Abstract
The functional importance of the amino terminus of the Helicobacter pylori vacuolating cytotoxin (VacA) was investigated by analyzing the relative levels of vacuolation of HeLa cells transfected with plasmids encoding wild-type and mutant forms of the toxin. Notably, VacA's intracellular activity was found to be sensitive to small truncations and internal deletions at the toxin's amino terminus. Moreover, alanine-scanning mutagenesis revealed the first VacA point mutations (at proline 9 or glycine 14) that completely abolish the toxin's intracellular activity.
The vacuolating toxin (VacA) is an important virulence factor in Helicobacter pylori-mediated gastric disease (1, 2, 4, 5, 14, 15, 17, 18, 20, 22, 24, 25). Oral administration of purified VacA confers to mice protection against infection by toxin-inducing strains of H. pylori (17). However, wild-type VacA is not an appropriate vaccine candidate because the toxin induces acute gastric epithelial erosion and ulceration (21). In an effort to circumvent VacA cytotoxicity, Manetti et al. inactivated the toxin by formaldehyde treatment (16). Formaldehyde-treated VacA retained the abilities to induce titers of neutralizing antibodies in rabbits and to protect mice from challenges with mouse-adapted strains of H. pylori (16). Collectively, these results indicate that detoxified VacA may be a candidate for use in vaccines against H. pylori infection and disease.
While chemical inactivation of proteins for use in human vaccines has been historically effective, recent efforts have focused on genetically detoxifying proteins (7, 11, 19). The strategy of genetic detoxification involves identification of essential amino acids that can be altered by molecular biological procedures to eliminate toxin activity. Genetic manipulations have greater potential for maintaining the antigenicity of the altered protein than does treating toxins with modifying chemicals. Moreover, genetic detoxification provides the quality control necessary for generating consistent preparations of toxoid with identical protective properties. Finally, recombinant toxoids potentially can be incorporated into live attenuated bacterial or viral vaccines.
Although VacA is an excellent candidate for genetic detoxification, point mutations that ablate the toxin's cellular activity below detectable levels have not been identified. While VacA exhibits properties resembling those of the intracellularly acting AB toxins (3, 9, 13), neither a discrete biochemical activity nor an intracellular target has been identified. In the absence of a defined assay, we have employed a transient transfection system in mammalian cells to identify and characterize the minimal intracellularly active fragment of VacA (Fig. 1) that induces degenerative vacuolation (9, 26). Using this system, we initiated mutational analyses to identify discrete domains and residues that are important for toxin function. Our earlier studies revealed that truncation of only 17 residues inactivated VacA (26), consistent with an independent report stating that a VacA mutant lacking the first 10 residues was inactive in the host cell cytosol (10). Moreover, analysis of mutants with large deletions (>20 residues) at the amino terminus demonstrated that an internal deletion of residues 6 to 26 fully ablated toxin activity, and a dominant-negative phenotype was demonstrated in the presence of wild-type toxin (23). Because these data suggested that the VacA amino terminus is important for toxin activity, we have conducted a more-detailed mutational analysis of this region (Fig. 1).
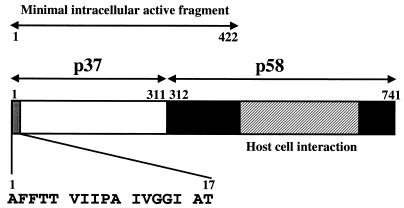
FIG. 1.
Domain structure of mature VacA. VacA is composed of a discrete amino-terminal domain (p37) (white bar) and a carboxyl-terminal domain (p58) (black bar). The minimal VacA fragment that induces cellular vacuolation when directly expressed within mammalian cells comprises residues 1 to 422. The putative VacA receptor-binding domain has been localized to the central region of p58 (hatched region). The amino acid sequence of the VacA amino-terminal region (residues 1 to 17) analyzed for its importance to intracellular VacA-mediated vacuolation is highlighted (gray bar).
