Abstract
Background
For patients with primary antibody deficiency, the first line of therapy is replacement with immunoglobulin (Ig) products. Prior to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, Ig products did not contain antibodies with specificity for this virus, and there have been limited data on the antibodies present in the Ig products in current use.
Objective
To quantitatively examine SARS-CoV-2 antibodies in current Ig products.
Methods
We examined 142 unique lots of 11 different Ig products intended for intravenous and/or subcutaneous delivery for IgG-binding activities against recombinant SARS-CoV-2 receptor binding domain, spike, and nucleocapsid proteins by enzyme-linked immunosorbent assays. In addition, to assess functionality, 48 of these unique lots were assessed for their ability to inhibit the variants SARS-CoV-2 Ancestral, Alpha, Beta, Delta, and Omicron spike binding to angiotensin-converting enzyme 2 (ACE2).
Results
Significantly increased antibody values were observed for products manufactured after the year 2020 (expiration dates 2023–2024), as compared with Ig products before 2020 (prepandemic). Sixty percent and 85% of the Ig products with expiration dates of 2023 and 2024 were positive for antibody to SARS-CoV-2 proteins, respectively. The area under the curve values were significantly higher in products with later expiration dates. Later dates of expiration were also strongly correlated with inhibition of ACE2-binding activity; however, a decline in inhibition activity was observed with later variants.
Conclusions
Overall, more recent Ig products (expiration dates 2023–2025) contained significantly higher binding and inhibition activities against SARS-CoV-2 proteins, compared with earlier, or prepandemic products. Normal donor SARS-CoV-2 antibodies are capable of inhibiting ACE2-binding activities and may provide a therapeutic benefit for patients who do not make a robust vaccine response.
Key words: Primary antibody deficiency immunoglobulin (Ig) products, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Enzyme-linked immunosorbent assays (ELISA), Recombinant SARS-CoV-2 receptor binding domain (RBD), Spike proteins
What is already known about this topic? Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with inborn errors of immunity is associated with increased morbidity/mortality; however, little is known about the content of SARS-CoV-2 antibodies in immunoglobulin products used as replacement therapy.
What does this article add to our knowledge? Immunoglobulin products with expiration dates between 2023 and 2025 were found to have both significantly higher binding activities, as well as angiotensin-converting enzyme 2 (ACE2) inhibition activity against Ancestral, Alpha, Beta, Delta, and Omicron SARS-CoV-2 proteins, in comparison with prepandemic products.
How does this study impact current management guidelines? When present, polyclonal SARS-CoV-2 antibodies in more recently collected immunoglobulin products may provide a therapeutic benefit for patients who do not make a robust vaccine response.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a single-stranded RNA virus that causes coronavirus disease 2019 (COVID-19), a disease with variable presentation ranging from a mild common cold to severe respiratory failure.1 , 2 As of March 2022, this virus had officially infected more than 440 million people and led to approximately 20 million official COVID-19 deaths globally.3 , 4 An increased risk for severe disease and death has been noted among elderly patients and persons with preexisting medical conditions.5 To date, mass vaccination using highly effective SARS-CoV-2 vaccines appears essential in protection against severe disease.6
Although there are a wealth of data to support the effectiveness and safety of the newly developed SARS-CoV-2 vaccines, initial clinical trials enrolled mostly healthy volunteers. Data regarding immunity in various patient populations previously excluded in these clinical trials continue to be gathered.6 Patients with inborn errors of immunity (IEIs) have been a focus of interest for several reasons. First, recent reports have suggested that patients with IEIs are at increased risk of developing severe COVID-19.6, 7, 8, 9 The underlying immune abnormalities impair the ability of IEI patients to develop protective immunity, potentially leading to worse survival. Most IEIs will also impair vaccine responses; however, a recent study of 26 patients with immune defects, including 13 patients with common variable immunodeficiency, suggested that some were able to develop both specific antibody responses and peptide-specific T-cell responses to the BNT162b2 COVID-19 mRNA vaccine.6
For patients with a diagnosis of primary antibody deficiency, the first-line therapy is replacement with immunoglobulin (Ig) products.10 Immunoglobulin products are typically prepared from the plasma of between 1,000 and 15,000 donors per lot and are prepared over the course of a year, with Ig products having a 3-year expiration date from the time of bottle filling.11 Prior to the COVID-19 pandemic, human Ig preparations did not contain detectable SARS-CoV-2 antibodies.12 There have been limited data presented on whether Ig products produced since the start of the COVID-19 pandemic now contain protective SARS-CoV-2 antibodies. In this study, we analyzed 125 lots of Ig products currently used as replacement therapy in our patients, for IgG binding against recombinant SARS-CoV-2 receptor binding domain (RBD), spike, and nucleocapsid protein (NP). In addition, for 48 lots, we examined whether these inhibited spike protein of SARS-CoV-2 Ancestral, Alpha, Beta, Delta, and Omicron variants from binding to angiotensin-converting enzyme 2 (ACE2). From our analyses, we conclude that Ig products with expiration dates of 2023 to 2024, indicating later plasma collections, are likely to contain relevant SARS-CoV-2 antibodies. This may provide useful therapeutic information for patients who do not produce robust vaccine responses.
