Abstract

Due to the contamination and global warming problems, it is necessary to search for alternative environmentally friendly energy sources. In this area, hydrogen is a promising alternative. Hydrogen is even more promising, when it is obtained through water electrolysis operated with renewable energy sources. Among the possible devices to perform electrolysis, proton exchange membrane (PEM) electrolyzers appear as the most promising commercial systems for hydrogen production in the coming years. However, their massification is affected by the noble metals used as electrocatalysts in their electrodes, with high commercial value: Pt at the cathode where the hydrogen evolution reaction occurs (HER) and Ru/Ir at the anode where the oxygen evolution reaction (OER) happens. Therefore, to take full advantage of the PEM technology for green H2 production and build up a mature PEM market, it is imperative to search for more abundant, cheaper, and stable catalysts, reaching the highest possible activities at the lowest overpotential with the longest stability under the harsh acidic conditions of a PEM. In the search for new electrocatalysts and considering the predictions of a Trasatti volcano plot, rhenium appears to be a promising candidate for HER in acidic media. At the same time, recent studies provide evidence of its potential as an OER catalyst. However, some of these reports have focused on chemical and photochemical water splitting and have not always considered acidic media. This review summarizes rhenium-based electrocatalysts for water splitting under acidic conditions: i.e., potential candidates as cathode materials. In the various sections, we review the mechanism concepts of electrocatalysis, evaluation methods, and the different rhenium-based materials applied for the HER in acidic media. As rhenium is less common for the OER, we included a section about its use in chemical and photochemical water oxidation and as an electrocatalyst under basic conditions. Finally, concluding remarks and perspectives are given about rhenium for water splitting.
Keywords: rhenium, HER, OER, water splitting, electrocatalysis, PEM, hydrogen, nanostructures
1. Introduction
The quest for alternative fossil fuels has led to the rise of hydrogen. This element is the simplest and most abundant in the universe and, in its molecular form (H2), has higher energy per mass unit than common fuels.1 As an energy vector, it can be used to convert, store, and then release energy, impacting different high-energy demanding sectors, including the chemical industry and climatization. To date, almost all hydrogen is produced from fossil fuels, which is associated with enormous CO2 emissions.2 In this regard, the generation of H2 through electrolysis driven by renewable energies, such as wind and solar power, appears as a possible blueprint for a future energy portfolio,3 leading to sustainable and efficient production of high-purity H2: the green hydrogen.
Regardless of the electrolyte media, a theoretical cell voltage of 1.23 V is required between the anode and cathode to split water into H2 and O2.4 However, the kinetically sluggish two-electron-transfer hydrogen evolution reaction (HER) and four-proton–electron-coupled oxygen evolution reaction (OER) require high overpotentials η, which dramatically lower the performance of water electrolyzers. Therefore, kinetically efficient electrocatalysts are necessary to reduce the energy barrier and overpotentials.5,6
Nowadays, there are three major competitive technologies in terms of water electrolysis (Figure 1).
Figure 1.
Schematic illustration of the three most competitive electrolysis technologies for green hydrogen production depending on the type of electrolyte and operation temperature: (a) alkaline, (b) solid oxide, and (c) PEM.
Alkaline electrolysis is the oldest and most mature technology, already implemented in industrial-scale projects.7 However, it requires a corrosive electrolyte, produces low-pressure gases, and requires a purification stage of the produced H2.8 Solid oxide electrolysis is still at the validation stage but promises high energy efficiency if it is combined with a heat source and a stable power supply.9 It has limitations that must be improved, like a limited lifetime due to repeated thermal cycles and very high operating temperatures (500–900 °C).10 Finally, proton exchange membrane (PEM) electrolyzers are reaching maturity thanks to their compactness and land footprint utilization. Among other advantages, they have a low environmental impact, low maintenance, reliable operation, fast response, low operation temperatures (20–80 °C), high efficiency, ability to generate at high pressure, and allow the production of ultrapure H2 (99.99%) and O2 as a byproduct.11−16 They are currently the most promising commercial systems for sustainable and efficient hydrogen production by 2030.17
Although PEM electrolyzers are technologically advanced, they are not widely deployed at an industrial scale due to the high costs associated with the noble-metal electrocatalysts used to manufacture the membrane electrode assemblies.18,19 Therefore, the search for more efficient, robust, and low-cost HER and OER electrocatalysts has been the subject of exploration for the last decades to realize H2 mass production via electrochemical water splitting (EWS). Highly active electrocatalysts are based on noble metals such as Pt, or Pt and Ru,20 for the cathodic HER and IrO2/RuO2 for the anodic OER.21−23 These catalysts comprise platinum-group metals (PGM) which belong to the rarest materials on earth, with uneven geographical distribution, challenging extracting processes, and high commercial value compared to other metals.24,25 So, to take full advantage of PEM technology for green H2 production and build up a mature market, it is imperative to search for cheaper and more abundant metals and catalysts for both the HER and OER, achieving the lowest overpotentials and highest stabilities possible during operation in acid media.
According to the Sabatier principle,26 an excellent HER catalyst should interact neither too strongly nor too weakly with the adsorbed H* intermediate.27 Among the most crucial inspirations behind the design of new HER catalysts are the so-called volcano plots that correlate the exchange current density with the chemisorption energy of hydrogen on different materials. This trend was first reported for metals by Trasatti in the 1970s,28 with Pt near the apex of the volcano (Figure 2).
Figure 2.

Trasatti’s HER volcano plot relating the activity with the M–H interaction energy. Reprinted with permission from ref (29). Copyright 2017 Springer Nature.
Since then, the interaction of H* with the surface and the correlation with a catalyst’s activity has been pivotal in the roadmap of hydrogen generation.30 Considering the trends of the volcano plot, earth-abundant transition-metal electrocatalysts (TMEs) like Cu, Co, Fe, and Ni have been widely explored as promising candidates for cathodes due to their high electrical conductivity, abundant reserves, and economical prices, but they are mostly used in basic media. Commonly TMEs are combined with anions, as the hybridization of the metal d orbitals with the s and p orbitals of the anion broadens the d band of the parent metal.31 Charge transfer from the metal center to the heteroatom can alter the electronic properties of the metal centers and, thus, the hydrogen bonding energy.32,33 For example, TME phosphides,34−38 sulfides,39−41 nitrides,42 borides,43 and chalcogenides44−46 have shown good HER activity under acidic conditions, comparable to that of the benchmark commercial Pt/C cathode, when comparing the geometrical surface normalized activities. However, TMEs and their anionic derivatives still suffer from low corrosion stability.
Rhenium has attracted researchers in search of new alternatives as electrocatalysts due to its exceptional plasticity, mechanical strength, and corrosion resistance.47 This element was identified for the first time in 1908 by Ogawa in Japan, who named it nipponium. Ogawa incorrectly put the new element in the position of the present technetium (Tc, Z = 43) in the periodic table of chemical elements. In 1925, Noddack, Noddack, and Berg isolated the element with Z = 75 from platinum ores extracted from the Rhine River in Germany. The researchers named the element rhenium, a derivation from the Latin term for the river, Rhenus.48,49 Ogawa’s mistake was corrected when X-ray spectroscopy measurements were made on his nipponium sample before his sudden death in 1930, but the findings were not published.50
The late discovery of rhenium and its complicated initial extraction severely limited its initial study and application. But it gradually gained a vital role in catalysis47 and superalloys.51 Nowadays, the extraction process of rhenium is well established.52 The primary rhenium source is molybdenite (MoS2)—a byproduct of copper mining—and the cleaning and treatment of the molybdenum concentrate. The largest rhenium reserves in the world are located in Chile,53 which harbors half of the global rhenium production. Rhenium has been suggested for the HER but with contradictory results. According to Trasatti’s volcano plot (Figure 2), bulk-state metallic Re should perform similarly to Pt but has been shown experimentally to require high overpotentials (>200–300 mV at 10 mA cmgeo–2, where cmgeo–2 shows the surface unit based on the geometrical surface).54 Moreover, most initial reports that claimed comparable performances of Re and Pt were actually rhenized surfaces, i.e., a mixture of rhenium oxides.55 Then, in 1965 Joncich and co-workers56 studied commercial rhenium wires (99.99% purity) for the HER. The authors obtained an exchange current density of approximately 7.5 × 10–6 A cm–2, 3 orders of magnitude lower than that in the work reported by Pecherskaya and Stender.55
Like HER electrocatalysts, developing materials for the OER with high activity and durability in acidic media significantly impacts PEM device efficiency and cost-effectiveness. PGM-based metal and metal oxide electrocatalysts have been extensively investigated for the OER.57,58 RuOx and IrOx have been used as benchmarks in developing active OER electrocatalysts.59−61 Despite their superior performance, these scarce material costs hamper PEM electrolyzers’ industrial applications.62−65 Moreover, despite their efficiency, RuO2 and IrO2 have low stability, and they cannot avoid an electronic phase transition along with gradual dissolution at higher anodic potentials.62
Inexpensive materials based on TME oxides and hydroxides have emerged as promising catalyst candidates for the OER,66−70 but their drawback is usually poor electroconductivity and low stability under acidic conditions. In contrast, nanostructures of chalcogenides and phosphides have a promising potential for bifunctional electrocatalysts toward the overall EWS. However, most of these studies related to new OER electrocatalysts were obtained in alkaline electrolytes. Expensive metal Ir and Ru oxides are currently the only known electrocatalysts with balanced activity and stability in the acidic environment of PEM electrolyzers.61,71−74 A rarely explored element for the design of an OER electrocatalyst is Re; only a few recent studies have reported the activity of Re-based systems for the OER with enhanced overall electrochemical performance mostly under alkaline conditions. Furthermore, some reports discussed the chemical and photochemical OER activities of Re-based catalysts.
This review summarizes the most critical advances for rhenium-based HER electrocatalysts, with a focus on acid media, as well as rhenium-based OER electrocatalysts, which mainly have been studied under basic conditions. The mechanisms behind the HER and OER are briefly summarized, along with the most common experimental techniques for electrocatalyst characterization (Section 2). To date, in the case of the HER, different material strategies have been explored, such as alloying and nanostructuring (Sections 3–6). Section 7 summarizes the few reports related to rhenium and OER. Since the number of publications is very low regarding OER, we included reports related to chemical, photochemical, and electrochemical water oxidation. Finally, a brief discussion is presented regarding the importance of computational tools in advancing rhenium-based electrocatalysts (Section 8). All reported potentials are referred to the reversible hydrogen electrode (RHE), while the overpotentials (η10), if not mentioned otherwise, were taken at a current density of 10 mA cmgeo–2. Overpotential values at current densities different than 10 mA cmgeo–2, for example, 2 mA·cmgeo–2, are identified with the specific current density value as a subscript, i.e., η2. Whenever possible, a table summarizes the properties of the modified electrodes, such as overpotential, mass activity, and mass loading, at the end of each section.
2. Mechanisms and Evaluation Approaches for HER and OER Catalysts
A detailed analysis of the HER and OER mechanisms is necessary to understand and improve the design of PEM electrolyzers. The HER half-reaction (2H+ + 2e– → H2) in acidic media proceeds by a proton discharge step, known as the Volmer reaction, initiated by the formation of adsorbed hydrogen intermediates (H*) on the electrode surface via the reduction of protons.75−78 After the formation of H*, H2(g) is generated by two possible mechanisms: the Tafel step (chemical desorption), the Heyrovsky step (electrochemical desorption), or both. The Tafel reaction is the rate-determining step when the surface coverage is high, while the Heyrovsky path needs the reaction of the adsorbed H* intermediates to generate hydrogen because of the low surface coverage of H* (Figure 3a). An analysis of the Tafel slope can help elucidate the process’s kinetics.79,80
Figure 3.

(a) Mechanism of hydrogen evolution on the surface of an electrode in acidic solutions. (b) OER mechanism under acid conditions. The oxygen evolution proceeds by the generation of a peroxide (M–OOH) intermediate (black line). The green line represents another route for the direct formation of O2 by the interaction of M–O oxo intermediates.
The OER half-reaction is more energy-consuming than the HER half-reaction, since four-proton coupled electron transfers are necessary. The most accepted OER mechanism involves an electrochemical oxidation pathway, as Figure 3b depicts.66,69,81−83 Under acid electrolyte conditions, the first step consists of the electrode surface (M) and the oxidation of H2O to give M–OH*. Then, M–OH* transforms into MO* after removing the proton and electron. MO* may produce O2 by the reaction of two MO* species, with a high thermodynamic energy barrier,11,12,84 or by the conversion of MO* into MOOH* species via one-electron oxidation, reacting with H2O under acidic conditions. Finally, another one-electron-transfer process generates O2 and the initial active site (M),13,85 closing the continuous cycle.
Activity, efficiency, and stability are the primary parameters for evaluating a catalyst. In the case of the HER and OER electrocatalysts, the often-used activity indicators are overpotential at a specific current, exchange current density, and turnover frequency (TOF); these will be explained in Section 2.1. Section 2.2 will discuss measurement methods for the electrochemically active surface area of the electrodes. The charge efficiency of electrochemical HER or OER is identified by Faradaic efficiency; we will discuss this specification in Section 2.3. Finally, Section 2.4 is dedicated to the stability evaluations of HER and OER catalysts.
2.1. Activity Descriptors
The thermodynamic potential of a redox reaction (E) is calculated by the Nernst equation (eq 1), where R is the molar gas constant, T is the temperature, F is the Faraday constant, n is the number of electrons transferred during the redox reaction, ar and ao are the activities of reduced and oxidized species, respectively, and m and k are their stoichiometric coefficients. Finally, E° is the standard redox potential under standard conditions (298.15 K and 1 atm).86
| 1 |
Hence, considering the OER and HER according to eqs 2 and 3, respectively, the thermodynamic potentials of these two half-reactions are related to the partial pressure of O2 or H2 and the pH of the solution. Therefore, to maintain the equilibrium potential at standard values (e.g., 0 V vs RHE for the HER and 1.23 V vs RHE for the OER) and ensure accurate activity measurements, the electrochemical performance of the catalysts should be determined in O2- or H2-saturated electrolytes (O2 in the case of the OER and H2 for the HER).87 However, to determine the Faradaic efficiency of the reactions (see section 2.3), the generated gases should be probed in inert-gas-saturated electrolytes. Consequently, the results obtained from the two experiments may not be equal. Furthermore, due to the pH dependence of the HER and OER, buffer solutions should be used to avoid pH shifts during the experiment.
| 2 |
| 3 |
In addition to the thermodynamic potential of a reaction, an extra voltage is required to overcome various kinetic barriers and drive appreciable currents. This additional potential is referred to as the overpotential.
In solar-driven water splitting, the overpotential necessary to generate a current density of 10 mA per 1 cm2 of the geometrical area of an electrode (cm2geo) is the most used benchmark value, equating to ∼12% efficiency for the photoelectrochemical water splitting.88 However, comparing activities only based on the geometrical surface area of the electrodes is not an acceptable assessment criterion, underlined by the effect of the catalyst’s mass loading or increased active surface area on the geometrical-normalized current density.89 Therefore, the specific activity of the catalysts using the real active surface area should be reported to overcome these issues. Methods to measure the electrochemically active surface area of the electrodes will be discussed in section 2.2.
In addition to the specific activity of a catalyst using its actual surface area, the activity (in other words, current density) normalized by the mass, i.e., the mass activity, is used to benchmark the performance. In this metric, the collected current in mA cm–2geo is divided by the mass loading of the catalyst in mgcatalyst cm–2geo. The problem with this metric is that one cannot guarantee that all of the active sites in the loaded catalyst are directly engaged in the reaction. The observed current will, in any case, only relate to active sites directly exposed to the electrolyte solution and involved in the electrochemical reaction. Furthermore, the catalyst’s accurate mass could drop due to electrode detachment/leaching throughout the operation. Accordingly, utilizing the loading measured before the reaction to normalize the current response across the whole reaction time is not ideal and is an approximation.
The exchange current (j0) is another primary assessment criterion of the electrochemical performance of water-splitting electrocatalysts. j0 is defined as the current that flows across the catalytic interface at the equilibrium potential of the target reaction, acquired by extrapolating the Tafel plot’s linear segment.90 The Tafel plot depicts the relationship between logarithmic j and η, and the linear part of the plot can be fitted to eq 4.
| 4 |
Accordingly, careful data acquisition and calculation of the Tafel plot is a critical step in correctly extracting j0. Dynamic polarization curves obtained from linear sweep voltammetry (LSV) or cyclic voltammetry (CV) techniques are the most widely spread methods to establish Tafel plots. However, these techniques do not represent a steady state and also involve double-layer capacitance. Therefore, to get a reliable j0 value from a Tafel plot, one should use potentiostatic/galvanostatic techniques as steady-state representing methods.91 Last but not least, j0 should be investigated based on the real electrochemically active surface area of the catalysts to display the intrinsic activity of the catalyst rather than apparent-activity-representative geometrical current density.
Although it is often underused due to its challenging accurate calculations, the most scientifically interesting activity metric is the turnover frequency (TOF), corresponding to the number of produced molecules (H2 in the case of the HER and O2 for the OER) evolved per time unit per number of participating catalytic sites at a specific overpotential.92 However, determining the exact number of active sites is difficult. In addition, applying geometrical current density decreases the accuracy of the calculated TOF, making a precise estimation of the catalyst’s electrochemically active surface area (ECSA) highly sought after.90,92 The following section will discuss the most frequently used techniques in determining the ECSA.
Among all the activity parameters, overpotential at a fixed current density (η10), the Tafel slope, and the catalyst loading are considered fundamental indicators of catalytic activity and can be used as a comparison criteria. Other activity parameters can also be determined, such as exchange current density, turnover frequency, and mass activity. However, a direct comparison with these parameters is not straightforward due to the catalysts’ differences in morphology, topography, and particle sizes.
2.2. Catalyst’s Surface Area Estimation
Generally, the most frequently applied techniques to measure the active surface area of electrocatalysts are as follows.87,90,93,94
-
(i)
hydrogen underpotential deposition (HUPD) (highly specific in applicable cases)
-
(ii)
CO stripping (highly specific in applicable cases)
-
(iii)
measuring nonfaradaic double-layer capacitance (Cdl) (nonspecific, always applicable)
-
(iv)
gas physisorption analysis of powder catalysts using the Brunauer–Emmett–Teller (BET) method
Each technique has advantages and ambiguities, addressed in the following paragraphs.
