Abstract
Background:
Paramagnetic rim lesions (PRLs) and slowly expanding lesions (SELs) have been posited as markers of chronic active lesions (CALs).
Objective:
To assess the lesion-level concordance of PRLs and SELs in MS and to characterize changes in brain tissue integrity in CALs over time.
Methods:
MRIs were analyzed from a substudy of AFFINITY [NCT03222973], a phase 2 trial of opicinumab in relapsing MS. Assessments included (1) identification of SELs based on longitudinal MRIs over 72 weeks, and identification of PRLs on susceptibility-weighted imaging (SWI) filtered phase images at week 72; (2) evaluation of subject-level correlation of SEL and PRL counts, volumes, and degree of lesion-level overlap between SELs and PRLs; and (3) characterization of tissue integrity over time in overlapping and non-overlapping SELs and PRLs.
Results:
In 41 subjects, 119 chronic PRLs and 267 SELs were detected. Of 119 (39.5%) chronic PRLs, 47 co-localized with a SEL; 46/267 (17.2%) SELs co-localized with a PRL. PRLs co-localized with SELs showed expansion and worsening microstructural damage over time. SELs with and without co-localization with PRLs showed ongoing tissue damage.
Conclusions:
Chronic MS lesions identified as both PRL and SEL were associated with the most severe accumulation of tissue damage.
Trial Registration:
AFFINITY [NCT03222973].
Keywords: Chronic active lesions, multiple sclerosis, MRI, opicinumab, paramagnetic lesions, slow expanding lesions
Introduction
In multiple sclerosis (MS), chronic active lesions (CALs) represent a pathological hallmark of progressive MS biology and chronic inflammation. CALs are characterized by the presence of activated microglia and/or macrophages at the demyelinating lesion edge1,2 and may also harbor, at their core, a perivascular cuff of central nervous system tissue-resident B and T CD8+ lymphocytic infiltrates.3,4 CALs are thought to be a meaningful contributor to chronic active demyelination and disability progression unrelated to new acute focal inflammation.5,6 Several approaches for in vivo imaging of CALs have recently been developed.7–14
Paramagnetic rim lesions (PRLs) have been proposed as potential in vivo markers of CALs. PRLs can be identified using susceptibility-weighted imaging (SWI) based on the presence of paramagnetic rims, thought to correspond to iron-laden macrophages and/or microglia at the lesion edge.8,10–13 PRLs can be imaged at 7-Tesla (T),8,10,11 3 T, 15 or even 1.5 T, 16 and can provide a feasible imaging marker in multicenter studies and clinical trials.9,15 PRLs represent a more destructive lesion phenotype and have been observed in patients with relapsing and progressive disease; increased PRL counts are correlated with greater T2-lesion burden and reduced brain volume, and may provide prognostic value for long-term disability.9,17,18
Slowly expanding lesions (SELs), as defined on brain MRI, have also emerged as a potential in vivo marker of CALs, detectable on longitudinal T1-weighted (T1w) and T2-weighted (T2w) MRIs. 14 SELs are observed in all clinical subtypes of MS and represent a subset of non-enhancing T2-lesions with greater microstructural damage and ongoing demyelination and/or axonal loss over time.14,19,20 SELs have also been shown to correlate with disability and be predictive of progression in relapsing and secondary progressive populations.21–23
In this study, we aim to better understand the relationship between PRLs and SELs by considering patient-level and lesion-level correspondence, as well as assessing the evolution over time of tissue damage in PRLs and SELs based on changes in normalized magnetization transfer ratio (nMTR), normalized T1w intensity (nT1), radial diffusivity (RD), and fractional anisotropy (FA).
Methods
Cohort and MRI data
Brain MRIs were acquired in AFFINITY [NCT03222973], a phase 2 trial of opicinumab in relapse-onset MS with an initial blinded, placebo-controlled period (part 1) followed by an open-label extension study (part 2). Patient inclusion criteria and imaging parameters can be found in the Supplemental Online Content. Analysis was restricted to the subset of 41 trial participants with an SWI acquisition at week 72 and scans at all time points in part 1 of the trial (baseline and weeks 24, 48, and 72).
The AFFINITY clinical trial was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice (GCP) guidelines, the European Union Clinical Trial Directive in relation to GCP in the conduct of clinical trials on investigational medicinal products, and the Declaration of Helsinki. All investigators obtained the required approvals from independent ethics committees and institutional review boards. All participants provided written informed consent.
Identification of PRLs
Filtered phase images were generated from the raw multi-echo gradient recalled echo data using a combination of spatial and temporal phase unwrapping, followed by high-pass filtering of the resultant unwrapped phase images.24,25 PRLs were manually identified using week 72 study scans by a single expert rater (DF) and subsequently by a second expert rater (DA), followed by a comparison and consensus adjudication by the two raters. PRLs were specifically identified based on the presence of discrete hypointense rims visible on the filtered phase image that co-localized with T2-lesions. Rims could be complete or partial but were required to span a minimum of two consecutive slices in the axial direction and be visible on both coronal and sagittal image views. Areas of T2-lesion associated with a given phase rim (T2-associated PRLs) were automatically generated by filling in the portion inside the identified rim that overlaps with a previously generated T2-lesion mask. In cases where a rim was partial, the completion of the rim was approximated using a bounding box around the partial rim and subsequently restricting the ROI associated with the PRL to areas within the T2-lesion mask. PRLs associated with either gadolinium-enhancing lesions at baseline or new T2-lesions detected at weeks 24, 28, and 72 (PRLs forming on study) were considered separately from those associated with non-enhancing T2-lesions present at baseline (chronic PRLs). T2-associated PRLs were used to define the region of interest associated with a given PRL in subsequent analyses when considering overlap with SELs, longitudinal evolution of PRLs, and T2-lesion volume associated with PRLs.