Methods
Study design
Immunoglobulin products
We collected 225 samples of 11 different brands of Ig products, with expiration dates of 2020 to 2025, intended for intravenous (IV) or subcutaneous (SC) use, either at the Mount Sinai Therapeutic Infusion Center or for home infusions. Of the 225 samples, 142 were from unique lots; where there were multiple samples from the same lot, mean values for these lots were used for these analyses. Twenty-six of these samples had expiration dates in 2020 or earlier (prepandemic), and the remaining 199 samples had expiration dates of 2021 or later
Expression and purification of recombinant SARS-CoV-2 proteins
Samples were first tested to determine the IgG-binding activity against recombinant SARS-CoV-2 RBD, SARS-CoV2 spike, and NP by enzyme-linked immunosorbent assay (ELISA), as described in detail by Stadlbauer et al.13 Recombinant SARS-CoV-2 proteins were produced using a mammalian cell protein expression system. Codon-optimized spike ectodomain, RBD, and NP gene sequences (GenBank: MN908947) were cloned into a mammalian expression vector, pCAGGs. Proteins were expressed using the Expi293 Expression System (Thermo Fisher Scientific), according to the manufacturer’s instructions. For spike and RBD proteins, cell culture supernatants were collected and clarified by centrifugation at 4,000× g, filtered, and purified with Ni2+-nitriloacetic acid agarose (QIAGEN). For NP, cell pellets were collected, resuspended in high salt buffer (1M Tris-HCl, pH 8.0; 0.5M ethylenediaminetetraacetic acid pH 8.0; 5M NaCl; Triton X-100 and distilled water) and lysed using a sonicator. Lysed cells were then centrifuged and the supernatant was purified with Ni2+-nitriloacetic Agarose (QIAGEN). The purified proteins were concentrated using Amicon Ultracell (Merck) centrifugation units, and the buffer was changed to phosphate-buffered saline (PBS) (pH 7.4). Proteins were stored at –80°C until use.
Enzyme-linked immunosorbent assays
Immulon 4 HBX 96-well microtiter plates (Thermo Fisher Scientific) were coated overnight at 4°C with recombinant proteins (100 ng/well) in PBS (pH 7.4). Well contents were discarded and blocked with 200 μL of 3% nonfat milk (AmericanBio) in PBS containing 0.1% Tween-20 (PBST) for 1 hour at room temperature (RT). After blocking, 100 μL of Ig samples diluted (starting at total Ig concentration of 10 mg/mL and serially diluted 2-fold) with 1% nonfat milk in PBST was added to each well for reaction at RT for 2 hours. After washing with PBST 3 times, 50 μL of horseradish peroxidase-labeled goat anti-human IgG antibody (Sigma-Aldrich) diluted 3,000-fold with 1% nonfat milk in PBST was added to each well, and incubated at RT for 1 hour. After washing with PBST 3 times, 100 μL of SIGMAFAST o-phenylenediamine dihydrochloride substrate solution (Sigma-Aldrich) was added to each well for the reaction at RT for 10 minutes. Reaction was stopped by addition of 50 μL of 3M hydrochloric acid (HCl). Optical density at 490 nm was measured using Synergy 4 (BioTek) plate reader. Eight wells on each plate received no primary antibody (blank wells), and the optical density in those wells was used to assess background. A detailed protocol for the ELISA can be found here.13
Angiotensin-converting enzyme 2 blocking assay
Immunoglobulin products were tested for their ability to block the interaction between SARS-CoV-2 Ancestral, Alpha, Beta, Delta, and Omicron variant spike and ACE2 proteins, using a multiplexed electrochemiluminescence detection system in 96-well plates (MSD V-PLEX® SARS-CoV-2 Plate 25). For testing, Ig products were diluted in a range from 1:10 to 1:2,560 and the percent inhibition was calculated for each individual variant, setting a diluent control at 0%.14
Statistical analyses
Area under the binding curve (AUC) was calculated by deducting the average of blank values plus 3 times SD of the blank values for ELISA analyses. Cut-off values were determined by the AUC of prepandemic samples using a typical method for negative control samples of the collective AUC mean + 3 SD. If the AUC value of the sample was above the cut-off value, the sample was counted as positive. The AUC cut-off values for RBD, spike, and NP were 10.60, 18.90, and 14.20,respectively. All data were analyzed in GraphPad Prism 9. In addition, 1-way analysis of variance with Kruskal-Wallis test was used for multiple comparison, and Mann-Whitney test for comparison between 2 groups.
