Abstract
Introduction
The incidence of long COVID is substantial, even in people with mild to moderate acute COVID-19. The role of early viral kinetics in the subsequent development of long COVID is largely unknown, especially in individuals who were not hospitalized for acute COVID-19.
Methods
Seventy-three non-hospitalized adult participants were enrolled within approximately 48 hours of their first positive SARS-CoV-2 RT-PCR test, and mid-turbinate nasal and saliva samples were collected up to 9 times within the first 45 days after enrollment. Samples were assayed for SARS-CoV-2 using RT-PCR and additional SARS-CoV-2 test results were abstracted from the clinical record. Each participant indicated the presence and severity of 49 long COVID symptoms at 1-, 3-, 6-, 12-, and 18-months post-COVID-19 diagnosis. Time from acute COVID-19 illness onset to SARS-CoV-2 RNA clearance greater or less than 28 days was tested for association with the presence or absence of each of 49 long COVID symptoms at 90+ days from acute COVID-19 symptom onset.
Results
Self-reported brain fog and muscle pain at 90+ days after acute COVID-19 onset were negatively associated with viral RNA clearance within 28 days of acute COVID-19 onset with adjustment for age, sex, BMI ≥ 25, and COVID vaccination status prior to COVID-19 (brain fog: aRR 0.46, 95% CI 0.22-0.95; muscle pain: aRR 0.28, 95% CI 0.08-0.94). Participants reporting higher severity brain fog or muscle pain at 90+ days after acute COVID-19 onset were less likely to have cleared SARS-CoV-2 RNA within 28 days. The acute viral RNA decay trajectories of participants who did and did not later go on to experience brain fog 90+ days after acute COVID-19 onset were distinct.
Discussion
This work indicates that at least two long COVID symptoms - brain fog and muscle pain – at 90+ days from acute COVID-19 onset are specifically associated with prolonged time to clearance of SARS-CoV-2 RNA from the upper respiratory tract during acute COVID-19. This finding provides evidence that delayed immune clearance of SARS-CoV-2 antigen or greater amount or duration of viral antigen burden in the upper respiratory tract during acute COVID-19 are directly linked to long COVID. This work suggests that host-pathogen interactions during the first few weeks after acute COVID-19 onset have an impact on long COVID risk months later.
Keywords: COVID-19, long COVID, post-acute COVID-19 syndrome, post-acute sequelae of SARS-CoV-2 infection, brain fog, cognitive dysfunction, pain, reservoir
1. Introduction
The incidence of long coronavirus disease (COVID) is substantial, even in people who did not require hospitalization for acute COVID-19 (1). The United States Census Household Pulse Survey of December 2022 estimates that 28% of U.S. adults who ever had COVID-19 experienced long COVID symptoms lasting 3 months or longer (2). Among the most common and debilitating symptoms are pain and a lack of mental clarity or problems with concentration, also called “brain fog” (3, 4). Viral antigen persistence in tissues may be one mechanism of long COVID (5, 6). However, few longitudinal studies with well-characterized viral dynamics in the acute phase have assessed the relationship between viral persistence and long COVID symptoms, especially in people with mild to moderate acute COVID-19 who represent typical disease severity in the general population. Here, we examine the association between time to viral RNA clearance from the upper respiratory tract (URT) during acute COVID-19 and specific clinical symptoms of long COVID at 90 or more days from acute COVID-19 illness onset in a cohort of people diagnosed with symptomatic COVID-19 in the outpatient setting.
2. Methods
A convenience sample of non-hospitalized adults with a first positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) PCR test within the previous 48 h from the Johns Hopkins Health System and their household contacts were prospectively enrolled between 21 April 2020 and 28 October 2021 ( Supplementary Figure 1 ) (7). Participants provided verbal consent after documentation of understanding, using a consent waiver with an alteration of informed consent, as most were isolating or quarantining at home. The study was conducted in either English or Spanish. This study protocol and verbal consent were approved by the Johns Hopkins University School of Medicine Institutional Review Board.