Identification of SELs
SELs were identified as areas of T2-lesions pre-existing at baseline that showed constant and concentric local expansion from baseline to week 72, as measured on T1w and T2w MRI using a previously described method. 14 Briefly, SEL candidates were automatically identified as contiguous areas of the baseline T2-lesion mask 26 of ⩾10 voxels in size (30 mm3) that showed local expansion from baseline to week 72, where expansion was determined using Jacobian integration 27 considering both the T1w and T2w images. SEL candidates were then filtered to ensure that (1) expansion was constant and gradual over all time points and (2) expansion followed a radial inside-out pattern. Manual verification of automatic SEL detection was limited to visual inspection of the non-linear deformations (over time) and the resultant Jacobian determinants.
Analysis of co-localization between PRLs and SELs
SEL masks were resampled from native resolution (1 × 1 × 3 mm) to the higher resolution of the SWI images (0.8 mm3) before determining lesion-level overlap between chronic PRLs (identified at week 72 and associated with a non-enhancing T2-lesion at baseline) and SELs (identified based on interval from baseline to week 72). An individual SEL was considered co-localized with a PRL (SEL+/PRL+) if there was any overlap with a T2-associated PRL label, otherwise it was considered not co-localized with a PRL (SEL+/PRL−). Similarly, an individual PRL was considered co-localized with an SEL (PRL+/SEL+) if the T2-associated PRL label overlapped with an SEL label, otherwise it was considered not co-localized with an SEL (PRL+/SEL−). PRL+/SEL+ and SEL+/PRL+ were assessed separately because SEL+/PRL+ and the corresponding PRL+/SEL+ generally do not have exactly matching boundaries, given differences in image resolution and the nature of the SEL detection algorithm, where SEL boundaries are determined from the Jacobian determinant (Figure 1).
Figure 1.
Assessment of co-localization between PRLs and SELs and identification of PRL+/SEL− and SEL+/PRL− phenotypes. (a) T1-weighted image. (b) T2-weighted image. (c) Filtered SWI-phase image. (d) T2-lesion mask at baseline. (e) Manual PRL annotation at week 72. (f) T2-associated PRL at week 72. (g) SELs detected based on interval from baseline to week 72. (h) Voxelwise overlap of T2-associated PRLs and SELs (red = PRL only, blue = SEL only, yellow = both). (i) Co-localized PRL+/SEL+ mask (dark red). (j) Co-localized SEL+/PRL+ mask (dark blue). (k) PRL+/SEL− lesions (light red). (l) SEL+/PRL− lesions (light blue). Note that lesion boundaries are slightly different in (i) and (j) due to differences in PRL and SEL detection methods.
PRL: paramagnetic rim lesion; SEL: slowly expanding lesion; SWI: susceptibility-weighted imaging; T2-assoc PRL: T2-lesion associated with a given paramagnetic rim.
Longitudinal evolution of PRLs and SELs by respective chronic lesion phenotype based on co-localization status
PRLs and SELs were grouped into four subcategories based on co-localization status (PRL+/SEL+, PRL+/SEL−, SEL+/PRL+, SEL+/PRL−). The longitudinal evolution from baseline to week 72 was analyzed for each lesion subcategory, for the following MRI contrasts: nT1, nMTR, RD, and FA. nMTR and nT1 values of 1 can be interpreted as corresponding to median white matter; 1 unit of nMTR or nT1 can be interpreted as corresponding to the difference between white and gray matter. 28 Details of the normalization procedure for MTR and T1w images are included in the Supplemental Online Content. All MRI images were resampled to the higher resolution (0.8 mm3) of the SWI images. In the context of chronic MS lesions, decreases in nMTR, nT1, or FA, and increases in RD, are associated with decreases in myelin and/or axonal density or integrity. 28 The overall baseline T2-lesion mask was also analyzed to provide a reference lesion evolution.
Results
Patient characteristics
Forty-one patients with MS were included in the analysis, with median (interquartile range [IQR]) age of 40 (30–45) years, median Expanded Disability Status Scale (EDSS) score of 2.5 (2–4.5), and median disease duration of 5.0 (2–10) years at baseline. Their median T2-lesion volume at baseline was 4.8 (2.4–9.3) mL; median normalized brain volume was 1457 (1409 – 1531) mL. Additional patient characteristics are presented in Supplemental Table e1.
Characterization of PRL and SEL severity in the context of other MS disease covariates
Altogether, 131 PRLs and 267 SELs were detected in the 41 subjects included in the study. Twelve of 131 PRLs were associated with either gadolinium-enhancing lesions at baseline (n = 2) or new T2-lesions that formed at weeks 24, 48, or 72 (n = 10). Only chronic PRLs associated with non-enhancing T2-lesions pre-existing at baseline (n = 119) were considered in the analysis of co-localization with SELs, as SEL detection was restricted to pre-existing non-enhancing T2-lesions at baseline. Patients had a median (IQR) of 1 (0 – 5) PRL and 2 (1 – 9) SELs. Median volumes associated with chronic PRLs and SELs were 117 mm3 (0 – 557) and 169 mm3 (52 – 934), respectively. Median (IQR) percentage of baseline T2-lesion volume associated with chronic PRLs and SELs was 1.3% (0.0% – 6.6%) and 4.3% (1.8% – 11.3%), respectively. SEL and chronic PRL counts and volumes were significantly correlated at the patient level (Pearson correlation, both r = 0.58). SEL and PRL counts were also both significantly and positively associated with patient-level T2-lesion volume at baseline (r = 0.74 and r = 0.75, respectively), and negatively associated with normalized brain volume (r =−0.46 and r =−0.44, respectively). No evidence of significant association between PRL or SEL counts with EDSS or age at baseline was observed. Patient demographics and disease covariates stratified by PRL and SEL counts are presented in Supplemental Table e1.
