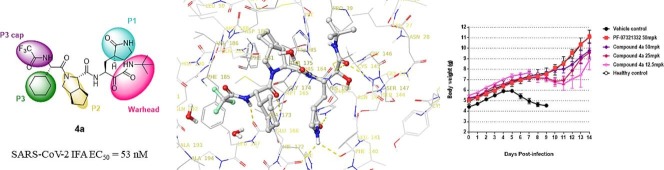
Graphical abstract
Keywords: SARS-CoV-2, 3-chymotrypsin-like cysteine protease, α-ketoamide
Abstract
The outbreak of SARS-CoV-2 has caused global crisis on health and economics. The multiple drug-drug interaction risk associated with ritonavir warrants specialized assessment before using Paxlovid. Here we report a multiple-round SAR study to provide a novel bicyclic[3.3.0]proline peptidyl α-ketoamide compound 4a, which is endowed with excellent antiviral activities and pharmacokinetic properties. Also, in vivo HCoV-OC43 neonatal mice model demonstrated compound 4a has good in vivo efficacy. Based on these properties, compound 4a worth further SAR optimization with the goal to develop compounds with better pharmacokinetic properties and finally to realize single agent efficacy in human.
Although three years have passed since the first patient reported, COVID-19 is still causing a global health emergency.1, 2 Billions of cases and millions of fatalities have been reported worldwide, indicating the urgent need of antiviral drugs.3 Remdesivir,4 which interferes with the function of RNA-dependent RNA polymerase, was recommended by World Health Organization (WHO) in 2020. However, it needs to be administered intravenously, which limits its widespread use during the pandemic. Oral SARS-CoV-2-specific therapeutics are urgently needed to prevent more severe disease, hospitalization and death.
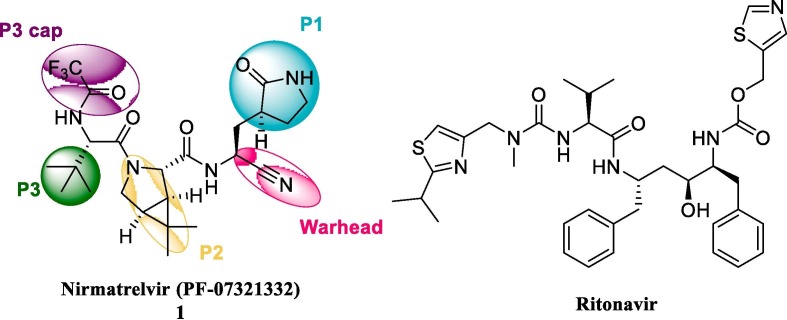
3-chymotrypsin-like cysteine protease enzyme (3CLpro/Mpro) plays an essential role in coronavirus replication. It can cleave the polyproteins at 11 different sites to yield shorter, non-structural proteins vital to viral replication.5, 6 Paxlovid, targeting 3C-like protease,7 was granted Emergency Use Authorization in December 2021 as therapy of nonhospitalized patients (adults and children 12 years or older). Paxlovid consists of two components, nirmatrelvir (1) and ritonavir.8, 9 Nirmatrelvir is a 3CLpro inhibitor and has low nanomolar inhibition potency against the COVID-19. Ritonavir is a protease inhibitor and potent inhibitor of enzyme (CYP3A4) responsible for the metabolism of nirmatrelvir, as a result, it enables higher peak level and more prolonged half-life of nirmatrelvir (Fig. 1 ).
Fig. 1.
Chemical structure of nirmatrelvir and ritonavir.
However, combination with ritonavir requires cautious use with other medications because interfering with CYP3A4 enzyme would increase drug concentration in serum of other medicines, which may result in unexpected side effects. Paxlovid should also not be given to patients on pharmacological agents that act as CYP inducers. These can lead to drastically reduced levels of nirmatrelvir, resulting in worse therapeutic effect.
