Abstract
Long COVID-19 (LC-19) is a condition that has affected a high percentage of the population that recovered from the initial disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). LC-19 diagnosis is currently poorly defined because of its variable, multisystem, episodic symptoms, and lack of uniformity in the critical time points associated with the disease. Considering the number of cases, workers’ compromised efficiency or inability to return to their duties can affect organizations and impact economies. LC-19 represents a significant burden on multiple levels and effectively reduces quality of life. These factors necessitate the establishment of firm parameters of diagnoses to provide a foundation for ongoing and future studies of clinical characteristics, epidemiology, risk factors, and therapy. In this scoping review, we conducted a literature search across multiple publication sites to identify papers of interest regarding the diagnosis of LC-19. We identified 225 records of interest and categorized them into seven categories. Based on our findings, there are only 11 original papers that outline the diagnostic process in detail with little overlap. This scoping review highlights the lack of consensus regarding the definition and, thereby, the LC-19 diagnosis processes. Due to no clear directive and considering the many unknowns surrounding the natural history of the disease and further recovery/sequelae from COVID-19, continued discussion and agreement on a definition/diagnosis will help future research and management of these patients.
Keywords: Long COVID-19, Diagnosis, Guidelines
1. Introduction
Since its emergence and detection in Wuhan, China, in late 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread to virtually every corner of the globe, costing more than six million lives (World Health Organization, 2023). According to the International Monetary Fund, the Coronavirus Disease 2019 (COVID-19) has resulted in policy changes due to the economic responses governments have taken to limit the human and economic impact of the pandemic (International Monetary Fund, 2020). Cases of COVID-19 are characterized by respiratory symptoms, fever, and gastrointestinal problems (Larsen et al., 2020). The symptoms are highly variable in length and severity, ranging from asymptomatic to severe pneumonia with multiorgan failure requiring hospitalization and ventilation (Di Gennaro et al., 2020; Li et al., 2021; Tian et al., 2020). Patients also present with a range of other symptoms, including dizziness, headache, impaired consciousness, taste & smell impairment, and nerve pain, suggesting central nervous system (CNS) involvement (Mao et al., 2020). Most cases of COVID-19 resolve in 1–2 weeks, with patients returning to their pre-COVID functional capabilities (Wu et al., 2020). In 2021, new variants of concern emerged, some of which showed increased infectivity and increased ability to bypass protection afforded by vaccines or prior infections (Harvey et al., 2021).
As the primary sickness continued to ravage the world, a new class of patients began appearing in the hospitals. Patients had a wide array of symptoms, including but not limited to myalgia, dyspnea, abnormal chest imaging and pulmonary function tests, and cardiovascular issues (Daitch et al., 2022). These people had recovered from an initial COVID-19 infection and were currently clinically negative but had unresolved continuing health issues. Patients present with a wide range of persistent symptoms months after the initial infection. Most patients were women, elderly, or had pre-existing conditions, and most involved vague symptoms (Carfi et al., 2020; Davis et al., 2021; Huang et al., 2021). These cases were initially not considered, especially since they often involved older people who are voiceless in the public arena, and their complaints were attributed to hypochondria or psychological (WebMD, 2022). These patients are now being diagnosed with long-haul COVID-19, LC-19, or post-acute sequelae of COVID-19 (PASC).