Mid-turbinate nasal swabs and saliva (URT samples) were collected at 1, 3, 5, 7, 14, 21, and 28 days from enrollment. Some participants had the option of study sampling on days 5, 10, 35, and 42 from enrollment. Mid-turbinate nasal swabs were collected in 600-µl volumes of viral transport media, and 200-µl volumes of saliva were collected. Collections at 1, 3, 5, 7, 10, 14, 21, 35, and 42 days after study enrollment were self-collected into study-provided vials with guidance by phone or video call from the study staff and frozen immediately. Collections at 28 days after study enrollment were performed primarily in-person at a research clinic visit with the study staff guiding or performing the collection. SARS-CoV-2 RT-PCR testing was performed on all samples (Abbott m2000 platform, Abbott Park, IL, USA) according to the manufacturer’s instructions as described previously (8). Discordant results from samples collected on the same day (e.g., positive nasal, negative saliva) were recorded as URT positive. The adequacy of self-collected samples was confirmed via RT-PCR for GAPDH human gene expression utilizing a TaqMan gene expression assay for the first 1,338 samples (8). Briefly, eluates from the Abbott m2000 were analyzed by the NanoDrop One (Thermo Fisher Scientific, Waltham, MA, USA) to determine total RNA concentration, and all samples were normalized to 1 ng/µl of total RNA prior to analysis for GAPDH. Normalized samples were utilized to synthesize cDNA in 10-µl volumes utilizing Superscript IV RT (Thermo Fisher) according to the manufacturer’s instructions. After cDNA synthesis, samples were assessed for the presence of gapDH utilizing RT-PCR (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. Positive reactions were defined as reactions having a cycle threshold value <40. One thousand two hundred twenty-seven of 1,338 self-collected samples were positive for human GAPDH, and all negative reactions started from eluates with <1 µl, indicating insufficient material for NanoDrop analysis or cDNA synthesis. COVID-19 PCR testing results from the clinical electronic medical records were also included in this analysis.
At 1, 3, 6, 12, and 18 months post-COVID-19 diagnosis, participants were asked, “Have you had any of the following symptoms in the past week?” and selected one of the following response options—”Not at all,” “A little bit,” “Somewhat,” “Very much,” or “Quite a bit”—for each of the 49 long COVID symptoms ( Table 1 ). Thirty-eight symptoms were derived from the FLU-PRO© Plus (9), and 11 additional symptoms were added based on the frequency of report in a Long COVID Patient-Led Research Collaborative survey with input from the patient-researcher authors (3). Similar to other symptoms, brain fog was assessed with the question “Have you had problems with concentration or ‘brain fog’ in the past week?”.
Table 1.
List of the 49 long COVID symptoms assessed in surveys at 1, 3, 6, 12, and 18 months from enrollment.
| Category | Symptom |
|---|---|
| Body/systemic | Fever (>100.4°F/>38.0°C or suspected but temperature unknown) |
| Low-grade fever (99.0-100.3°F/37.2-37.9°C) | |
| Chills or shivering | |
| Felt cold | |
| Felt hot | |
| Sweating | |
| Head congestion | |
| Constant thirst | |
| Lack of appetite | |
| Body aches or pain | |
| Joint pain | |
| Muscle pain | |
| Leg swelling | |
| Sleeping more than usual | |
| Trouble sleeping or insomnia | |
| Weak or tired or fatigued | |
| Neurologic | Headache |
| Felt dizzy | |
| Problems with balance | |
| Confusion | |
| Memory problems | |
| Problems with concentration or “brain fog” | |
| Seizure | |
| Hallucinations or lucid dreaming | |
| Anxiety | |
| Ringing in the ears | |
| Eye | Teary or watery eyes |
| Sore or painful eyes | |
| Eyes sensitive to light | |
| Nose | Runny or dripping nose |
| Congested or stuffy nose | |
| Sinus pressure | |
| Throat | Scratchy or itchy throat |
| Sore or painful throat | |
| Difficulty swallowing | |
| Chest/respiratory | Trouble breathing/shortness of breath |
| Dry or hacking cough | |
| Wet or loose cough | |
| Chest congestion | |
| Chest pain or pressure, chest tightness or burning in the chest | |
| Heart palpitations; funny or fast heartbeat | |
| Smell/taste | Full or partial loss of taste |
| Full or partial loss of smell | |
| Gastrointestinal | Felt nauseous (feeling like you want to throw up) |
| Vomiting | |
| Stomachache | |
| Diarrhea | |
| Skin | Tingling, numbness, coldness, or other unusual sensation in the skin |
| Rash |
Participants were asked, “Have you had any of the following symptoms in the past week?” and selected one of the following response options—”Not at all,” “A little bit,” “Somewhat,” “Very much,” or “Quite a bit”—for each symptom.