With the goal of overcoming those issues associated with the combination use of PK booster ritonavir while retaining great antiviral activity, herein we would like to report on the discovery of a novel series of α-ketoamide based potent 3CLpro inhibitors that may be used as single anti-Covid agent without the need to be used along with ritonavir.10
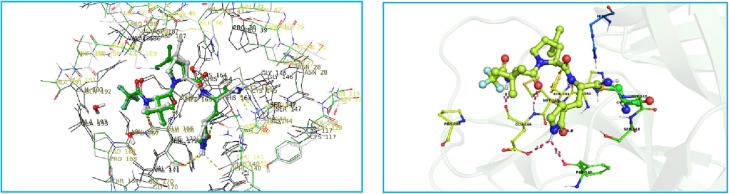
At the beginning of our study, we first studied the binding mode of nirmatrelvir by docking it to a published co-crystal structure of GC-376 with the SARS-CoV-2 Mpro (PDB: 6WTT).11 This established docking model was found aligned well with later published nirmatrelvir co-crystal structure (PDB: 7VH8).12 The γ-lactam ring in nirmatrelvir forms hydrogen-bond interactions with Glu166 and His163. The nitrile warhead forms a reversible covalent thioimidate adduct with the Cys145 (Fig. 2 ). The dimethyl cyclopropanyl group at P2 and the tert-butyl group at P3 show hydrophobic interactions with the binding pockets, which are suitable for more structure–activity relationship studies.
Fig. 2.
a) Predicted binding mode of nirmatrelvir and SARS-CoV-2 Mpro (PDB: 6WTT). Pink dashes are potential hydrogen bonds. b) Nirmatrelvir’s co-crystal structure with SARS-CoV-2 Mpro12.
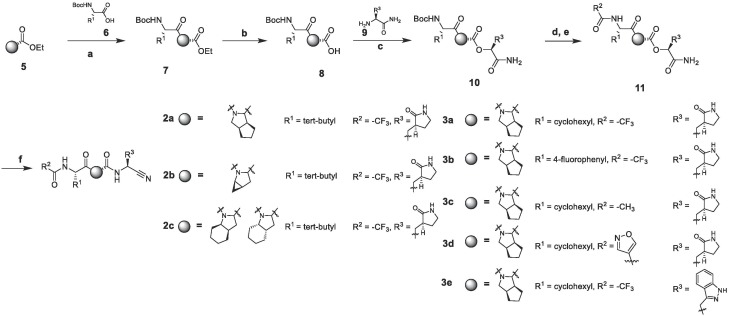
We first started to explore with different greasy fragments to fill up the hydrophobic P2 pocket. Compounds 2a, 2b, 2c were selected as our initial SAR exploration targets. The syntheses of 2a-c are shown in Scheme 1 . SARS-CoV-2 Mpro enzymatic potencies, HCoV OC43 CPE assay and SARS-CoV-2 IFA test were examined and compared with nirmatrelvir. HCoV OC43 CPE assay was selected because its 3CL protease has high homology with SARS-CoV-2 Mpro.13
Scheme 1.
Synthesis of bicyclic[3.3.0]proline peptidyl cyano compounds.a Reagents and conditions: a) HATU, DIEA, 6, DMF; b) LiOH·H2O, THF/H2O; c) EDCI, HOBt, DIEA, 9; d) TFA, DCM; f) TFAA, pyridine, THF; For compound 3c d) TFAA, pyridine, THF; e) TFA, DCM; f) Acetyl chloride, TEA, DMAP; For compound 3d: d) TFA, DCM; e) EDCI, HOBt, DIEA, 1, 2-oxazole-3-carboxylic acid,2-butanone; f) TFAA, pyridine, THF a The two isomers of compound 2c were not isolated.
As shown in Table 1 , among P2 modified compounds, compound 2a was found to be the most potent one with Mpro enzymatic IC50 value of 43 nM, HCoV OC43 CPE EC50 of 136 nM and SARS-CoV-2 IFA EC50 value of 64 nM. In contrary, compounds 2b and 2c were both found to be inactive.
Table 1.
Antiviral activities of P1, P2, P3, P3 cap modification compounds and nirmatrelvir.
| Assays | Compounds | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nirmatrelvir(1) | 2a | 2b | 2ca | 3a | 3b | 3c | 3d | 3e | |
| Mpro enzyme IC50 (nM) | 14 | 43 | 356 | >1000 | 47 | 165 | 551 | 135 | >1000 |
| HCoV OC43 CPE EC50 (nM) | 70 | 136 | 5806 | >10000 | 112 | 677 | 2778 | 573 | 4887 |
| SARS-CoV-2 IFA EC50 (nM) | 27 | 64 | – | – | 43 | – | – | – | – |
The two isomers were tested together and showed no antiviral activities.