Amino-terminal truncations of VacA.
HeLa cells were transfected with pET-20b harboring genes encoding either the fully-active VacA polypeptide (residues 1 to 741), cloned from the 60190 toxigenic strain of H. pylori, or mutagenized fragments of VacA fused to green fluorescence protein (GFP). All of the mutant forms of VacA used in this study were constructed by using a QuikChange site-directed mutagenesis kit (Stratagene) and confirmed by sequencing the entire DNA sequence across the open reading frame (Thermo Sequence; Amersham Life Science). All plasmids were amplified in Escherichia coli and purified prior to transfection experiments. HeLa cells were first infected with recombinant vaccinia virus (vT7) bearing the gene for phage T7 RNA polymerase (9, 12). Twenty hours after transfection, the HeLa cells were analyzed for vacuolation by quantifying the cellular uptake of neutral red (25). Using this system, 50 to 80% of the cells clearly demonstrated GFP fluorescence. In HeLa cells transfected with VacA-GFP, vacuolation was observed only in those cells demonstrating GFP fluorescence.
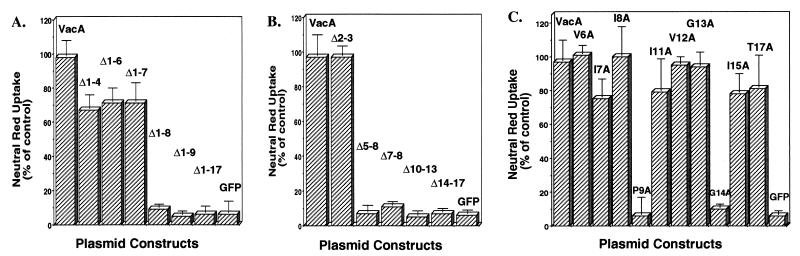
To define which residues at the VacA amino terminus are essential, plasmids expressing VacA fragments with amino-terminal truncations of 4, 6, 7, 8, 9, and 17 amino acids were constructed. Analysis of HeLa cells transfected with these plasmids revealed that truncating the amino terminus by seven residues or less did not diminish the ability of VacA to mediate vacuolation from within the host cell cytosol (Fig. 2A). In sharp contrast, the deletion of eight or more residues from the VacA amino terminus resulted in protein fragments that were unable to induce detectable vacuolation (Fig. 2A). Collectively, these results indicate that nearly the entire VacA amino terminus is required for the toxin to mediate intracellular vacuolation.
FIG. 2.
Mutational analysis of the VacA amino terminus. HeLa cells were transfected with pET20b plasmids expressing VacA mutants. After 20 h, the cells were assayed for uptake of neutral red. Data are expressed as percentages of neutral red uptake by HeLa cells transfected with a plasmid expressing full-length VacA-GFP. The negative control in these experiments was GFP alone. The data from three separate experiments performed at least in triplicate were averaged. (A) Truncation analysis: neutral red uptake of HeLa cells transfected with plasmids expressing VacA fragments comprising residues 1 to 741, 5 to 741, 7 to 741, 8 to 741, 9 to 741, 10 to 741, or 18 to 741, each fused to GFP, or GFP alone. (B) Internal deletion analysis: neutral red uptake of HeLa cells transfected with plasmids expressing VacA fragments comprising residues 1 to 741 with internal deletions of residues 2 and 3, 5 to 8, 7 and 8, 10 to 13, or 14 to 17, each, fused to GFP, or GFP alone. (C) Alanine-scanning mutagenesis of the VacA amino terminus: neutral red uptake of HeLa cells transfected with plasmids expressing VacA fragments comprising residues 1 to 741, fused to GFP, each with one of the following substitutions: V6A, I7A, I8A, P9A, I11A, V12A, G13A, G14A, I15A, and T17A. Error bars indicate standard deviations.