Results
Overall antibody activity
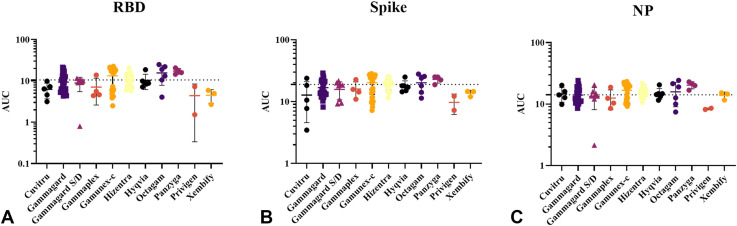
A total of 142 unique lots of Ig samples from 11 different Ig brands were analyzed in these studies (Table I ). First, using ELISA, 125 lots were examined for antibody binding to RBD, spike, and NP components. Gamunex-C, Octagam, and Panzyga were positive in RBD, spike, and NP ELISAs because their mean values exceeded the cut-off values for all 3 proteins (Figure 1 ). Hizentra and Hyqvia were positive for RBD and NP antibodies (Figure 1). With no clear trend emerging between brands, we then separated the samples based on expiration dates and route of administration.
Table I.
Immunoglobulin brand characteristics∗
| Brand | Number of lots tested | Site of administration | Route of administration | Date of expiration |
|---|---|---|---|---|
| Cuvitru | 5 | Home infusion | SC | 2023–2024 |
| Gammagard | 31 | Home infusion and infusion center | IV | 2020–2024 |
| Gammagard SD | 8 | Home infusion and infusion center | IV | 2020–2023 |
| Gammaplex | 4 | Home infusion | IV | 2023–2024 |
| Gamunex-C | 35 | Home infusion and infusion center | IV (mostly) and SC | 2020–2024 |
| Hizentra | 21 | Home infusion | SC | 2021–2023 |
| Hyqvia | 6 | Home infusion | SC | 2022–2023 |
| Octagam | 6 | Infusion center | IV | 2020–2023 |
| Panzyga | 4 | Home infusion | IV | 2023–2024 |
| Privigen | 2 | Home infusion | SC | 2022 |
| Xembify | 3 | Home infusion | IV | 2023–2024 |
Prepandemic samples correspond to an expiration year of 2020.
Figure 1.
Products with expiration dates ranging from 2020 or earlier to 2024 were analyzed for IgG-binding activities, against recombinant (A) SARS-CoV-2 RBD, (B) spike, and (C) NP using ELISA. Data points represent the AUC signifying binding activity. A number of brands had a mean binding activity above the cut-off values, denoting a positive result. Although all products did not have a positive result according to the mean, a majority of brands (9 of 11) had some samples that tested above the cut-off values. Error bars indicate mean and SD. Cut-off values are mean + 3 SD of AUC of prepandemic samples.
Immunoglobulin products by expiration date
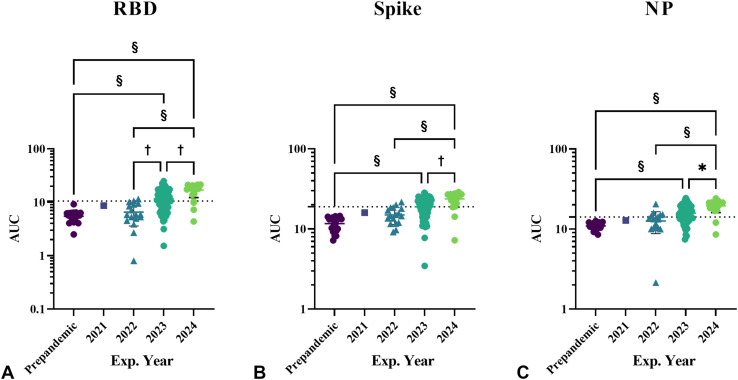
Products were first stratified based on date of expiration of 2020 or before (n = 20), 2021 (n = 1), 2022 (n = 17), 2023 (n = 64), and 2024 (n = 23). Significantly increased AUC values were observed in products with expiration dates of 2023 (RBD 11.59 ± 5.23; spike 18.95 ± 5.29; and NP 15.80 ± 4.80 [P < .0001]) and 2024 (RBD 17.03 ± 4.78; spike 23.77 ± 5.03; and NP 19.44 ± 3.40 [P < .0001]), compared with the Ig products with expiration dates in 2020, previously tested12 (Figure 2 ). Although there was a slight increase in the AUC values for products with expiration dates 2021 and 2022, compared with products from 2020 (prepandemic), these results were not statistically significant.
Figure 2.
