Among studies included in the systematic review, few reported on changes in serum glucose or lipid concentrations, perhaps one of the most concerning side effects.
In light of the above findings, there remains a need to balance efficacy with the propensity for undesirable effects such as weight gain with elevated doses. 49 However, data from the systematic review generally provide tentative evidence for good tolerability of high-dose olanzapine, in keeping with findings from case series and other open-label studies.25,26,50
Ethical considerations
In some countries, high-dose olanzapine is considered off-label use. Concerns with prescribing medication off-label include the risk of adverse events and possible litigation. Prescribing off-label is not uncommon, but we have a duty of care to ensure patient safety while doing so. In the case of high-dose olanzapine, this should involve regular physical monitoring, taking into account patient age, comorbidities and co-prescribed medication. It should involve informing the patient when a medication is prescribed for off-label use, thorough discussion of risks and benefits and shared decision-making with the patient. When prescribing off-label, it is advisable to continue to base our recommendations on evidence-based data in the areas of efficacy, pharmacokinetics, interactions and tolerability.
Strengths
Strengths of this systematic review were the inclusion of five RCTs and one double-blind crossover trial.27–32 To the best of our knowledge, this is the first systematic review of high-dose olanzapine in TRS to include studies with an adequate definition of TRS and to provide tolerability data. Some, but not all, studies included in our review were large scale and of long duration, up to 3 years, providing efficacy measures beyond acute response.27–29,31 Studies that examined olanzapine at a fixed high-dose added strength to our overall findings.27,30,32 Two RCTs defined treatment resistance as per the Kane criteria31,32,38 and a further three based their definition on the Kane criteria, with clearly explained and validated alterations.27,29,30 Other outcomes, such as cognitive performance, were an informative addition in a number of trials. All information for the systematic review was extracted and cross-checked by three study authors.
Limitations
The literature in this area is sparse and the methodological designs different, with study heterogeneity evident. Due to study heterogeneity, particularly pertaining to study designs and statistical techniques, meta-analysis was not feasible. However, we were able to provide qualitatively important results through a synthesis of findings across trials. The review is notable for the small sample sizes in some included studies,30,32,33,36 with combined total n = 60, mean n = 15, and a range of 13 to 18 participants for these studies. Four of the trials were non-randomised, non-controlled open label, with associated limitations including lack of blinding. However, all used a strict definition of TRS and provided longitudinal results beyond acute response.33–36 Side effects were reported across included studies, and compared with efficacy measures, there was less heterogeneity, allowing for data synthesis of side effect rates to be completed and a prevalence rate calculated. This provides a reasonably robust understanding of the degree and frequency of adverse effects associated with high-dose olanzapine use in RCTs and open-label studies. Indirect factors, including the beneficial effect of increased patient–doctor interaction in the setting of a clinical study, particularly in unblinded open-label studies, should always be taken into account as having the potential to bias reported outcomes. The potential for investigator bias was reported in one of the open-label studies. 33
None of the included studies provided data on plasma olanzapine concentrations, or when clozapine was the comparator, clozapine concentrations. Furthermore, none of the included studies stratified outcomes by moderators of plasma olanzapine (or clozapine) concentrations such as sex, ethnicity or smoking status. The use of plasma concentrations to guide dosing and to mitigate side effect burden is a potentially important component to high-dose olanzapine treatment. Plasma concentrations would also provide a useful indication as to whether optimal clozapine treatment is received, which would be important for making accurate comparisons between clozapine and potential alternatives. Therapeutic drug monitoring should, therefore, be factored into future trials.
None of the included studies provided data on QTc duration, a dose-dependent side effect of olanzapine, with patients on higher doses with putative increased risk of QTc prolongation and its associated complications.
None of the included studies assessed quality of life or patient/carer perception of high-dose olanzapine treatment. This may be accounted for by the lack of more recent studies and should be an included outcome measure in future RCTs.