Longitudinal properties of co-localized SELs and PRLs versus SELs without paramagnetic rims and PRLs without slow expansion
Forty-seven of 119 PRLs (39.5%) overlapped with an SEL (PRL+/SEL+), whereas only 46 of 267 SELs (17.2%) overlapped with a PRL (SEL+/PRL+). For PRL+/SEL+ lesions, mean voxelwise overlap with SELs was 43.3% while for SEL+/PRL+, mean voxelwise overlap with PRLs was 43.7%. Smaller PRLs (<60 mm3) were much less likely to overlap with SELs (3 of 30 PRLs or 10%) while more than half (33 of 59 or 56%) of PRLs larger than 100 mm3 had overlap with SELs. PRLs and SELs both showed greater tissue damage at baseline as compared with overall T2-lesions, as demonstrated by lower mean nMTR, nT1, and FA, and higher RD, although these differences only reached statistical significance when comparing PRLs with overall T2-lesions for nMTR, nT1, and RD, and SELs with overall T2-lesions for RD and FA (Supplemental Table e2). PRLs also had significantly lower mean nT1 and nMTR at baseline as compared with SELs (Supplemental Table e2). PRLs and SELs both showed a significant reduction in nMTR, nT1, and FA and an increase in RD over 72 weeks (Supplemental Table e2). However, when compared with the change over 72 weeks in overall T2-lesions, the difference was significant in all four MRI contrasts (nMTR, nT1, FA, and RD) for SELs, but only in RD for PRLs (Supplemental Table e2).
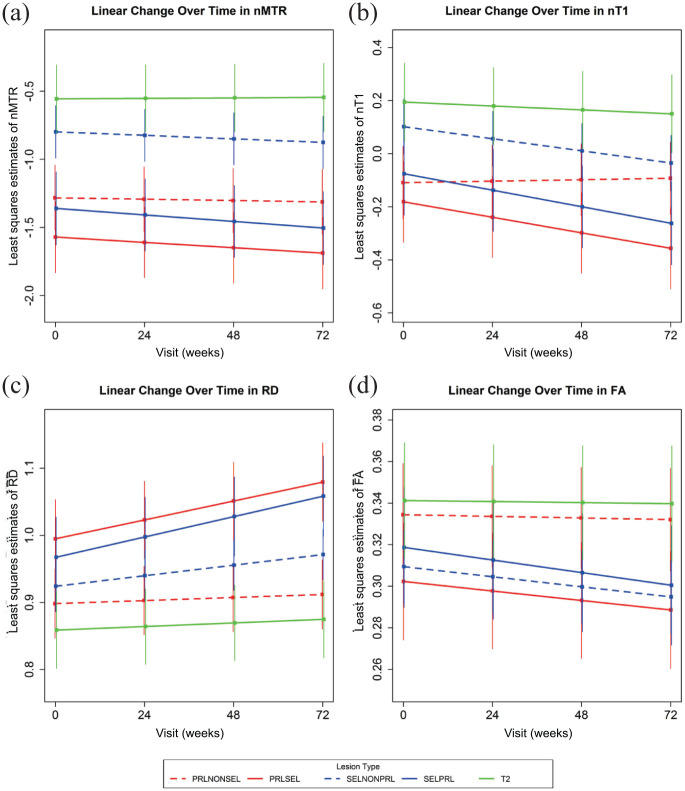
When PRLs were subcategorized into PRL+/SEL+ and PRL+/SEL−, both PRL subtypes showed more pronounced tissue damage cross-sectionally, as measured by reduced baseline values of nMTR and nT1, compared with overall T2-lesions; however, only PRL+/SEL+ lesions showed a significant increase in RD and a significant decrease in FA as compared with overall T2-lesions (Table 1, Figure 2, Supplemental Figure e1). PRL+/SEL+ lesions showed evidence of ongoing tissue destruction over 72 weeks, as characterized by changes in nMTR, nT1, FA, and RD (Table 1, Figure 2, Supplemental Figures e2 and e3), whereas PRL+/SEL− lesions appeared longitudinally stable with no significant change in nMTR, nT1, FA, and RD over 72 weeks (Table 1, Figure 2, Supplemental Figures e2 and e3). Changes over time in PRL+/SEL− lesions were not significantly different from change in overall T2-lesions, except for nT1, which showed an increase (relative improvement in tissue integrity) in PRL+/SEL− lesions compared with overall T2-lesions (Table 1).
Table 1.
Comparison of baseline and longitudinal change in tissue damage in (A) PRLs that do and do not co-localize with SELs and (B) SELs that do and do not co-localize with PRLs.
| nMTR | nT1 | RD | FA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EST | P value | EST | P value | EST | P value | EST | P value | |||
| A | Baseline | PRL+/SEL+ | −1.5868 | n/a | −0.1916 | n/a | 0.9976 | n/a | 0.3022 | n/a |
| PRL+/SEL− | −1.3218 | n/a | −0.1183 | n/a | 0.8991 | n/a | 0.3340 | n/a | ||
| T2 lesion | −0.5669 | n/a | 0.1955 | n/a | 0.8579 | n/a | 0.3417 | n/a | ||
| Difference at baseline | (PRL+/SEL+) − (PRL+/SEL−) | −0.2650 | 0.1806 | −0.0733 | 0.3597 | 0.0985 | 0.0022 | −0.0317 | 0.0458 | |
| (PRL+/SEL+) − (T2 lesion) | −1.0199 | <0.0001 | −0.3871 | <0.0001 | 0.1397 | 0.0002 | −0.0394 | 0.0323 | ||
| (PRL+/SEL−) − (T2 lesion) | −0.7549 | <0.0001 | −0.3138 | 0.0003 | 0.0412 | 0.2331 | −0.0077 | 0.6516 | ||
| Change over 72 weeks | PRL+/SEL+ | −0.1178 | 0.0062 | −0.1755 | <0.0001 | 0.0847 | <0.0001 | −0.0137 | 0.0025 | |
| PRL+/SEL− | −0.0299 | 0.4024 | 0.0165 | 0.3491 | 0.0137 | 0.0878 | −0.0024 | 0.5287 | ||
| T2 lesion | 0.0113 | 0.8054 | −0.0441 | 0.0520 | 0.0161 | 0.1236 | −0.0015 | 0.7594 | ||
| Difference in change over 72 weeks | (PRL+/SEL+) − (PRL+/SEL−) | −0.0879 | 0.1162 | −0.1920 | <0.0001 | 0.0710 | <0.0001 | −0.0113 | 0.0541 | |
| (PRL+/SEL+) − (T2 lesion) | −0.1291 | 0.0405 | −0.1314 | <0.0001 | 0.0686 | <0.0001 | −0.0122 | 0.0675 | ||
| (PRL+/SEL−) − (T2 lesion) | −0.0413 | 0.4789 | 0.0606 | 0.0350 | −0.0024 | 0.8550 | −0.0009 | 0.8883 | ||