(i) HUPD. In this method, voltammetry peaks of the submonolayer H atom’s adsorption and desorption at the catalyst surface are measured. After eliminating the double-layer currents, the HUPD area is integrated with both positive and negative scan directions and averaged.93 Finally, the resultant Coulombic charge is translated to the ECSA, using the charge associated with a monolayer of hydrogen on the metallic electrode surface per unit surface area (e.g., 210 μC cm–2 for Pt).93,95 The experiment must be performed in an Ar-saturated solution with constant Ar delivery during the measurement to remove dissolved O2 and prevent overlapping of the oxygen reduction reaction (ORR) with the ECSA estimation. This approach has been applied widely for pure metal electrocatalysts such as Ir, Rh, and Pt, as well as their alloys.96,97 However, alloying may drastically modify the chemisorption of atomic hydrogen, resulting in a considerable decrease of adsorbed hydrogen and increasing ambiguities in the estimated ECSA.98 It is worth noting that even the surface structure of the pure metallic electrodes could cause inaccuracy in a HUPD-derived ECSA.87 For example, remarkable differences were detected in the apparent charge for H-desorption on pristine Pt surfaces, ranging from 176 μC cm–2disk on polycrystalline Pt to 220 μC cm–2disk on Pt (533).87
(ii) CO stripping voltammetry. In this protocol, the Coulombic charge of oxidative removal of a submonolayer of CO from the electrode surface is determined (eq 5).87,93,99 Therefore, the ability of the surface under study toward CO oxidation is the primary condition in using this technique.
| 5 |
To this end, after degassing the solution using Ar, the catalyst surface adsorbs a submonolayer of CO by saturating the electrolyte with CO and removing the excess unadsorbed CO by subsequent Ar bubbling.100 Then, the Coulombic charge for stripping the CO monolayer is calculated by voltammetry and converted to the surface area by assuming a specific charge, e.g., 420 μC cm–2Pt for Pt.101 It is essential to consider that alloying can cause ambiguity in the obtained ECSA. This error occurs due to the different binding strengths of CO on the alloy surface than on the pure metal, making the alloy’s specific charge different from the values obtained for the pure metal.102
(iii) Non-Faradaic electrochemical double-layer capacitance. According to eq 6, non-Faradaic double-layer capacitance (Cdl in F cm–2) can be obtained from the linear relationship of i versus v, where i (A cm–2) and v (V s–1) are the current and scan rate, respectively.103,104
| 6 |
Cdl is then converted to the ECSA using a specific capacitance (Cs in F cm–2 for a standard with 1 cm2 of real surface area) (eq 7), which can be the origin of the inaccuracy of the estimated ECSA (cm2real) since the exact specific capacitance is usually unknown and material-specific.103
| 7 |
Furthermore, the possible contribution of current generated from ion transfer processes or adsorption of ions on the electrode surface in Cdl can amplify the errors in the approximated surface area.105,106 These phenomena are a significant error source in metal oxide and metal chalcogenide Cdl calculations.105,106 Moreover, in the case of arrays or composites, species that are not directly involved in the catalytic reaction could contribute to the total Cdl, hence interfering with the estimated ECSA.
(iv) Gas sorption methods. BET theory107 based on the physisorption of gas molecules (such as N2) on a solid surface is commonly used to determine the specific surface area of powder materials. However, in the case of a grown film on a substrate, this method may not be applicable, since the material must be scratched from the substrate surface, which requires fabricating many electrodes to provide a sufficient amount of powder. Additionally, scratching may change the real surface area.
It is relevant to state that the method determines the total specific surface area; however, in some cases, not all of the gas sorption sites may be active toward the electrochemical reaction. On the other hand, only the species in contact with the electrolyte may be electrochemically active; hence, the total specific surface area is not ideal for an intrinsic electrochemical activity evaluation.
2.3. Faradaic Efficiency
In an electrochemical reaction, any side reaction with a thermodynamic potential with an absolute value less than the voltage under study can potentially contribute to the collected current. For example, easily oxidizable carbon components can undergo an oxidative carbon corrosion reaction at OER-sufficient overpotentials.108 Therefore, the direct correlation of the acquired current to the Faradaic efficiency of the target reaction is not always given and should be avoided without further investigation of the formed products. Consequently, detecting the actual amount of the product and reporting the Faradaic efficiency of the OER or HER (eq 8)109 at the recorded overpotential are critical steps in evaluating an electrocatalyst’s performance. Meanwhile, assessing the reaction’s TOF using the acquired current without considering the Faradaic efficiency could conclude a value far from the realistic TOF.92
| 8 |
n(experimental) is the experimentally detected amount of the product gas, and n(theoretical) attributes the theoretical amount of the generated gas (eq 9):109
| 9 |
Q is the number of total charges that pass during the electrochemical reaction, n is the number of electrons involved in the reaction, and F is the Faraday constant.
2.4. Stability
The long-term stability of a water-splitting catalyst under an operational environment is a precondition for its future application. For both HER and OER catalysts, long-term stability is widely measured by subjecting the catalyst to a chronoamperometric (CA) or chronopotentiometric (CP) experiment for a few hours. Furthermore, subjecting the catalyst to several cycles of CV is another technique to examine its robustness. These measurements can be conducted in a rotating-disk-electrode (RDE) configuration, and changes in the acquired potential (CP) or current (CA and CV) are correlated to the catalyst instability.110 However, it has been revealed that even an RDE cannot prevent the active sites’ shielding by generating gas bubbles and subsequently decreasing the electrochemical response of the system, which is indeed a measurement artifact rather than catalyst degradation.110,111
In addition to assessing a catalyst’s electrochemical response, identifying any corrosion products in the electrolyte using proper methods, such as inductively coupled plasma mass spectrometry (ICP-MS), is critical. Catalyst corrosion and dynamic surface reconstructions could even elevate catalyst activity by generating more active species or creating a rougher surface with more active sites exposed to the electrolyte. Therefore, the aging experiments and postcatalytic characterizations shed light not only on the stability of a catalyst but also on understanding real active sites for a rational catalyst design. Despite the current debate on surface reconstruction under OER conditions, this process under HER settings has been less often explored, including in the Re-based HER community.112,113
The typical three-electrode cells are currently the predominant approach for testing the activity and stability of catalysts, and PEM systems are seldom utilized to evaluate catalysts in contemporary studies. This could be because membrane electrode assembly investigations require gram-grade catalyst powder and take a long time to fabricate. Meanwhile, the lengthy electrochemical testing time (often several days) makes the method highly time-consuming.114 However, standard three-electrode experiments are dissimilar to the industrial exchange membrane electrolyzers. As a result, future research should concentrate on developing improved membrane electrodes and non-noble-metal catalysts and test them at the PEM level to represent potential industrial applications.
3. Rhenium Bulk Substrates Used in Early Electrochemical Studies
Nowadays, most of the studies regarding rhenium are related to bulk surfaces of solid commercial electrodes54,115−118 or rhenium surfaces obtained electrochemically on different substrates, such as Pt,119−121 Au,119 Cu,122 ITO,123 and Si.124,125 The first result of the application of a rhenium electrode for HER was reported in 1975 by Miles and co-workers115,116 that described higher catalytic activity than Os, Ru, and Ni and lower activity than Pd, Pt, Rh, and Ir in 0.1 M H2SO4 with an HER overpotential (ηHER) value of 544 mV at η2, respectively. It was not until 2016 that Garcia–Garcia et al.54 reported an onset potential for H2 of 350 mV, with an overpotential of 470 mV at η2, being the first promising data on the catalytic activity of Re in an acidic medium (0.5 M H2SO4). The same group117 concluded that Re0 follows the Sabatier principle and suggested that HER follows the Volmer–Heyrovsky mechanism, with a Tafel slope of ∼67 mV dec–1. Unfortunately, no further reports on bulk metallic Re have been published to date.
The electrosynthesis of metallic rhenium on a working electrode has been very complex, mainly because there are several reduction processes to obtain Re0 from the most common precursor ReO4–, generating a mixture of rhenium oxides, mainly ReO2 and ReO3.122−124,126 These oxides have also displayed interesting catalytic properties. However, it has been suggested that they act mainly as proton exchange coatings; thus, the registered catalytic properties correspond to the working electrode, Pt.127 Additionally, Re is one of the hardest metals to electrodeposit due to its low overpotential, causing a competition reaction with the HER.117,119,121,124 This phenomenon has also been described by Muñoz and co-workers,125 who studied the nucleation and growth mechanism of metallic Re grown on Si(100) with corrected equations of the theoretical models considering the evolution of hydrogen during the nucleation and growth of Re.
It has been described that the use of high concentrations of the precursor ion ReO4– promotes the formation of rhenium oxide.123,128 Cao et al. reported obtaining Re0 without rhenium oxides using low concentrations of ReO4– and high concentrations of H2SO4.122 To deposit metallic rhenium from solutions with high concentrations of ReO4– it is necessary to add high concentrations of salts, like sodium sulfate, in addition to H2SO4. The salt ions displace the HER process to a more negative potential due to the solvation effect of the ions in the solution.129 Furthermore, the catalytic properties of rhenium-based materials—Re0 and rhenium oxides—have not been thoroughly studied121 and need to be further explored.
4. Rhenium Chalcogenides, Phosphides, and Borides
4.1. Sulfides
One of the most studied next-generation transition-metal dichalcogenides (TMDs) is molybdenum disulfide (MoS2), with a two-dimensional (2D) hexagonal layered structure and one of the most low-cost and most abundant dichalcogenides on Earth.130,131 Its structure is not catalytically homogeneous; the edges are the most active sites toward the HER, while the basal planes are inert.132,133 Chemical methods have improved this property by incorporating a percentage of rhenium. Chhetri’s research group134 used LSV, CV, and electrochemical impedance spectroscopy (EIS) techniques on MoS2 electrocatalysts in fullerene structures (IF-MoS2) and rhenium-modified MoS2-fullerene composites (IF-RexMo1–xS2). The results demonstrated that a modification with Re as low as 100 ppm in IF-MoS2 decreased the overpotential by 60–90 mV compared to IF-MoS2 and increased the electrocatalytic activity by 60 times compared to the MoS2 with a mass loading of 0.707 mg cm–2. Consequently, the combined synergistic effect of Re doping and the fullerene structure not only changes the intrinsic nature of the MoS2 but also increases its reactivity, possibly by modifying the electronic environment due to the oxophilicity of Re. Yang et al.133 reported another strategy for MoS2 using its normally inert basal planes of the 2D layers of the 2H phase. The authors incorporated a certain percentage of Re by chemical methods, transforming the material into a stable T-phase tetragonal structure. Finally, the following decreasing overpotentials were obtained for solid solution samples with increasing Re content of the type RexMo1–xS2: Re0.04Mo0.96S2 (382 mV), Re0.55Mo0.45S2 (147 mV), and Re0.75Mo0.25S2 (365 mV), with a mass loading of 0.285 mg cm–2 on a glassy-carbon electrode. In addition, the authors included four control samples, Pt (61 mV), pure 2H-MoS2 (475 mV), distorted-tetragonal rhenium sulfide (ReS2, 438 mV), and 1T-MoS2 intercalated with lithium (216 mV), and compared their respective Tafel slopes with the most active Re0.55Mo0.45S2 sample, resulting in 22, 134, 200, 77, and 56 mV dec–1, respectively. This study demonstrated that a Re0.04Mo0.96S2 alloy/solid solution could have superior performance to MoS2, being a promising catalyst to replace Pt in an acidic medium.
Another study published in 2019 tested Re-doped MoS2 flower-like microspheres synthesized by a hydrothermal protocol. The new material exhibited lower charge transfer resistance than MoS2, decreasing the η10 overpotential from 326 to 210 mV when considering a loading of 14.7% Re in acidic media.130 Then, in 2020 Kwak and co-workers131 synthesized Re1–xMoxS2 in various compositions and evaluated the electrocatalytic performance of HER by LSV in a typical three-electrode configuration at pH 0. The authors concluded that Re0.5Mo0.5S2 had the best electrocatalytic activity in all studied samples, obtaining an η10 overpotential of 98 mV and a Tafel slope of 54 mV dec–1 in 0.5 M H2SO4, with a mass loading of 0.39 mg cm–2. In contrast, ReS2 and MoS2 (211 and 187 mV) were less close to the value of the commercial 20 wt % Pt/C catalyst (27 mV and 30 mV dec–1).
ReS2 has been recognized as a next-generation transition-metal dichalcogenide material for HER high-efficiency electrocatalysis.135−137 In 2016, Wang et al.138 used LSV to compare the catalytic properties of ReS2-modified glassy-carbon electrodes with ReS2 and MoS2, obtaining η10 overpotentials of 453 and 336 mV, respectively. At the same time, Gao and co-workers139 synthesized via chemical vapor deposition (CVD) ReS2 nanosheets with vertical orientation perpendicular to the growth substrate (Au foil). The HER was evaluated in an acid medium (0.5 M H2SO4), reporting a Tafel slope of 85 mV dec–1 and an overpotential lower than 100 mV but with a very high mass loading of 3.3 mg cm–2. In 2017 Zhao et al.140 compared the HER electrocatalysis of nanolayers and bulk ReS2 and MoS2 prepared by a β-cyclodextrin-assisted aqueous exfoliation method. In acid media, the systems presented the following η10 overpotentials: MoS2 nanosheets 277 mV, ReS2 nanosheets 498 mV, bulk MoS2 586 mV, and bulk ReS2 639 mV. In turn, the exfoliated nanolayers had lower Tafel slopes than the bulk crystals, with values of 101, 136, 194, and 161 mV dec–1, respectively. Also, exfoliated ReS2 was synthesized and tested in an acidic medium. The catalysts exhibited high chemical stability toward oxidation even after the exfoliation. The better performance strongly depended on electrochemical pretreatment at a high reductive potential (as high as −2 V vs RHE). The overpotential toward HER was 530 mV with a Tafel slope of 115 mV dec–1.141
In 2020 Pang and co-workers142 applied metallic Re and ReS2 nanosheets supported on carbon cloth (CC) by using a heat treatment at 700 °C, using either H2 (Re/ReS2-7H/CC) or N2 for the calcination process (ReS2-7N/CC). The sample obtained with H2 (Re/ReS2-H) presented a lower overpotential of 42 mV in an acid medium (0.5 M H2SO4) and a small Tafel slope of 36 mV dec–1 (at a catalyst mass loading of 0.378 mg cm–2), even lower than that Pt foil (52 mV dec–1), and remarkable stability (Figure 4). The new catalyst had superior electrocatalytic performance compared to the commercial 20 wt % Pt/C electrode.
Figure 4.
(a) High-resolution TEM images of Re/ReS2-7H/CC. The lattice fringes of 0.20 (b) and 0.21 nm (c) are ascribed to the (006) plane of ReS2 and (101) plane of Re, respectively, concluding that H2 treatment can partially reduce ReS2 to metallic Re. (d) LSV plots, (e) Tafel plots, and (f) chronopotentiometric curve of Re/ReS2-7H/CC with a constant current density of 10 mA cm–2 for 100 h (without iR correction). Adapted with permission from ref (142). Copyright 2020 John Wiley and Sons.
A novel experimental work was proposed by Huang et al.143 for the fabrication of ReS2 with high crystallinity through CVD using a carbon structure derived from a sylvestris wood sheet (CSW) as a support to substrate working electrode, which was carbonized at high temperature. In the first instance, the ReS2/CSW electrode was annealed at different temperatures (700, 750, and 800 °C), and their catalytic activity was evaluated by LSV in 0.5 M H2SO4 electrolyte. It was determined that ReS2/CSW-750 °C exhibited the best electrochemical performance with an overpotential η10 of 260 mV and a Tafel slope of ∼189 mV dec–1. According to the authors, this type of Re- and wood-based electrode design could be very interesting for green hydrogen production since wood is abundant on earth, environmentally friendly, and renewable.
Two-dimensional ReS2 has aroused immense interest as an electrocatalyst for the HER. Atomic engineering has been proposed as a new perspective to improve the catalytic performance of ReS2 by modifying the 2D electronic structure using strain engineering and intercalated atoms. Thus, monolayers of TMDs have been explored to optimize active electronic states through intrinsic charge engineering.144 The authors modified ReS2 by this technique, achieving a lower overpotential (147 mV) than for unmodified ReS2 (223 mV) and MoS2 (191 mV). Also, the Tafel slope decreased to 82 mV dec–1 compared to ReS2 without modification, indicating that active Re–Re bonds around vacancies could optimize S–H bonding. Another study evaluated the electrodeposition of ReS2 on 2D- and 3D-printed carbon electrodes.145 For these electrodes, the η10 overpotentials and Tafel slopes were 413 mV and 195 mV dec–1 for 2D-ReS2 and 288 mV at and 147 mV dec–1 for 3D-ReS2, respectively, with a catalyst mass loading of around 1.7 mg cm–2.
Another way to increase the stability and performance of electrocatalysts consists of adding moderate quantities of doping atoms. In 2021, Wang et al.146 reported an electrode material based on ultrathin Mo-doped ReS2 nanolayers assembled on carbon fabric decorated with carbon nanowire arrays (Mo/ReS2@CA/CC), showing an HER η10 overpotential in an acidic medium (0.5 M H2SO4) of 101 mV and a Tafel slope of 40 mV dec–1, with a mass loading of 2.1 mg cm–2. In another study, Liu and co-workers147 synthesized a hierarchical F-doped ReS2 structure composed of ultrathin few-layer nanosheets by a fluorination treatment. According to the authors, this procedure creates vacancies in the basal planes of ReS2, exposing more in-plane active sites and establishing ion transfer channels. At the same time, F-doping enhances the activity of the catalytic sites of ReS2 and accelerates electron transport. The authors concluded that the new material (with 0.34 mg cm–2 mass loading) optimizes the catalytic efficiency, achieving an η10 overpotential of 142 mV, a Tafel slope of 64 mV dec–1, and enhanced stability due to synergistic effects. Fe doping of ReS2 has also been reported to produce superior electrocatalytic activity toward HER, reducing the Tafel slope to 63 mV dec–1 compared to that of ReS2 of 87 mV dec–1.148 Xu and co-workers149 reported a one-step hydrothermal method to synthesize sub-50 nm hierarchical Mo-doped ReS2 nanolayers with numerous hierarchical, defect-rich, few-layered nanospheres and diameters below 50 nm. Electrochemical measurements in an acid medium demonstrated that a 10% Mo-ReS2 catalyst with a mass loading of 0.285 mg cm–2 presented an overpotential of 81 mV, a Tafel slope of 62 mV dec–1, and 50 h stability.
Chalcogenide TMDs and graphene and their oxides have been combined recently due to their attractive properties. Gao et al.150 synthesized 2D ReS2 semiconducting nanosheets of a few layers directly on reduced graphene oxide (rGO) by a hydrothermal method and compared them with ReS2 nanosheets. The ReS2/rGO hybrid demonstrated enhanced electrocatalytic activity for the HER in acidic media because rGO possesses highly conductive and porous networks which allow electrolyte infiltration, efficient charge transfer, and provide an active edge site, resulting in a Tafel slope of 107 mV dec–1, lower than that of ReS2 (153 mV dec–1, with a catalyst mass loading of 0.282 mg cm–2). The new electrocatalyst presented a higher current density of 5.2 mA cm–2 at 250 mV (vs RHE) compared to ReS2, which showed a value of 3.1 mA cm–2 at the same potential. Another study reported the synthesis of ultrasmall ReS2 nanoparticles hybridized with reduced graphene oxide (ReS2/rGO).151 ReS2 exhibited many catalytically active sites responsible for the adsorption processes of hydrogen atoms. At the same time, rGO allows for increasing electrical conductivity. The nanocomposite was evaluated as an HER electrocatalyst in acid media, recording a Tafel slope of 67 mV dec–1 and an overpotential of 148 mV at one of the lowest mass loading values (0.13 mg cm–2) and highest mass activities (Table 1) reported for sulfide-based rhenium catalysts. In addition, the study at different pH values demonstrated higher performance in acid conditions due to faster HER kinetics and better coverage of the catalyst surface. Feng and co-workers152 developed vortex flow chemical vapor deposition (VFCVD) to synthesize vertical arrays of ReS2/ReO2 on a flexible graphene-polyimide film (G-PI), concluding that the G-PI has excellent mechanical and electrical properties as well as good conductivity. The flexible substrate can be used in a wide temperature range from −200 to +300 °C under strongly acidic conditions and with good corrosion resistance. The HER electrocatalytic properties of the new electrocatalysts were investigated in an acid medium (0.5 M H2SO4), showing a Tafel slope of 65 mV dec–1 and an η10 overpotential of 150 mV. More recently, Yi et al.153 produced a single-atom catalyst (SAC), in which independent and active Pt atoms were uniformly anchored on N-, B-, and F-doped ReS2 and Mo2CTx MXene as supports (Pt/NBF-ReS2/Mo2CTx), shown in Figure 5a,b. In an acidic medium and with a catalyst mass loading of 0.56 mg cm–2, this material presented a low Tafel slope of 24 mV dec–1 and an η10 overpotential of only 29 mV, showing a high HER performance comparable with that of commercial Pt/C (Figure 5c,d).