Compound 2a was further evaluated through in vitro ADME and in vivo PK tests. As shown in Table 2 , for ADME, both nirmatrelvir and 2a displayed significant species different stability profiles and seemed quite unstable in mice. Compound 2a showed comparable liver microsome and hepatocytes stability with nirmatrelvir, along with slightly better plasma stability. For rat PK results, which should be more consistent with human according to the in vitro ADME data, compound 2a showed similar oral exposure albeit with better bioavailability. However, the T1/2 value determined for 2a was still relatively short (Table 3 ).
Table 2.
ADME and rodents PK properties of compounds 2a, 3a and nirmatrelvir.
| Assay Type | nirmatrelvir | 2a | 3a | ||||
|---|---|---|---|---|---|---|---|
| In vitro stabilitya | Plasma stability (Remaining% at 2 h) | 91, 84, 74 | 102, 100, 106 | 100, 92, 108 | |||
| Microsome stability (CLint(liver), mL/min/kg) |
329, <17.3, 29 | 262, <17.3, 13 | 215, <17.3, 19 | ||||
| Hepatocytes stability (CLint(liver), mL/min/kg) |
169, <29.9, <17.8 | 435, 32, <17.8 | 455, 51, <17.8 | ||||
| Dose route | PK parameterb | mouse | rat | mouse | rat | mouse | rat |
| iv | CL (mL min−1 kg−1) | 30.2 | 42.0 | 79.3 | 75.4 | 92.2 | 62.7 |
| Vss (L/kg) | 0.456 | 1.24 | 0.640 | 1.25 | 1.01 | 1.34 | |
| T1/2 (h) | 0.714 | 0.770 | 0.111 | 0.239 | 0.188 | 0.378 | |
| po | Cmax (μM) | 2.72 | 2.14 | 0.79 | 2.98 | 1.16 | 3.47 |
| AUC (μM × h) | 3.58 | 2.38 | 0.553 | 2.31 | 0.478 | 2.92 | |
| F (%) | 32.7 | 29.9 | 13.3 | 52 | 14.2 | 57.6 | |
In vitro stability data is listed in the sequence of mouse, rat, human.
Mice were administered at 3 mg/kg for iv group and 10 mg/kg for po group. Rats were administered at 2 mg/kg for iv group, and 10 mg/kg for po group. The vehicle was saline for all administration groups.
Table 3.
Antiviral potency of compound 4a in different assays.
| results | |||||
|---|---|---|---|---|---|
| Compound | Nirmatrelvir(1) | 4a | 4b | 4c | 4d |
| Mpro enzyme IC50 (nM) | 14 | 34 | 104 | 50 | 77 |
| HCoV OC43 CPE EC50 (nM) | 70 | 146 | – | 421 | 444 |
| SARS-CoV-2 IFA EC50 (nM) | 27 | 53 | – | – | – |
To continue effort towards discovery of potent 3CL inhibitors endowed with better pharmacokinetic properties, we began another round of SAR optimization on P3 and P3 cap. We kept octahydrocyclopenta[c] pyrrole moiety in compound 2a and designed a series of compounds and ranked them according to docking results. Through this exercise, compounds 3a and 3b were first selected for synthesis and evaluation.
To our satisfaction, compound 3a exhibited comparable antiviral potencies to that displayed by compound 2a (Table 1). On another hand, compound 3b was found to be 4-fold weaker in enzymatic potency relative to 2a. Several modifications in P3 cap (3c and 3d) with high docking scores were then tested. However, they both showed 3–10 folds loss in enzymatic and cell potencies, suggesting the trifluoromethyl group in nirmatrelvir may picked up extra hydrogen-halogen bonds other than pure hydrophobic interactions at P3 cap position (Table 1). 2-isoxazole group in compound 3d maintained some of the hydrogen bonds while trimethyl group in compound 3c did not have this interaction. This may explain the difference of antiviral potencies with 3c and 3d.
We then evaluated DMPK profiles of 3a and found that it had comparable PK properties as compound 2a (Table 2). Once again, significant species difference and relatively inferior PK profiles of 3a existed.