Products were analyzed by expiration year, for IgG binding activities against (A) recombinant SARS-CoV-2 RBD, (B) spike, and (C) NP using ELISA. Data points represent area under the ELISA curve, separated by product expiration year. These was a significant increase in AUC in Ig products with expiration dates of 2023 compared with prepandemic products. The increasing trend in AUC continued across all 3 protein assays for expiration years 2023 and 2024. Error bars indicate mean and SD. Exp., Expiration. ∗P < .05; †P <.01; ‡P < .001; §P < .0001. A 1-way analysis of variance with Kruskal-Wallis test was performed for statistical analysis
Immunoglobulin products by brand
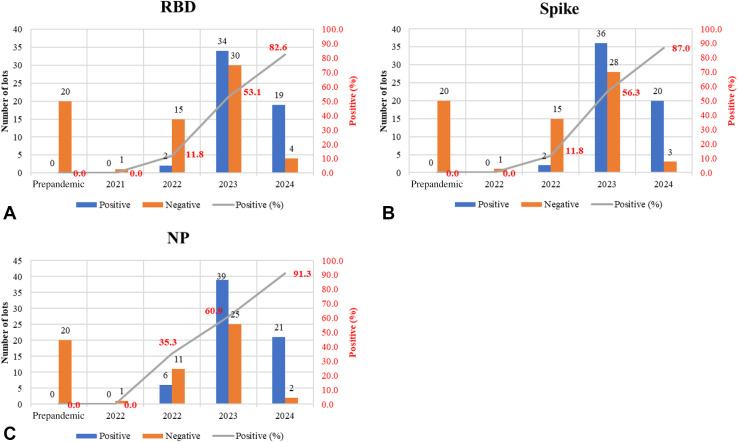
Five IV products including Gammagard, Gammagard SD, Gammaplex, Gamunex-C, and Panzyga and 3 SC products including Cuvitru, Hizentra, and Hyqvia were found to have anti–SARS-CoV-2 antibody, whereas the tested samples of 3 other available brands, Octagam, Privigen, and Xembify, with expiration dates ranging 2022 to 2024 had no detectable anti–SARS-CoV-2 antibody. An upward trend, linking percent positive samples and the expiration year, began to emerge for the years 2023 and 2024; 60% of lots with expiration in 2023, and 85% of lots with expiration in 2024, contained antibody to SARS-CoV-2 proteins (Figure 3 ). It should be noted that, whereas the number of lots for the expiration year 2024 (23) is fewer than 2023 (64), the percentage of positive lots is greater in 2024 (85%) than in 2023 (60%) (Figure 3).
Figure 3.
Products analyzed by expiration year for IgG binding activities against (A) recombinant SARS-CoV-2 RBD, (B) spike, and (C) NP using ELISA. Graph represents the number of samples testing positive and negative for binding to SARS-CoV-2 proteins. A clear upward trend emerges, with approximately 60% and 85% of the Ig products with expiration dates of 2023 and 2024 testing positive for binding to SARS-CoV-2 proteins, respectively.
Immunoglobulin products by route of infusion (SC vs IV preparations)
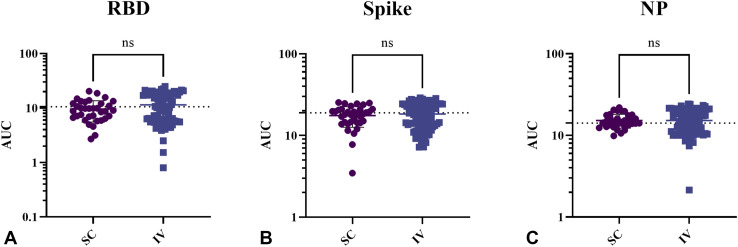
Analysis of products by route of administration was also examined, given differences in the manufacturing processes. In these analyses, the IV products were Gammagard, Gammagard SD, Gammaplex, Gammunex-C, Octagam, Panzyga, and Privigen; the SC products were Cuvitru, Hizentra, Hyqvia, and Xembify. However, there were no significant differences between SC and IV products (Figure 4 ): IV versus SC Ig (RBD 11.42 ± 6.33 vs 9.58 ± 4.1 [P < .07]), (spike 18.3 ± 6.3 vs 17.57 ± 4.96 [P < .48]), and (NP 15.24 ± 4.82 vs 15.21 ± 2.95 [P < .97]).
Figure 4.
Products by route of administration analyzed for IgG binding activities against recombinant SARS-CoV-2 proteins. (A) RBD, (B) spike, and (C) NP using ELISA. Data points represent area under the ELISA curve, demarcated by route of infusion. The AUC values were higher in IV products than in SC products across all 3 protein assays. Error bars indicate mean and SD. ns, Not significant.