Table 1. Summary of the HER Performance of Rhenium-Based Sulfides.
| system | mass loading (mg cm–2) | mass activity (A g–1) | overpotential (η10 in mV at 10 mA cm–2a) | Tafel slope (mV dec–1) | ref |
|---|---|---|---|---|---|
| IF-RexMo1–xS2 | 0.707 | 14.14 | 677 | 136 | (134) |
| Re0.04Mo0.96S2 | 0.285 | 35.09 | 382 | 180 | (133) |
| Re0.55Mo0.45S2 | 0.285 | 35.09 | 147 | 56 | |
| Re0.75Mo0.25S2 | 0.285 | 35.09 | 365 | 136 | |
| Re-doped MoS2 | 0.285 | 35.09 | 210 | 78 | (130) |
| Mo0.5Re0.5S | 0.39 | 25.64 | 98 | 54 | (131) |
| ReS2 | 336 | (138) | |||
| ReS2/Au | 2.3 | 4.340 | 100 | 84 | (139) |
| ReS2 nanosheets | 498 | 136 | (140) | ||
| Bulk ReS2 | 639 | 161 | |||
| ReS2 | 360 | 142 | (141) | ||
| ReSe2 | 430 | 230 | |||
| ReS2 exf | 530 | 115 | |||
| ReSe2 exf | 630 | 130 | |||
| Re/ReS2-7H/CC | 0.378 | 26.45 | 42 | 36 | (142) |
| ReS2/CSW-700 °C | 357 | 304 | (143) | ||
| ReS2/CSW-750 °C | 260 | 189 | |||
| ReS2/CSW-800 °C | 299 | 193 | |||
| 2D-Re–ReS2 | 147 | 69 | (144) | ||
| ReS2 | 223 | 151 | |||
| 3D-ReS2 | 1.83 | 5.460 | 413 | 195 | (145) |
| 2D-ReS2 | 1.67 | 5.980 | 288 | 147 | |
| Mo/ReS2@CA/CC | 2.1 | 4.760 | 101 | 40 | (146) |
| ReS2 | 0.34 | 29.41 | 395 | 178 | (147) |
| ReS2-F5.93 | 142 | 64 | |||
| Fe-ReS2@N-CNF-5 | 1 | 10.00 | 242 | 63 | (148) |
| ReS2 | 0.285 | 35.09 | 174 | 82 | (149) |
| 5% Mo-ReS2 | 130 | 64 | |||
| 10% Mo-ReS2 | 81 | 62 | |||
| 15% Mo-ReS2 | 147 | 84 | |||
| ReS2/rGO | 0.282 | 18.44 | 250 | 107 η5.2 | (150) |
| ReS2 | 250 | 152 η3.1 | |||
| ReS2/rGO | 0.13 | 76.92 | 148 | 67 | (151) |
| ReS2/ReO2 | 150 | 65 | (152) | ||
| Pt/NBF-ReS2/Mo2CTx | 0.56 | 17.85 | 29 | 24 | (153) |
Current density at which the overpotential was measured is explicitly mentioned when it differs from 10 mA cm–2.
Figure 5.
(a) High-resolution TEM images and (b) high-resolution HAADF-STEM images of Pt/NBF-ReS2/Mo2CTx. (c) Polarization curves of Pt/NBF-ReS2/Mo2CTx and other references with a scan rate of 5 mV s–1 in 0.5 M H2SO4 and (d) the corresponding Tafel plots. Adapted with permission from ref (153). Copyright 2021 Elsevier.
Table 1 summarizes the information described for each catalyst in this section. Since TOF values are usually not reported in the literature, we summarized the catalysts’ mass loading and mass activity in the tables when the data were available.
4.2. Selenides
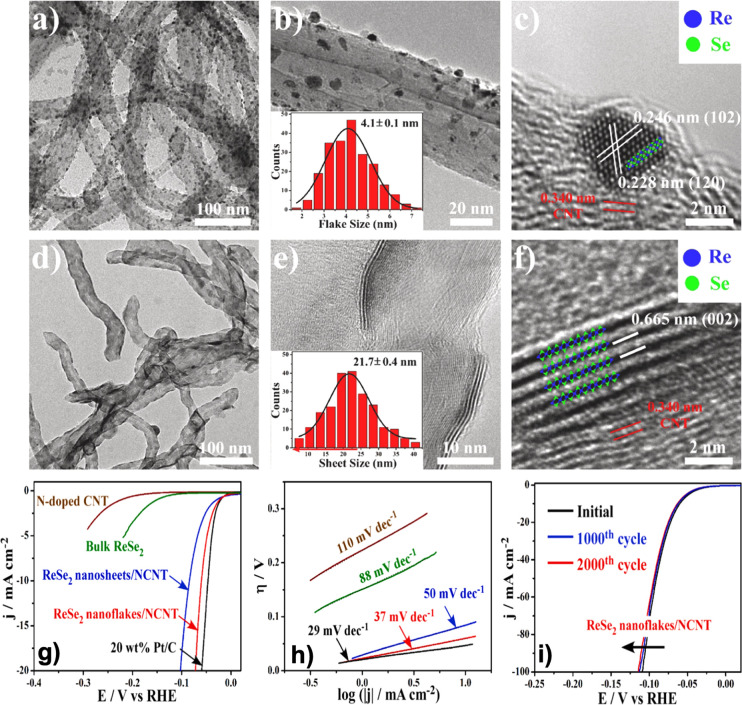
Rhenium selenide (ReSe2) studies are less common than those of ReS2 in the context of HER electrocatalysis. ReSe2 has a direct band gap structure in all stacking forms among the monolayer, multilayer, and bulk phases. In this regard, Sun et al.137 obtained ReSe2–x nanosheets via electronic structure modulation from abundant Se vacancies, tuned via a hot-injection colloidal process. The authors evaluated the nanosheets as a catalyst for the HER in an acid medium (0.5 M H2SO4). The results showed a Tafel plot of 67 mV dec–1 and an overpotential of 102 mV. The TMDs, including the ReSe2, were characterized by anisotropy, a feature that allows for varying properties according to the direction in which they are examined. Usually, this material adopts the 1T″ phase (a distorted form of the trigonal 1T phase) with triclinic symmetry, giving rise to the in-plane anisotropy. A more evident example of the influence of ReSe2 anisotropy was reported by Kwak and co-workers.154 These authors synthesized Re1–xMoxSe2 alloy nanosheets using a hydrothermal reaction. The substitutional Mo atoms aggregated in the 1T″ ReSe2 phase with Se vacancies. The incorporation of the 1T′ phase makes the alloy nanosheets more metallic than the end compositions. The electrochemical test showed that Re1–xMoxSe2 alloy nanosheets exhibit a Tafel slope of 42 mV dec–1 and an η10 overpotential of 77 mV. Also, in materials formed by various layers of one or more metals, it is possible to observe the effect of anisotropy. For example, 1T′-ReSe2 is comprised of Se–Re–Se in three atomic layers, where covalent bonds link the Re and Se atoms. This material has plane anisotropy and forms a 1T′ phase. Zhuang and co-workers155 synthesized by a polymer-assisted strategy sub-5 nm-sized 1T′-ReSe2 nanoflakes/sheets supported on nitrogen-doped multiwalled carbon nanotubes (CNTs) (Figure 6a–f). In this work, the authors found that the small size of the nanoflakes increases the exposed surface area of the electrode, enhancing the HER catalytic activity. The 1T′-ReSe2 nanoflake/N-CNT catalyst was evaluated with a mass loading of 0.5 mg cm–2 in an acid medium and exhibited a low overpotential of 60 mV, along with a downward Tafel slope of 37 mV dec–1, a high exchange current density of 0.3 mA cm–2, and good stability, demonstrating the potential of ReSe2 for the HER (Figure 6g–i).
Figure 6.
Morphology of 1T′-ReSe2 nanoflakes/sheets on N-doped multiwalled carbon nanotubes (CNTs). (a, b) TEM images of CNT-supported nanoflakes with a size of 4.1 ± 0.1 nm. (c) High-resolution TEM side-view image of the nanoflakes on CNT. (d, e) TEM images of nanosheets with a size of 21.7 ± 0.4 nm on the CNT surface. (f) HRTEM image of nanosheets on CNT. The insets of (c) and (f) are the crystalline structure models of 1T′-ReSe2. Blue spheres denote rhenium atoms, and green spheres are selenium atoms. (g) Polarization curves for HER in 0.5 M H2SO4 at a glassy-carbon electrode modified with various catalysts, (h) the corresponding Tafel plots, and (i) polarization curves of 1T′-ReSe2 nanoflakes/CNT after various cycles. Adapted with permission from ref (155). Copyright 2019 Elsevier.
In material science, it is necessary to synthesize systems with tunable properties. In this regard, heteroatom doping is an excellent strategy to obtain multicomponent materials, improve the conductivity, modify structural stability, and improve the catalytic behavior for HER. A good example in that context was reported by Xia et al.,156 who synthesized by a solvothermal method 3D nanosheets derived from sulfur-doped rhenium selenide (ReSe2(1–x)S2x) supported on carbon fiber paper (CFP). The catalyst ReSe1.78S0.22/CFP was evaluated for the HER in an acid medium with a mass loading of 0.44 mg cm–2. This material exhibited good performance, with a Tafel slope of 84 mV dec–1 and an η10 overpotential of 123 mV. In this case, the electrochemical performance was related to the quantity of sulfur as a dopant.
Unfortunately, similarly to ReS2, ReSe2 has the disadvantage of not being a good electrical conductor. However, it is compatible with various carbon species (carbon cloth, graphene, graphene oxide, and carbon nanotubes). Carbon-based materials could improve the electron transport between the electrode and the active sites when they are used as supports, competing with Pt-based catalysts. As a first example of the importance of using carbonaceous species as ReSe2 supports, we can mention Liu and co-workers,157 who synthesized ultrathin ReSe2 nanosheets supported on 3D graphene foam (GF) using CVD. The ReSe2/GF catalyst was evaluated for the HER in an acid medium (0.5 M H2SO4) with 0.1 mg cm–2 catalyst mass loading, one of the lowest values reported for rhenium selenides. The results showed high activity for the HER, with a Tafel slope of 68 mV dec–1, close to that of a Pt/C reference catalyst (41 mV dec–1), and an η10 overpotential of 106 mV. GF enhances the conductivity and controls the size and distribution of active nanosheets. Then, Li and co-workers explored the positive effect of using a porous carbon cloth (PCC) as a support for ultrahigh-density ReSe2 nanoflake electrodes.158 This catalyst/electrode was evaluated for the HER in an acid medium (0.5 M H2SO4) and revealed a Tafel slope of 64 mV dec–1 and an η10 overpotential of 140 mV. According to the authors, more edges and surfaces of 2D catalyst can be exposed perpendicular to the substrate, positively affecting the HER.
ReSe2 can have different morphologies, such as nanosheets, microspheres, nanoflakes, and nanodots. In this way, the exposed active sites can be increased, affecting the charge transfer phenomena. Li and co-workers159 explored the synthesis of vertically aligned ReSe2 nanosheets supported on carbon cloth by CVD. The HER measurements were carried out in an acid medium, revealing a low Tafel slope of 69 mV dec–1 and an η10 overpotential of 265 mV. Qi and co-workers160 synthesized ReSe2 microspheres constituted by the assembly of few-layer nanosheets by a hydrothermal reaction. The authors evaluated the ReSe2-microsphere catalysts in an acid medium, obtaining a small Tafel slope of 67.5 mV dec–1 and a low η10 overpotential of 80 mV. Yan et al.161 prepared ReSe2 nanoflakes perpendicularly anchored on reduced graphene oxide (rGO) by a hydrothermal synthesis. This catalyst was evaluated for the HER in an acid medium (0.5 M H2SO4), and the results showed a small Tafel slope of 41 mV dec–1 and a low overpotential of 145.3 mV with one of the best mass activities for selenide-based rhenium catalysts. Finally, Lai and co-workers162 prepared 1T′-phase ReS2xSe2(1–x) (x = 0–1) nanodots by chemical vapor transport (CVT). The prepared material was loaded on a glassy-carbon electrode, showing a Tafel slope of 50 mV dec–1 and a low overpotential of 84 mV toward the HER in an acidic medium (0.5 M H2SO4). According to the authors, this performance is related to an S vacancy on the nanodot surface, essential for hydrogen adsorption on the active sites. Table 2 summarizes the information described for each catalyst in this section.
Table 2. Summary of the HER Performance of Rhenium-Based Selenides.
| system | mass loading (mg cm–2) | mass activity (A g–1) | overpotential (η10 in mV at 10 mA cm-2a) | Tafel slope (mV dec–1) | ref |
|---|---|---|---|---|---|
| ReSe2–x nanosheets | 102 | 67 | (137) | ||
| Re1–xMoS2 nanosheets | 77 | 42 | (154) | ||
| 1T′-ReSe2 nanoflakes | 0.5 | 20.00 | 60 | 37 | (155) |
| ReSe2(1–x)S2x/CFP | 0.44 | 22.72 | 84 | 123 | (156) |
| ReSe2/GF | 0.1 | 100 | 106 | 68 | (157) |
| ReSe2 nanoflakes/PCC | 140 | 64 | (158) | ||
| ReSe2 nanosheets/carbon cloth | 265 | 69 | (159) | ||
| ReSe2 microspheres | 67.5 | 80 | (160) | ||
| ReSe2 nanoflakes/rGO | 0.21 | 47.61 | 145.3 | 40.7 | (161) |
| ReS2xSe2(1–x) nanodots | 84 | 50.1 | (162) |
Current density at which the overpotential was measured is explicitly mentioned when it differs from 10 mA cm–2.
4.3. Phosphides and Borides
Another eye-attracting research has been the application of metal phosphides due to their wide range of compositions and tuning of the electronic structure.164,165 Previous studies have revealed that P can significantly improve the electrocatalytic performance by extracting electron density from the metal atom, attracting positively charged protons from the electrolyte during the HER process.166 Only a few studies have focused on the synthesis and application of rhenium phosphides on HER. In 2019 and for the first time, Sun et al. designed Re2P and Re3P4 nanoparticles (NP) anchored in N,P-doped vesicular carbon (NPVC) via pyrolysis.167 Re3P4 NP had the highest catalytic activity, close to that of the commercial Pt/C reference catalyst. Concretely, with a mass loading of 0.143 mg cm–2, the Tafel slope was 38 mV dec–1, and the η10 overpotential was only 40 mV in acidic media (0.5 M H2SO4), one of the best results reported for non-Pt-based electrocatalysts, demonstrating the potential of Re3P4 NPs. In the case of Re3P4, only one report published in 2022 described the effect of metal doping, i.e., doping Re3P4/N,P-doped carbon with Ru, thereby decreasing the overpotential in 0.5 M H2SO4 (88 mV) as well as the Tafel slope (53.18 mV dec–1) when compared to undoped Re3P4, using a mass loading of 1.6 mg cm–2. The carbon support played a crucial role in regulating the electrocatalytic activity by preventing the aggregation of the nanoparticles.163
In the case of borides, Guo et al. explored a family of 12 monometallic diborides containing transition metals from group IV to group VIII elements, synthesized via a molten-salt-assisted method. ReB2 presented a nanosheet structure and derived electrodes with an η10 overpotential of approximately 160 mV, higher than that obtained for the best-performing RuB2 catalyst, with a value of 35 mV.168Table 3 summarizes the information described for each catalyst in this section.
Table 3. Summary of the HER Performance of Rhenium-Based Phosphides and Borides.
| system | mass loading (mg cm–2) | mass activity (Ag–1) | overpotential (η10 in mV at 10 mA cm-2a) | Tafel slope (mV dec–1) | ref |
|---|---|---|---|---|---|
| Re3P4 NP | 0.143 | 69.9 | 40 | 38 | (167) |
| Ru-doped Re3P4/N,P-doped carbon | 1.6 | 6.30 | 88 | 53.18 | (163) |
| ReB2 | 0.46 | 21.7 | 160 | (168) |
Current density at which the overpotential was measured is explicitly mentioned when it differs from 10 mA cm–2.
5. Rhenium Metal Alloys
The efficiency of traditional electrodes for water splitting is far from optimal. Moreover, noble metals with high catalytic activity have a high market value due to their high demand and scarcity. The volcano plot shows that the elements with the best performance in the hydrogen evolution are generally scarce in the Earth’s crust, such as iridium, palladium, platinum, and other metal elements of the iron subgroup, such as iron, nickel, and cobalt. Greeley and co-workers169 used computational calculations and an experimental approximation to design new alloys of noble metals. They concluded that a wide variety of platinum alloys with transition metals have good performance for the HER with low overpotentials. The authors highlighted two alloys, in particular, Pt–Au and Pt–Re, which appear as tentative alternatives to Pt due to their high hardness, corrosion resistance, and high bath stabilization for electrochemical deposition.170 Using alloys of two or more elements affects production costs and generates new prospects for catalysis.
Ahn’s group171 investigated a non-noble-metal alloy, electrodepositing Co and Re over carbon paper (CP). An XRD analysis showed that the Co peak shifted to a lower angle of the Re HCP crystal structure, demonstrating the formation of CoRe alloys. The material performed better than rhenium oxides in acid media, with an overpotential of 45 mV and a 40 mV dec–1 Tafel slope (Figure 7), using 1.64 mg cm-2 catalyst mass loading of the catalyst (37% Re in the alloy). As seen for other examples, the higher the rhenium concentration, the better the catalytic activity.
Figure 7.
(a, b) Field emission scanning electron microscopy (FESEM) images of CoRe with an electrodeposition time of 600 s at different magnifications and HER performance measurement of CoRe/CP and ReOx/CP in 0.5 M H2SO4, (c) LSV curves of each catalyst at room temperature with a scan rate of 5 mV s–1 and the corresponding (d) Tafel plot of each catalyst. Adapted with permission from ref (171). Copyright 2021 Elsevier.
Ni-based alloys mixed with Zn, P, Mo, V, W, and Fe are reported as a way of developing Ni electrodes used for hydrogen and oxygen reactions.172 These materials have lower overpotential concerning the HER compared with Ni electrodes, related to the higher active surface area. However, nickel alloys must be used with caution, as easy oxidation to Ni2+ can proceed. Zabinski and co-workers170 used NH4ReO4 and Ni(NH2SO3)2 to electrodeposit a NiRe alloy over a copper disk and study its performance for the HER. The experimental conditions included different bath temperatures, current density, and bath composition. At high temperatures (70 °C), less rhenium was deposited, resulting in finer and homogeneous grains, while lower temperatures (20 °C) resulted in globular and rough deposits. An increase of the rhenium content in the alloy (23 atom % or more) improved the activity for hydrogen evolution.