Internal deletions within the VacA amino terminus.
We next tested the hypothesis that amino acids just downstream of residue 7 are essential for activity by designing a series of internal deletion mutant-GFP fusion constructs. These VacA mutants had small deletions of residues 2 and 3, 5 to 8, 7 and 8, 10 to 13, or 14 to 17. Analysis of transfected cells revealed that VacA mutants with residues 5 to 8, 7 and 8, 10 to 13, or 14 to 17 deleted were unable to induce intracellular vacuolation (Fig. 2B), while deletion of residues 2 and 3 had no detectable effect on VacA activity, confirming the truncation results. Collectively, the results of the truncation and deletion analyses demonstrate that VacA-induced cellular vacuolation is sensitive to small perturbations at the toxin's amino terminus.
Alanine scanning of the VacA amino terminus.
To identify amino acids at the VacA amino terminus that are important for activity, we specifically probed residues 6 to 17 by alanine-scanning mutagenesis (Fig. 1). Each of the selected residues was individually changed to alanine, thereby eliminating all amino acid side chain interactions involving atoms beyond the β-carbon (8). This is an especially powerful approach for screening a large set of amino acids because substitution of alanine is considered the least-perturbing substitution of the 20 natural amino acids. Analysis of HeLa cells transfected with plasmids encoding each point mutant revealed that substitution of alanine for Pro-9 or Gly-14 resulted in a mutant form of VacA that was unable to induce intracellular vacuolation (Fig. 2C). Notably, these are the first VacA point mutations that have been determined to essentially eliminate toxin-induced vacuolation of mammalian cells. In the absence of a known intracellular biochemical activity for VacA, it is not presently possible to ascertain why an alanine substitution at either residue 9 or 14 eliminates toxin activity. Although Western blot analysis revealed that both of these mutant forms were expressed as full-length proteins in HeLa cells (data not shown), further work will be required to delineate whether VacA is destabilized by substitutions at residue 9 or 14.
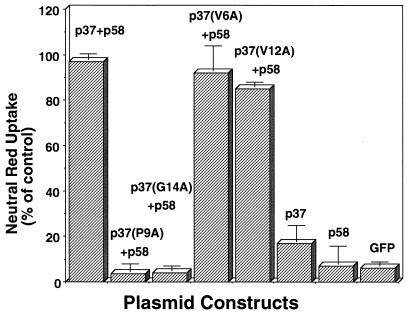
Neither p37 (P9A) nor p37 (G14A) functionally complements p58.
Earlier investigations showed that the VacA amino-terminal and carboxyl-terminal domains, called p37 and p58, respectively, demonstrate functional complementation when coexpressed as discrete fragments in HeLa cells (26). To test whether a substitution at residue 9 or 14 disrupts functional complementation, we substituted alanine for proline 9 or glycine 14 in a VacA fragment consisting of p37 alone. When coexpressed with p58 in transfected HeLa cells, neither p37 (P9A) nor p37 (G14A) functionally complemented p58 (Fig. 3), further confirming the importance of residues 9 and 14 to VacA intracellular activity. V6A and V12A, two point mutations that had no apparent effect on VacA-mediated intracellular vacuolation (Fig. 2C), were also constructed in the p37 fragment alone. In contrast to p37 (P9A) and p37 (G14A), both of these mutant forms of p37 functionally complemented p58 when coexpressed with p58 in transfected HeLa cells (Fig. 3).
FIG. 3.
Effects of p37 point mutations on p37-p58 functional complementation. HeLa cells were transfected with separate plasmids expressing VacA fragments comprising residues 1 to 311 (p37) or 312 to 741 (p58) or with GFP alone. Also shown are data for HeLa cells cotransfected with plasmids encoding either wild-type p37, p37 (V6A), p37 (P9A), p37 (V12A), or p37 (G14A), along with separate plasmids encoding p58 fused to GFP. After 20 h, the cells were assayed for uptake of neutral red. Data are expressed as percentages of neutral red uptake by HeLa cells transfected with a plasmid expressing full-length VacA-GFP. The data from three separate experiments performed at least in triplicate were averaged. Error bars indicate standard deviations.