As for P1 SAR, we decided to employ indazole moiety. Docking model showed indazole could maintained the same set of hydrogen bonds as γ-lactam within P1 pocket. However the phenol ring clashed slightly to the P3 moiety within the molecule which led to a lower binding score. Triggered by indazole’s anti-viral properties showed in other programs, compound 3e was still synthesized and tested. Disappointedly, this compound did not possess any activity in bioassays evaluate.
After initial examination of SAR on P1, P2, P3 and P3 cap moieties, we obtained several novel compounds (e.g., 2a & 3a, all containing nitrile group as warhead) displaying comparable potencies as nirmatrelvir. However, none of these compounds showed improvement in pharmacokinetic properties both in terms of oral exposure level (AUC) and T1/2 value. Therefore, we turned our attention to warhead alteration (nitrile group as employed by PF-series). Towards that end, aldehyde and α, β-unsaturated ester are two commonly used warheads in antiviral medicines, but both aldehyde and α, β-unsaturated ester may cause unexpected toxicity and instability problems. After careful literature search, we were delighted to find the α-ketoamide bearing HCV protease inhibitor Telaprevir reported by Eli Lilly group.14, 15, 16, 17 α-Ketoamides have been shown to possess better pharmacokinetic properties such as improved membrane permeability and enhanced stability toward plasma esterases.18, 19 It has also been used in exploring broad-spectrum inhibitors of coronavirus and enterovirus replication.20 Inspired by these findings, we decided to introduce α-ketoamide as the warhead for our anti-Covid-19 3CL inhibitor program.21
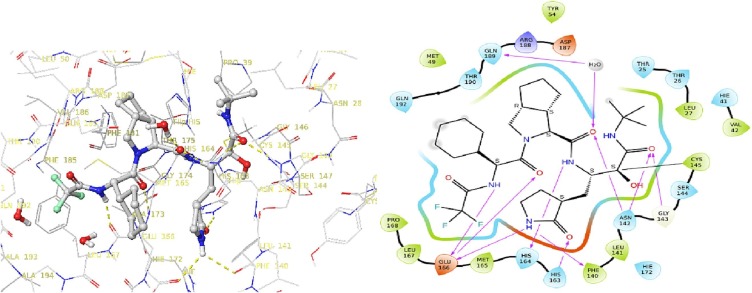
To explore the warhead pocket of α-ketoamide bearing Covid-19 3CL protease inhibitor series, we docked various types of P1′ groups and found a greasy pocket that could accommodate appropriate substituent group to gain extra binding. Therefore, we decided to install tert-butyl group on the warhead moiety as seen in compound 4a (see Scheme 2 ). The docking results of 4a with viral enzyme are outlined in Fig. 3 . The α-carbonyl group of α-ketoamide could form a reversible hemi-thioketal with Cys145, which is similar to the role of cyano group in nirmatrelvir. Moreover, the amide group of α-ketoamide may form additional hydrogen bonds with Asn142 and Gly143. The hydrogen bonds and hydrophobic interactions on other sites are also maintained (Fig. 4 ).
Scheme 2.
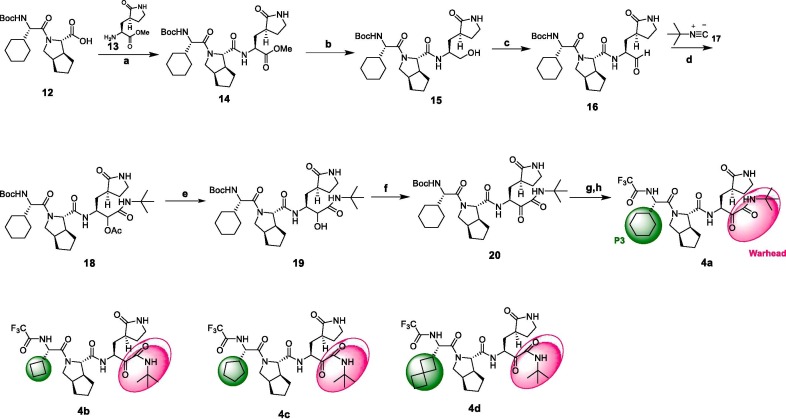
Synthesis of compounds 4a-4d. Reagents and conditions: a) T3P, DIEA, 13, DCM, 67%; b) LiBH4, THF, 94%; c) DMP, DCM, 94%; d) HOAc, 17, DCM, 84%; e) K2CO3, MeOH/H2O, 100%; f) DMP, DCM, 96%; g) HCl/EA, THF; h) TFAA, pyridine, 53% for 2 steps.