Gamburg et al.173 also tested a Ni–Re alloy and concluded that the high catalytic activity of the alloy is associated with a high degree of structural disordering. On exposure of Ni–Re alloys to high temperatures,174 the catalytic activity decreases due to the reduction of Re6+ to Re0. These results confirmed the important role of rhenium oxide as a catalyst. Kuznetsov and co-workers175 also studied the HER with a NiReP cathode prepared via electrodeposition. The structural disorders offer oxyphilic sites on the surface and facilitate the dissociation of water molecules. Unlike the electrodeposits seen previously, a more significant presence of Re, Ni, and P oxides and hydroxides was observed, accompanied by a lower amount of rhenium deposited over the electrode. As was previously evaluated, criteria such as the proportion of metal in the alloy, deposition time, current intensity, and degree of structural disorder directly affect the HER. Still, the resistance of these electrodes to harsh conditions must also be considered.
6. Rhenium Oxides
Besides the nanosystems based on chalcogenides and phosphides, rhenium has also been applied as nanoparticles (NPs). To date, limited reports have been published on the activity of Re NPs on the HER. One of the first works was reported in 2012 and explored nanopyramids of Re. The authors described the first carbon-induced nanofaceting of a Re single crystal by annealing at 700 K in acetylene followed by annealing under vacuum at 1100 K. Rhenium structures corresponded to faceted three-sided nanopyramids but showed a high overpotential (>200 mV at ∼η1).176 On the other hand, the electrosynthesis of rhenium oxide nanoislands (200–600 nm) was explored by Vargas-Uscategui and co-workers177 by using pulsed current electrodeposition over a transparent conductive oxide substrate (indium tin doped oxide, ITO) in an alkaline aqueous electrolyte. Compared to ITO, rhenium oxide nanoislands presented a better electrocatalytic performance in 0.45 M H2SO4 (∼285 mV at η100). The authors concluded that a higher abundance of the Re7+ species causes a greater HER rate than that generated by the Re4+ state. Later, in 2017, Cheng et al.178 covered silicon nanowires (45–90 nm diameter) with different metals, like Os, Re, and Rh, among others. The Re/Si nanowires presented an overpotential of 248 mV but had lower activity against the HER than Os/Si (43 mV) and Pt/C (25 mV). In the same year, another study improved the performance of Re/Si nanowires by changing the Re:Si ratio, resulting in a Tafel slope of 81 mV dec–1 and an overpotential of 100 mV in 0.5 M H2SO4.179 The authors confirmed the partial oxidation of Re NP by XPS to Re4+ and attributed the improved electrocatalytic performance against the HER mainly to the amorphous structure of Re NPs and a synergistic effect between Re and Si. As the conditions of the XPS experiment were not totally detailed by the authors, it is not clear if the oxidation of the sample occurred after its synthesis by the ambient oxygen. In 2019, Kim and co-workers synthesized and characterized spherical superstructures of rhenium ReO2 NPs of 200–500 nm held together by an amorphous-carbon phase.180 These Re/C clusters deposited on glassy carbon have a small overpotential (133 mV), a Tafel slope near 50 mV dec–1, and good stability under acidic, neutral, and basic conditions. In addition, they exhibit excellent durability over the entire pH range, confirming that Re-based nanomaterials can become less expensive and durable alternatives to the currently used Pt-based HER catalysts. In the same year, Wu and co-workers reported the thermal synthesis of ReO3 NP with oxygen vacancies produced by plasma exposure.181 The NP supported on carbon paper exhibited an overpotential of 138 mV and good electrocatalytic stability for 20 h at 10 mA cm–2 in an acidic electrolyte (0.5 M H2SO4) that can be correlated to the high active surface area, abundant oxygen vacancies, and good conductivity. However, the mass activity was quite low compared to those of other studies in this section (Table 4).
Table 4. Summary of the HER Performance of Rhenium-Based Nanoparticles.
| system | mass loading (mg cm–2) | mass activity (A g–1) | overpotential (η10 in mV at 10 mA cm–2a) | Tafel slope (mV dec–1) | ref |
|---|---|---|---|---|---|
| Re nanopyramids | >200 at ∼η1 | (176) | |||
| ReO2 nanoislands | 285 | (177) | |||
| Re/Si nanowires | 0.257 | 39.9 | 248 | (178) | |
| Re/Si nanowires | 0.411 | 24.3 | 100 | 81 | (179) |
| ReO2NP clusters | 0.283 | 35.3 | 133 | 50 | (180) |
| ReO3NP | 3.34 | 2.99 | 138 | (181) | |
| ReNP@DNA | 0.2 | 50.0 | 152 | (182) |
Current density at which the overpotential was measured is explicitly mentioned when it differs from 10 mA cm–2.
In 2020, the Kundu group employed the backing of biomolecule deoxyribonucleic acid (DNA) as a scaffold for the in situ reduction of ammonium perrhenate to 5 nm large Re NPs. The authors confirmed the presence of Re0 and Re6+ and reported an overpotential of 152 mV.182 The cycling study showed the catalysts’ activation, evidenced by the calculated electrochemical surface area. The post-HER study revealed the stable nature of chain-like structures of Re@DNA along with exposed sites after continuous cathodization. Table 4 summarizes the information described for each catalyst in this section.
7. Rhenium for OER
In this section, we summarize the reports on the water oxidation activity of Re-based catalysts, assessed with various methods from chemical and photochemical to (photo)electrochemical techniques. In the case of Re-based OER catalysts, most of the electrochemical studies have been performed in alkaline solutions.
Re, with an atomic number of 75, is a heavier congener of Mn, the only element capable of oxygen evolution in Nature. The oxygen-evolving complex (OEC) in photosystem II (PSII), CaMn4O5, catalyzes water oxidation in the natural photosynthesis process.183 Hundreds of research articles on the development of manganese-based OER catalysts and many review papers on the subject exist, and some key reviews have been cited here.184−189 However, despite a rich body of literature on the water oxidation activity of manganese-based catalysts, the number of reports on Re-based OER is minimal. Perhaps one reason is the different chemistries of the two elements190 as well as the relatively young history of Re,48,50 which left the water oxidation activity of Re oxide based materials unexplored until the late 1980s.
Finally, it was in 1989 that Farina and co-workers, for the first time, examined the oxygen evolution activity of a series of rhenium oxides in the presence of Ce4+ or [Ru(bipy)]3+ oxidants.191 The results suggested that ReO2 was the most efficient water-oxidizing catalyst among the studied rhenium oxides. The study showed that catalyst corrosion during water oxidation is the possible reason for the lowest O2 yields for high Ce4+:RexOn ratios.191
Shortly afterward, kinetic studies by Mills and Russell established a poor O2 production yield for ReO3 (62%) in the presence of Ce(IV) as an oxidant, which was comparable to those of MnO2 (50% or 61%, depending on the preparation method), Ir2O3 (75%), Rh2O3 (67%), and heat-treated oxides of Os and Ir, namely IrO2·yH2O (74%) and OsO2·yH2O (61%).192 In contrast, a series of microcrystalline powders of Ru and Ir oxides with a high surface area had shown the highest O2 production yields (80% ≤ O2). The investigated materials were classified according to the related O2 production yield, e.g., inactive catalyst (O2 yield 0%), poorly active (O2% <80), and functional (80% ≤ O2).192 Additionally, similar to the report by Farina and co-workers,191 the authors detected ReO3 corrosion in the presence of the oxidant.192 In the recent decade, anodic corrosion of the OER catalysts and interface studies in electrochemical water oxidation reactions have been given considerable attention to pave the road for developing suitable catalysts for large-scale water-splitting applications.112,193−195
In 2018 Suzuki et al. studied the visible-light-responsive oxygen-evolving photoactivity of M3ReO8 (M = Y, La, Nd, Sm, Eu, Gd, Dy, and Yb) loaded with IrO2 cocatalyst.196 The water oxidation activity of the catalysts was assessed by dispersing the photocatalysts in an aqueous AgNO3 solution. While the charges on the main cations in active photocatalyst materials usually range from +1 to +6, this work revealed active semiconductors with heptavalent cations (Re(VII)).196 The report suggested that partially filled f orbital electrons limit the photocatalytic activity. However, this is not the case for the half-filled Gd3+ ion, and so Gd3ReO8–IrO2 and Y3ReO8–IrO2 showed the highest O2 evolution among the reported M3ReO8 catalysts (Figure 8a).196 Interestingly, a similar trend was previously noted for the RVO4 (R = Y, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu) system, with the highest activities for the YVO4 (Y3+: [Kr]4f0), GdVO4 (Gd3+: [Xe]4f7, half-filled), and LuVO4 (Lu3+: [Xe]4f14) samples.197 Surprisingly, though La3ReO8 was expected to have an appropriate band gap for water oxidation under visible light, due to the low chemical stability of the catalyst in aqueous solution, the O2 evolution rate on La3ReO8–IrO2 gradually decreased over time (Figure 8b).196 PXRD and a morphological analysis of the post-mortem photocatalyst confirmed the instability of the material (Figure 8c–e).196
Figure 8.
(a) O2 evolution performance of IrO2–M3ReO8 photocatalysts from aqueous AgNO3 solution for 10 h under visible light irradiation. (b) Visible-light-responsive oxygen evolution of IrO2-M3ReO8 (M = Y, La, or Gd) in aqueous AgNO3 solution over time. (c) XRD patterns of M3ReO8 (M = Y, La, or Gd) before and after O2 evolution or stirring in distilled water. (d) SEM image of La3ReO8. and (e) SEM image of IrO2–La3ReO8 after light irradiation in aqueous AgNO3 solution. Adapted with permission from ref (196). Copyright 2013 Royal Society of Chemistry.
ReS2, with a conduction band minimum (CBM) above the water reduction level and a valence band maximum (VBM) below the water oxidation potential (for monolayer ReS2 the calculated CBM and VBM values are −6.15 and −4.26 eV, respectively, relative to the vacuum level), has attracted attention as photochemical water splitting catalyst, with a particular interest in HER (Figure 9a).198,199
Figure 9.
(a) Band edge positions of multilayer ReS2 relative to the vacuum level. Reprinted with permission from ref (199). Copyright 1999 Royal Society of Chemistry. (b) LSV of ReS2/n-Si and ReS2/p-Si photoelectrodes under chopped illumination and (c) fabrication of a NiCo-LDH/ReS2/n-PSi photoanode. Adapted with permission from ref (200). Copyright 2013 Royal Society of Chemistry. (d) OER activity of Re-NiFeOH/N-CC samples with different Re contents at 1 M KOH and (e) catalytic performance of Re-NiFeOH/N-CC with various Re contents in the OER and HER. Adapted with permission from ref (201). Copyright 2022 Elsevier.
For example, Zhao et al. developed a p–n junction of ReS2/n-PSi by CVD of ReS2 nanosheets (NS) on an n-type pyramid patterned Si (PSi) substrate as a high-performance photoanode for the OER (Figure 9c). The pyramidal pattern of Si provides a 3D antireflection structure. Finally, a NiCo layered double hydroxide (LDH) was electrodeposited on the ReS2/n-PSi to enhance the photocurrent as a cocatalyst and protective layer.200 The catalytic activity of the electrode was measured in a 0.5 M Na2SO4 aqueous solution. Compared to the ReS2/p-Si heterostructure with p–p junction, the ReS2/n-Si demonstrated a boosted OER activity due to the better carrier separation properties of the p–n junction generated by n-type Si and p-ReS2 (Figure 9b). However, ReS2/p-Si showed better performance in the hydrogen production reaction.
ReS2 and its combination with OER-active materials (e.g., ReS2/NiFe LDH) have also been investigated as a bifunctional OER and HER electrocatalyst.142,153,202 Pang et al. have developed a bifunctional water-splitting catalyst composed of sulfur-defect-rich ReS2 nanosheets and metallic Re on carbon cloth (Re/ReS2/CC). The electrode was fabricated by a hydrothermal reaction of NH4ReO4, HO-NH2·HCl, and thiourea on HNO3-treated CC followed by a heat treatment under a H2/N2 gas mixture. The catalyst showed an OER overpotential of 290 mV at 10 mA cm–2 under alkaline conditions, attributed to increased active sites and decreased adsorption energy of intermediates due to the presence of S defects on the surface. A two-electrode system containing Re/ReS2/CC as both anode and cathode has displayed a cell voltage of 1.3 V in 1 M KOH electrolyte.142 S defects have also played a role in the rapid charge transfer property of a Ni-doped ReS2 OER catalyst developed by Das et al.203 The latter catalyst had an overpotential of 270 mV at 10 mA cm–2 in an alkaline electrochemical water oxidation reaction.
In addition, Re has also been used as a dopant to enhance the water-splitting activity. For example, a recent report from Pumera’s group demonstrated improved electron transfer properties of MoSe2 and WSe2 by Re doping, which promotes the OER photocurrent in alkaline solution under UV light illumination.204 A decreased band gap and increased number of charge carriers by providing more electrons into the conductive band in the investigated Re-doped layered diselenides were further supported by DFT calculations. Finally, the Re-doped samples offered an improved OER onset potential compared to the undoped counterpart materials and required a lower potential to reach a current density of 10 mA cm–2.
On the other hand, Re insertion in NiFeOH/nitrogen-functionalized carbon cloth has led to an active bifunctional water-splitting catalyst (Re-NiFeOH/N-CC), delivering a current density of 300 mA cm–2 at a cell voltage of 1.88 V in a 1 M KOH solution.201 To fabricate the electrode, the N-functionalized CC was prepared by HNO3 treatment and subsequent electrodeposition of NiFe-LDH on the N-CC substrate. Afterward, Re was doped over or inside the NiFe-LDH structure using the CV technique. It was found that the amount of Re increases on increasing the cycle number from 2 to 5 or 10 cycles. The Re-NiFeOH/N-CC synthesized with 2 cycles shows an OER activity similar to that of NiFeOH/N-CC (Figure 9d,e). However, an excess amount of Re diminishes the OER activity of the catalyst, described by a decreased number of the catalytically active sites due to replacing Fe3+ ions or lowered direct contact of the hydroxide ions with nickel and iron sites (Figure 9d,e).201
Another recent example of Re as a dopant for an oxygen-evolving catalyst is an Re-doped Ni3S2 nanoarray deposited on N-doped graphene (NG) modified nickel foam (NF) as the substrate (Re–Ni3S2/NG/NF),205 a multifunctional catalyst possessing OER, HER, and ORR activity. Owing to Re’s role in modulating the local electronic structure and optimizing the adsorption of hydrogen- and oxygen-containing intermediates on the Re–Ni3S2 surface, as well as improving the electrochemically active surface area of the catalyst, the final symmetrical water electrolyzer containing Re–Ni3S2/NG/NF as both cathode and anode in a 1 M KOH solution reached a current density of 10 mA cm–2 at a cell potential of 1.58 V. A mass loading of about 1.2 mg cm–2 for Re–Ni3S2 on the NG/NF electrode was reported; therefore, one can calculate a mass activity of 4.16 A g–1 for the whole system (under the assumption of Re–Ni3S2 as the only active species and considering 1.2 mg cm–2 × 2 of Re–Ni3S2 mass loading for the entire system).205
As we pointed out above, despite an increasing trend in the number of reports on Re-based OER catalysts and applications in the past few years, the number of publications is still limited, and there is a lot of room for further research. Accordingly, the systematic development of efficient Re-based OER-active catalysts should be intensified to deliver additional crucial insights. In the meantime, in-depth evaluations of the real catalytic species hold the key to carefully interpreting the results and thus developing efficient and robust catalysts.
8. Computational Studies
Computational calculations have accelerated the screening and performance prediction of HER and OER catalysts. In a seminal study, Nørskov and co-workers206 introduced the computational hydrogen electrode model, with the hydrogen adsorption free energy ΔGH computed from density functional theory (DFT) to accurately estimate the catalytic activity of the HER catalyst by the connection of ΔGH and the electrochemical exchange currents. Accordingly, the ideal HER catalyst should have a ΔGH* value close to zero with a high j0. The work of Nørskov demonstrated the importance of computational descriptions to reduce the trial and error that has historically been the norm for experimental HER electrocatalysis. Since then, computational methods have predicted many candidates’ catalytic activity, saving time and costs.207−215 As in the HER, the interaction of the OER intermediates OH*, O*, and OOH* can be successfully calculated by DFT.216 This scheme has accelerated OER catalysts’ screening and performance prediction.217−219
As stated before, the electrochemical performance of transition-metal electrocatalysts can be considerably influenced by less electropositive elements such as S, Se, and P. The charge transfer from the metal to the heteroatom can alter the electronic properties of the metal centers, resulting in dissimilar hydrogen or oxygen binding energies.32,33 In the case of rhenium, computational studies are very scarce. Sun and co-workers167 compared Re, ReP2, and Re3P4NP supported on N,P-doped vesicular carbon (NPVC). DFT calculations indicated that Re3P4|NPVC can generate a closer thermoneutral behavior (|ΔGH*| → 0) compared to the other studied systems; it boosts the kinetics during the overall HER process and, at the same time, the abundant electrons transferred from NPVC to Re3P4 can facilitate H* desorption to become H2. However, to date, there have been no computational reports to systematically analyze the effect of anions on Re as a metal center. Then, understanding at an atomic level the environment around Re after the orbital hybridization with anions is crucial for exploiting new electrocatalysts and correlating the electrocatalytic activity. In the case of OER, computational doping analyses are less common due to the mechanism’s higher complexity than the HER and point to a better electrocatalytic performance by increasing active sites.220,221
On the other hand, trace-level doping can induce charge transfer from the dopant to the host matrix, modulating the intermediate adsorption energy of H* and increasing the performance of the electrocatalyst.222,223 Few studies have described the effect of doping rhenium with other metals by computational tools, and most works have only referred to the HER. For example, Fe doping results in superior electrochemical activity compared with the pristine ReS2, attributed to the refined energy levels of ReS2 nanosheets after low-valent transition-metal doping.148,224 The calculated ΔGH* value for Mo-ReS2 is much lower than that of pure ReS2, with a density of states (DOS) profile showing a metallic electronic structure attributed to the impurity states of Mo atoms, which would be beneficial to the charge transport in the Mo-ReS2 catalysts, accelerating charge transfer for the HER. With the sustained development of computational capabilities, ab initio molecular dynamics (AIMD) can offer a robust platform to explore the stability of new electrocatalysts and HER and OER mechanistic aspects over time.225,226 Now, most of the studies involving the variable of time are still managed under parametrized force fields, which neglect the explicit electronic contribution. AIMD, in combination with experimental methods, can lead to the design of better-performing catalysts in a shorter time.
9. Future Perspectives
PEM electrolyzers are the most promising commercial systems for high-purity and efficient electrochemical water splitting. However, the high costs and scarcity of the noble-metal electrocatalysts used as electrodes make it necessary to search for more efficient, robust, and low-cost materials. Rhenium, with an important production in Chile and few known applications, appears to be a potential candidate for H2 and O2 generation from water. Although lab-scale catalyst characterization and durability experiments have been performed in different operation conditions than for industrial water electrolyzers and PEM devices, they are highly beneficial for the quick characterization of novel electrocatalysts and prescreening their long-term performance. Later, laboratory data can be translated to future PEM applications by conducting membrane electrode assembly tests.
As seen in the works published in the last few years, decreasing the particle size could become an exciting alternative to develop a new group of catalysts for the HER, which can displace in the short term those based on platinum. At this point, it is imperative to explore the most active oxidation state of rhenium, as there have only been a few studies evaluating the electrocatalytic activity each of the different rhenium oxides with all the possible oxidation states of the metal. The substrate’s stability and conductivity should also be analyzed before and after the hydrogen production, since changes in the oxidation state of rhenium and morphology can affect the performance of the cathode. In the case of OER, catalysts are often unstable toward anodic corrosion due to the highly oxidizing condition of the water oxidation reaction. To this end, a wide range of in situ and operando studies of active species, from laboratory testing methods to operando synchrotron-based techniques and in situ electron microscopy and a combination of the results with advanced computational studies can accelerate the knowledge-based designing of active Re-based OER catalysts.