Although the mechanism of p37-p58 complementation has not been elucidated, one model states that these two fragments interact in trans to mediate vacuolating activity (26). To begin testing this model, we investigated whether p37 (P9A) or p37 (G14A) could block functional complementation of wild-type p37 and p58. In these competition studies, we triple-transfected HeLa cells with plasmids encoding p37 and p58 as well as p37 (P9A), p37 (G14A), or GFP. When transfecting cells with three plasmids, encoding p37, p58, or GFP, we scored transfection efficiency by counting those cells that were both fluorescent (indicating expression of GFP) and vacuolated (indicating the expression of both p37 and p58). Notably, in these experiments, the transfection efficiency ranged from 40 to 80%, which is similar to that found in cells transfected with one or two plasmids. While expression of GFP had no detectable effect on p37-p58 complementation (Table 1), HeLa cells transfected with an excess of plasmids expressing either p37 (P9A) or p37 (G14A) demonstrated markedly reduced cellular vacuolation. In cells transfected with a sixfold molar excess of a plasmid encoding mutant p37 (P9A), the level of vacuolation was reduced to 27% of that of cells coexpressing only p37 and p58. Mutant p37 (G14A) was even more effective at blocking p37-p58 complementation; in cells transfected with a sixfold molar excess of a plasmid encoding p37 (G14A), the level of vacuolation was reduced to less than 10% of that of HeLa cells expressing p37 and p58 only. The finding that mutant p37 partially blocks p37-p58 functional complementation supports a model involving direct interactions between the two domains. Moreover, these results suggest that a mutation at residue 9 or 14 apparently does not entirely eliminate the ability of p37 and p58 to interact within target cells.
TABLE 1.
Mutant forms of p37 block functional complementation of wild-type p37 and p58
| Relative molar amt of plasmids expressinga:
|
Neutral red uptake (mean OD530–410 ± SD)b | Normalized vacuolating activity (%)c | ||||
|---|---|---|---|---|---|---|
| p37 | p58 | GFP | p37 (p9A) | p37 (G14A) | ||
| 1.0 | 1.0 | 0.17 | 0.92 ± 0.042 | 100 | ||
| 1.0 | 1.0 | 1.0 | 0.91 ± 0.055 | 98 | ||
| 1.0 | 1.0 | 6.0 | 0.93 ± 0.029 | 102 | ||
| 1.0 | 1.0 | 0.17 | 0.90 ± 0.091 | 96 | ||
| 1.0 | 1.0 | 1.0 | 0.86 ± 0.078 | 88 | ||
| 1.0 | 1.0 | 6.0 | 0.57 ± 0.044 | 27 | ||
| 1.0 | 1.0 | 0.17 | 0.88 ± 0.004 | 92 | ||
| 1.0 | 1.0 | 1.0 | 0.82 ± 0.072 | 79 | ||
| 1.0 | 1.0 | 6.0 | 0.48 ± 0.030 | 8 | ||
| 1.0 | 1.0 | 0.92 ± 0.045 | 100 | |||
| 1.0 | 0.46 ± 0.035 | 4 | ||||
| 0.44 ± 0.017 | 0 | |||||
Equimolar amounts of pET20b-p37 and pET20b-p58 were used in each transfection experiment. To each reaction, pET20b-p37 (P9A), pET20b-p37 (G14A), or pET20b-GFP was added in the relative molar amount indicated. The total DNA added to each transfection reaction was 2 to 6 μg.
Neutral red uptake was analyzed as previously described (6). OD530–410, optical density at 530 to 410 nm.