Time to SARS-CoV-2 RNA clearance was defined as the number of days between acute COVID-19 illness onset (recorded at study enrollment) and the midpoint between a participant’s last positive URT RNA test and the following negative URT PCR test. Time to viral RNA clearance was classified as a binary variable: within or beyond 28 days. This timepoint was chosen because the median viral RNA clearance time obtained from a Kaplan–Meier analysis of participants who completed acute sampling and at least one survey 1+ months from acute COVID-19 illness onset was 27.5 days. Additionally, the last required acute study sampling day was study day 28.
For each of the 49 long COVID symptoms, we estimated the relative risk of reporting that symptom 90+ days from acute COVID-19 onset by time to viral RNA clearance within 28 days using log-binomial regression (10) and adjusting for age, sex, BMI over/under 25, and COVID vaccination status prior to acute COVID-19 (1+ vaccines vs. none) (Stata/SE 17, StataCorp, College Station, TX, USA). The maximum severity of each symptom was defined as the highest score from 1 (“Not at all”) to 5 (“Quite a bit”) for that symptom that each participant reported 90 or more days after acute COVID-19 illness onset and was used as a continuous predictor in an adjusted logistic regression model to estimate the association of maximum severity of symptoms in the late post-acute phase and acute viral RNA clearance. We used functional data analysis methods to visualize the curve of the RT-PCR cycle threshold values over time by grouping participants with or without brain fog at 90 or more days from symptom onset.
3. Results
Seventy-three participants with symptomatic, PCR-confirmed acute COVID-19, age ≥18, with ≥2 URT samples within 28 days of enrollment, and who completed at least one comprehensive symptom survey ≥90 days from acute COVID-19 onset were included in this analysis ( Supplementary Figure 1 ). The participants had a median of 3 study days with a positive PCR test prior to a study day with a negative PCR test, and 75% of the participants had ≥5 study days with PCR tests available for analysis. The median time to viral RNA clearance was 17 days for the 51 participants who had a negative PCR test within 21 days of their last positive PCR test (interquartile ratio, IQR, 14.75-22.75). Twenty-nine (40%) participants met this cohort’s definition of long COVID by reporting in the survey at least once at study months 3, 6, 12, or 18 that they had not returned to their usual pre-COVID health status and also complained of at least one symptom.
The median age of the participants was 52 years (IQR, 42-60), 63% were women, 27% self-reported as non-Hispanic African American, 52% self-reported as non-Hispanic White, and 16% self-reported as Hispanic ( Table 2 ). All participants tested positive for COVID-19 in the outpatient setting, and two (2.7%) ultimately required hospitalization during acute COVID-19. Of the participants, 81% were unvaccinated prior to COVID-19, 5% had incomplete primary vaccine series, and 14% had received a complete primary vaccine series prior to COVID-19 ( Table 2 ).
Table 2.