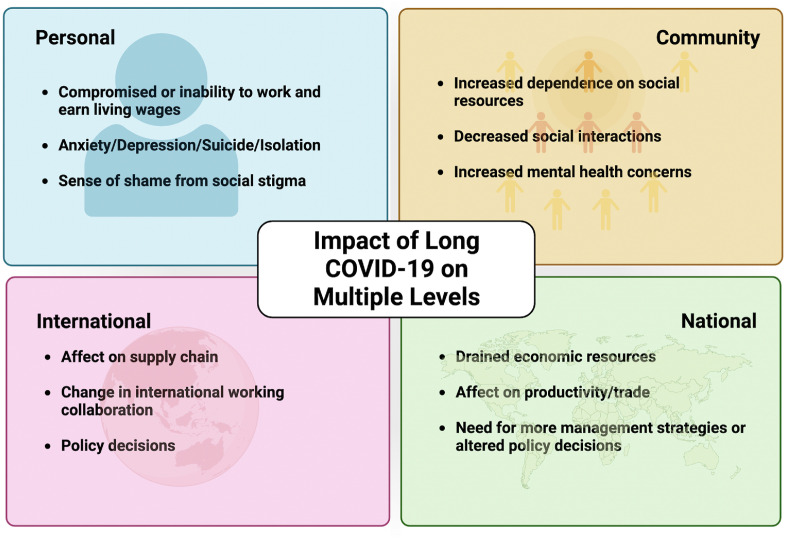
Currently, Long COVID-19 (LC-19) is defined as a condition following recovery from acute COVID-19 infection or unresolved COVID-19 illness with symptoms not attributable to any other condition. Typical symptoms include fatigue, shortness of breath, cough, chest pain, neurological issues, and many others. Opinions about the timing are not unilateral, with some clinicians believing it starts four weeks to four months after the acute illness (Sisó-Almirall et al., 2021). In contrast, other studies only stipulate a symptom duration of >6 weeks as defining LC-19 (Duerlund et al., 2022). The WHO-led Delphi study defines the timing as three months from the onset of the acute disease, with symptoms lasting at least two months (Soriano et al., 2022). Furthermore, LC-19 symptoms can present in an episodic nature, where symptoms resolve and return over varying time points (Brown and O’Brien, 2021). Clinicians have a general process for LC-19 diagnosis through evaluation of symptoms and exclusion of other conditions to reach a conclusion. However, no uniform guidelines are in place for LC-19 to aid in standardized and earlier detection. While this general process is typically followed, since LC-19 manifests in various forms and a vast array of clinical presentations, diagnosing it is challenging due to multiple definitions and diagnostic standard. Late diagnosis of this disease can have a detrimental effect at various levels; individual, community, national, and international, as depicted in Fig. 1 . To minimize this impact, there is a need to standardize the definition and diagnosis of LC-19 worldwide to improve data sharing and comparison, and development of appropriate treatments and clinical management strategies.
Fig. 1.
Impact of Long COVID-19 at various levels to display the importance of early detection and minimize its impact. Created with BioRender.com.
This scoping review aims to present a summary of the relevant records in current literature on LC-19 diagnosis, followed by categorization and analysis of this data. Furthermore, we critically assessed the 11 papers of interest that elucidate the steps of LC-19 diagnosis, bringing to light the need for consistency and clear consensus about the process (Greenhalgh et al., 2020; Kim et al., 2022; Munblit et al., 2022; Raveendran, 2021; Shah et al., 2021; SIGN, 2022; Sisó-Almirall et al., 2021; Soriano et al., 2022; Yale Medicine, 2020; Yelin et al., 2022).