As not all of the studies mention the critical indicators of catalytic activity, it is necessary to establish standardized protocols to compare the performance of different investigations to accelerate the screening and optimization of catalysts. Due to the difference in mass loadings, or even the absence of information about catalyst supports, methods in preparation of working electrodes, and reaction conditions, it is not easy to accurately compare and judge the performance of various materials. Moreover, the rhenium percentage in each catalyst is rarely mentioned, adding one more concern when comparing different systems. Therefore, it is necessary to provide a complete set of information on the electrocatalytic behavior, such as electrolyte, loading amount of catalysts, overpotential, Tafel slope, exchange current density, durability, activities normalized by both electrode area and mass loading, TOF, and FE at the observed overpotential.
Acknowledgments
The authors acknowledge the funding support of Fondecyt-ANID No. 1230426. Funding of the Freiburg Rising Star Academy by the German Federal Ministry of Education and Research (BMBF) in the frame of the "Research in Germany" initiative and the Excellence Cluster livMatS, funded by the ClusterDeutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy – EXC-2193/1 – 390951807, is gratefully acknowledged as well as funding of the Saltus! program by the University of Freiburg and the Eva Mayr-Stihl foundation and funding of the Momentum program by the Volkwagen Foundation.
Author Contributions
CRediT: Andrés M.R. Ramírez conceptualization (equal), funding acquisition (equal), supervision (equal), writing-original draft (equal), writing-review & editing (equal); Sima Heidari writing-original draft (equal), writing-review & editing (equal); Ana Vergara writing-original draft (equal), writing-review & editing (equal); Miguel Villicaña Aguilera writing-original draft (equal), writing-review & editing (equal); Paulo Preuss writing-original draft (equal), writing-review & editing (equal); María Belén Camarada conceptualization (equal), funding acquisition (equal), supervision (equal), writing-original draft (equal), writing-review & editing (equal); Anna Fischer conceptualization (equal), funding acquisition (equal), supervision (equal), writing-original draft (supporting), writing-review & editing (equal).
The authors declare no competing financial interest.
References
- Davis S. J.; Lewis N. S.; Shaner M.; Aggarwal S.; Arent D.; Azevedo I. L.; Benson S. M.; Bradley T.; Brouwer J.; Chiang Y.-M.; Clack C. T. M.; Cohen A.; Doig S.; Edmonds J.; Fennell P.; Field C. B.; Hannegan B.; Hodge B.-M.; Hoffert M. I.; Ingersoll E.; Jaramillo P.; Lackner K. S.; Mach K. J.; Mastrandrea M.; Ogden J.; Peterson P. F.; Sanchez D. L.; Sperling D.; Stagner J.; Trancik J. E.; Yang C.-J.; Caldeira K. Net-zero emissions energy systems. Science 2018, 360 (6396), eaas9793 10.1126/science.aas9793. [DOI] [PubMed] [Google Scholar]
- Bermudez J.M.; Hannula I.; Hydrogen; IEA: 2021. [Google Scholar]
- Hou J.; Wu Y.; Zhang B.; Cao S.; Li Z.; Sun L. Rational Design of Nanoarray Architectures for Electrocatalytic Water Splitting. Adv. Funct. Mater. 2019, 29 (20), 1808367. 10.1002/adfm.201808367. [DOI] [Google Scholar]
- You B.; Sun Y. Innovative Strategies for Electrocatalytic Water Splitting. Acc. Chem. Res. 2018, 51 (7), 1571–1580. 10.1021/acs.accounts.8b00002. [DOI] [PubMed] [Google Scholar]
- Wang H.; Lee H.-W.; Deng Y.; Lu Z.; Hsu P.-C.; Liu Y.; Lin D.; Cui Y. Bifunctional non-noble metal oxide nanoparticle electrocatalysts through lithium-induced conversion for overall water splitting. Nat. Commun. 2015, 6 (1), 1–8. 10.1038/ncomms8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You B.; Tang M. T.; Tsai C.; Abild-Pedersen F.; Zheng X.; Li H. Enhancing Electrocatalytic Water Splitting by Strain Engineering. Adv. Mater. 2019, 31 (17), 1807001. 10.1002/adma.201807001. [DOI] [PubMed] [Google Scholar]
- Yu Z.-Y.; Duan Y.; Feng X.-Y.; Yu X.; Gao M.-R.; Yu S.-H. Clean and Affordable Hydrogen Fuel from Alkaline Water Splitting: Past, Recent Progress, and Future Prospects. Adv. Mater. 2021, 33 (31), 2007100. 10.1002/adma.202007100. [DOI] [PubMed] [Google Scholar]
- Dutta S.; Hussain C.M.. Sustainable Fuel Technologies Handbook; Academic Press: 2020. [Google Scholar]
- Tang E.; Wood T.; Brown C.; Casteel M.; Pastula M.; Richards M.; Petri R.. Solid Oxide Based Electrolysis and Stack Technology with Ultra-High Electrolysis Current Density and Efficiency; FuelCell Energy, Inc.: 2018. [Google Scholar]
- Elder R.; Cumming D.; Mogensen M.B.. High Temperature Electrolysis. In Carbon Dioxide Utilisation; Styring P., Quadrelli E. A.; Armstrong K., Eds.; Elsevier: 2015; Chapter 11, pp 183–209. [Google Scholar]
- Ju H.; Badwal S.; Giddey S. A comprehensive review of carbon and hydrocarbon assisted water electrolysis for hydrogen production. Appl. Energy 2018, 231, 502–533. 10.1016/j.apenergy.2018.09.125. [DOI] [Google Scholar]
- Nikolaidis P.; Poullikkas A. A comparative overview of hydrogen production processes. Renewable Sustainable Energy Rev. 2017, 67, 597–611. 10.1016/j.rser.2016.09.044. [DOI] [Google Scholar]
- Grigoriev S. A.; Millet P.; Fateev V. N. Evaluation of carbon-supported Pt and Pd nanoparticles for the hydrogen evolution reaction in PEM water electrolysers. J. Power Sources 2008, 177 (2), 281–285. 10.1016/j.jpowsour.2007.11.072. [DOI] [Google Scholar]
- Millet P.; Ngameni R.; Grigoriev S. A.; Mbemba N.; Brisset F.; Ranjbari A.; Etiévant C. PEM water electrolyzers: From electrocatalysis to stack development. Int. J. Hydrogen Energy 2010, 35 (10), 5043–5052. 10.1016/j.ijhydene.2009.09.015. [DOI] [Google Scholar]
- Babic U.; Suermann M.; Büchi F. N.; Gubler L.; Schmidt T. J. Critical Review—Identifying Critical Gaps for Polymer Electrolyte Water Electrolysis Development. J. Electrochem. Soc. 2017, 164 (4), F387–F399. 10.1149/2.1441704jes. [DOI] [Google Scholar]
- Paidar M.; Fateev V.; Bouzek K. Membrane electrolysis—History, current status and perspective. Electrochim. Acta 2016, 209, 737–756. 10.1016/j.electacta.2016.05.209. [DOI] [Google Scholar]
- Shiva Kumar S.; Himabindu V. Hydrogen production by PEM water electrolysis – A review. Mater. Sci. Energy Technol. 2019, 2 (3), 442–454. 10.1016/j.mset.2019.03.002. [DOI] [Google Scholar]
- Hubert M. A.; King L. A.; Jaramillo T. F. Evaluating the Case for Reduced Precious Metal Catalysts in Proton Exchange Membrane Electrolyzers. ACS Energy Lett. 2022, 7 (1), 17–23. 10.1021/acsenergylett.1c01869. [DOI] [Google Scholar]
- Yao D.; Gu L.; Zuo B.; Weng S.; Deng S.; Hao W. A strategy for preparing high-efficiency and economical catalytic electrodes toward overall water splitting. Nanoscale 2021, 13 (24), 10624–10648. 10.1039/D1NR02307A. [DOI] [PubMed] [Google Scholar]
- Cheng J.; Zhang H.; Chen G.; Zhang Y. Study of IrxRu1–xO2 oxides as anodic electrocatalysts for solid polymer electrolyte water electrolysis. Electrochim. Acta 2009, 54 (26), 6250–6256. 10.1016/j.electacta.2009.05.090. [DOI] [Google Scholar]
- Santana M. H. P.; De Faria L. A. Oxygen and chlorine evolution on RuO2 + TiO2 + CeO2 + Nb2O5 mixed oxide electrodes. Electrochim. Acta 2006, 51 (17), 3578–3585. 10.1016/j.electacta.2005.09.050. [DOI] [Google Scholar]
- Baglio V.; Di Blasi A.; Denaro T.; Antonucci V.; Aricò A. S.; Ornelas R.; Matteucci F.; Alonso G.; Morales L.; Orozco G.; Arriaga L. G. Synthesis, characterization and evaluation of IrO2-RuO 2 electrocatalytic powders for oxygen evolution reaction. J. New Mater. Electrochem. Syst. 2008, 105–108. [Google Scholar]
- Kötz R.; Stucki S. Stabilization of RuO2 by IrO2 for anodic oxygen evolution in acid media. Electrochim. Acta 1986, 31 (10), 1311–1316. 10.1016/0013-4686(86)80153-0. [DOI] [Google Scholar]
- Du L.; Zhang G.; Sun S. Proton Exchange Membrane (PEM) Fuel Cells with Platinum Group Metal (PGM)-Free Cathode. Automotive Innovation 2021, 4 (2), 131–143. 10.1007/s42154-021-00146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke C.; Suermann M.; Bensmann B.; Hanke-Rauschenbach R. Is iridium demand a potential bottleneck in the realization of large-scale PEM water electrolysis?. Int. J. Hydrogen Energy 2021, 46 (46), 23581–23590. 10.1016/j.ijhydene.2021.04.174. [DOI] [Google Scholar]
- Sabatier P. Hydrogénations et déshydrogénations par catalyse. Berichte der deutschen chemischen Gesellschaft 1911, 44 (3), 1984–2001. 10.1002/cber.19110440303. [DOI] [Google Scholar]
- Yu P.; Wang F.; Shifa T. A.; Zhan X.; Lou X.; Xia F.; He J. Earth abundant materials beyond transition metal dichalcogenides: A focus on electrocatalyzing hydrogen evolution reaction. Nano Energy 2019, 58, 244–276. 10.1016/j.nanoen.2019.01.017. [DOI] [Google Scholar]
- Trasatti S. Work function, electronegativity, and electrochemical behaviour of metals: III. Electrolytic hydrogen evolution in acid solutions. J. Electroanal. Chem. Interfacial Electrochem. 1972, 39 (1), 163–184. 10.1016/S0022-0728(72)80485-6. [DOI] [Google Scholar]
- Roger I.; Shipman M. A.; Symes M. D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1 (1), 0003. 10.1038/s41570-016-0003. [DOI] [Google Scholar]
- Sheng W.; Myint M.; Chen J. G.; Yan Y. Correlating the hydrogen evolution reaction activity in alkaline electrolytes with the hydrogen binding energy on monometallic surfaces. Energy. Environ. Sci. 2013, 6 (5), 1509–1512. 10.1039/c3ee00045a. [DOI] [Google Scholar]
- Boppella R.; Tan J.; Yun J.; Manorama S. V.; Moon J. Anion-mediated transition metal electrocatalysts for efficient water electrolysis: Recent advances and future perspectives. Coord. Chem. Rev. 2021, 427, 213552. 10.1016/j.ccr.2020.213552. [DOI] [Google Scholar]
- Ojha K.; Saha S.; Dagar P.; Ganguli A. K. Nanocatalysts for hydrogen evolution reactions. Phys. Chem. Chem. Phys. 2018, 20 (10), 6777–6799. 10.1039/C7CP06316D. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Kelly T. G.; Chen J. G.; Mustain W. E. Metal Carbides as Alternative Electrocatalyst Supports. ACS Catal. 2013, 3 (6), 1184–1194. 10.1021/cs4001249. [DOI] [Google Scholar]
- Boppella R.; Tan J.; Yang W.; Moon J. Homologous CoP/NiCoP Heterostructure on N-Doped Carbon for Highly Efficient and pH-Universal Hydrogen Evolution Electrocatalysis. Adv. Funct. Mater. 2019, 29 (6), 1807976. 10.1002/adfm.201807976. [DOI] [Google Scholar]
- Wang X.; Kolen’ko Y. V.; Bao X. Q.; Kovnir K.; Liu L. One-step synthesis of self-supported nickel phosphide nanosheet array cathodes for efficient electrocatalytic hydrogen generation. Angew. Chem. 2015, 127 (28), 8306–8310. 10.1002/ange.201502577. [DOI] [PubMed] [Google Scholar]
- Li W.; Xiong D.; Gao X.; Song W.-G.; Xia F.; Liu L. Self-supported Co-Ni-P ternary nanowire electrodes for highly efficient and stable electrocatalytic hydrogen evolution in acidic solution. Catal. Today 2017, 287, 122–129. 10.1016/j.cattod.2016.09.007. [DOI] [Google Scholar]
- Callejas J. F.; Read C. G.; Roske C. W.; Lewis N. S.; Schaak R. E. Synthesis, characterization, and properties of metal phosphide catalysts for the hydrogen-evolution reaction. Chem. Mater. 2016, 28 (17), 6017–6044. 10.1021/acs.chemmater.6b02148. [DOI] [Google Scholar]
- Anantharaj S.; Ede S. R.; Sakthikumar K.; Karthick K.; Mishra S.; Kundu S. Recent trends and perspectives in electrochemical water splitting with an emphasis on sulfide, selenide, and phosphide catalysts of Fe, Co, and Ni: a review. ACS Catal. 2016, 6 (12), 8069–8097. 10.1021/acscatal.6b02479. [DOI] [Google Scholar]
- Li W.; Wang X.; Xiong D.; Liu L. Efficient and durable electrochemical hydrogen evolution using cocoon-like MoS2 with preferentially exposed edges. Int. J. Hydrogen Energy 2016, 41 (22), 9344–9354. 10.1016/j.ijhydene.2016.03.209. [DOI] [Google Scholar]
- Wang Y.; Zhao Y.; Ding X.; Qiao L. Recent advances in the electrochemistry of layered post-transition metal chalcogenide nanomaterials for hydrogen evolution reaction. J. Energy Chem. 2021, 60, 451–479. 10.1016/j.jechem.2021.01.021. [DOI] [Google Scholar]
- Staszak-Jirkovsky J.; Malliakas C. D.; Lopes P. P.; Danilovic N.; Kota S. S.; Chang K. C.; Genorio B.; Strmcnik D.; Stamenkovic V. R.; Kanatzidis M. G.; Markovic N. M. Design of active and stable Co-Mo-Sx chalcogels as pH-universal catalysts for the hydrogen evolution reaction. Nat. Mater. 2016, 15, 197. 10.1038/nmat4481. [DOI] [PubMed] [Google Scholar]
- Han N.; Liu P.; Jiang J.; Ai L.; Shao Z.; Liu S. Recent advances in nanostructured metal nitrides for water splitting. J. Mater. Chem. A 2018, 6 (41), 19912–19933. 10.1039/C8TA06529B. [DOI] [Google Scholar]
- Xu N.; Cao G.; Chen Z.; Kang Q.; Dai H.; Wang P. Cobalt nickel boride as an active electrocatalyst for water splitting. J. Mater. Chem. A 2017, 5 (24), 12379–12384. 10.1039/C7TA02644G. [DOI] [Google Scholar]
- Li W.; Wang S.; Li Y.; Ma C.; Huang Z.; Wang C.; Li J.; Chen Z.; Liu S. One-step hydrothermal synthesis of fluorescent nanocrystalline cellulose/carbon dot hydrogels. Carbohydr. Polym. 2017, 175, 7–17. 10.1016/j.carbpol.2017.07.062. [DOI] [PubMed] [Google Scholar]
- Amorim I.; Xu J.; Zhang N.; Yu Z.; Araújo A.; Bento F.; Liu L. Dual-phase CoP– CoTe2 nanowires as an efficient bifunctional electrocatalyst for bipolar membrane-assisted acid-alkaline water splitting. Chem. Eng. J. 2021, 420, 130454. 10.1016/j.cej.2021.130454. [DOI] [Google Scholar]
- Xu K.; Wang F.; Wang Z.; Zhan X.; Wang Q.; Cheng Z.; Safdar M.; He J. Component-controllable WS(2(1-x))Se(2x) nanotubes for efficient hydrogen evolution reaction. ACS Nano 2014, 8, 8468. 10.1021/nn503027k. [DOI] [PubMed] [Google Scholar]
- Hämäläinen J.; Mizohata K.; Meinander K.; Mattinen M.; Vehkamäki M.; Räisänen J.; Ritala M.; Leskelä M. Rhenium Metal and Rhenium Nitride Thin Films Grown by Atomic Layer Deposition. Angew. Chem., Int. Ed. 2018, 57 (44), 14538–14542. 10.1002/anie.201806985. [DOI] [PubMed] [Google Scholar]
- Noddack W. Die Ekamangane. Naturwissenschaften 1925, 13, 567–574. 10.1007/BF01558746. [DOI] [Google Scholar]
- Santos G. M. A tale of oblivion: Ida Noddack and the universal abundance of matter. Notes Rec. 2014, 68 (4), 373–389. 10.1098/rsnr.2014.0009. [DOI] [Google Scholar]
- Yoshihara H. K. Nipponium as a new element (Z = 75) separated by the Japanese chemist, Masataka Ogawa: a scientific and science historical re-evaluation. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 2008, 84 (7), 232–245. 10.2183/pjab.84.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.; Makineni S. K.; Liebscher C. H.; Dehm G.; Rezaei Mianroodi J.; Shanthraj P.; Svendsen B.; Bürger D.; Eggeler G.; Raabe D.; Gault B. Unveiling the Re effect in Ni-based single crystal superalloys. Nat. Commun. 2020, 11 (1), 389. 10.1038/s41467-019-14062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; He X.; Zhou Y.; Guo J.; Han S.; Wang H.; Li Y.; Tan M. Resources, application and extraction status of rhenium. Precious Met. 2014, 35 (2), 77–81. [Google Scholar]
- Sanz J.; Tomasa O.; Jimenez-Franco A.; Sidki-Rius N.. Rhenium (Re) [Z. = 75], Elements and Mineral Resources; Springer International: 2022; pp 173–175. [Google Scholar]