Calculated by comparing neutral red uptake with that in experiments using equimolar amounts of pET20b-p37-GFP and pET20b-p58-GFP (100%) or nontransfected HeLa cells (0%).
In summary, we have provided evidence to support the hypothesis that residues at the VacA amino terminus are important for toxin-mediated intracellular activity. Collectively, our results demonstrate that nearly the entire amino terminus is essential for VacA activity (10, 26). Moreover, these studies have led to the discovery of the first VacA point mutations that completely abolish the toxin's intracellular activity. Further work with these inactive mutants may yield stable, nontoxic immunogens for incorporation into vaccines that are effective at protecting against gastric ulcer- and cancer-causing strains of H. pylori.
Acknowledgments
We thank Patrick Callaerts for assistance with fluorescence microscopy to confirm expression of VacA-GFP fusions in transfected HeLa cells. We also thank Art Vailas and Daniel Martinez, who provided access to their laboratory's Dynatech MR5000 microtiter plate reader.
This work was supported in part by the National Institutes of Health (RO1 AI45928), a Welch Foundation award (1557904), an American Heart Association grant (1558565), an Oak Ridge Junior Faculty Enhancement Award, and two University of Houston PEER grants (1127260 and 1127264).
REFERENCES
- 1.Atherton J C. H. pylori virulence factors. Br Med Bull. 1998;54:105–120. doi: 10.1093/oxfordjournals.bmb.a011662. [DOI] [PubMed] [Google Scholar]
- 2.Atherton J C, Peek R M, Jr, Tham K T, Cover T L, Blaser M J. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997;112:92–99. doi: 10.1016/s0016-5085(97)70223-3. [DOI] [PubMed] [Google Scholar]
- 3.Cover T L. An intracellular target for Helicobacter pylori vacuolating toxin. Trends Microbiol. 1998;6:127–128. doi: 10.1016/s0966-842x(98)01231-1. [DOI] [PubMed] [Google Scholar]
- 4.Cover T L. The vacuolating cytotoxin of Helicobacter pylori. Mol Microbiol. 1996;20:241–246. doi: 10.1111/j.1365-2958.1996.tb02612.x. [DOI] [PubMed] [Google Scholar]
- 5.Cover T L, Blaser M J. Helicobacter pylori infection, a paradigm for chronic mucosal inflammation: pathogenesis and implications for eradication and prevention. Adv Intern Med. 1996;41:85–117. [PubMed] [Google Scholar]
- 6.Cover T L, Puryear W, Perez-Perez G I, Blaser M J. Effect of urease on HeLa cell vacuolation induced by Helicobacter pylori cytotoxin. Infect Immun. 1991;59:1264–1270. doi: 10.1128/iai.59.4.1264-1270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cropley I, Douce G, Roberts M, Chatfield S, Pizza M, Marsili I, Rappuoli R, Dougan G. Mucosal and systemic immunogenicity of a recombinant, non-ADP-ribosylating pertussis toxin: effects of formaldehyde treatment. Vaccine. 1995;13:1643–1648. doi: 10.1016/0264-410x(95)00134-m. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham B C, Wells J A. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989;244:1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- 9.de Bernard M, Arico B, Papini E, Rizzuto R, Grandi G, Rappuoli R, Montecucco C. Helicobacter pylori toxin VacA induces vacuole formation by acting in the cell cytosol. Mol Microbiol. 1997;26:665–674. doi: 10.1046/j.1365-2958.1997.5881952.x. [DOI] [PubMed] [Google Scholar]