Fig. 3.
Predicted binding mode of compound 4a; a potential reversible covalent Cys145 adduct is formed with the α-ketoamides in compound 4a.
Fig. 4.
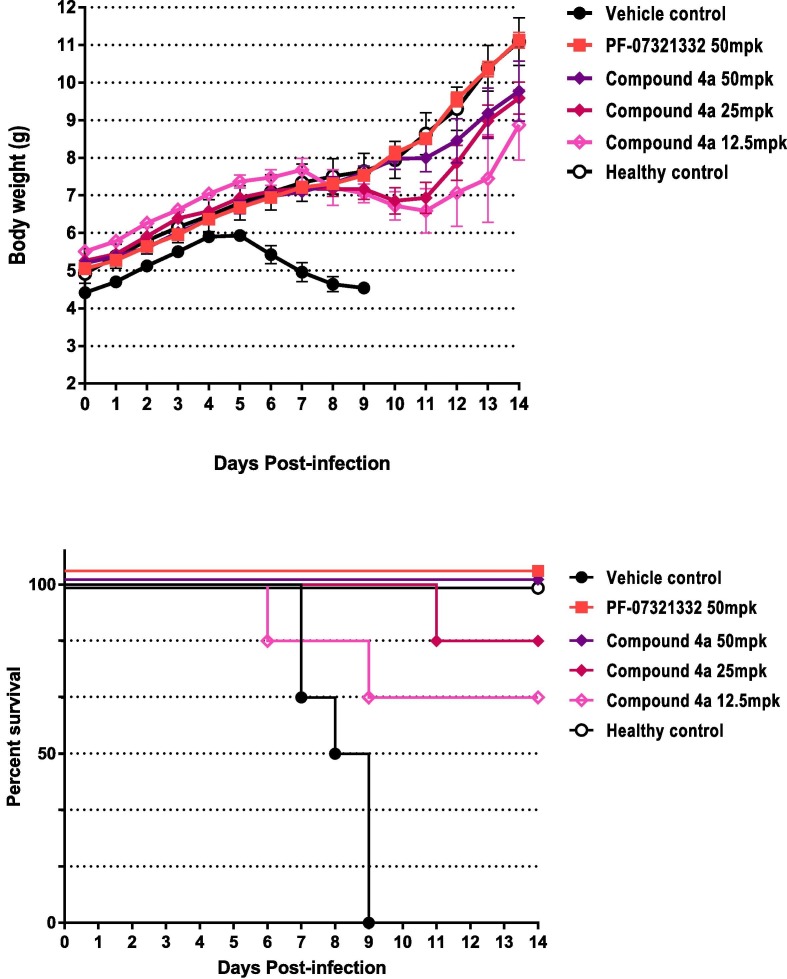
Survival Percentage- dosing relationship curve and body weight change-{Citation}dosing relationship Curve.
The synthesis of the α-ketoamide 4a is outlined in Scheme 2. Starting from the acid precursor 12 described in Scheme 2, compound 16 was obtained through condensation and oxidation state adjustment. Reaction with tert-butyl isocyanide 17 and then de-acylation provided the keto amide precursor 19. Oxidation of compound 19 with DMP, followed by deprotection and trifluoroacetylation provided the desired compound 4a. After finished the synthesis of compound 4a, more modifications on P3 were done. Compounds 4b, 4c and 4d were then synthesized according to the route displayed.
Consistent with the docking model prediction, α-ketoamide inhibitor 4a, 4b, 4c, 4d had good enzymatic potencies with IC50 values ranging from 30 to 100 nM. Compound 4a also exhibits 2-fold weaker yet still impressive potencies in different cell and viral assays compared with nirmatrelvir. Encouraged by the promising antiviral potency demonstrated by compound 4a and 4c, we continued to evaluate ADME/PK profiles on these compounds (see Table 4 ).
Table 4.