Some of the trials were industry sponsored, with three RCTs and one open-label study funded by pharmaceutical companies.28,29,32,35 The potential impact of funding bias must be kept in mind when interpreting results across studies. In two of the industry-sponsored trials, treatment intolerant cases were not included,28,29 but this was not reported in the other two studies.31,35 The potential impact of the inclusion of treatment intolerant patients must be considered, in that a mix of TRS and treatment-intolerant cases may provide ambiguous outcomes, potentially obscuring the increased efficacy of a treatment for TRS. In one of the industry-sponsored RCTs, a suboptimal mean clozapine dose of 303.6 mg was attained, potentially precluding an optimal clozapine response at higher therapeutic doses. 28
It is also important to note the challenges of blinding trials that include clozapine. To achieve adequate blinding, weekly monitoring of white blood cells was done in all treatment arms.28,29,31 A potential limitation of this method is that it could lead to an underestimation of the acceptability, retention and efficacy of the comparison treatments because blood testing for those treatments would not represent clinical reality. 51
Notwithstanding the above limitations, our findings suggest that olanzapine, at higher than standard doses, is a safe and effective treatment alternative for TRS where clozapine use is not appropriate.
While this study focused on high-dose olanzapine as a treatment alternative to clozapine, for future research, it may be beneficial to assess the effectiveness and tolerability of other high-dose antipsychotics in TRS, including those that are administered as long-acting injectable antipsychotics, such as paliperidone and aripiprazole, with some evidence to support their use in high doses for the treatment-resistant population. 52
Conclusion
Our findings provide tentative support for the use of high-dose olanzapine in TRS, though the evidence base is modest. Clozapine remains the first-line treatment and the comparative studies have thus far been limited by lack of clozapine optimisation in the protocols. However, olanzapine demonstrates efficacy and tolerability, providing increased consideration for its use at doses above 20 mg/day when clozapine use is contraindicated, is not tolerated or with patient refusal prohibiting its use. It remains clear from this review that the use of high-dose olanzapine in TRS is under researched, with no studies in the past 9 years, thus limiting clinical interpretations from available evidence. Additional studies investigating the efficacy of high-dose olanzapine in TRS are required to overcome present limitations and provide more robust findings. A future RCT of high-dose olanzapine compared with clozapine would be beneficial, with a larger sample size, clearly defined and characterised TRS with clear documentation of previous antipsychotics used, dose and duration and confirmed adherence. Furthermore, a fixed-dose study of standard- and high-dose olanzapine in TRS would be recommended to investigate differential dose response in TRS. Plasma olanzapine concentrations should be measured to correlate measures with efficacy and adverse effects. Careful evaluation of cardiometabolic adverse effects is required including weight gain and it will be important to record QTc intervals on electrocardiograms (ECGs). Studies with higher dosing such as greater than 30 mg/day could be considered for dosing strategy in future RCTs, though with the need to balance potentially improved efficacy with putative dose–response impact on treatment-emergent adverse events.
Acknowledgments
Authors contacted for additional information included:
Dr. Juan Carlos Gómez Perez and Dr. Antonio Ciudad, in reference to an open-label study. 25
Dr. Jean-Marie Batail, in reference to an open-label study. 26
Appendix 1
Searches
PubMed
Search: ((Olanzapine AND (high-dose OR high dose) AND treatment resistant schizophrenia)) OR (Olanzapine[TI] AND (high*-dos*[TI] OR high* dos*[TI]))
= 64 results
Scopus
Search: Olanzapine AND (high-dose OR high dose) AND (treatment-resistant schizophrenia OR treatment resistant schizophrenia)
= 91 results
Google Scholar
Advanced search: Find articles: with all of the words in the title of the article – Olanzapine treatment resistant schizophrenia
= About 72 results
Footnotes
ORCID iD: Louisa Gannon  https://orcid.org/0000-0003-2137-2631
https://orcid.org/0000-0003-2137-2631
Contributor Information
Louisa Gannon, Department of Psychiatry, University College Dublin, Dublin, Ireland; Department of Psychiatry, St. Vincent’s University Hospital, Dublin, Ireland.