Participants’ demographics, body mass index, and pre-COVID vaccination status.
| Time to viral RNA clearance from the URT >28 days from acute COVID-19 onset (n = 30) | Time to viral RNA clearance from the URT ≤28 days from acute COVID-19 onset (n = 43) | ||
|---|---|---|---|
| Age at enrollment, median (IQR), years | 54 (46-65) | 50 (40-58) | |
| Female, no. (%) | 18 (60) | 28 (65) | |
| Race and ethnicity, no. (%) | Non-Hispanic African American | 9 (30) | 11 (26) |
| Non-Hispanic White | 15 (50) | 23 (53) | |
| Non-Hispanic other | 1 (3) | 2 (5) | |
| Hispanic | 5 (17) | 7 (16) | |
| BMI ≥ 25 kg/m2, no. (%) | 25 (83) | 29 (67) | |
| Prior diagnosis of hypertension, no. (%) | 14 (47) | 12 (28) | |
| Prior diagnosis of diabetes, no. (%) | 7 (23) | 6 (14) | |
| Prior diagnosis of anxiety, depression, or bipolar, no. (%) | 5 (17) | 15 (35) | |
| Median time to viral RNA clearance (IQR), days | 74 (37-132) | 16 (14-21) | |
| Hospitalized during acute COVID-19, no. (%) | 2 (7) | 0 (0) | |
| COVID vaccination status prior to COVID-19, no. (%) | No vaccine doses | 29 (97) | 30 (70) |
| Incomplete primary vaccination series | 0 (0) | 4 (9) | |
| Complete primary vaccination series | 1 (3) | 9 (21) |
URT, upper respiratory tract encompassing mid-turbinate nasal swab and saliva; BMI, body mass index.
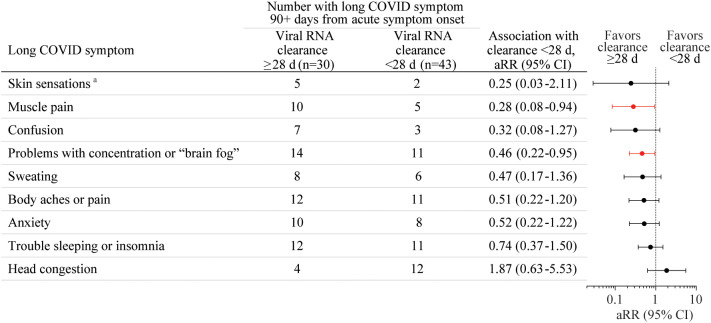
The association of the presence of each of the 49 long COVID symptoms ( Table 1 ) at 90+ days post-acute COVID-19 onset with time to SARS-CoV-2 RNA clearance from the URT within 28 days was first tested without adjustment using log-binomial regression analyses. Eleven symptoms with p-values <0.2 were tested using log-binomial regression analyses with adjustment for age, sex, BMI ≥25, and COVID vaccination status prior to acute COVID-19. Brain fog and muscle pain at 90+ days after acute COVID-19 illness onset were negatively associated with viral RNA clearance within 28 days with these adjustments [ Figure 1 , brain fog: adjusted risk ratio (aRR) 0.46, 95% CI 0.22-0.95; muscle pain: aRR 0.28, 95% CI 0.08-0.94]. The model failed to converge for two rarely reported symptoms: leg swelling and problems with balance. Leg swelling at 90+ days from acute COVID-19 onset was negatively associated with viral RNA clearance within 28 days of acute COVID-19 onset in an unadjusted analysis (RR 0.17, 95% CI 0.04-0.76) and in an analysis adjusted only for age and sex (aRR 0.21, 95% CI 0.05-0.82). Problems with balance at 90+ days from acute COVID-19 onset were negatively associated with viral RNA clearance within 28 days of acute COVID-19 onset only in an analysis adjusted for age, sex, and vaccination status (aRR 0.47, 95% CI 0.25-0.90) but not in a univariate analysis. There were non-significant trends toward negative associations of body aches or pain, anxiety, confusion, and sweating with viral RNA clearance within 28 days after adjustment for age, sex, BMI ≥25, and COVID vaccination status prior to acute COVID-19 ( Figure 1 ). No other associations of long COVID symptoms with viral RNA clearance time within 28 days reached statistical significance.