2. Strategy for scoping
2.1. Study selection/literature search
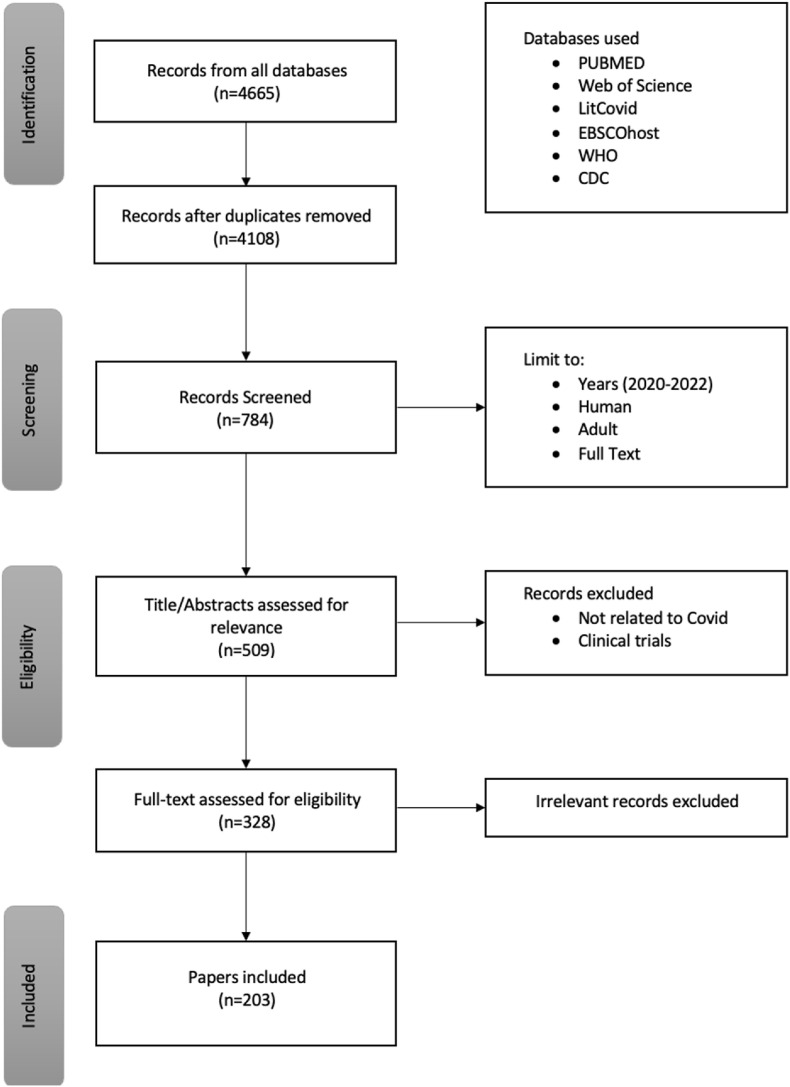
A search was conducted on PubMed, EBSCOhost, Web of Science, Websites of the Centers for Disease Control and Prevention and World Health Organization, Cochrane Library via Wiley, and other current scientific publications. Data was identified, screened, and included in the study based on criteria presented in the flow diagram in Fig. 2 . Detailed inclusion and exclusion criteria were developed to identify publications relevant to the stated purpose of the review according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews, PRISMA-ScR. (Tricco et al., 2018).
Fig. 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram pathway to filter records from database output for inclusion in our study.
2.2. Search parameters
Long COVID, Long COVID-19, Long Hauler, Long Haul COVID, PACS, Post SARS-COV2 syndrome, Diagnosis.
2.3. Data identification
Records from all databases were identified, and duplicates removed. The search was limited to records from December 2020 to December 2022, full text in English, humans, and adults (ages 19 and up). The titles and abstracts were then screened to remove unrelated records and clinical trials. Eligibility was determined independently by two reviewers, and in cases of conflict, a consensus was achieved by discussion or input from a third reviewer. Eligible records were scrutinized thoroughly before inclusion in the final study.
2.4. Identification, screening, and inclusion in study
4665 records were identified through a search from all databases using the keywords. Screening included the removal of duplicates, limiting records to December 2020 to December 2022, humans, adults, and full text, which resulted in 784 records. Further screening to remove articles unrelated to COVID diagnosis, and clinical trials caused another 275 records to be excluded leaving 509 publications of interest. A review of the remaining records’ titles and abstracts resulted in removing irrelevant records. Finally, 328 records were considered eligible/relevant, and the full text was evaluated before inclusion in our study.
2.5. Data extraction and analysis
Working independently, the two reviewers collated the LC-19 papers into seven categories: papers detailing diagnostic guidelines, symptoms, metabolic studies, other diseases/conditions, therapy, biomarkers/predicative/risks, and health inequity. Based on these distinct categories, we evaluated the publications of interest according to their number and subject.