John Reynolds, Department of Psychiatry, Mayo University Hospital, Castlebar, Ireland.
Martin Mahon, Department of Psychiatry, Connolly Hospital, Dublin, Ireland.
Fiona Gaughran, National Psychosis Service, South London and Maudsley NHS Foundation Trust, London, UK; Department of Psychosis Studies, Institute of Psychiatry Psychology and Neuroscience, Kings College London, London, UK; National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, UK.
John Lally, Department of Psychiatry, School of Medicine and Medical Science, University College Dublin, Belfield, Dublin, D04 V1W8, Ireland; Department of Psychiatry, Mater Misericordiae University Hospital, Dublin, Ireland; Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), King’s College London, London, UK.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Louisa Gannon: Data curation; Methodology; Project administration; Visualisation; Writing – original draft.
John Reynolds: Data curation; Methodology; Project administration; Supervision; Writing – review & editing.
Martin Mahon: Writing – review & editing.
Fiona Gaughran: Writing – review & editing.
John Lally: Conceptualisation; Data curation; Methodology; Project administration; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Not applicable.
References
- 1.Lally J, Ajnakina O, Di Forti M, et al. Two distinct patterns of treatment resistance: clinical predictors of treatment resistance in first-episode schizophrenia spectrum psychoses. Psychol Med 2016; 46: 3231–3240. [DOI] [PubMed] [Google Scholar]
- 2.Lally J, Gaughran F. Treatment resistant schizophrenia: review and a call to action. Ir J Psychol Med 2019; 36: 279–291. [DOI] [PubMed] [Google Scholar]
- 3.Mizuno Y, McCutcheon RA, Brugger SP, et al. Heterogeneity and efficacy of antipsychotic treatment for schizophrenia with or without treatment resistance: a meta-analysis. Neuropsychopharmacology 2020; 45: 622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siskind D, McCartney L, Goldschlager R, et al. Clozapine v. First- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry 2016; 209: 385–392. [DOI] [PubMed] [Google Scholar]
- 5.Barnes TR, Drake R, Paton C, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: Updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2020; 34: 3–78. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen J, Young C, Ifteni P, et al. Worldwide differences in regulations of clozapine use. CNS Drugs 2016; 30: 149–161. [DOI] [PubMed] [Google Scholar]
- 7.Hasan A, Falkai P, Wobrock T, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry 2012; 13: 318–378. [DOI] [PubMed] [Google Scholar]
- 8.Jablensky A, Castle DJ, Dark F, et al. The 2016 RANZCP guidelines for the management of schizophrenia and related disorders: what’s next. Australas Psychiatry 2017; 25: 600–602. [DOI] [PubMed] [Google Scholar]
- 9.Keepers GA, Fochtmann LJ, Anzia JM, et al. The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry 2020; 177: 868–872. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi H, Takekita Y, Hori H, et al. Pharmacological treatment algorithms for the acute phase, agitation, and maintenance phase of first-episode schizophrenia: Japanese Society of Clinical Neuropsychopharmacology treatment algorithms. Hum Psychopharmacol 2021; 36: e2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Japanese Society of Neuropsychopharmacology. Japanese Society of Neuropsychopharmacology: ‘guideline for pharmacological therapy of schizophrenia’. Neuropsychopharmacol Rep 2021; 41: 266–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flanagan RJ, Lally J, Gee S, et al. Clozapine in the treatment of refractory schizophrenia: a practical guide for healthcare professionals. Br Med Bull 2020; 135: 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 2019; 394: 939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013; 382: 951–962. [DOI] [PubMed] [Google Scholar]