Inhibitory activity
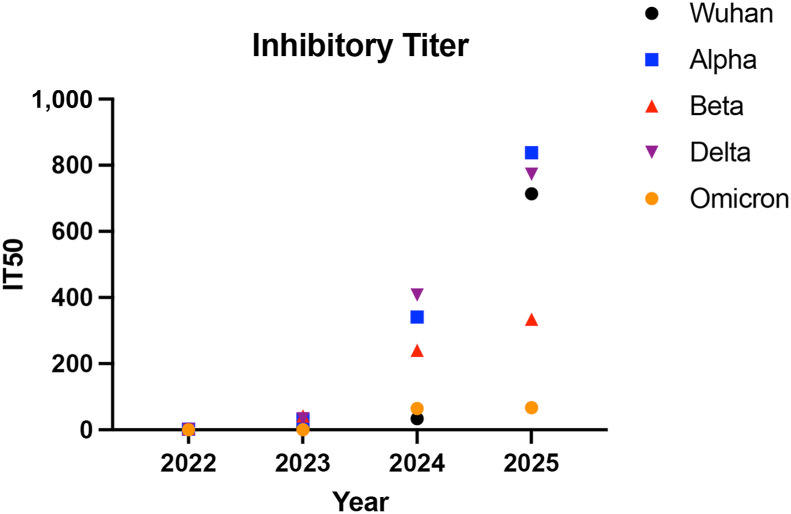
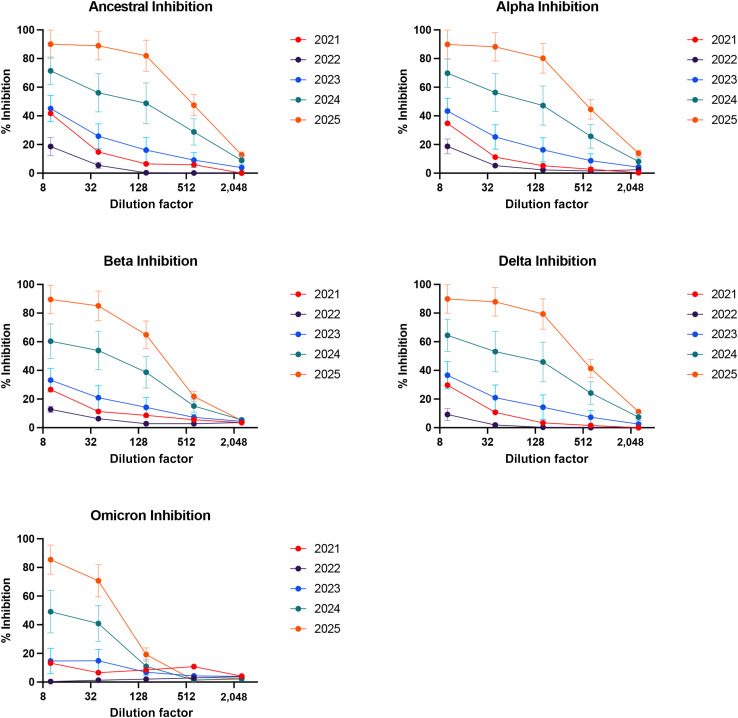
A total of 48 unique lots were then analyzed for their ability to block the interaction between SARS-CoV-2 Ancestral, Alpha, Beta, Delta, and Omicron spike variants and ACE2 protein. These data are summarized in Figure 5 . Demonstrating this for a particular expiration year (2023), using a dilution factor (1:10) for all products, marked differences in inhibition against different strains were seen: in the Ancestral strain (45%), Alpha strain (43%), Beta (32%), Delta strain (38%), and Omicron (15%). Of note, the lowest inhibition activity was seen against the Omicron strain, in lots expiring in 2023 (Figure E1; available in this article’s Online Repository at www.jaci-inpractice.org). The most variability in the individual lots was seen in products with expiration dates 2024 (Figure E1). We calculated the inhibitory titer 50 using neutralization curves generated that were then expressed by year and variant, a higher titer translated to more dilute samples being able to neutralize 50% of the spike-ACE2 protein. Overall, one clear trend that emerged was an increased inhibition activity with later expiration dates for all viral strains (Figure 5 and Figure E1). It is important to note overall, although the inhibition activity increased with increasing expiration date, the overall inhibition activity against the Omicron variant was low in comparison with earlier strains (Figures 5 and 6 15).
Figure 5.
Neutralization activity assessment using inhibitory titer 50 (IT 50) in products sorted by expiration date against COVID-19 variants. The IT 50 values were derived from neutralization curves, with higher titers indicating greater neutralizing capacity at higher dilutions for 50% of spike-ACE2 protein interactions. The IT 50 values for products expiring in 2021 were excluded owing to limited product quantity.
Figure E1.
Products with different expiration dates analyzed for inhibition activity at different dilution factors of immunoglobulin samples using angiotensin-converting enzyme 2 assay against different strains of coronavirus including the Ancestral, Alpha, Beta, Delta, and Omicron strains. Data points represent the percentage inhibition activity for product grouped based on the expiration date. Error bars indicating the standard error of the mean for product with expiration dates ranging between 2023 and 2025.
Figure 6.