- Garcia-Garcia R.; Rivera J. G.; Antaño-Lopez R.; Castañeda-Olivares F.; Orozco G. Impedance spectra of the cathodic hydrogen evolution reaction on polycrystalline rhenium. Int. J. Hydrogen Energy 2016, 41 (8), 4660–4669. 10.1016/j.ijhydene.2016.01.010. [DOI] [Google Scholar]
- Pecherskaya A.; Stender V. Potentials of the evolution of hydrogen in acid solutions. Zh. Fiz. Khim. 1950, 24, 856–9. [Google Scholar]
- Joncich M.; Stewart L.; Posey F. Hydrogen overvoltage on rhenium and niobium electrodes. J. Electrochem. Soc. 1965, 112 (7), 717. 10.1149/1.2423674. [DOI] [Google Scholar]
- Reier T.; Nong H. N.; Teschner D.; Schlögl R.; Strasser P. Electrocatalytic Oxygen Evolution Reaction in Acidic Environments – Reaction Mechanisms and Catalysts. Adv. Energy Mater. 2017, 7 (1), 1601275. 10.1002/aenm.201601275. [DOI] [Google Scholar]
- Ma Z.; Zhang Y.; Liu S.; Xu W.; Wu L.; Hsieh Y.-C.; Liu P.; Zhu Y.; Sasaki K.; Renner J. N.; et al. Reaction mechanism for oxygen evolution on RuO2, IrO2, and RuO2@ IrO2 core-shell nanocatalysts. J. Electroanal. Chem. 2018, 819, 296–305. 10.1016/j.jelechem.2017.10.062. [DOI] [Google Scholar]
- Reier T.; Oezaslan M.; Strasser P. Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal. 2012, 2 (8), 1765. 10.1021/cs3003098. [DOI] [Google Scholar]
- Lee Y.; Suntivich J.; May K. J.; Perry E. E.; Shao-Horn Y. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 2012, 3 (3), 399–404. 10.1021/jz2016507. [DOI] [PubMed] [Google Scholar]
- McCrory C. C. L.; Jung S.; Peters J. C.; Jaramillo T. F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135 (45), 16977–16987. 10.1021/ja407115p. [DOI] [PubMed] [Google Scholar]
- Lv L.; Yang Z.; Chen K.; Wang C.; Xiong Y. 2D layered double hydroxides for oxygen evolution reaction: from fundamental design to application. Adv. Energy Mater. 2019, 9 (17), 1803358. 10.1002/aenm.201803358. [DOI] [Google Scholar]
- Wang Q.; Xu C.-Q.; Liu W.; Hung S.-F.; Bin Yang H.; Gao J.; Cai W.; Chen H. M.; Li J.; Liu B. Coordination engineering of iridium nanocluster bifunctional electrocatalyst for highly efficient and pH-universal overall water splitting. Nat. Commun. 2020, 11 (1), 4246. 10.1038/s41467-020-18064-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz L. C.; Dickens C. F.; Nishio K.; Hikita Y.; Montoya J.; Doyle A.; Kirk C.; Vojvodic A.; Hwang H. Y.; Norskov J. K.; Jaramillo T. F. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science 2016, 353 (6303), 1011–1014. 10.1126/science.aaf5050. [DOI] [PubMed] [Google Scholar]
- Reier T.; Pawolek Z.; Cherevko S.; Bruns M.; Jones T.; Teschner D.; Selve S.; Bergmann A.; Nong H. N.; Schlögl R.; Mayrhofer K. J. J.; Strasser P. Molecular Insight in Structure and Activity of Highly Efficient, Low-Ir Ir–Ni Oxide Catalysts for Electrochemical Water Splitting (OER). J. Am. Chem. Soc. 2015, 137 (40), 13031–13040. 10.1021/jacs.5b07788. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y.; Sato E. Electrocatalytic properties of transition metal oxides for oxygen evolution reaction. Mater. Chem. Phys. 1986, 14 (5), 397–426. 10.1016/0254-0584(86)90045-3. [DOI] [Google Scholar]
- Man I. C.; Su H. Y.; Calle-Vallejo F.; Hansen H. A.; Martínez J. I.; Inoglu N. G.; Kitchin J.; Jaramillo T. F.; Nørskov J. K.; Rossmeisl J. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem. 2011, 3 (7), 1159–1165. 10.1002/cctc.201000397. [DOI] [Google Scholar]
- Burke M. S.; Enman L. J.; Batchellor A. S.; Zou S.; Boettcher S. W. Oxygen evolution reaction electrocatalysis on transition metal oxides and (oxy) hydroxides: activity trends and design principles. Chem. Mater. 2015, 27 (22), 7549–7558. 10.1021/acs.chemmater.5b03148. [DOI] [Google Scholar]
- Fabbri E.; Habereder A.; Waltar K.; Kötz R.; Schmidt T. J. Developments and perspectives of oxide-based catalysts for the oxygen evolution reaction. Catal. Sci. Technol. 2014, 4 (11), 3800–3821. 10.1039/C4CY00669K. [DOI] [Google Scholar]
- Kim J. S.; Kim B.; Kim H.; Kang K. Recent progress on multimetal oxide catalysts for the oxygen evolution reaction. Adv. Energy Mater. 2018, 8 (11), 1702774. 10.1002/aenm.201702774. [DOI] [Google Scholar]
- McCrory C. C.; Jung S.; Ferrer I. M.; Chatman S. M.; Peters J. C.; Jaramillo T. F. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 2015, 137 (13), 4347–4357. 10.1021/ja510442p. [DOI] [PubMed] [Google Scholar]
- Kim J.; Shih P.-C.; Tsao K.-C.; Pan Y.-T.; Yin X.; Sun C.-J.; Yang H. High-performance pyrochlore-type yttrium ruthenate electrocatalyst for oxygen evolution reaction in acidic media. J. Am. Chem. Soc. 2017, 139 (34), 12076–12083. 10.1021/jacs.7b06808. [DOI] [PubMed] [Google Scholar]
- Gao J.; Tao H.; Liu B. Progress of Nonprecious-Metal-Based Electrocatalysts for Oxygen Evolution in Acidic Media. Adv. Mater. 2021, 33 (31), 2003786. 10.1002/adma.202003786. [DOI] [PubMed] [Google Scholar]
- Frydendal R.; Paoli E. A.; Chorkendorff I.; Rossmeisl J.; Stephens I. E. Toward an active and stable catalyst for oxygen evolution in acidic media: Ti-stabilized MnO2. Adv. Energy Mater. 2015, 5 (22), 1500991. 10.1002/aenm.201500991. [DOI] [Google Scholar]
- Guo Y.; Park T.; Yi J. W.; Henzie J.; Kim J.; Wang Z.; Jiang B.; Bando Y.; Sugahara Y.; Tang J.; Yamauchi Y. Nanoarchitectonics for Transition-Metal-Sulfide-Based Electrocatalysts for Water Splitting. Adv. Mater. 2019, 31 (17), 1807134. 10.1002/adma.201807134. [DOI] [PubMed] [Google Scholar]
- Li X.; Hao X.; Abudula A.; Guan G. Nanostructured catalysts for electrochemical water splitting: current state and prospects. J. Mater. Chem. A 2016, 4, 11973–12000. 10.1039/C6TA02334G. [DOI] [Google Scholar]
- Zou X.; Zhang Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44 (15), 5148–5180. 10.1039/C4CS00448E. [DOI] [PubMed] [Google Scholar]
- Zhu W.; Bian Z.; Lu Y.. Environmental control system for pig farm based on mobile coordinator routing algorithm, Precision Livestock Farming 2019 - Papers Presented at the 9th European Conference on Precision Livestock Farming; ECPLF: 2019; pp 851–857.
- Doyle R.L.; Lyons M.E.. The oxygen evolution reaction: mechanistic concepts and catalyst design, Photoelectrochemical solar fuel production; Springer: 2016; pp 41–104. [Google Scholar]
- Shinagawa T.; Garcia-Esparza A. T.; Takanabe K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5 (1), 1–21. 10.1038/srep13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockris J. O. M. Kinetics of Activation Controlled Consecutive Electrochemical Reactions: Anodic Evolution of Oxygen. J. Chem. Phys. 1956, 24 (4), 817–827. 10.1063/1.1742616. [DOI] [Google Scholar]
- Wade W. H.; Hackerman N. Anodic phenomena at an iron electrode. Trans. Faraday Soc. 1957, 53, 1636–1647. 10.1039/tf9575301636. [DOI] [Google Scholar]
- Bockris J. O. M.; Otagawa T. The Electrocatalysis of Oxygen Evolution on Perovskites. J. Electrochem. Soc. 1984, 131 (2), 290–302. 10.1149/1.2115565. [DOI] [Google Scholar]
- Lu F.; Zhou M.; Zhou Y.; Zeng X. First-Row Transition Metal Based Catalysts for the Oxygen Evolution Reaction under Alkaline Conditions: Basic Principles and Recent Advances. Small 2017, 13 (45), 1701931. 10.1002/smll.201701931. [DOI] [PubMed] [Google Scholar]
- Grigoriev S. A.; Porembsky V. I.; Fateev V. N. Pure hydrogen production by PEM electrolysis for hydrogen energy. Int. J. Hydrogen Energy 2006, 31 (2), 171–175. 10.1016/j.ijhydene.2005.04.038. [DOI] [Google Scholar]
- Bard A. J.; Stratmann M.; Calvo E. J.. Encyclopedia of electrochemistry; Wiley-VCH: 2003. 10.1002/9783527610426. [DOI] [Google Scholar]
- Wei C.; Rao R. R.; Peng J.; Huang B.; Stephens I. E. L.; Risch M.; Xu Z. J.; Shao-Horn Y. Recommended Practices and Benchmark Activity for Hydrogen and Oxygen Electrocatalysis in Water Splitting and Fuel Cells. Adv. Mater. 2019, 31 (31), 1806296 10.1002/adma.201806296. [DOI] [PubMed] [Google Scholar]
- Pinaud B. A.; Benck J. D.; Seitz L. C.; Forman A. J.; Chen Z.; Deutsch T. G.; James B. D.; Baum K. N.; Baum G. N.; Ardo S.; Wang H.; Miller E.; Jaramillo T. F. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy. Environ. Sci. 2013, 6 (7), 1983. 10.1039/c3ee40831k. [DOI] [Google Scholar]
- Hansen J. N.; Prats H.; Toudahl K. K.; Mørch Secher N.; Chan K.; Kibsgaard J.; Chorkendorff I. Is There Anything Better than Pt for HER?. ACS Energy Lett. 2021, 6 (4), 1175–1180. 10.1021/acsenergylett.1c00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y.; Zheng Y.; Jaroniec M.; Qiao S. Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44 (8), 2060–2086. 10.1039/C4CS00470A. [DOI] [PubMed] [Google Scholar]
- Anantharaj S.; Noda S.; Driess M.; Menezes P. W. The Pitfalls of Using Potentiodynamic Polarization Curves for Tafel Analysis in Electrocatalytic Water Splitting. ACS Energy Lett. 2021, 1607–1611. 10.1021/acsenergylett.1c00608. [DOI] [Google Scholar]
- Anantharaj S.; Karthik P. E.; Noda S. The Significance of Properly Reporting Turnover Frequency in Electrocatalysis Research. Angew. Chem., Int. Ed. 2021, 60 (43), 23051–23067. 10.1002/anie.202110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasatti S.; Petrii O. A. Real surface area measurements in electrochemistry. J. Electroanal. Chem. 1992, 327 (1–2), 353–376. 10.1016/0022-0728(92)80162-W. [DOI] [Google Scholar]
- Wei C.; Sun S.; Mandler D.; Wang X.; Qiao S. Z.; Xu Z. J. Approaches for measuring the surface areas of metal oxide electrocatalysts for determining their intrinsic electrocatalytic activity. Chem. Soc. Rev. 2019, 48 (9), 2518–2534. 10.1039/C8CS00848E. [DOI] [PubMed] [Google Scholar]
- Biegler T.; Rand D. A. J.; Woods R. Limiting oxygen coverage on platinized platinum; Relevance to determination of real platinum area by hydrogen adsorption. J. Electroanal. Chem. Interfacial Electrochem. 1971, 29 (2), 269–277. 10.1016/S0022-0728(71)80089-X. [DOI] [Google Scholar]
- El Sawy E. N.; Birss V. I. Nano-porous iridium and iridium oxide thin films formed by high efficiency electrodeposition. J. Mater. Chem. 2009, 19 (43), 8244. 10.1039/b914662h. [DOI] [Google Scholar]
- Łosiewicz B.; Jurczakowski R.; Lasia A. Kinetics of hydrogen underpotential deposition at iridium in sulfuric and perchloric acids. Electrochim. Acta 2017, 225, 160–167. 10.1016/j.electacta.2016.12.116. [DOI] [Google Scholar]
- Stamenkovic V. R.; Fowler B.; Mun B. S.; Wang G.; Ross P. N.; Lucas C. A.; Marković N. M. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 2007, 315 (5811), 493–497. 10.1126/science.1135941. [DOI] [PubMed] [Google Scholar]
- Su M.; Dong J.-C.; Le J.-B.; Zhao Y.; Yang W.-M.; Yang Z.-L.; Attard G.; Liu G.-K.; Cheng J.; Wei Y.-M.; Tian Z.-Q.; Li J.-F. In Situ Raman Study of CO Electrooxidation on Pt(hkl) Single-Crystal Surfaces in Acidic Solution. Angew. Chem., Int. Ed. 2020, 59 (52), 23554–23558. 10.1002/anie.202010431. [DOI] [PubMed] [Google Scholar]
- Unmüssig T.; Melke J.; Fischer A. Synthesis of Pt@TiO2 nanocomposite electrocatalysts for enhanced methanol oxidation by hydrophobic nanoreactor templating. Phys. Chem. Chem. Phys. 2019, 21 (25), 13555–13568. 10.1039/C9CP00502A. [DOI] [PubMed] [Google Scholar]
- Rudi S.; Cui C.; Gan L.; Strasser P. Comparative Study of the Electrocatalytically Active Surface Areas (ECSAs) of Pt Alloy Nanoparticles Evaluated by Hupd and CO-stripping voltammetry. Electrocatalysis 2014, 5 (4), 408–418. 10.1007/s12678-014-0205-2. [DOI] [Google Scholar]
- Bandarenka A. S.; Varela A. S.; Karamad M.; Calle-Vallejo F.; Bech L.; Perez-Alonso F. J.; Rossmeisl J.; Stephens I. E. L.; Chorkendorff I. Design of an Active Site towards Optimal Electrocatalysis: Overlayers, Surface Alloys and Near-Surface Alloys of Cu/Pt(111). Angew. Chem. 2012, 124 (47), 12015–12018. 10.1002/ange.201205314. [DOI] [PubMed] [Google Scholar]
- Trasatti S.; Petrii O. A. Real surface area measurements in electrochemistry. Pure Appl. Chem. 1991, 63 (5), 711–734. 10.1351/pac199163050711. [DOI] [Google Scholar]
- Benck J. D.; Chen Z.; Kuritzky L. Y.; Forman A. J.; Jaramillo T. F. Amorphous Molybdenum Sulfide Catalysts for Electrochemical Hydrogen Production: Insights into the Origin of their Catalytic Activity. ACS Catal. 2012, 2 (9), 1916–1923. 10.1021/cs300451q. [DOI] [Google Scholar]
- Yoon Y.; Yan B.; Surendranath Y. Suppressing Ion Transfer Enables Versatile Measurements of Electrochemical Surface Area for Intrinsic Activity Comparisons. J. Am. Chem. Soc. 2018, 140 (7), 2397–2400. 10.1021/jacs.7b10966. [DOI] [PubMed] [Google Scholar]
- Chen Q.-S.; Solla-Gullón J.; Sun S.-G.; Feliu J. M. The potential of zero total charge of Pt nanoparticles and polycrystalline electrodes with different surface structure: The role of anion adsorption in fundamental electrocatalysis. Electrochim. Acta 2010, 55 (27), 7982–7994. 10.1016/j.electacta.2010.03.050. [DOI] [Google Scholar]
- Brunauer S.; Emmett P. H.; Teller E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60 (2), 309–319. 10.1021/ja01269a023. [DOI] [Google Scholar]
- Melder J.; Kwong W. L.; Shevela D.; Messinger J.; Kurz P. Electrocatalytic Water Oxidation by MnOx /C: In Situ Catalyst Formation, Carbon Substrate Variations, and Direct O2 /CO2Monitoring by Membrane-Inlet Mass Spectrometry. ChemSusChem 2017, 10 (22), 4491–4502. 10.1002/cssc.201701383. [DOI] [PubMed] [Google Scholar]
- Mosa I. M.; Biswas S.; El-Sawy A. M.; Botu V.; Guild C.; Song W.; Ramprasad R.; Rusling J. F.; Suib S. L. Tunable mesoporous manganese oxide for high performance oxygen reduction and evolution reactions. J. Mater. Chem. A 2016, 4 (2), 620–631. 10.1039/C5TA07878D. [DOI] [Google Scholar]
- El-Sayed H. A.; Weiß A.; Olbrich L. F.; Putro G. P.; Gasteiger H. A. OER Catalyst Stability Investigation Using RDE Technique: A Stability Measure or an Artifact?. J. Electrochem. Soc. 2019, 166 (8), F458–F464. 10.1149/2.0301908jes. [DOI] [Google Scholar]
- Fathi Tovini M.; Hartig-Weiß A.; Gasteiger H. A.; El-Sayed H. A. The Discrepancy in Oxygen Evolution Reaction Catalyst Lifetime Explained: RDE vs MEA - Dynamicity within the Catalyst Layer Matters. J. Electrochem. Soc. 2021, 168 (1), 014512. 10.1149/1945-7111/abdcc9. [DOI] [Google Scholar]
- Li Y.; Du X.; Huang J.; Wu C.; Sun Y.; Zou G.; Yang C.; Xiong J. Recent Progress on Surface Reconstruction of Earth-Abundant Electrocatalysts for Water Oxidation. Small 2019, 15 (35), 1901980 10.1002/smll.201901980. [DOI] [PubMed] [Google Scholar]
- Zeng Y.; Zhao M.; Huang Z.; Zhu W.; Zheng J.; Jiang Q.; Wang Z.; Liang H. Surface Reconstruction of Water Splitting Electrocatalysts. Adv. Energy Mater. 2022, 12 (33), 2201713. 10.1002/aenm.202201713. [DOI] [Google Scholar]
- Lazaridis T.; Stühmeier B. M.; Gasteiger H. A.; El-Sayed H. A. Capabilities and limitations of rotating disk electrodes versus membrane electrode assemblies in the investigation of electrocatalysts. Nat. Catal. 2022, 5 (5), 363–373. 10.1038/s41929-022-00776-5. [DOI] [Google Scholar]
- Miles M. H. Evaluation of Electrocatalysts for Water Electrolysis in Alkaline-Solutions. J. Electroanal. Chem. 1975, 60 (1), 89–96. 10.1016/S0022-0728(75)80205-1. [DOI] [Google Scholar]
- Miles M. H.; Thomason M. A. Periodic Variations of Overvoltages for Water Electrolysis in Acid Solutions from Cyclic Voltammetric Studies. J. Electrochem. Soc. 1976, 123 (10), 1459–1461. 10.1149/1.2132619. [DOI] [Google Scholar]