ADME and rodents PK properties of compounds 4a and 4c.
| Assay Type | 4a | 4c | ||
|---|---|---|---|---|
| In vitro stabilitya | Plasma stability (Remaining% at 2 h) | 106, 96, 102 | - | |
| Microsome stability (CLint(liver), mL/min/kg) |
212, 25, 33 | - | ||
| Hepatocytes stability (CLint(liver), mL/min/kg) |
1023, 42, <17.8 | - | ||
| Dose route | PK parameter b | mouse | rat | mouse |
| iv | CL (mL min−1 kg−1) | 2.50 | 2.55 | 5.06 |
| Vss (L/kg) | 1.27 | 0.929 | 0.911 | |
| T1/2 (h) | 6.24 | 4.36 | 3.27 | |
| po | Cmax (μM) | 4.81 | 3.79 | 4.80 |
| AUC (μM × h) | 33.0 | 37.4 | 16.8 | |
| F (%) | 32 | 37.4 | 30.8 | |
In vitro stability data is listed in the sequence of mouse, rat, human.
Mice were administered at iv 3 mg/kg and po 10 mg/kg. Rats were administered at iv 2 mg/kg and po 10 mg/kg. Vehicle was 40% PEG400 + 60 %saline for all groups. -: not tested.
In the subsequent ADME and PK evaluations, compound 4a also displayed excellent plasma stability, liver microsome and hepatocytes stability data indicated 4a is very stable in human plasma. For PK results, compound 4a has longer T1/2, lower clearance, higher plasma exposure and moderate oral bioavailability in both mice and rat. Predicted human clearance is also very low, which is about 2.9 ml/min/kg using simple allometric scaling method. Compound 4c also exhibited significant improvement in pharmacokinetic parameters compared with nirmatrelvir, but it is slightly inferior to 4a. Overall, α-ketoamide compounds exhibit excellent PK properties with much lower clearance and longer half-life than the nitrile-series, compounds 2a and 3a.
Owing to its impressive anti-COVID-19 activities and desirable DMPK profile, compound 4a was further evaluated in in vivo efficacy study. HCoV-OC43 neonatal mice model was used as a surrogate model for COVID-19 infection. This model was chosen because of the following considerations: 1) high viral sequence homology between HCoV-OC43 and SARS-CoV-2 Mpro, and 2) laboratory execution convenience (BSL-2 vs. BSL-3).
Compound 4a was administered via intraperitoneal injection at three doses. The results showed that none of the mice (0/6) in vehicle group survived at Day 9. All mice (6/6) in healthy group, nirmatrelvir and high dosing group of compound 4a survived at day 14. Five out of six mice survived in medium dosing group while four out of six mice survived in low dosing group, indicating a dose dependent in vivo efficacy being observed with compound 4a. The body weight change curve also showed this dose-dependent tendency.
In conclusion, we have discovered a series of bicyclic[3.3.0]proline peptidyl α-ketoamide compounds through multiple rounds of SAR optimization. The most promising one, compound 4a, displayed impressive potencies and much better PK profile which has higher oral exposure and longer T1/2. Based on these properties, compound 4a is warranted further SAR optimization with the goal to achieve single agent efficacy in human (without the need to co-administered with Ritonavir). RAY1216 21, successor of compound 4a, finally realized with this goal and have recently been approved for NDA in China. The details of RAY1216 SAR investigation will be disclosed in the subsequent manuscript in the near future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank many WuXi Apptec colleagues who have contributed for this program. In particular, we would like to thank Dr. Ming Jiang, Enhui Hao, Yu Han, Zhongsheng Yao, Yongxin Wang, Min Zhang, Haijing Huang, Qiao Liao, Ziyu Wan for their efforts on compounds synthesis; Fusen Lin and Fubiao Xiao for carrying out in vitro and in vivo efficacy studies; Yijing Gu for conducting ADME and pharmacokinetic studies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bmcl.2023.129324.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21. Lancet Lond Engl. 2022;399(10334):1513-1536. [DOI] [PMC free article] [PubMed]
- 2.Estimating global, regional, and national daily and cumulative infections with SARS-CoV-2 through Nov 14, 2021: a statistical analysis. Lancet Lond Engl. 2022;399(10344):2351-2380. [DOI] [PMC free article] [PubMed]