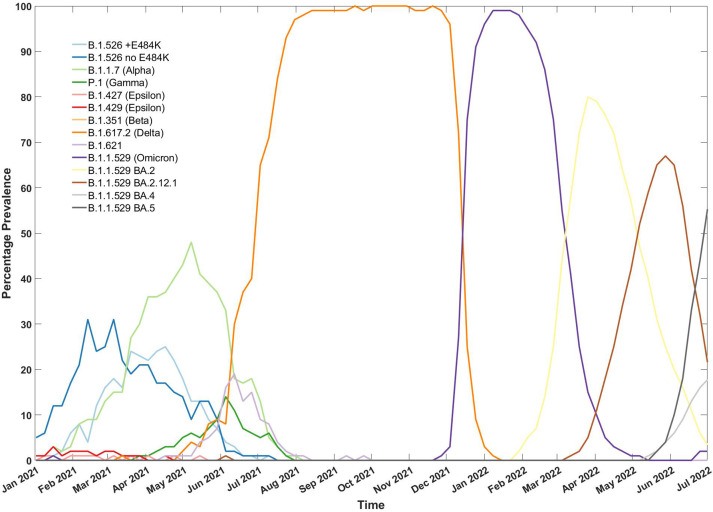
Prevalence of COVID-19 variants in circulation in New York City from January 2021 to July 2022. Legend indicates each genetic variant of SARS-CoV-2 in circulation, followed by World Health Organization nomenclature in parentheses where available. (Data generated from the NYC Department of Health and Mental Hygiene (NYC Department of Health and Mental Hygiene. Hygiene. Coronavirus Disease 2019 (COVID-19) in New York City (NYC) 2022. Accessed July 14. 2022. https://github.com/nychealth/coronavirus-data.15)
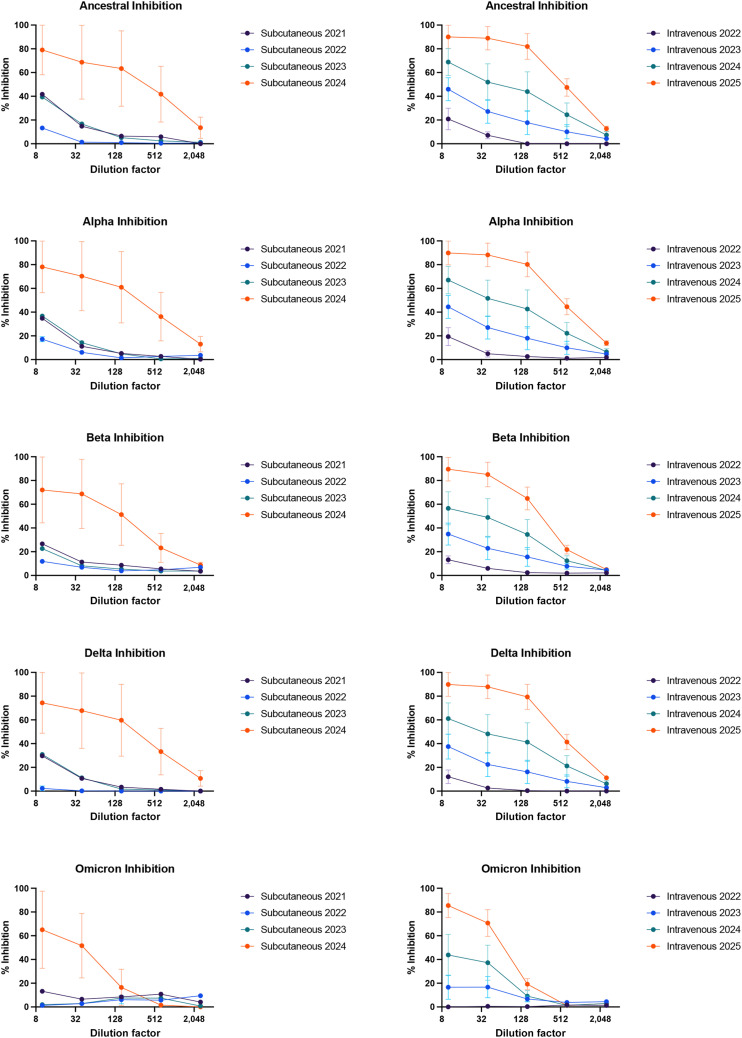
Analysis of products by the intended route of administration was also examined against each of the SARS-CoV-2 variants (Figure E2; available in this article’s Online Repository at www.jaci-inpractice.org). The 10% products used for IV infusion, compared with the 20% products used in SC delivery (diluted to the same Ig content), had more inhibition activity, regardless of expiration dates. For example, comparisons between IV and SC for products with expiration dates in 2024 showed an inhibition activity at 90% and 80%, respectively, against the Ancestral strain. This pattern was also observed for the other 4 strains. Generally, inhibition was also lower for both SC and IV products against the most recent strain (Omicron) in comparison with earlier strains of the virus (Figure E2).
Figure E2.
Products by route of administration (subcutaneous vs intravenous) grouped by expiration dates analyzed for inhibition activity at different dilution factors of immunoglobulin samples using angiotensin-converting enzyme 2 assay against different strains of coronavirus including the Ancestral, Alpha, Beta, Delta, and Omicron strains. Data points represent the percentage inhibition activity for product groups based on expiration dates for both subcutaneous and intravenous. Error bars indicate the standard error of the mean for products with expiration dates ranging between 2024 and 2025.