- Garcia-Garcia R.; Ortega-Zarzosa G.; Rincón M. E.; Orozco G. The Hydrogen Evolution Reaction on Rhenium Metallic Electrodes: A Selected Review and New Experimental Evidence. Electrocatalysis 2015, 6 (3), 263–273. 10.1007/s12678-014-0240-z. [DOI] [Google Scholar]
- Rivera J. G.; Garcia-Garcia R.; Coutino-Gonzalez E.; Orozco G. Hydrogen evolution reaction on metallic rhenium in acid media with or without methanol. Int. J. Hydrogen Energy 2019, 44 (50), 27472–27482. 10.1016/j.ijhydene.2019.08.212. [DOI] [Google Scholar]
- Zerbino J. O.; Luna A. M. C.; Zinola C. F.; Mendez E.; Martins M. E. A comparative study of electrochemical and optical properties of rhenium deposited on gold and platinum. J. Brazil Chem. Soc. 2002, 13 (4), 510–515. 10.1590/S0103-50532002000400016. [DOI] [Google Scholar]
- Huang Q.; Lyons T. W. Electrodeposition of rhenium with suppressed hydrogen evolution from water-in-salt electrolyte. Electrochem. Commun. 2018, 93, 53–56. 10.1016/j.elecom.2018.06.003. [DOI] [Google Scholar]
- Méndez E.; Cerdá M.a.F.; Castro Luna A. M.; Zinola C. F.; Kremer C.; Martins M.a.E. Electrochemical behavior of aqueous acid perrhenate-containing solutions on noble metals: critical review and new experimental evidence. J. Colloid Interface Sci. 2003, 263 (1), 119–132. 10.1016/S0021-9797(03)00165-6. [DOI] [PubMed] [Google Scholar]
- Cao H. Z.; Chai D. G.; Wu L. K.; Zheng G. Q. Communication—A Mechanistic Study on Electrodeposition of Rhenium from Acidic Solution of Ammonium Perrhenate. J. Electrochem. Soc. 2017, 164 (13), D825–D827. 10.1149/2.0871713jes. [DOI] [Google Scholar]
- Hahn B. P.; May R. A.; Stevenson K. J. Electrochemical deposition and characterization of mixed-valent rhenium oxide films prepared from a perrhenate solution. Langmuir 2007, 23 (21), 10837–10845. 10.1021/la701504z. [DOI] [PubMed] [Google Scholar]
- Schrebler R.; Cury P.; Suarez C.; Munoz E.; Vera F.; Cordova R.; Gomez H.; Ramos-Barrado J. R.; Leinen D.; Dalchiele E. A. Study of the electrodeposition of rhenium thin films by electrochemical quartz microbalance and X-ray photoelectron spectroscopy. Thin Solid Films 2005, 483 (1–2), 50–59. 10.1016/j.tsf.2004.12.061. [DOI] [Google Scholar]
- Munoz E. C.; Schrebler R. S.; Orellana M. A.; Cordova R. Rhenium electrodeposition process onto p-Si(100) and electrochemical behaviour of the hydrogen evolution reaction onto p-Si/Re/0.1 M H2SO4 interface. J. Electroanal. Chem. 2007, 611 (1–2), 35–42. 10.1016/j.jelechem.2007.07.023. [DOI] [Google Scholar]
- Eliaz N.; Gileadi E., Induced Codeposition of Alloys of Tungsten, Molybdenum and Rhenium with Transition Metals. In Modern Aspects of Electrochemistry; Vayenas C. G., White R. E., Gamboa-Aldeco M. E., Eds. Springer New York: 2008; pp 191–301. [Google Scholar]
- Szabó S.; Bakos I.. Study of rhenium deposition onto Pt surface with electrochemical methods. In: Studies in Surface Science Catalysis;Delmon B., Jacobs P. A., Maggi R., Martens J. A., Grange P., Poncelet G., Eds.; Elsevier: 1998; pp 269–276. [Google Scholar]
- Szabó S.; Bakos I. Electrodeposition of rhenium species onto a gold surface in sulfuric acid media. J. Solid State Electrochem. 2004, 8 (3), 190–194. 10.1007/s10008-003-0425-5. [DOI] [Google Scholar]
- Cao H. Z.; Hu L. L.; Zhang H. B.; Hou G. Y.; Tang Y. P.; Zheng G. Q. The Significant Effect of Supporting Electrolytes on the Galvanic Deposition of Metallic Rhenium. Int. J. Electrochem Sc 2020, 15 (7), 6769–6777. 10.20964/2020.07.53. [DOI] [Google Scholar]
- Aliaga J.; Vera P.; Araya J.; Ballesteros L.; Urzua J.; Farias M.; Paraguay-Delgado F.; Alonso-Nunez G.; Gonzalez G.; Benavente E. Electrochemical Hydrogen Evolution over Hydrothermally Synthesized Re-Doped MoS2 Flower-Like Microspheres. Molecules 2019, 24 (24), 4631. 10.3390/molecules24244631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak I. H.; Debela T. T.; Kwon I. S.; Seo J.; Yoo S. J.; Kim J. G.; Ahn J. P.; Park J.; Kang H. S. Anisotropic alloying of Re1-xMoxS2 nanosheets to boost the electrochemical hydrogen evolution reaction. J. Mater. Chem. A 2020, 8 (47), 25131–25141. 10.1039/D0TA09299A. [DOI] [Google Scholar]
- Shi W. W.; Wang Z. G.; Fu Y. Q. Rhenium doping induced structural transformation in mono-layered MoS2 with improved catalytic activity for hydrogen evolution reaction. J. Phys. D-Appl. Phys. 2017, 50 (40), 405303. 10.1088/1361-6463/aa85c9. [DOI] [Google Scholar]
- Yang S. Z.; Gong Y. J.; Manchanda P.; Zhang Y. Y.; Ye G. L.; Chen S. M.; Song L.; Pantelides S. T.; Ajayan P. M.; Chisholm M. F.; Zhou W. Rhenium-Doped and Stabilized MoS2 Atomic Layers with Basal-Plane Catalytic Activity. Adv. Mater. 2018, 30 (51), 1803477. 10.1002/adma.201803477. [DOI] [PubMed] [Google Scholar]
- Chhetri M.; Gupta U.; Yadgarov L.; Rosentsveig R.; Tenne R.; Rao C. N. R. Beneficial effect of Re doping on the electrochemical HER activity of MoS2 fullerenes. Dalton Trans. 2015, 44 (37), 16399–16404. 10.1039/C5DT02562A. [DOI] [PubMed] [Google Scholar]
- Zhuo Z.; Zhu J.; Wei A.; Liu J.; Luo N.; Zhao Y.; Liu Z. Electrocatalytic performance of ReS2 nanosheets in hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47 (4), 2293–2303. 10.1016/j.ijhydene.2021.10.120. [DOI] [Google Scholar]
- Fujita T.; Ito Y.; Tan Y.; Yamaguchi H.; Hojo D.; Hirata A.; Voiry D.; Chhowalla M.; Chen M. Chemically Exfoliated ReS2 nanosheets. Nanoscale 2014, 6 (21), 12458–12462. 10.1039/C4NR03740E. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Meng J.; Ju H.; Zhu J.; Li Q.; Yang Q. Electrochemical activity of 1T′ structured rhenium selenide nanosheets via electronic structural modulation from selenium-vacancy generation. J. Mater. Chem. A 2018, 6 (45), 22526–22533. 10.1039/C8TA06889E. [DOI] [Google Scholar]
- Wang L.; Sofer Z.; Luxa J.; Sedmidubsky D.; Ambrosi A.; Pumera M. Layered rhenium sulfide on free-standing three-dimensional electrodes is highly catalytic for the hydrogen evolution reaction: Experimental and theoretical study. Electrochem. Commun. 2016, 63, 39–43. 10.1016/j.elecom.2015.11.011. [DOI] [Google Scholar]
- Gao J.; Li L.; Tan J. W.; Sun H.; Li B. C.; Idrobo J. C.; Singh C. V.; Lu T. M.; Koratkar N. Vertically Oriented Arrays of ReS2 Nanosheets for Electrochemical Energy Storage and Electrocatalysis. Nano Lett. 2016, 16 (6), 3780–3787. Article 10.1021/acs.nanolett.6b01180. [DOI] [PubMed] [Google Scholar]
- Zhao W. C.; Tan X. X.; Jiang J. Y.; Liu F. J.; Mu T. C. Highly Efficient, Green, and Scalable beta-Cyclodextrin-Assisted Aqueous Exfoliation of Transition-Metal Dichalcogenides: MoS2 and ReS2 Nanoflakes. Chem. - Asian J. 2017, 12 (10), 1052–1056. 10.1002/asia.201700355. [DOI] [PubMed] [Google Scholar]
- Luxa J.; Marvan P.; Lazar P.; Sofer Z. Chalcogenide vacancies drive the electrocatalytic performance of rhenium dichalcogenides. Nanoscale 2019, 11 (31), 14684–14690. 10.1039/C9NR03281A. [DOI] [PubMed] [Google Scholar]
- Pang Q. Q.; Niu Z. L.; Yi S. S.; Zhang S.; Liu Z. Y.; Yue X. Z. Hydrogen-Etched Bifunctional Sulfur-Defect-Rich ReS2/CC Electrocatalyst for Highly Efficient HER and OER. Small 2020, 16 (34), 2003007. 10.1002/smll.202003007. [DOI] [PubMed] [Google Scholar]
- Huang W. T.; Su S. Q.; Liu Y. W.; Li J.; Wang M. J.; Hou Z. P.; Gao X. S.; Wang X.; Notzel R.; Zhou G. F.; Zhang Z.; Liu J. M. Wood-derived electrode supporting CVD-grown ReS(2) for efficient and stable hydrogen production. J. Mater. Sci. 2021, 56 (2), 1551–1560. 10.1007/s10853-020-05248-4. [DOI] [Google Scholar]
- Zhou Y.; Song E. H.; Zhou J. D.; Lin J. H.; Ma R. G.; Wang Y. W.; Qiu W. J.; Shen R. X.; Suenaga K.; Liu Q.; Wang J. C.; Liu Z.; Liu J. J. Auto-optimizing Hydrogen Evolution Catalytic Activity of ReS2 through Intrinsic Charge Engineering. ACS Nano 2018, 12 (5), 4486–4493. 10.1021/acsnano.8b00693. [DOI] [PubMed] [Google Scholar]
- Ng S.; Iffelsberger C.; Sofer Z.; Pumera M. Tunable Room-Temperature Synthesis of ReS2 Bicatalyst on 3D-and 2D-Printed Electrodes for Photo- and Electrochemical Energy Applications. Adv. Funct. Mater. 2020, 30 (19), 1910193. 10.1002/adfm.201910193. [DOI] [Google Scholar]
- Wang J. C.; He J. J.; Odunmbaku G. O.; Zhao S.; Gou Q. Z.; Han G.; Xu C. H.; Frauenheim T.; Li M. Regulating the electronic structure of ReS2 by Mo doping for electrocatalysis and lithium storage. Chem. Eng. J. 2021, 414, 128811. 10.1016/j.cej.2021.128811. [DOI] [Google Scholar]
- Liu Y. G.; Li H. J.; Li J. F.; Ma X. S.; Cui Z. M.; Gao D. Q.; Tang Z. H. Fluorination activates the basal plane HER activity of ReS2: a combined experimental and theoretical study. J. Mater. Chem. A 2021, 9 (25), 14451–14458. 10.1039/D1TA03258E. [DOI] [Google Scholar]
- Lai F.; Chen N.; Ye X.; He G.; Zong W.; Holt K. B.; Pan B.; Parkin I. P.; Liu T.; Chen R. Refining Energy Levels in ReS2 Nanosheets by Low-Valent Transition-Metal Doping for Dual-Boosted Electrochemical Ammonia/Hydrogen Production. Adv. Funct. Mater. 2020, 30 (11), 1907376. 10.1002/adfm.201907376. [DOI] [Google Scholar]
- Xu J.; Fang C. J.; Zhu Z. Q.; Wang J. W.; Yu B. S.; Zhang J. J. Nanoscale engineering and Mo-doping of 2D ultrathin ReS(2)nanosheets for remarkable electrocatalytic hydrogen generation. Nanoscale 2020, 12 (32), 17045–17052. 10.1039/D0NR03693E. [DOI] [PubMed] [Google Scholar]
- Gao H.; Yue H. H.; Qi F.; Yu B.; Zhang W. L.; Chen Y. F. Few-layered ReS2 nanosheets grown on graphene as electrocatalyst for hydrogen evolution reaction. Rare Metals 2018, 37 (12), 1014–1020. 10.1007/s12598-018-1121-z. [DOI] [Google Scholar]
- Askari M. B.; Salarizadeh P. Ultra-small ReS2 nanoparticles hybridized with rGO as cathode and anode catalysts towards hydrogen evolution reaction and methanol electro-oxidation for DMFC in acidic and alkaline media. Synth. Met. 2019, 256, 116131. 10.1016/j.synthmet.2019.116131. [DOI] [Google Scholar]
- Feng Q. L.; Li M.; Wang T. X.; Chen Y. P.; Wang X. J.; Zhang X. D.; Li X. B.; Yang Z. C. Y.; Feng L. P.; Zheng J. B.; Xu H.; Zhai T. Y.; Jiang Y. M. Low-temperature growth of Three dimensional ReS2/ReO2 metal-semiconductor heterojunctions on Graphene/polyimide film for enhanced hydrogen evolution reaction. Appl. Catal., B 2020, 271, 118924. 10.1016/j.apcatb.2020.118924. [DOI] [Google Scholar]
- Yi M.; Li N.; Lu B.; Li L.; Zhu Z.; Zhang J. Single-atom Pt decorated in heteroatom (N, B, and F)-doped ReS2 Grown on Mo2CTx for efficient pH-universal hydrogen evolution reaction and flexible Zn-air batteries. Energy Storage Mater. 2021, 42, 418–429. 10.1016/j.ensm.2021.07.048. [DOI] [Google Scholar]
- Kwak I. H.; Kwon I. S.; Debela T. T.; Abbas H. G.; Park Y. C.; Seo J.; Ahn J.-P.; Lee J. H.; Park J.; Kang H. S. Phase Evolution of Re1-xMoxSe2 Alloy Nanosheets and Their Enhanced Catalytic Activity toward Hydrogen Evolution Reaction. ACS Nano 2020, 14 (9), 11995–12005. 10.1021/acsnano.0c05159. [DOI] [PubMed] [Google Scholar]
- Zhuang M.; Xu G.-L.; Gan L.-Y.; Dou Y.; Sun C.-J.; Ou X.; Xie Y.; Liu Z.; Cai Y.; Ding Y.; Abidi I. H.; Tyagi A.; Amine K.; Luo Z. Sub-5 nm edge-rich 1T′-ReSe2 as bifunctional materials for hydrogen evolution and sodium-ion storage. Nano Energy 2019, 58, 660–668. 10.1016/j.nanoen.2019.01.093. [DOI] [Google Scholar]
- Xia Y.; Huang J.; Wu W.; Zhang Y.; Wang H.; Zhu J.; Yao J.; Xu L.; Sun Y.; Zhang L.; Lu R.; Xiong J.; Zou G. Sulfur-Doped Rhenium Selenide Vertical Nanosheets: A High-Performance Electrocatalyst for Hydrogen Evolution. ChemCatChem. 2018, 10 (19), 4424–4430. 10.1002/cctc.201800757. [DOI] [Google Scholar]
- Liu Z.; Ou X.; Zhuang M.; Li J.; Hossain M. D.; Ding Y.; Wong H.; You J.; Cai Y.; Abidi I. H.; Tyagi A.; Shao M.; Yuan B.; Luo Z. Confinement-Enhanced Rapid Interlayer Diffusion within Graphene-Supported Anisotropic ReSe2 Electrodes. ACS Appl. Mater. Interfaces. 2019, 11 (34), 31147–31154. 10.1021/acsami.9b08157. [DOI] [PubMed] [Google Scholar]
- Li J.; Liu Y.; Liu C.; Huang W.; Zhang Y.; Wang M.; Hou Z.; Wang X.; Jin M.; Zhou G.; Gao X.; Zhang Z.; Liu J. Enhanced charge transport in ReSe2-based 2D/3D electrodes for efficient hydrogen evolution reaction. Chem. Commun. 2020, 56 (2), 305–308. 10.1039/C9CC08076G. [DOI] [PubMed] [Google Scholar]
- Li J.; Zhou Q.; Yuan C.; Cheng P.; Hu X.; Huang W.; Gao X.; Wang X.; Jin M.; Nötzel R.; Zhou G.; Zhang Z.; Liu J. Direct growth of vertically aligned ReSe2 nanosheets on conductive electrode for electro-catalytic hydrogen production. J. Colloid Interface Sci. 2019, 553, 699–704. 10.1016/j.jcis.2019.06.073. [DOI] [PubMed] [Google Scholar]
- Qi F.; Wang X.; Zheng B.; Chen Y.; Yu B.; Zhou J.; He J.; Li P.; Zhang W.; Li Y. Self-assembled chrysanthemum-like microspheres constructed by few-layer ReSe2 nanosheets as a highly efficient and stable electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2017, 224, 593–599. 10.1016/j.electacta.2016.12.097. [DOI] [Google Scholar]
- Yan Y.; Xu S.; Li H.; Selvam N. C. S.; Lee J. Y.; Lee H.; Yoo P. J. Perpendicularly anchored ReSe2 nanoflakes on reduced graphene oxide support for highly efficient hydrogen evolution reactions. Chem. Eng. J. 2021, 405, 126728. 10.1016/j.cej.2020.126728. [DOI] [Google Scholar]
- Lai Z.; Chaturvedi A.; Wang Y.; Tran T. H.; Liu X.; Tan C.; Luo Z.; Chen B.; Huang Y.; Nam G.-H.; Zhang Z.; Chen Y.; Hu Z.; Li B.; Xi S.; Zhang Q.; Zong Y.; Gu L.; Kloc C.; Du Y.; Zhang H. Preparation of 1T′-Phase ReS2xSe2(1-x) (x = 0–1) Nanodots for Highly Efficient Electrocatalytic Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2018, 140 (27), 8563–8568. 10.1021/jacs.8b04513. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Zhao Y.; Liu H.; Shao M.; Chen Z.; Ma T.; Wu Z.; Wang L. N, P-doped carbon supported ruthenium doped Rhenium phosphide with porous nanostructure for hydrogen evolution reaction using sustainable energies. J. Colloid Interface Sci. 2022, 606, 1874–1881. 10.1016/j.jcis.2021.08.077. [DOI] [PubMed] [Google Scholar]
- Shi Y.; Zhang B. Correction: Recent advances in transition metal phosphide nanomaterials: synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45 (6), 1781–1781. 10.1039/C6CS90013E. [DOI] [PubMed] [Google Scholar]
- Popczun E. J.; McKone J. R.; Read C. G.; Biacchi A. J.; Wiltrout A. M.; Lewis N. S.; Schaak R. E. Nanostructured Nickel Phosphide as an Electrocatalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2013, 135 (25), 9267–9270. 10.1021/ja403440e. [DOI] [PubMed] [Google Scholar]
- Carenco S.; Portehault D.; Boissière C.; Mézailles N.; Sanchez C. Nanoscaled Metal Borides and Phosphides: Recent Developments and Perspectives. Chem. Rev. 2013, 113 (10), 7981–8065. 10.1021/cr400020d. [DOI] [PubMed] [Google Scholar]
- Sun F.; Wang Y.; Fang L.; Yang X.; Fu W.; Tian D.; Huang Z.; Li J.; Zhang H.; Wang Y. New vesicular carbon-based rhenium phosphides with all-pH range electrocatalytic hydrogen evolution activity. Appl. Catal., B 2019, 256, 117851. 10.1016/j.apcatb.2019.117851. [DOI] [Google Scholar]
- Guo F.; Wu Y.; Ai X.; Chen H.; Li G.-D.; Chen W.; Zou X. A class of metal diboride electrocatalysts synthesized by a molten salt-assisted reaction for the hydrogen evolution reaction. Chem. Commun. 2019, 55 (59), 8627–8630. 10.1039/C9CC03638E. [DOI] [PubMed] [Google Scholar]