Figure 1.
The association of the presence of each of the 49 long coronavirus disease (COVID) symptoms at 90+ days after acute COVID-19 illness onset with SARS-CoV-2 RNA clearance from the upper respiratory tract (URT, mid-turbinate nasal and/or saliva) within 28 days from acute COVID-19 onset was tested without adjustment using log-binomial regression analyses. Nine long COVID symptoms with p-values <0.2 were tested using log-binomial regression analyses with adjustment for age, sex, BMI over/under 25, and COVID vaccination status prior to acute COVID-19 (1+ vaccines vs. none), with results shown here. aRR, adjusted risk ratio, d, day. aTingling, numbness, coldness, or other unusual sensation in the skin.
The acute viral RNA decay trajectories of participants who did and did not later go on to experience brain fog 90 or more days from acute COVID-19 illness onset were distinct ( Supplementary Figure 2 ). Participants reporting higher severity brain fog or muscle pain at 90 or more days from acute COVID-19 illness onset were less likely to have cleared SARS-CoV-2 RNA within 28 days. In logistic regression models adjusted for age, sex, and BMI ≥25, with each 1-level increase of maximum reported brain fog or muscle pain on a scale of 1-5, the odds of clearance in 28 days decreased (brain fog: aOR 0.58, 95% CI 0.36-0.94; muscle pain: aOR 0.44, 95% CI 0.26-0.76).
4. Discussion
We demonstrate here that post-acute brain fog and muscle pain are associated with prolonged time to clearance of SARS-CoV-2 RNA from the upper respiratory tract during acute COVID-19. This finding provides evidence that delayed immune clearance of SARS-CoV-2 antigen and/or greater amount or duration of viral antigen burden in the upper respiratory tract during acute COVID-19 is directly linked to long COVID. This work indicates that host–pathogen interactions during the first few weeks after COVID-19 symptom onset have an impact on long COVID risk months later.
Currently, the biological mechanisms underlying long COVID and its myriad symptoms are unclear. There are several different hypotheses: persistent immune dysregulation following acute COVID-19, persistent SARS-CoV-2 virions or antigen (“reservoirs”) triggering chronic inflammation, reactivation of other viruses such as the Epstein–Barr virus (EBV), autoimmunity triggered by acute illness, endothelial dysfunction and/or microthrombi impairing organ function, metabolic or mitochondrial dysfunction on the cellular level, autonomic nervous system injury, lingering or evolving organ injury from acute COVID-19, and microbiota alterations, among others (11). A recent comprehensive autopsy study provided evidence for an early viremic phase during acute COVID-19 that spreads infectious virus throughout the body and demonstrated convincingly that SARS-CoV-2 RNA can persist for >30 days in multiple extrapulmonary sites (12).
There have been a few studies that link long COVID with persistent SARS-CoV-2 antigen detected in the late post-acute time period, notably spike protein in plasma (13), SARS-CoV-2 proteins in plasma neuron- and astrocyte-derived extracellular vesicles (14), spike protein in monocytes (15), and fecal RNA shedding (16). However, there has been just one study to our knowledge linking virus kinetics or detection during acute COVID-19 with long COVID; this study associated the presence or absence of SARS-CoV-2 RNA in the plasma at the time of COVID-19 diagnosis and hospitalization with one or more symptoms of long COVID several months later (17). However, this study was limited to people with severe acute COVID-19 (17), and it may be that the presence of SARS-CoV-2 RNAemia at hospital presentation is an indicator of the severity of acute COVID-19, which is already known to be a risk factor for long COVID (1).
Our study provides robust support for the hypothesis that viral kinetics during the acute phase of illness is associated with long COVID by using frequent longitudinal sampling beginning within days of acute COVID-19 onset to determine the duration of viral RNA shedding from the URT. Additionally, since long COVID may comprise one or more syndromes with unique mechanisms, we assessed individual symptoms from multiple organs or domains affected by long COVID to determine whether particular syndromes or symptom clusters of long COVID are associated with viral kinetics in the acute phase. We studied a population that primarily had mild–moderate COVID-19 and, thus, is more representative of the general population of people who had COVID-19 or long COVID and limits the amount by which the severity of acute illness could confound our results. A final strength of this cohort is the racial and ethnic diversity of participants. A weakness of the study is that the intensity of study coordination involved in repeated longitudinal sampling and follow-up in acute and post-acute COVID-19 limited the number of participants enrolled.