Discussion
Immunization remains the paramount intervention for the prevention of infectious diseases. Nevertheless, data concerning the efficacy of the SARS-CoV-2 vaccine in immunocompromised populations, including patients with IEIs, are still under investigation. In 1 study, the humoral and cellular immune response to the mRNA-based BNT162b2 COVID-19 vaccine in a cohort of 26 adult patients with IEIs was examined. These data showed that the majority of this subset of patients with IEIs were able to respond to the vaccine with significant levels of neutralizing antibodies, T-cell responses (evaluating for interleukin-2 and interferon-gamma), or both.6 Whereas Ig replacement is the first-line therapy in management of antibody defects,10 prepandemic Ig preparations did not contain detectable SARS-CoV-2 antibodies.12 One study examined 13 samples from 5 commercial Ig preparations from prepandemic donors; these samples were blindly screened using a semiquantitative U.S. Food and Drug Administration–approved and validated ELISA. Nine of 13 preparations (69.2%) from 2 different manufacturers were antibody-positive, based on the defined cut-off positivity; however, the authors concluded prepandemic Ig donors had prepandemic cross-reactive antibodies derived from seasonal coronaviruses.16
In our study, we assessed 11 distinct Ig replacement products, finding 8 of the products contained detectable anti–SARS-CoV-2 antibodies. This presence correlated with the products' expiration dates and, consequently, their collection dates. Products with expiration dates between 2023 and 2024 demonstrated a significantly higher likelihood of containing anti–SARS-CoV-2 antibodies. Furthermore, the expiration date served as a predictor of a product's inhibitory activity, with later expiration dates associated with increased inhibitory activity. One exception involved products with 2021 and 2022 expiration dates, which deviated from the general trend of increasing inhibition with later expiration dates. This discrepancy could be attributed to the limited number of samples with 2022 expiration dates available for testing. During the pandemic, various strains emerged sequentially: ancestral, Alpha, Beta, Delta, and Omicron. We observed a substantial decline in inhibitory activity against these strains for products with expiration dates prior to 2025. This decrease may suggest that successive strains exhibit diminished binding to the wild-type (ancestral) virus, thereby attenuating their ability to inhibit the propagation of newer strains, such as the Omicron variant. However, this decline in inhibitory activity was not evident for products with expiration dates in 2025, particularly for more recent strains like Omicron, implying the potential inclusion of Omicron-specific antibodies in the latest Ig products.
We noted that that the 10% products used in IV administration had more ACE2 inhibitory binding activity (at comparable IgG dilutions) than the 20% products used in SC treatments. These differences could be due to differences in the manufacturing processes, storage conditions, or the fact that there were fewer SC samples available for analyses than IV products. With regard to antibody binding, differences in IV versus SC products were not statistically significant. The main determinant in these assays was the expiration date of the product.
The following are limitations of our study. First, we were not able to test the same numbers of lots of each product, potentially skewing our results. The difference in the numbers of lots distributed among products was primarily due to reliance on collection from infusion center stock and products obtained from patients. The brand of product for each patient on home infusion is typically dependent on clinical needs as well as insurance coverage. Additional lots may have more antibody than the levels noted. Second, package inserts listed that product may be stored for 36 months at 36°F to 46°F; however, if a product was stored at 77°F, the shelf life would be 6 months. For products used in the Infusion Center, these conditions would be expected; however, for products used in the home, different storage conditions used could theoretically alter antibody content.
Our findings suggest that Ig products are likely to provide increasing therapeutic benefits for patients with loss of antibody production.
Other studies support our results, including 1 that evaluated 14 samples of Ig products, detecting neutralizing COVID-19 antibody content without substantial differences between manufacturers.17 Another study found that 2 years into the pandemic, the amount of antibodies to SARS-CoV-2 varied considerably among commercial Ig batches obtained from 3 commercial producers. In batches with high concentrations of antibodies directed against the original virus strain, passive immunity to the Omicron variant appeared to be insufficient.18
Although the clinical implications and benefit of this therapeutic remain uncertain, as mass vaccination continues, and infections continue to appear with the Delta (B.1.617.2) and Omicron (B.1.1.529) variants, we expect the useful SARS-CoV-2 antibodies to continue to increase in commercial Ig products. However, Omicron has evaded neutralization by many of the commercially available therapeutic monoclonal antibodies and the subvariants BQ.1.1 and XBB have been shown to completely escape neutralization by any approved prophylactic or therapeutic monoclonal antibody treatments. Polyclonal Ig products may be a potential solution to fill this gap.19
Acknowledgments
We would like to thank our patients and our dedicated medical teams.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
K. Sano is supported by the Japanese Society for the Promotion of Science (JSPS) Overseas Research Fellowship. Work in the Krammer laboratory was partially funded by the Centers of Excellence for Influenza Research and Surveillance (CEIRS; contract #HHSN272201400008C), the CEIRR (contract #75N93021C00014), by the Collaborative Influenza Vaccine Innovation Centers (CIVICs; contract #75N93019C00051), and by institutional funds. This study was supported by the Serological Sciences Network (SeroNet) in part with Federal funds from the National Cancer Institute, National Institutes of Health (NIH), under contract no. 75N91019D00024, task order no. 75N91021F00001; NIH AI 101093, AI-086037, AI-48693; and the David S. Gottesman Immunology Chair.