| B | Baseline | SEL+/PRL+ | −1.3715 | n/a | −0.0816 | n/a | 0.9701 | n/a | 0.3173 | n/a |
| SEL+/PRL− | −0.8121 | n/a | 0.1064 | n/a | 0.9250 | n/a | 0.3097 | n/a | ||
| T2 lesion | −0.5669 | n/a | 0.1955 | n/a | 0.8579 | n/a | 0.3417 | n/a | ||
| Difference at baseline | (SEL+/PRL+) − (SEL+/PRL−) | −0.5594 | <0.0001 | −0.1880 | 0.0074 | 0.0451 | 0.1078 | 0.0075 | 0.5967 | |
| (SEL+/PRL+) − (T2 lesion) | −0.8046 | <0.0001 | −0.2771 | 0.0034 | 0.1122 | 0.0031 | −0.0244 | 0.1897 | ||
| (SEL+/PRL−) − (T2 lesion) | −0.2452 | 0.0399 | −0.0891 | 0.2305 | 0.0671 | 0.0249 | −0.0319 | 0.0304 | ||
| Change over 72 weeks | SEL+/PRL+ | −0.1444 | 0.0009 | −0.1867 | <0.0001 | 0.0912 | <0.0001 | −0.0182 | 0.0001 | |
| SEL+/PRL− | −0.0776 | 0.0001 | −0.1370 | <0.0001 | 0.0471 | <0.0001 | −0.0145 | <0.0001 | ||
| T2 lesion | 0.0113 | 0.8054 | −0.0441 | 0.0520 | 0.0161 | 0.1236 | −0.0015 | 0.7594 | ||
| Difference in change over 72 weeks | (SEL+/PRL+) − (SEL+/PRL−) | −0.0668 | 0.1618 | −0.0497 | 0.0348 | 0.0441 | <0.0001 | −0.0036 | 0.4690 | |
| (SEL+/PRL+) − (T2 lesion) | −0.1557 | 0.0140 | −0.1427 | <0.0001 | 0.0751 | <0.0001 | −0.0167 | 0.0129 | ||
| (SEL+/PRL−) − (T2 lesion) | −0.0889 | 0.0762 | −0.0929 | 0.0002 | 0.0310 | 0.0065 | −0.0130 | 0.0146 | ||
Overall T2 lesions are used as the reference for both (A) and (B). Lower values of nMTR, nT1, and FA, and higher values of RD, signify worsening tissue integrity. Baseline estimates were derived from an MMRM with weeks since baseline modeled as categorical. Change over 72 weeks was derived from an MMRM with weeks since baseline modeled as linear continuous. When looking at change over 72 weeks, statistical significance relates to difference from 0 (no change), whereas for difference at baseline and difference in change over 72 weeks statistical significance relates to differences between lesion subtypes. Bold numbers indicate P-value < 0.05.
EST: estimate; FA: fractional anisotropy; MMRM: mixed-effect models of repeated measurement; n/a: not applicable; nMTR: normalized magnetization transfer ratio; nT1: normalized T1 intensity; PRL: paramagnetic rim lesions RD: radial diffusivity; SEL: slowly expanding lesion.
Figure 2.
Evolution of tissue damage in PRLs and SELs based on lesion-level correspondence. PRLs are subcategorized into those that overlap with SELs (PRL+/SEL+, solid red line) and those that do not (PRL+/SEL−, dashed red line). SELs are subcategorized into those that overlap with PRLs (SEL+/PRL+, solid blue line) and those that do not (SEL+/PRL−, dashed blue line). Overall T2 lesions are included as a reference (solid green line). Least-squares estimates and [95%] confidence intervals of change over time are derived from MMRM with weeks since baseline modeled as linear continuous. (A) nMTR, (B) nT1, (C) RD, and (D) FA.
FA: fractional anisotropy; MMRM: mixed-effect model of repeated measurement; nMTR: normalized magnetization transfer ratio; nT1: normalized T1 intensity; PRL: paramagnetic rim lesion; RD: radial diffusivity; SEL: slowly expanding lesion.
When SELs were subcategorized into SEL+/PRL+ and SEL+/PRL−, SEL+/PRL+ lesions showed significantly lower nMTR and nT1 at baseline compared with SEL+/PRL− lesions and overall T2-lesions (Table 2, Figure 2, Supplemental Figure e1). Both SEL subtypes showed significantly increased RD at baseline compared with overall T2-lesions, and although both SEL subtypes also showed reduced FA compared with overall T2-lesions, this difference was only statistically significant for SEL+/PRL−. Longitudinally, both SEL subtypes showed significant decreases in nMTR, nT1, and FA and increases in RD over 72 weeks, although this change was more pronounced in SEL+/PRL+ compared with SEL+/PRL−. When compared with changes over 72 weeks in overall T2-lesions, all changes in both SEL subtypes were significantly greater, except for changes in nMTR for SEL+/PRL−, which did not reach statistical significance (Table 2).
Table 2.
Comparison of baseline and longitudinal change in tissue damage in SELs that do and do not co-localize with PRLs.