- 3.Couzin-Frankel J. Antiviral pills could change pandemic’s course. Science. 2021;374:799–800. doi: 10.1126/science.acx9605. [DOI] [PubMed] [Google Scholar]
- 4.Siegel D., Hui H.C., Doerffler E., et al. Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. J Med Chem. 2017;60:1648–1661. doi: 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- 5.Pillaiyar T., Manickam M., Namasivayam V., Hayashi Y., Jung S.H. An Overview of Severe Acute Respiratory Syndrome-Coronavirus (SARS-CoV) 3CL Protease Inhibitors: Peptidomimetics and Small Molecule Chemotherapy. J Med Chem. 2016;59:6595–6628. doi: 10.1021/acs.jmedchem.5b01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin Z., Du X., Xu Y., et al. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 7.Owen D.R., Allerton C.M.N., Anderson A.S., et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586–1593. doi: 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- 8.Hammond J., Leister-Tebbe H., Gardner A., et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. New Engl J Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li P., Wang Y., Lavrijsen M., et al. SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res. 2022;32:322–324. doi: 10.1038/s41422-022-00618-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiaoxin Chen, Xiaodong Huang, Qinhai Ma, et al. Inhibition mechanism and antiviral activity of an α-ketoamide based SARS-CoV-2 main protease inhibitor. bioRxiv. Published online January 1, 2023:2023.03.09.531862.
- 11.Ma C., Sacco M.D., Hurst B., et al. Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 2020;30:678–692. doi: 10.1038/s41422-020-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y., Fang C., Zhang Q., et al. Crystal structure of SARS-CoV-2 main protease in complex with protease inhibitor PF-07321332. Protein Cell. 2022;13:689–693. doi: 10.1007/s13238-021-00883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang M., Park R., Park Y.I., et al. EGCG, a green tea polyphenol, inhibits human coronavirus replication in vitro. Biochem Biophys Res Commun. 2021;547:23–28. doi: 10.1016/j.bbrc.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S.H., Lamar J., Yip Y., et al. P1 and P1; Optimization of [3,4]-Bicycloproline P2 Incorporated Tetrapeptidyl α-Ketoamide Based HCV Protease Inhibitors. Lett Drug Des Discov. 2005;2:118–123. [Google Scholar]
- 15.Victor F., Lamar J., Snyder N., et al. P1 and P3 optimization of novel bicycloproline P2 bearing tetrapeptidyl alpha-ketoamide based HCV protease inhibitors. Bioorg Med Chem Lett. 2004;14:257–261. doi: 10.1016/j.bmcl.2003.09.075. [DOI] [PubMed] [Google Scholar]
- 16.Yip Y., Victor F., Lamar J., et al. Discovery of a novel bicycloproline P2 bearing peptidyl alpha-ketoamide LY514962 as HCV protease inhibitor. Bioorg Med Chem Lett. 2004;14:251–256. doi: 10.1016/j.bmcl.2003.09.074. [DOI] [PubMed] [Google Scholar]
- 17.Lamar J., Victor F., Snyder N., et al. Novel P4 truncated tripeptidyl α-ketoamides as HCV protease inhibitors. Bioorg Med Chem Lett. 2004;14:263–266. doi: 10.1016/j.bmcl.2003.09.076. [DOI] [PubMed] [Google Scholar]
- 18.Stöckigt J., Antonchick A.P., Wu F., Waldmann H. The Pictet-Spengler reaction in nature and in organic chemistry. Angew Chem (Int Ed English) 2011;50:8538–8564. doi: 10.1002/anie.201008071. [DOI] [PubMed] [Google Scholar]
- 19.Harbeson S.L., Abelleira S.M., Akiyama A., et al. Stereospecific synthesis of peptidyl alpha-keto amides as inhibitors of calpain. J Med Chem. 1994;37:2918–2929. doi: 10.1021/jm00044a013. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L., Lin D., Kusov Y., et al. α-Ketoamides as Broad-Spectrum Inhibitors of Coronavirus and Enterovirus Replication: Structure-Based Design, Synthesis, and Activity Assessment. J Med Chem. 2020;63:4562–4578. doi: 10.1021/acs.jmedchem.9b01828. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Wang J, Huang J, et al. Ketoamide derivative and application thereof. 2023;(CN 202111057236 A).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.