- Greeley J.; Nørskov J. K.; Kibler L. A.; El-Aziz A. M.; Kolb D. M. Hydrogen Evolution Over Bimetallic Systems: Understanding the Trends. ChemPhysChem 2006, 7 (5), 1032–1035. 10.1002/cphc.200500663. [DOI] [PubMed] [Google Scholar]
- Zabinski P.; Franczak A.; Kowalik R. Electrodeposition of Functional Ni-Re Alloys for Hydrogen Evolution. ECS Trans. 2012, 41 (33), 39–48. 10.1149/1.3702411. [DOI] [Google Scholar]
- Kim H.; Kim J.; Han G. H.; Guo W.; Hong S.; Park J.; Ahn S. H. Electrodeposited rhenium-cobalt alloy with high activity for acidic hydrogen evolution reaction. J. Ind. Eng. Chem. 2021, 95, 357–366. 10.1016/j.jiec.2021.01.008. [DOI] [Google Scholar]
- Pashova V.; Mirkova L.; Monev M. Electrocatalytic Materials of NiCoRe Electrodeposited Alloy for Alkaline Water Electrolysis. ECS Trans. 2010, 25 (35), 395. 10.1149/1.3414032. [DOI] [Google Scholar]
- Gamburg Y. D.; Zhulikov V. V.; Lyakhov B. F. Electrodeposition, properties, and composition of rhenium-nickel alloys. Russ. J. Electrochem. 2016, 52 (1), 78–82. 10.1134/S1023193515120058. [DOI] [Google Scholar]
- Kuznetsov V. V.; Gamburg Y. D.; Zhulikov V. V.; Batalov R. S.; Filatova E. A. Re-Ni cathodes obtained by electrodeposition as a promising electrode material for hydrogen evolution reaction in alkaline solutions. Electrochim. Acta 2019, 317, 358–366. 10.1016/j.electacta.2019.05.156. [DOI] [Google Scholar]
- Kuznetsov V. V.; Gamburg Y.; Zhulikov V. V.; Krutskikh V. M.; Filatova E. A.; Trigub A. L.; Belyakova O. A. Electrodeposited NiMo, CoMo, ReNi, and electroless NiReP alloys as cathode materials for hydrogen evolution reaction. Electrochim. Acta 2020, 354, 136610. 10.1016/j.electacta.2020.136610. [DOI] [Google Scholar]
- Yang X.; Koel B. E.; Wang H.; Chen W.; Bartynski R. A. Nanofaceted C/Re(11): Fabrication, Structure, and Template for Synthesizing Nanostructured Model Pt Electrocatalyst for Hydrogen Evolution Reaction. ACS Nano 2012, 6 (2), 1404–1409. 10.1021/nn204615j. [DOI] [PubMed] [Google Scholar]
- Vargas-Uscategui A.; Mosquera E.; Chornik B.; Cifuentes L. Electrocatalysis of the hydrogen evolution reaction by rhenium oxides electrodeposited by pulsed-current. Electrochim. Acta 2015, 178, 739–747. 10.1016/j.electacta.2015.08.065. [DOI] [Google Scholar]
- Cheng Y.; Fan X.; Liao F.; Lu S.; Li Y.; Liu L.; Li Y.; Lin H.; Shao M.; Lee S.-T. Os/Si nanocomposites as excellent hydrogen evolution electrocatalysts with thermodynamically more favorable hydrogen adsorption free energy than platinum. Nano Energy 2017, 39, 284–290. 10.1016/j.nanoen.2017.07.009. [DOI] [Google Scholar]
- Yang L.; Lu S.; Wang H.; Shao Q.; Liao F.; Shao M. The self-activation and synergy of amorphous Re nanoparticle - Si nanowire composites for the electrocatalytic hydrogen evolution. Electrochim. Acta 2017, 228, 268–273. 10.1016/j.electacta.2017.01.048. [DOI] [Google Scholar]
- Kim M.; Yang Z.; Park J. H.; Yoon S. M.; Grzybowski B. A. Nanostructured Rhenium-Carbon Composites as Hydrogen-Evolving Catalysts Effective over the Entire pH Range. ACS Appl. Nano Mater. 2019, 2 (5), 2725–2733. 10.1021/acsanm.9b00236. [DOI] [Google Scholar]
- Wu W.; Yao J.; Liu S.; Zhao L.; Xu L.; Sun Y.; Lou Y.; Zhao J.; Choi J.-H.; Jiang L.; Wang H.; Zou G. Nanostructured hexagonal ReO3 with oxygen vacancies for efficient electrocatalytic hydrogen generation. Nanotechnology 2019, 30 (35), 355701. 10.1088/1361-6528/ab214c. [DOI] [PubMed] [Google Scholar]
- Karthick K.; Subhashini S.; Teepikha M.; Kumar R.; Sethuram Markandaraj S.; Kundu S. Employing DNA scaffold with rhenium electrocatalyst for enhanced HER activities. Appl. Surf. Sci. 2020, 528, 147049. 10.1016/j.apsusc.2020.147049. [DOI] [Google Scholar]
- Umena Y.; Kawakami K.; Shen J.-R.; Kamiya N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473 (7345), 55–60. 10.1038/nature09913. [DOI] [PubMed] [Google Scholar]
- Melder J.; Bogdanoff P.; Zaharieva I.; Fiechter S.; Dau H.; Kurz P. Water-Oxidation Electrocatalysis by Manganese Oxides: Syntheses, Electrode Preparations, Electrolytes and Two Fundamental Questions. Z. Phys. Chem. 2020, 234 (5), 925–978. 10.1515/zpch-2019-1491. [DOI] [Google Scholar]
- Najafpour M. M.; Allakhverdiev S. I. Manganese compounds as water oxidizing catalysts for hydrogen production via water splitting: From manganese complexes to nano-sized manganese oxides. Int. J. Hydrogen Energy 2012, 37 (10), 8753–8764. 10.1016/j.ijhydene.2012.02.075. [DOI] [Google Scholar]
- Jiao F.; Frei H. Nanostructured cobalt and manganese oxide clusters as efficient water oxidation catalysts. Energy. Environ. Sci. 2010, 3 (8), 1018. 10.1039/c002074e. [DOI] [Google Scholar]
- Park S.; Lee Y. H.; Choi S.; Seo H.; Lee M. Y.; Balamurugan M.; Nam K. T. Manganese oxide-based heterogeneous electrocatalysts for water oxidation. Energy. Environ. Sci. 2020, 13 (8), 2310–2340. 10.1039/D0EE00815J. [DOI] [Google Scholar]
- Najafpour M. M.; Renger G.; Hołyńska M.; Moghaddam A. N.; Aro E.-M.; Carpentier R.; Nishihara H.; Eaton-Rye J. J.; Shen J.-R.; Allakhverdiev S. I. Manganese Compounds as Water-Oxidizing Catalysts: From the Natural Water-Oxidizing Complex to Nanosized Manganese Oxide Structures. Chem. Rev. 2016, 116 (5), 2886–2936. 10.1021/acs.chemrev.5b00340. [DOI] [PubMed] [Google Scholar]
- Oliver N.; Avramov A. P.; Nürnberg D. J.; Dau H.; Burnap R. L. From manganese oxidation to water oxidation: assembly and evolution of the water-splitting complex in photosystem II. Photosynth. Res. 2022, 152, 107. 10.1007/s11120-022-00912-z. [DOI] [PubMed] [Google Scholar]
- Rouschias G. Recent advances in the chemistry of rhenium. Chem. Rev. 1974, 74 (5), 531–566. 10.1021/cr60291a002. [DOI] [Google Scholar]
- Farińa A.; Hernández S.; Román E. Catalytic oxidation of water mediated by rhenium oxides. J. Chem. Soc., Dalton Trans. 1989, (5), 849–851. 10.1039/DT9890000849. [DOI] [Google Scholar]
- Mills A.; Russell T. Comparative study of new and established heterogeneous oxygen catalysts. J. Chem. Soc., Faraday Trans. 1991, 87 (8), 1245. 10.1039/ft9918701245. [DOI] [Google Scholar]
- Chen J.; Chen H.; Yu T.; Li R.; Wang Y.; Shao Z.; Song S. Recent Advances in the Understanding of the Surface Reconstruction of Oxygen Evolution Electrocatalysts and Materials Development. Electrochem. Energy Rev. 2021, 4 (3), 566–600. 10.1007/s41918-021-00104-8. [DOI] [Google Scholar]
- Balaghi S. E.; Mehrabani S.; Mousazade Y.; Bagheri R.; Sologubenko A. S.; Song Z.; Patzke G. R.; Najafpour M. M. Mechanistic Understanding of Water Oxidation in the Presence of a Copper Complex by In Situ Electrochemical Liquid Transmission Electron Microscopy. ACS Appl. Mater. Interfaces. 2021, 13 (17), 19927–19937. 10.1021/acsami.1c00243. [DOI] [PubMed] [Google Scholar]
- Abdi Z.; Balaghi S. E.; Sologubenko A. S.; Willinger M.-G.; Vandichel M.; Shen J.-R.; Allakhverdiev S. I.; Patzke G. R.; Najafpour M. M. Understanding the Dynamics of Molecular Water Oxidation Catalysts with Liquid-Phase Transmission Electron Microscopy: The Case of Vitamin B 12. ACS Sustainable Chem. Eng. 2021, 9 (28), 9494–9505. 10.1021/acssuschemeng.1c03539. [DOI] [Google Scholar]
- Suzuki H.; Tomita O.; Higashi M.; Abe R. The first example of an oxide semiconductor photocatalyst consisting of a heptavalent cation: visible-light-induced water oxidation on M 3 ReO 8. J. Mater. Chem. A 2018, 6 (5), 1991–1994. 10.1039/C7TA10185F. [DOI] [Google Scholar]
- Oshikiri M.; Ye J.; Boero M. Inhomogeneous RVO 4 Photocatalyst Systems (R = Y, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu). J. Phys. Chem. C 2014, 118 (16), 8331–8341. 10.1021/jp410565e. [DOI] [Google Scholar]
- Rahman M.; Davey K.; Qiao S. Z. Advent of 2D Rhenium Disulfide (ReS2): Fundamentals to Applications. Adv. Funct. Mater. 2017, 27 (10), 1606129. 10.1002/adfm.201606129. [DOI] [Google Scholar]
- Liu H.; Xu B.; Liu J. M.; Yin J.; Miao F.; Duan C.-G.; Wan X. G. Highly efficient and ultrastable visible-light photocatalytic water splitting over ReS2. Phys. Chem. Chem. Phys. 2016, 18 (21), 14222–14227. 10.1039/C6CP01007E. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Song W.; Li Z.; Zhang Z.; Zhou G. A new strategy: fermi level control to realize 3D pyramidal NiCo-LDH/ReS2 /n-PSi as a high-performance photoanode for the oxygen evolution reaction. J. Mater. Chem. C 2022, 10 (10), 3848–3855. 10.1039/D1TC05863K. [DOI] [Google Scholar]
- Roy S. B.; Jung E.; Kim K. H.; Patil A.; Chun S.-H.; Park J. H.; Jun S. C. Simultaneous integration of low-level rhenium (Re) doping and nitrogen-functionalized 3D carbon backbone into nickel-iron hydroxide (NiFeOH) to amplify alkaline water electrolysis at high current densities. Chem. Eng. J. 2022, 435, 135184. 10.1016/j.cej.2022.135184. [DOI] [Google Scholar]
- Han X.; Li N.; Kang Y. B.; Dou Q.; Xiong P.; Liu Q.; Lee J. Y.; Dai L.; Park H. S. Unveiling Trifunctional Active Sites of a Heteronanosheet Electrocatalyst for Integrated Cascade Battery/Electrolyzer Systems. ACS Energy Lett. 2021, 6 (7), 2460–2468. 10.1021/acsenergylett.1c00936. [DOI] [Google Scholar]
- Das T. K.; Ping T.; Mohapatra M.; Anwar S.; Gopinath C. S.; Jena B. K. Concerted effect of Ni-in and S-out on ReS2 nanostructures towards high-efficiency oxygen evolution reaction. Chem. Commun. 2022, 58 (22), 3689–3692. 10.1039/D1CC07030D. [DOI] [PubMed] [Google Scholar]
- Urbanová V.; Antonatos N.; Plutnar J.; Lazar P.; Michalička J.; Otyepka M.; Sofer Z.; Pumera M. Rhenium Doping of Layered Transition-Metal Diselenides Triggers Enhancement of Photoelectrochemical Activity. ACS Nano 2021, 15 (2), 2374–2385. 10.1021/acsnano.0c04437. [DOI] [PubMed] [Google Scholar]
- Han X.; Li N.; Xiong P.; Kang Y.; Dou Q.; Liu Q.; Li W.; Lee J. Y.; Park H. S. Rhenium induced electronic structure modulation of Ni3S2/N-doped graphene for efficient trifunctional electrocatalysis. Composites, Part B 2022, 234, 109670. 10.1016/j.compositesb.2022.109670. [DOI] [Google Scholar]
- Nørskov J. K.; Bligaard T.; Logadottir A.; Kitchin J. R.; Chen J. G.; Pandelov S.; Stimming U. Trends in the Exchange Current for Hydrogen Evolution. J. Electrochem. Soc. 2005, 152 (3), J23. 10.1149/1.1856988. [DOI] [Google Scholar]
- Yang T. T.; Patil R. B.; McKone J. R.; Saidi W. A. Revisiting trends in the exchange current for hydrogen evolution. Catal. Sci. Technol. 2021, 11 (20), 6832–6838. 10.1039/D1CY01170G. [DOI] [Google Scholar]
- Nørskov J. K.; Bligaard T.; Rossmeisl J.; Christensen C. H. Towards the computational design of solid catalysts. Nat. Chem. 2009, 1 (1), 37–46. 10.1038/nchem.121. [DOI] [PubMed] [Google Scholar]
- Seh Z. W.; Kibsgaard J.; Dickens C. F.; Chorkendorff I.; Nørskov J. K.; Jaramillo T. F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355 (6321), eaad4998 10.1126/science.aad4998. [DOI] [PubMed] [Google Scholar]
- Sultan S.; Ha M.; Kim D. Y.; Tiwari J. N.; Myung C. W.; Meena A.; Shin T. J.; Chae K. H.; Kim K. S. Superb water splitting activity of the electrocatalyst Fe3Co(PO4)4 designed with computation aid. Nat. Commun. 2019, 10 (1), 5195. 10.1038/s41467-019-13050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor T. A. A.; Pedersen J. K.; Winther S. H.; Castelli I. E.; Jacobsen K. W.; Rossmeisl J. High-Entropy Alloys as a Discovery Platform for Electrocatalysis. Joule 2019, 3 (3), 834–845. 10.1016/j.joule.2018.12.015. [DOI] [Google Scholar]
- Baghban A.; Habibzadeh S.; Zokaee Ashtiani F. On the evaluation of hydrogen evolution reaction performance of metal-nitrogen-doped carbon electrocatalysts using machine learning technique. Sci. Rep. 2021, 11 (1), 21911. 10.1038/s41598-021-00031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran K.; Ulissi Z. W. Active learning across intermetallics to guide discovery of electrocatalysts for CO2 reduction and H2 evolution. Nat. Catal. 2018, 1 (9), 696–703. 10.1038/s41929-018-0142-1. [DOI] [Google Scholar]
- Hammer B.; Nørskov J.K.. Theoretical surface science and catalysis—calculations and concepts; Academic Press: 2000; Advances in Catalysis, pp 71–129. [Google Scholar]
- Xu H.; Cheng D.; Cao D.; Zeng X. C. A universal principle for a rational design of single-atom electrocatalysts. Nat. Catal. 2018, 1 (5), 339–348. 10.1038/s41929-018-0063-z. [DOI] [Google Scholar]
- Kim D. Y.; Ha M.; Kim K. S. A universal screening strategy for the accelerated design of superior oxygen evolution/reduction electrocatalysts. J. Mater. Chem. A 2021, 9 (6), 3511–3519. 10.1039/D0TA02425B. [DOI] [Google Scholar]
- Jain A.; Wang Z.; Nørskov J. K. Stable Two-Dimensional Materials for Oxygen Reduction and Oxygen Evolution Reactions. ACS Energy Lett. 2019, 4 (6), 1410–1411. 10.1021/acsenergylett.9b00876. [DOI] [Google Scholar]
- Su H.-Y.; Gorlin Y.; Man I. C.; Calle-Vallejo F.; Nørskov J. K.; Jaramillo T. F.; Rossmeisl J. Identifying active surface phases for metal oxide electrocatalysts: a study of manganese oxide bi-functional catalysts for oxygen reduction and water oxidation catalysis. Phys. Chem. Chem. Phys. 2012, 14 (40), 14010–14022. 10.1039/c2cp40841d. [DOI] [PubMed] [Google Scholar]
- Shinde A.; Jones R. J.; Guevarra D.; Mitrovic S.; Becerra-Stasiewicz N.; Haber J. A.; Jin J.; Gregoire J. M. High-Throughput Screening for Acid-Stable Oxygen Evolution Electrocatalysts in the (Mn-Co-Ta-Sb) O x Composition Space. Electrocatalysis 2015, 6 (2), 229–236. 10.1007/s12678-014-0237-7. [DOI] [Google Scholar]
- Zhang M.; Li H.; Chen J.; Yi L.; Shao P.; Xu C.-Y.; Wen Z. Nitrogen-doped graphite encapsulating RuCo nanoparticles toward high-activity catalysis of water oxidation and reduction. Chem. Eng. J. 2021, 422, 130077. 10.1016/j.cej.2021.130077. [DOI] [Google Scholar]
- Xu X.; Xu H.; Cheng D. Design of high-performance MoS2 edge supported single-metal atom bifunctional catalysts for overall water splitting via a simple equation. Nanoscale 2019, 11 (42), 20228–20237. 10.1039/C9NR06083A. [DOI] [PubMed] [Google Scholar]
- Xiao W.; Zhang L.; Bukhvalov D.; Chen Z.; Zou Z.; Shang L.; Yang X.; Yan D.; Han F.; Zhang T. Hierarchical ultrathin carbon encapsulating transition metal doped MoP electrocatalysts for efficient and pH-universal hydrogen evolution reaction. Nano Energy 2020, 70, 104445. 10.1016/j.nanoen.2020.104445. [DOI] [Google Scholar]
- He Q.; Tian D.; Jiang H.; Cao D.; Wei S.; Liu D.; Song P.; Lin Y.; Song L. Achieving Efficient Alkaline Hydrogen Evolution Reaction over a Ni5P4 Catalyst Incorporating Single-Atomic Ru Sites. Adv. Mater. 2020, 32 (11), 1906972. 10.1002/adma.201906972. [DOI] [PubMed] [Google Scholar]
- Obodo K. O.; Ouma C. N. M.; Obodo J. T.; Braun M. Influence of transition metal doping on the electronic and optical properties of ReS2 and ReSe2 monolayers. Phys. Chem. Chem. Phys. 2017, 19 (29), 19050–19057. 10.1039/C7CP03455E. [DOI] [PubMed] [Google Scholar]
- Le J.-B.; Chen A.; Li L.; Xiong J.-F.; Lan J.; Liu Y.-P.; Iannuzzi M.; Cheng J. Modeling Electrified Pt(111)-Had/Water Interfaces from Ab Initio Molecular Dynamics. JACS Au 2021, 1 (5), 569–577. 10.1021/jacsau.1c00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg N.; Laasonen K. Ab Initio Electrochemistry: Exploring the Hydrogen Evolution Reaction on Carbon Nanotubes. J. Phys. Chem. C 2015, 119 (28), 16166–16178. 10.1021/acs.jpcc.5b04739. [DOI] [Google Scholar]