Our data are consistent with a few biologically plausible scenarios. First, both delayed clearance of viral antigen and long COVID are caused by another factor such as dysregulated or distracted immunity. This dysregulated immunity might cause long COVID by reactivating other latent viruses, triggering autoimmunity, causing direct organ damage, or delaying tissue repair from acute infection. Second, persistent expression of viral antigens, and/or a larger burden of initial antigen, might directly cause long COVID by sustaining tissue injury and inflammation. The latter mechanism is supported by early studies linking antiviral treatment of people during acute COVID-19 with reduced incidence of long COVID (18). More studies with longitudinal repeated sampling beginning from acute COVID-19 illness treated with and without antivirals through the post-acute phase are urgently needed to assess how host–pathogen interactions lead to long COVID.
Here, we report that at least two long COVID symptoms—brain fog and muscle pain—at 90+ days from acute COVID-19 onset are specifically associated with longer time to clearance of SARS-CoV-2 RNA from the upper respiratory tract during acute illness.
Data availability statement
The raw data supporting the conclusions of this article will be made available upon request, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Johns Hopkins University School of Medicine IRB. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
AA, TY, and YM: conception and design. AA, TY, and CH: statistical analysis and interpretation of data. PB and YM: funding acquisition. DT and YM: administrative, technical, or material support. All authors: writing, review, and critical revision of the manuscript for important intellectual content. AA and YM: study supervision. All authors contributed to the article and approved the submitted version.
OutSMART Study Group
M. Gabriela Varela Heslin, Sarika K. Mullapudi, Chamia Dorsey, Christine Payton, Sidney-Saint-Hilaire, Zihan Yang, Justin Chan, Razvan Azamfirei, Minyoung Jang, Taylor Church, Carolyn Reuland, Vismaya S. Bachu, Jennifer L. Townsend, Sara C. Keller, Jeanne C. Keruly, Justin P. Hardick, Madison Conte, and Thelio Sewell.
Acknowledgments
We express our gratitude to all the participants who donated time and samples for this study. We thank the following individuals for their assistance with this study: Diane M. Brown, Brittany Barnaba, Curtisha Charles, Michelle Prizzi, Oyinkansola Kusemiju, and Jaylynn R. Johnstone.
Funding Statement
PB and YM received support for this work from the Henry M. Jackson Foundation for the Advancement of Military Medicine (contract number 1007957) and the Sherrilyn and Ken Fisher Center for Environmental Infectious Diseases Discovery Program. AA is supported by the National Institutes of Health (grant number K08AI143391).
Conflict of interest
Author PB was employed by Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. YM has received research grant support through Johns Hopkins University from Hologic, Cepheid, Roche, ChemBio, Becton Dickinson, and miDiagnostics and has provided consultative support to Abbott.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1147549/full#supplementary-material
References
- 1. Xie Y, Bowe B, Al-Aly Z. Burdens of post-acute sequelae of covid-19 by severity of acute infection, demographics and health status. Nat Commun (2021) 12(1):6571. doi: 10.1038/s41467-021-26513-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Statistics NCfH . Long covid household pulse survey (2023). Available at: https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm.