- 10.de Bernard M, Burroni D, Papini E, Rappuoli R, Telford J, Montecucco C. Identification of the Helicobacter pylori VacA toxin domain active in the cell cytosol. Infect Immun. 1998;66:6014–6016. doi: 10.1128/iai.66.12.6014-6016.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douce G, Turcotte C, Cropley I, Roberts M, Pizza M, Domenghini M, Rappuoli R, Dougan G. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc Natl Acad Sci USA. 1995;92:1644–1648. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuerst T R, Niles E G, Studier F W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garner J A, Cover T L. Binding and internalization of the Helicobacter pylori vacuolating cytotoxin by epithelial cells. Infect Immun. 1996;64:4197–4203. doi: 10.1128/iai.64.10.4197-4203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghiara P, Marchetti M, Blaser M J, Tummuru M K R, Cover T L, Segal E D, Tompkins L S, Rappuoli R. Role of the Helicobacter pylori virulence factors vacuolating cytotoxin, CagA, and urease in a mouse model of disease. Infect Immun. 1995;63:4154–4160. doi: 10.1128/iai.63.10.4154-4160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leunk R, Johnson P, David B, Kraft W, Morgan D. Cytotoxin activity in broth culture filtrates of Campylobacter pylori. J Med Microbiol. 1988;26:93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- 16.Manetti R, Massari P, Marchetti M, Magagnoli C, Nuti S, Lupetti P, Ghiara P, Rappuoli R, Telford J L. Detoxification of the Helicobacter pylori cytotoxin. Infect Immun. 1997;65:4615–4619. doi: 10.1128/iai.65.11.4615-4619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 18.Phadnis S H, Ilver D, Janzon L, Normark S, Westblom T U. Pathological significance and molecular characterization of the vacuolating toxin gene of Helicobacter pylori. Infect Immun. 1994;62:1557–1565. doi: 10.1128/iai.62.5.1557-1565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts M, Bacon A, Rappuoli R, Pizza M, Cropley I, Douce G, Dougan G, Marinaro M, McGhee J, Chatfield S. A mutant pertussis toxin molecule that lacks ADP-ribosyltransferase activity, PT-9K/129G, is an effective mucosal adjuvant for intranasally delivered proteins. Infect Immun. 1995;63:2100–2108. doi: 10.1128/iai.63.6.2100-2108.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitt W, Haas R. Genetic analysis of the Helicobacter pylori vacuolating cytotoxin: structural similarities with the IgA protease type of exported protein. Mol Microbiol. 1994;12:307–319. doi: 10.1111/j.1365-2958.1994.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 21.Telford J L, Ghiara P, Dell'Orco M, Comanducci M, Burroni D, Bugnoli M, Tecce M F, Censini S, Covacci A, Xiang Z, et al. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994;179:1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Doorn L-J, Figueiredo C, Sanna R, Pena S, Midolo P, Ng E K W, Atherton J C, Blaser M J, Quint W G V. Expanding allelic diversity of Helicobacter pylori vacA. J Clin Microbiol. 1998;36:2597–2603. doi: 10.1128/jcm.36.9.2597-2603.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinion-Dubiel A D, McClain M S, Czajkowsky D M, Iwamoto H, Ye D, Cao P, Schraw W, Szabo G, Blanke S R, Shao Z, Cover T L. A dominant negative mutant of Helicobacter pylori vacuolating toxin (VacA) inhibits VacA-induced cell vacuolation. J Biol Chem. 1999;274:37736–37742. doi: 10.1074/jbc.274.53.37736. [DOI] [PubMed] [Google Scholar]
- 24.Wang H J, Kuo C H, Yeh A A, Chang P C, Wang W C. Vacuolating toxin production in clinical isolates of Helicobacter pylori with different vacA genotypes. J Infect Dis. 1998;178:207–212. doi: 10.1086/515600. [DOI] [PubMed] [Google Scholar]
- 25.Xiang Z, Censini S, Bayeli P F, Telford J L, Figura N, Rappuoli R, Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995;63:94–98. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye D, Willhite D C, Blanke S R. Identification of the minimal intracellular vacuolating domain of the Helicobacter pylori vacuolating toxin. J Biol Chem. 1999;274:9277–9282. doi: 10.1074/jbc.274.14.9277. [DOI] [PubMed] [Google Scholar]