- 15.McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry 2006; 163: 600–610. [DOI] [PubMed] [Google Scholar]
- 16.Samara MT, Dold M, Gianatsi M, et al. Efficacy, acceptability, and tolerability of antipsychotics in treatment-resistant schizophrenia: a network meta-analysis. JAMA Psychiatry 2016; 73: 199–210. [DOI] [PubMed] [Google Scholar]
- 17.Taylor DM. Clozapine for treatment-resistant schizophrenia: still the gold standard. CNS Drugs 2017; 31: 177–180. [DOI] [PubMed] [Google Scholar]
- 18.Leucht S, Crippa A, Siafis S, et al. Dose-response meta-analysis of antipsychotic drugs for acute schizophrenia. Am J Psychiatry 2020; 177: 342–353. [DOI] [PubMed] [Google Scholar]
- 19.Citrome L, Kantrowitz JT. Olanzapine dosing above the licensed range is more efficacious than lower doses: fact or fiction? Expert Rev Neurother 2009; 9: 1045–1058. [DOI] [PubMed] [Google Scholar]
- 20.Botts S, Littrell R, de Leon J. Variables associated with high olanzapine dosing in a state hospital. J Clin Psychiatry 2004; 65: 1138–1143. [DOI] [PubMed] [Google Scholar]
- 21.Souza JS, Kayo M, Tassell I, et al. Efficacy of olanzapine in comparison with clozapine for treatment-resistant schizophrenia: evidence from a systematic review and meta-analyses. CNS Spectr 2013; 18: 82–89. [DOI] [PubMed] [Google Scholar]
- 22.Howes OD, McCutcheon R, Agid O, et al. Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group consensus guidelines on diagnosis and terminology. Am J Psychiatry 2017; 174: 216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting – Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martín J, Gómez JC, García-Bernardo E, et al. Olanzapine in treatment-refractory schizophrenia: results of an open-label study. J Clin Psychiatry 1997; 58: 479–483. [PubMed] [Google Scholar]
- 26.Batail JM, Langrée B, Robert G, et al. Use of very-high-dose olanzapine in treatment-resistant schizophrenia. Schizophr Res 2014; 159: 411–414. [DOI] [PubMed] [Google Scholar]
- 27.Conley RR, Tamminga CA, Bartko JJ, et al. Olanzapine compared with chlorpromazine in treatment-resistant schizophrenia. Am J Psychiatry 1998; 155: 914–920. [DOI] [PubMed] [Google Scholar]
- 28.Tollefson GD, Birkett MA, Kiesler GM, et al. Double-blind comparison of olanzapine versus clozapine in schizophrenic patients clinically eligible for treatment with clozapine. Biol Psychiatry 2001; 49: 52–63. [DOI] [PubMed] [Google Scholar]
- 29.Volavka J, Czobor P, Sheitman B, et al. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry 2002; 159: 255–262. [DOI] [PubMed] [Google Scholar]
- 30.Conley RR, Kelly DL, Richardson CM, et al. The efficacy of high-dose olanzapine versus clozapine in treatment-resistant schizophrenia: a double-blind crossover study. J Clin Psychopharmacol 2003; 23: 668–671. [DOI] [PubMed] [Google Scholar]
- 31.Meltzer HY, Bobo WV, Roy A, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry 2008; 69: 274–285. [DOI] [PubMed] [Google Scholar]
- 32.Ermilov M, Gelfin E, Levin R, et al. A pilot double-blind comparison of d-serine and high-dose olanzapine in treatment-resistant patients with schizophrenia. Schizophr Res 2013; 150: 604–605. [DOI] [PubMed] [Google Scholar]
- 33.Dursun SM, Gardner DM, Bird DC, et al. Olanzapine for patients with treatment-resistant schizophrenia: a naturalistic case-series outcome study. Can J Psychiatry 1999; 44: 701–704. [DOI] [PubMed] [Google Scholar]
- 34.Lindenmayer JP, Volavka J, Lieberman J, et al. Olanzapine for schizophrenia refractory to typical and atypical antipsychotics: an open-label, prospective trial. J Clin Psychopharmacol 2001; 21: 448–453. [DOI] [PubMed] [Google Scholar]