| nMTR | nT1 | RD | FA | |||||
|---|---|---|---|---|---|---|---|---|
| EST | P value | EST | P value | EST | P value | EST | P value | |
| Baseline | ||||||||
| SEL+/PRL+ | −1.3715 | n/a | −0.0816 | n/a | 0.9701 | n/a | 0.3173 | n/a |
| SEL+/PRL− | −0.8121 | n/a | 0.1064 | n/a | 0.9250 | n/a | 0.3097 | n/a |
| T2 lesion | −0.5669 | n/a | 0.1955 | n/a | 0.8579 | n/a | 0.3417 | n/a |
| Difference at baseline | ||||||||
| (SEL+/PRL+) − (SEL+/PRL−) | −0.5594 | <0.0001 | −0.1880 | 0.0074 | 0.0451 | 0.1078 | 0.0075 | 0.5967 |
| (SEL+/PRL+) − (T2 lesion) | −0.8046 | <0.0001 | −0.2771 | 0.0034 | 0.1122 | 0.0031 | −0.0244 | 0.1897 |
| (SEL+/PRL−) − (T2 lesion) | −0.2452 | 0.0399 | −0.0891 | 0.2305 | 0.0671 | 0.0249 | −0.0319 | 0.0304 |
| Change over 72 weeks | ||||||||
| SEL+/PRL+ | −0.1444 | 0.0009 | −0.1867 | <0.0001 | 0.0912 | <0.0001 | −0.0182 | 0.0001 |
| SEL+/PRL− | −0.0776 | 0.0001 | −0.1370 | <0.0001 | 0.0471 | <0.0001 | −0.0145 | <0.0001 |
| T2 lesion | 0.0113 | 0.8054 | −0.0441 | 0.0520 | 0.0161 | 0.1236 | −0.0015 | 0.7594 |
| Difference in change over 72 weeks | ||||||||
| (SEL+/PRL+) − (SEL+/PRL−) | −0.0668 | 0.1618 | −0.0497 | 0.0348 | 0.0441 | <0.0001 | −0.0036 | 0.4690 |
| (SEL+/PRL+) − (T2 lesion) | −0.1557 | 0.0140 | −0.1427 | <0.0001 | 0.0751 | <0.0001 | −0.0167 | 0.0129 |
| (SEL+/PRL−) − (T2 lesion) | −0.0889 | 0.0762 | −0.0929 | 0.0002 | 0.0310 | 0.0065 | −0.0130 | 0.0146 |
Overall T2 lesions are used as the reference. Lower values of nMTR, nT1, and FA, and higher values of RD, signify worsening tissue integrity. Baseline estimates were derived from an MMRM with weeks since baseline modeled as categorical. Change over 72 weeks was derived from an MMRM with weeks since baseline modeled as linear continuous. When looking at change over 72 weeks, statistical significance relates to difference from 0 (no change), whereas for difference at baseline and difference in change over 72 weeks statistical significance relates to differences between lesion subtypes. Bold numbers indicate P-value < 0.05.
EST: estimate; FA: fractional anisotropy; MMRM: mixed-effect models of repeated measurement; n/a: not applicable; nMTR: normalized magnetization transfer ratio; nT1: normalized T1.
Discussion
PRLs represent the subset of chronic lesions with sufficient iron-laden microglia and/or macrophages to be detected on SWI and are characterized by more severe tissue damage. 11 In the current study, only a subset of PRLs showed appreciable expansion and/or ongoing evidence of tissue destruction over 72 weeks. Similar variability in the dynamics of PRLs over several years has also been observed in other studies.9,29,30 The life cycle of PRLs is still not fully understood, but it is likely that the presence of a persistent paramagnetic rim is determined at or soon after lesion onset, and that new T2-lesions with persistent paramagnetic rims have a more severe onset, in terms of larger lesion volumes and greater T1 hypointensity, compared with new T2-lesions without persistent paramagnetic rims.10,31 Observations of PRL lesions in the 2 years following lesion appearance have also demonstrated a reduction in lesion volume in the early post-acute phase. 10 In the current study, approximately 13% of the new T2-lesions that were observed on study (at weeks 24 and 48) had paramagnetic rims at week 72. 32 These new T2-lesions with persistent paramagnetic rims were larger and had greater decreases in nMTR, both at first observation and at week 72, compared with new T2-lesions without paramagnetic rims. 32 Although more severe, new T2-lesions with persistent paramagnetic rims did show some degree of resolution in the early post-acute phase, as seen by decreased volume and increases in nMTR and nT1 from first observation of the new T2-lesion. In recent studies looking at the long-term dynamics of PRLs, significant variability in lesion dynamics was observed both across lesions and across time for the same lesion; the paramagnetic rim was shown to become less pronounced over time, and in some cases, to disappear.29,30
Variability in PRL dynamics observed over a period of 72 weeks in this study may be partially explained by different stages in the life cycle of individual PRLs. New T2-lesions with persistent paramagnetic rims may secondarily adopt a slowly expanding dynamic after a post-acute partial-resolution phase, although the demonstration and exact time frame of this phenomenon remains to be established. Chronic PRLs that do not slowly expand over time and appear “inactive” from the perspective of MRI markers of ongoing tissue destruction may represent the tail end of the early post-acute phase or, alternatively, a much later chronic “inactive” lesion stage that may or may not be subsequent to a previous multiyear phase of SEL behavior (Figure 3). Although it has been postulated that lack of observable expansion in some CALs may be due to expansion at the lesion edge being offset by concurrent shrinkage at the lesion core due to severe tissue damage and structural collapse, 33 the relative stability of tissue integrity over time of non-expanding PRLs observed in this study did not support this concept. A more in-depth analysis of the dynamics of lesion core and rim with higher-resolution images is required to further explore this hypothesis. Although chronic PRLs represent a subset of lesions with greater chronic tissue damage, this might be at least partly a function of the severity of the original acute inflammatory event. 10 Further studies with long-term follow-up from lesion formation would provide additional insight into the life cycle of PRLs.
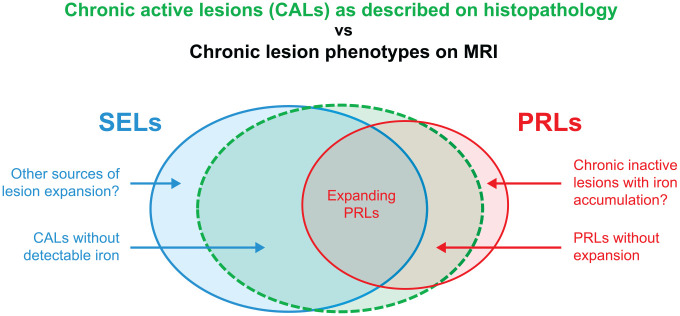
Figure 3.
Summary representation of CALs as described by histopathology versus imaging phenotypes of PRLs and SELs in MS.29,34
Figure is for representative purposes only, and the degree of overlap is not meant to convey a quantitative description of overlap between lesion types.