- 3. Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long covid in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine (2021) 38:101019. doi: 10.1016/j.eclinm.2021.101019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ceban F, Ling S, Lui LMW, Lee Y, Gill H, Teopiz KM, et al. Fatigue and cognitive impairment in post-Covid-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun (2022) 101:93–135. doi: 10.1016/j.bbi.2021.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brodin P, Casari G, Townsend L, O’Farrelly C, Tancevski I, Loffler-Ragg J, et al. Studying severe long covid to understand post-infectious disorders beyond covid-19. Nat Med (2022) 28(5):879–82. doi: 10.1038/s41591-022-01766-7 [DOI] [PubMed] [Google Scholar]
- 6. Mehandru S, Merad M. Pathological sequelae of long-haul covid. Nat Immunol (2022) 23(2):194–202. doi: 10.1038/s41590-021-01104-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blair PW, Brown DM, Jang M, Antar AAR, Keruly JC, Bachu VS, et al. The clinical course of covid-19 in the outpatient setting: a prospective cohort study. Open Forum Infect Dis (2021) 8(2):ofab007. doi: 10.1093/ofid/ofab007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Antar AAR, Yu T, Pisanic N, Azamfirei R, Tornheim JA, Brown DM, et al. Delayed rise of oral fluid antibodies, elevated bmi, and absence of early fever correlate with longer time to sars-Cov-2 rna clearance in a longitudinally sampled cohort of covid-19 outpatients. Open Forum Infect Dis (2021) 8(6):ofab195. doi: 10.1093/ofid/ofab195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richard SA, Epsi NJ, Pollett S, Lindholm DA, Malloy AMW, Maves R, et al. Performance of the influenza patient-reported outcome plus (Flu-pro plus) instrument in patients with coronavirus disease 2019. Open Forum Infect Dis (2021) 8(12):ofab517. doi: 10.1093/ofid/ofab517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wacholder S. Binomial regression in glim: estimating risk ratios and risk differences. Am J Epidemiol (1986) 123(1):174–84. doi: 10.1093/oxfordjournals.aje.a114212 [DOI] [PubMed] [Google Scholar]
- 11. Castanares-Zapatero D, Chalon P, Kohn L, Dauvrin M, Detollenaere J, Maertens de Noordhout C, et al. Pathophysiology and mechanism of long covid: a comprehensive review. Ann Med (2022) 54(1):1473–87. doi: 10.1080/07853890.2022.2076901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stein SR, Ramelli SC, Grazioli A, Chung JY, Singh M, Yinda CK, et al. Sars-Cov-2 infection and persistence in the human body and brain at autopsy. Nature (2022) 612(7941):758–63. doi: 10.1038/s41586-022-05542-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Swank Z, Senussi Y, Manickas-Hill Z, Yu XG, Li JZ, Alter G, et al. Persistent circulating sars-Cov-2 spike is associated with post-acute covid-19 sequelae. Clin Infect Dis (2022) 76 (3):e487–90. doi: 10.1093/cid/ciac722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peluso MJ, Deeks SG, Mustapic M, Kapogiannis D, Henrich TJ, Lu S, et al. Sars-Cov-2 and mitochondrial proteins in neural-derived exosomes of covid-19. Ann Neurol (2022) 91(6):772–81. doi: 10.1002/ana.26350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patterson BK, Francisco EB, Yogendra R, Long E, Pise A, Rodrigues H, et al. Persistence of sars cov-2 S1 protein in Cd16+ monocytes in post-acute sequelae of covid-19 (Pasc) up to 15 months post-infection. Front Immunol (2021) 12:746021. doi: 10.3389/fimmu.2021.746021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Natarajan A, Zlitni S, Brooks EF, Vance SE, Dahlen A, Hedlin H, et al. Gastrointestinal symptoms and fecal shedding of sars-Cov-2 rna suggest prolonged gastrointestinal infection. Med (N Y) (2022) 3(6):371–87 e9. doi: 10.1016/j.medj.2022.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Su Y, Yuan D, Chen DG, Ng RH, Wang K, Choi J, et al. Multiple early factors anticipate post-acute covid-19 sequelae. Cell (2022) 185(5):881–95 e20. doi: 10.1016/j.cell.2022.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xie Y, Choi T, Al-Aly Z. Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition. JAMA Intern Med (2023). doi: 10.1001/jamainternmed.2023.0743 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available upon request, without undue reservation.