- 35.Chiu NY, Yang YK, Chen PS, et al. Olanzapine in Chinese treatment-resistant patients with schizophrenia: an open-label, prospective trial. Psychiatry Clin Neurosci 2003; 57: 478–484. [DOI] [PubMed] [Google Scholar]
- 36.Kishi T, Suzuki T, Sekiguchi H, et al. Efficacy and tolerability of high dose olanzapine in Japanese patients with treatment-resistant schizophrenia. Asian J Psychiatr 2013; 6: 86–87. [DOI] [PubMed] [Google Scholar]
- 37.Kelly DL, Conley RR, Richardson CM, et al. Adverse effects and laboratory parameters of high-dose olanzapine vs. Ann Clin Psychiatry 2003; 15: 181–186. [DOI] [PubMed] [Google Scholar]
- 38.Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45: 789–796. [DOI] [PubMed] [Google Scholar]
- 39.Littrell KH, Johnson CG, Hilligoss NM, et al. Switching clozapine responders to olanzapine. J Clin Psychiatry 2000; 61: 912–915. [DOI] [PubMed] [Google Scholar]
- 40.Agid O, Arenovich T, Sajeev G, et al. An algorithm-based approach to first-episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry 2011; 72: 1439–1444. [DOI] [PubMed] [Google Scholar]
- 41.Conley RR, Tamminga CA, Kelly DL, et al. Treatment-resistant schizophrenic patients respond to clozapine after olanzapine non-response. Biol Psychiatry 1999; 46: 73–77. [DOI] [PubMed] [Google Scholar]
- 42.Launer M. High dose olanzapine in treatment resistant schizophrenia. Schizophrenia Research 1998; 29: 149–150. [DOI] [PubMed] [Google Scholar]
- 43.Kinon BJ, Volavka J, Stauffer V, et al. Standard and higher dose of olanzapine in patients with schizophrenia or schizoaffective disorder: a randomized, double-blind, fixed-dose study. J Clin Psychopharmacol 2008; 28: 392–400. [DOI] [PubMed] [Google Scholar]
- 44.Smith RC, Infante M, Singh A, et al. The effects of olanzapine on neurocognitive functioning in medication-refractory schizophrenia. Int J Neuropsychopharmacol 2001; 4: 239–250. [DOI] [PubMed] [Google Scholar]
- 45.Sim K, Su HC, Fujii S, et al. High-dose antipsychotic use in schizophrenia: a comparison between the 2001 and 2004 Research on East Asia Psychotropic Prescription (REAP) studies. Br J Clin Pharmacol 2009; 67: 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petersen AB, Andersen SE, Christensen M, et al. Adverse effects associated with high-dose olanzapine therapy in patients admitted to inpatient psychiatric care. Clin Toxicol 2014; 52: 39–43. [DOI] [PubMed] [Google Scholar]
- 47.Kinon BJ, Basson BR, Gilmore JA, et al. Long-term olanzapine treatment: weight change and weight-related health factors in schizophrenia. J Clin Psychiatry 2001; 62: 92–100. [PubMed] [Google Scholar]
- 48.Bronson BD, Lindenmayer JP. Adverse effects of high-dose olanzapine in treatment-refractory schizophrenia. J Clin Psychopharmacol 2000; 20: 382–384. [DOI] [PubMed] [Google Scholar]
- 49.Spertus J, Horvitz-Lennon M, Abing H, et al. Risk of weight gain for specific antipsychotic drugs: a meta-analysis. NPJ Schizophr 2018; 4: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mountjoy CQ, Baldacchino AM, Stubbs JH. British experience with high-dose olanzapine for treatment-refractory schizophrenia. Am J Psychiatry 1999; 156: 158–159. [DOI] [PubMed] [Google Scholar]
- 51.Wohlfarth T, Linszen D, Van Den Brink W. Blinding in clozapine trials: a problem and a potential solution. Int J Methods Psychiatr Res 2009; 18: 185–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernandez-Miranda JJ, Diaz-Fernandez S, Lopez-Munoz F. High-doses of second generation long-acting antipsychotics in the treatment of patients with severe resistant schizophrenia: a six-year mirror-image study. Psychiat Clin Psychopharmacol 2020; 30: 335–345. [Google Scholar]