CAL: chronic active lesion; PRL: paramagnetic rim lesion; SEL: slowly expanding lesion.
SELs are identified longitudinally based on lesion dynamics, as observed on MRI over the period of interest (72 weeks in this study), and represent a subset of lesions that demonstrate ongoing tissue damage over the observation period. The subset of SELs that co-localized with PRLs likely represents the subset of CALs with detectable iron at the lesion edge with ongoing chronic inflammation and demyelination and observable lesion expansion (Figure 3). Previous longitudinal studies of paramagnetic rim lesions have shown that rimless lesions tend to shrink, on average, rather than enlarge.29,30 These studies also show that a larger proportion of PRLs expand over time as compared with rimless lesions, but that a subset of rimless lesions do enlarge over time.29,30 Given that PRLs represent a small proportion of overall T2-lesions, when considered in absolute terms, most of the chronic lesions that expand over time are rimless, as also shown in the current study. SELs without paramagnetic rims (SEL+/PRL−), in contrast to PRL+/SEL−, showed more severe ongoing tissue destruction over 72 weeks compared with overall T2-lesions. These rimless SELs may in part represent CALs without detectable iron.11,34 Mechanisms of lesion expansion not consistent with the pathology-based definition of chronic active lesions cannot be excluded (Figure 3), especially for those SEL+/PRL− that do not exhibit pronounced demyelination at baseline but show ongoing tissue destruction over 72 weeks. In this respect, recent studies examined the accumulation of T2-lesions due to the conversion of diffusively abnormal white matter to focal white matter lesions, 35 and the conversion of diffusively abnormal white matter in perilesional tissue could in some cases be modeled as slow expansion of pre-existing lesions in the SEL detection framework. Further histological investigation of rimless MS lesions that slowly expand over time (SEL+/PRL−) is required to better understand the pathological correlates of these lesions.
Limitations
This study had several limitations. The trial population considered had specific inclusion criteria, where patients had to meet cutoffs for maximum mean nMTR and maximum mean RD within the overall T2-lesion mask, with the intended aim to enrich the trial with patients who had chronically demyelinated lesions that still had sufficient tissue integrity for potential remyelination. 36 Patients also had to be stable on their background disease-modifying therapies (DMTs) and relapse-free for 6 months. Background DMTs were non-randomized, as can be observed with a greater proportion of natalizumab-treated patients among those with a greater number of PRLs or SELs (Supplemental Table e1). Further studies are needed to determine whether the relatively low level of PRL/SEL overlap observed here is generalizable to broader populations of patients with MS.
SELs were detected at a lower resolution (1 × 1 × 3 mm) than PRLs (0.8 mm3), which may have contributed to the degree of spatial overlap observed between these lesion subtypes. PRLs were identified only on week 72 scans. Although only those PRLs corresponding to non-enhancing lesions present in the baseline scan were considered in this analysis, the presence of a paramagnetic rim was not explicitly confirmed prior to week 72 due to lack of susceptibility-weighted acquisitions. PRLs were identified on filtered phase images. Quantitative susceptibility mapping (QSM) may provide a more specific identification of paramagnetic rims that limits the effects of possible blooming artifacts.37,38 QSM may also provide more granular quantification of iron content in the lesion rim both cross-sectionally and over time. 39 Smaller PRLs (<60 mm3) had very low lesion-level correspondence (10%) with SELs. This is likely due to the minimum size criterion applied to SELs (⩾30 mm3) and also to the increased difficulty in reliably identifying smaller PRLs at field strengths and resolutions suitable for use in a clinical trial setting. The detection of SELs is sensitive to the time frame used (72 weeks in this study) and also to algorithm implementation and parameters as well as acquisition. A recently published study also comparing correspondence of SELs and PRLs had similar high-level findings but detected a much higher proportion of T2-lesions as SELs. 40
The statistical analysis performed for this study implicitly assumed lesion independence within subjects, although a sensitivity analysis considering subject-level correlation yielded consistent point estimates and clinical interpretation, albeit with wider confidence intervals (data not shown).
Conclusion
This study demonstrates that co-localized SELs and PRLs may identify the most destructive type of chronic MS lesions characterized by the most severe accumulation of active tissue damage over time. Furthermore, SEL+/PRL− and PRL+/SEL− may capture distinct phenotypes of MS lesions (Figure 3) and/or different time frames within the life cycle of chronic lesions, with significantly different cross-sectional and longitudinal properties: PRL+/SEL− showed more pronounced tissue damage at baseline compared with SEL+/PRL−, whereas SEL+/PRL− showed ongoing tissue destruction over 72 weeks, and PRL+/SEL− appeared stable over time. Taken together, these results highlight the need to jointly detect and quantify tissue damage over time in SELs and PRLs to properly inform the mechanism of action of therapies that aim to target the progressive biology of MS and, in particular, the potentially heterogeneous pathological correlates of smoldering inflammation and neurodegenerative pathways.
Supplemental Material
Supplemental material, sj-docx-3-msj-10.1177_13524585231162262 for Lesion-level correspondence and longitudinal properties of paramagnetic rim and slowly expanding lesions in multiple sclerosis by Colm Elliott, David A Rudko, Douglas L Arnold, Dumitru Fetco, Ahmed M Elkady, David Araujo, Bing Zhu, Arie Gafson, Zhe Tian, Shibeshih Belachew, Daniel P Bradley and Elizabeth Fisher in Multiple Sclerosis Journal
Supplemental material, sj-pdf-1-msj-10.1177_13524585231162262 for Lesion-level correspondence and longitudinal properties of paramagnetic rim and slowly expanding lesions in multiple sclerosis by Colm Elliott, David A Rudko, Douglas L Arnold, Dumitru Fetco, Ahmed M Elkady, David Araujo, Bing Zhu, Arie Gafson, Zhe Tian, Shibeshih Belachew, Daniel P Bradley and Elizabeth Fisher in Multiple Sclerosis Journal
Supplemental material, sj-pdf-2-msj-10.1177_13524585231162262 for Lesion-level correspondence and longitudinal properties of paramagnetic rim and slowly expanding lesions in multiple sclerosis by Colm Elliott, David A Rudko, Douglas L Arnold, Dumitru Fetco, Ahmed M Elkady, David Araujo, Bing Zhu, Arie Gafson, Zhe Tian, Shibeshih Belachew, Daniel P Bradley and Elizabeth Fisher in Multiple Sclerosis Journal
Acknowledgments
Biogen funded editorial support for the preparation of this manuscript, provided by Excel Scientific Solutions (Fairfield, CT, USA).