Conflicts of interest: The Icahn School of Medicine at Mount Sinai has filed patent applications relating to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) serological assays and NDV-based SARS-CoV-2 vaccines that list F. Krammer as co-inventor (V. Simon is also listed on the serological assay patent application as co-inventor). Mount Sinai has developed a company, Kantaro, to market serological tests for SARS-CoV-2. F. Krammer has consulted for Merck and Pfizer (before 2020); and is currently consulting for Pfizer, Seqirus, 3rd Rock Ventures and Avimex. The Krammer laboratory is also collaborating with Pfizer on animal models of SARS-CoV-2. The rest of the authors declare that they have no relevant conflicts of interest.
Online Repository
References
- 1.Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization WHO Coronavirus (COVID-19) Dashboard Geneva. https://covid19.who.int/table
- 4.Wang H., Paulson K.R., Pease S.A., Watson S., Comfort H., Zheng P., et al. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet. 2022;399:1513–1536. doi: 10.1016/S0140-6736(21)02796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., et al. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020;2:1069–1076. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagin D., Freund T., Navon M., Halperin T., Adir D., Marom R., et al. Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J Allergy Clin Immunol. 2021;148:739–749. doi: 10.1016/j.jaci.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyts I., Bucciol G., Quinti I., Neven B., Fischer A., Seoane E., et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol. 2021;147:520–531. doi: 10.1016/j.jaci.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho H.E., Mathew S., Peluso M.J., Cunningham-Rundles C. Clinical outcomes and features of COVID-19 in patients with primary immunodeficiencies in New York City. J Allergy Clin Immunol Pract. 2021;9:490–493.e2. doi: 10.1016/j.jaip.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin H., Moss R., Reed J.C., Hertzberg E., Cruz M.R., Akkoyun E., et al. IFN-gamma receptor 2 deficiency initial mimicry of multisystem inflammatory syndrome in children (MIS-C) J Allergy Clin Immunol Pract. 2021;9:989–992.e1. doi: 10.1016/j.jaip.2020.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonilla F.A., Khan D.A., Ballas Z.K., Chinen J., Frank M.M., Hsu J.T., et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136:1186–1205. doi: 10.1016/j.jaci.2015.04.049. e1-78. [DOI] [PubMed] [Google Scholar]
- 11.Jolles S., Sewell W.A., Misbah S.A. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol. 2005;142:1–11. doi: 10.1111/j.1365-2249.2005.02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M., et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26:1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stadlbauer D., Amanat F., Chromikova V., Jiang K., Strohmeier S., Arunkumar G.A., et al. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Curr Protoc Microbiol. 2020;57 doi: 10.1002/cpmc.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roltgen K., Nielsen S.C.A., Silva O., Younes S.F., Zaslavsky M., Costales C., et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell. 2022;185:1025–1040.e14. doi: 10.1016/j.cell.2022.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NYC Department of Health and Mental Hygiene Hygiene. Coronavirus Disease 2019 (COVID-19) in New York City (NYC) 2022. https://github.com/nychealth/coronavirus-data Accessed July 14. 2022.
- 16.Dalakas M.C., Bitzogli K., Alexopoulos H. Anti-SARS-CoV-2 Antibodies within IVIg preparations: cross-reactivities with seasonal coronaviruses, natural autoimmunity, and therapeutic implications. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.627285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsiger J.R., Weigang S., Walz A.C., Fuchs J., Daly M.L., Eggimann S., et al. Passive immunization against COVID-19 by anti-SARS-CoV-2 spike IgG in commercially available immunoglobulin preparations in severe antibody deficiency. J Allergy Clin Immunol Pract. 2022;10:2452–2455.e3. doi: 10.1016/j.jaip.2022.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindahl H., Klingstrom J., Da Silva Rodrigues R., Christ W., Chen P., Ljunggren H.G., et al. Neutralizing SARS-CoV-2 antibodies in commercial immunoglobulin products give patients with X-linked agammaglobulinemia limited passive immunity to the omicron variant. J Clin Immunol. 2022;42:1130–1136. doi: 10.1007/s10875-022-01283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q., Iketani S., Li Z., Liu L., Guo Y., Huang Y., et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 2023;186:279–286.e8. doi: 10.1016/j.cell.2022.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]