Footnotes
Author Contributions: C.E. had full access to all the study data and takes responsibility for the integrity of the data and the accuracy of the data analysis. Acquisition, analysis, and interpretation were by C.E., D.L.A., D.F., D.A., D.A.R., A.M.E., S.B., D.P.B., and E.F. Drafting of the manuscript was by C.E., S.B., and E.F. Statistical analysis was by Z.T. Administrative, technical, and material support was by D.A.R. and A.M.E. All the authors gave critical revision of manuscript for important intellectual content.
Data Sharing Statement: Anonymized participant data collected during the AFFINITY trial will be shared with qualified scientific researchers who provide a methodologically sound proposal. Proposals should be submitted through Vivli (https://vivli.org). To gain access, data requestors will need to sign a data sharing agreement. Data are made available for 1 year on a secure platform.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C.E. has received speaker honoraria from EMD Serono and is an employee of NeuroRx Research. D.L.A. has received grants from Biogen, Immunotec, and Novartis and consulting fees from Biogen, Celgene, Frequency Therapeutics, Genentech/Roche, Med-Ex Learning, Merck, Novartis, the Population Council, Queens University and Sanofi Aventis, and has an ownership interest in NeuroRx. D.A. has received personal compensation from NeuroRx Research. Z.T. is an employee of Biogen. B.Z., A.G., S.B., D.P.B., and E.F. are employees and shareholders of Biogen. D.A.R., D.F. and A.M.E. have nothing to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support for this study was provided by Biogen.
ORCID iDs: Colm Elliott  https://orcid.org/0000-0002-3004-523X
https://orcid.org/0000-0002-3004-523X
Douglas L Arnold  https://orcid.org/0000-0003-4266-0106
https://orcid.org/0000-0003-4266-0106
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Colm Elliott, NeuroRx Research, Montreal, QC, Canada.
David A Rudko, Department of Neurology and Neurosurgery, McGill University, Montreal, QC, Canada/McConnell Brain Imaging Centre, Montreal Neurological Institute and Hospital, Montreal, QC, Canada/Department of Biomedical Engineering, McGill University, Montreal, QC, Canada.
Douglas L Arnold, NeuroRx Research, Montreal, QC, Canada/Department of Neurology and Neurosurgery, McGill University, Montreal, QC, Canada.
Dumitru Fetco, NeuroRx Research, Montreal, QC, Canada/Department of Neurology and Neurosurgery, McGill University, Montreal, QC, Canada.
Ahmed M Elkady, Department of Neurology and Neurosurgery, McGill University, Montreal, QC, Canada/McConnell Brain Imaging Centre, Montreal Neurological Institute and Hospital, Montreal, QC, Canada/Department of Biomedical Engineering, McGill University, Montreal, QC, Canada.
David Araujo, NeuroRx Research, Montreal, QC, Canada/Department of Neurology and Neurosurgery, McGill University, Montreal, QC, Canada.
Bing Zhu, Biogen, Cambridge, MA, USA.
Arie Gafson, Biogen, Cambridge, MA, USA.
Zhe Tian, Biogen, Cambridge, MA, USA.
Shibeshih Belachew, Biogen, Cambridge, MA, USA.
Daniel P Bradley, Biogen, Cambridge, MA, USA.
Elizabeth Fisher, Biogen, Cambridge, MA, USA.
References
- 1.Frischer JM, Weigand SD, Guo Y, et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol 2015; 78(5): 710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuhlmann T, Ludwin S, Prat A, et al. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol 2017; 133(1): 13–24. [DOI] [PubMed] [Google Scholar]
- 3.Fransen NL, Hsiao CC, van der Poel M, et al. Tissue-resident memory T cells invade the brain parenchyma in multiple sclerosis white matter lesions. Brain 2020; 143(6): 1714–1730. [DOI] [PubMed] [Google Scholar]
- 4.Machado-Santos J, Saji E, Troscher AR, et al. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 2018; 141(7): 2066–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramaglia V, Rojas O, Naouar I, et al. The ins and outs of central nervous system inflammation — lessons learned from multiple sclerosis. Ann Rev Immunol 2021; 39(1): 199–226. [DOI] [PubMed] [Google Scholar]
- 6.Luchetti S, Fransen NL, van Eden CG, et al. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: A retrospective autopsy cohort analysis. Acta Neuropathol 2018; 135(4): 511–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthews PM. Chronic inflammation in multiple sclerosis—seeing what was always there. Nat Rev Neurol 2019; 15(10): 582–593. [DOI] [PubMed] [Google Scholar]
- 8.Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: A combined imaging and histopathological study at 7 Tesla. Brain 2011; 134(Pt. 12): 3602–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Absinta M, Sati P, Masuzzo F, et al. Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol 2019; 76(12): 1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest 2016; 126(7): 2597–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: Pathology and 7 T magnetic resonance imaging. Acta Neuropathol 2017; 133(1): 25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaunzner UW, Kang Y, Zhang S, et al. Quantitative susceptibility mapping identifies inflammation in a subset of chronic multiple sclerosis lesions. Brain 2019; 142(1): 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol 2021; 8(4): 877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott C, Wolinsky JS, Hauser SL, et al. Slowly expanding/evolving lesions as a magnetic resonance imaging marker of chronic active multiple sclerosis lesions. Mult Scler 2019; 25(14): 1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. Am J Neuroradiol 2018; 39(7): 1233–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: Comparison of visualization at 1.5-T and 3-T MRI. Am J Roentgenol 2022; 219(1): 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemond CC, Baek J, Ionete C, et al. Paramagnetic rim lesions are associated with pathogenic CSF profiles and worse clinical status in multiple sclerosis: A retrospective cross-sectional study. Mult Scler 2022; 28(13): 2046–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler 2022; 28(14): 2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elliott C, Arnold DL, Chen H, et al. Patterning chronic active demyelination in slowly expanding/evolving white matter MS lesions. Am J Neuroradiol 2020; 41(9): 1584–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calvi A, Tur C, Chard D, et al. Slowly expanding lesions relate to persisting black-holes and clinical outcomes in relapse-onset multiple sclerosis. Neuroimage Clin 2022; 35: 103048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott C, Belachew S, Wolinsky JS, et al. Chronic white matter lesion activity predicts clinical progression in primary progressive multiple sclerosis. Brain 2019; 142(9): 2787–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calvi A, Carrasco FP, Tur C, et al. Association of slowly expanding lesions on MRI with disability in people with secondary progressive multiple sclerosis. Neurology 2022; 98(17): e1783–e1793. [DOI] [PubMed] [Google Scholar]
- 23.Preziosa P, Pagani E, Meani A, et al. Slowly expanding lesions predict 9-year multiple sclerosis disease progression. Neurol Neuroimmunol Neuroinflamm 2022; 9(2): e1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schofield MA, Zhu Y. Fast phase unwrapping algorithm for interferometric applications. Opt Lett 2003; 28(14): 1194–1196. [DOI] [PubMed] [Google Scholar]
- 25.Robinson S, Schödl H, Trattnig S. A method for unwrapping highly wrapped multi-echo phase images at very high field: UMPIRE. Magn Reson Med 2014; 72(1): 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francis SJ. Automatic lesion identification in MRI of multiple sclerosis patients. MSc Thesis, McGill University, Montreal, QC, Canada, 2004. [Google Scholar]
- 27.Nakamura K, Guizard N, Fonov VS, et al. Jacobian integration method increases the statistical power to measure gray matter atrophy in multiple sclerosis. Neuroimage Clin 2014; 4: 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Granziera C, Wuerfel J, Barkhof F, et al. Quantitative magnetic resonance imaging towards clinical application in multiple sclerosis. Brain 2021; 144(5): 1296–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Long-term evolution of multiple sclerosis iron rim lesions in 7 T MRI. Brain 2021; 144(3): 833–847. [DOI] [PubMed] [Google Scholar]
- 30.Weber CE, Wittayer M, Kraemer M, et al. Long-term dynamics of multiple sclerosis iron rim lesions. Mult Scler Relat Disord 2022; 57: 103340. [DOI] [PubMed] [Google Scholar]
- 31.Wenzel N, Wittayer M, Weber CE, et al. MRI predictors for the conversion from contrast-enhancing to iron rim multiple sclerosis lesions. J Neurol 2022; 269(8): 4414–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elliott C, Belachew S, Fisher E, et al. MRI characteristics of phase rim lesions in chronic and recent acute MS lesions (4106). Neurology 2021; 96(Suppl. 15): 4106. [Google Scholar]
- 33.Sethi V, Nair G, Absinta M, et al. Slowly eroding lesions in multiple sclerosis. Mult Scler 2017; 23(3): 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Popescu BF, Frischer JM, Webb SM, et al. Pathogenic implications of distinct patterns of iron and zinc in chronic MS lesions. Acta Neuropathol 2017; 134(1): 45–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dadar M, Mahmoud S, Narayanan S, et al. Diffusely abnormal white matter converts to T2 lesion volume in the absence of MRI-detectable acute inflammation. Brain 2021; 145(6): 2008–2017. [DOI] [PubMed] [Google Scholar]
- 36.Cadavid D, Mellion M, Hupperts R, et al. Safety and efficacy of opicinumab in patients with relapsing multiple sclerosis (SYNERGY): A randomised, placebo-controlled, phase 2 trial. Lancet Neurol 2019; 18(9): 845–856. [DOI] [PubMed] [Google Scholar]
- 37.Marcille M, Hurtado Rúa S, Tyshkov C, et al. Disease correlates of rim lesions on quantitative susceptibility mapping in multiple sclerosis. Sci Rep 2022; 12(1): 4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang W, Sweeney EM, Kaunzner UW, et al. Quantitative susceptibility mapping versus phase imaging to identify multiple sclerosis iron rim lesions with demyelination. J Neuroimaging 2022; 32(4): 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zinger N, Ponath G, Sweeney E, et al. Dimethyl fumarate reduces inflammation in chronic active multiple sclerosis lesions. Neurol Neuroimmunol Neuroinflamm 2022; 9(2): e1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calvi A, Clarke MA, Prados F, et al. Relationship between paramagnetic rim lesions and slowly expanding lesions in multiple sclerosis. Mult Scler 2023; 29(3): 352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-3-msj-10.1177_13524585231162262 for Lesion-level correspondence and longitudinal properties of paramagnetic rim and slowly expanding lesions in multiple sclerosis by Colm Elliott, David A Rudko, Douglas L Arnold, Dumitru Fetco, Ahmed M Elkady, David Araujo, Bing Zhu, Arie Gafson, Zhe Tian, Shibeshih Belachew, Daniel P Bradley and Elizabeth Fisher in Multiple Sclerosis Journal
Supplemental material, sj-pdf-1-msj-10.1177_13524585231162262 for Lesion-level correspondence and longitudinal properties of paramagnetic rim and slowly expanding lesions in multiple sclerosis by Colm Elliott, David A Rudko, Douglas L Arnold, Dumitru Fetco, Ahmed M Elkady, David Araujo, Bing Zhu, Arie Gafson, Zhe Tian, Shibeshih Belachew, Daniel P Bradley and Elizabeth Fisher in Multiple Sclerosis Journal
Supplemental material, sj-pdf-2-msj-10.1177_13524585231162262 for Lesion-level correspondence and longitudinal properties of paramagnetic rim and slowly expanding lesions in multiple sclerosis by Colm Elliott, David A Rudko, Douglas L Arnold, Dumitru Fetco, Ahmed M Elkady, David Araujo, Bing Zhu, Arie Gafson, Zhe Tian, Shibeshih Belachew, Daniel P Bradley and Elizabeth Fisher in Multiple Sclerosis Journal