3. Results
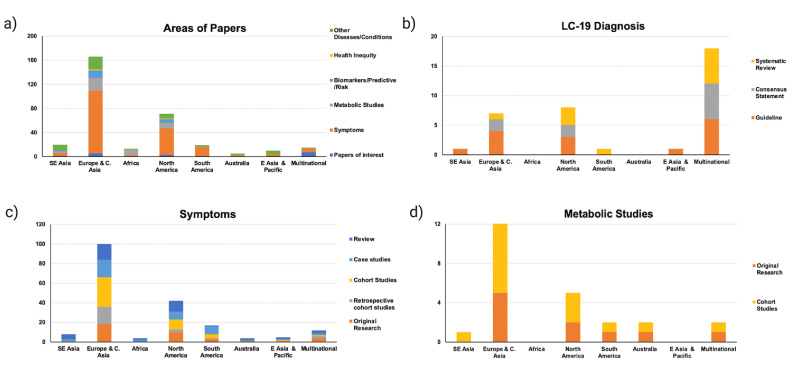
Table 1 details the number of records based on the types of publications. Original Research and case studies account for the largest proportion at nearly three-fourths (72%) of the publications. The substantial number of case studies (30%), which are unique cases, indicates the wide array of symptoms among patients. Papers focusing on discussions for guidelines for the diagnosis represent a mere 6% of the records included in our study.
Table 1.
The distribution of records based upon type of publication. Data points in groups are not exclusive.
| Type of Article | Total Number | Percentage |
|---|---|---|
| Guideline | 12 | 3.7 |
| Consensus Statement | 7 | 2.1 |
| Original Research | 139 | 42.4 |
| Retrospective cohort studies | 34 | 10.4 |
| Cohort Studies | 86 | 26.2 |
| Case Studies | 98 | 29.9 |
| Case report | 82 | 25.0 |
| Case series | 16 | 4.9 |
| Reviews | 45 | 13.7 |
| Systematic Review | 20 | 6.1 |
| Literature Review | 23 | 7.0 |
| Scoping Review | 2 | 0.6 |
| Perspective | 3 | 0.9 |
| Total | 328 | 100.0 |
Fig. 3 represents the data based on the geographical locations of the studies: Southeast Asia, Europe and Central Asia, Africa, North America, South America, Australia, East Asia and Pacific, and of Multinational origin. The total number of records in each category is depicted in Fig. 3a and shows an extremely high number (70%) published from Europe and North America. Papers detailing the symptoms of LC-19 constitute the largest category (52%), with the majority being case studies. 15% (50/328) of the papers in our analysis consisted of the impact of LC-19 on the diagnosis, presentation, and complications of other diseases/conditions. This number is probably a serious underestimation considering the complexities of LC-19 diagnosis. In Fig. 3b, we further delve into the publications detailing the diagnostic process according to the study characteristics. These were guidelines, consensus statements, original research, retrospective cohort studies, cohort studies, case studies, and reviews (systematic, literature, scoping). Among guidelines, in addition to the high number from Europe and North America, a substantial number resulted from collaborations between different countries (multinational). One of the seminal multinational collaborations was coordinated by the World Health Organization (WHO) involving more than one hundred countries worldwide to standardize the definition and diagnostic criteria of LC-19 (Soriano et al., 2022). Currently, most countries around the world utilize these WHO guidelines with minor modifications in diagnostic criteria. The graphs with data about symptoms (3c.) and metabolic studies (3d.) also show a similar trend of a high number of publications from Europe and North America.
Fig. 3.
The distribution of papers based upon categories and geographic areas of publications. (a) Number of records based on the area of the paper (b) Papers detailing diagnostic guidelines (c) Papers on the symptoms of LC-19 (d) Distribution of papers discussing metabolic studies. Data points in groups are not exclusive.
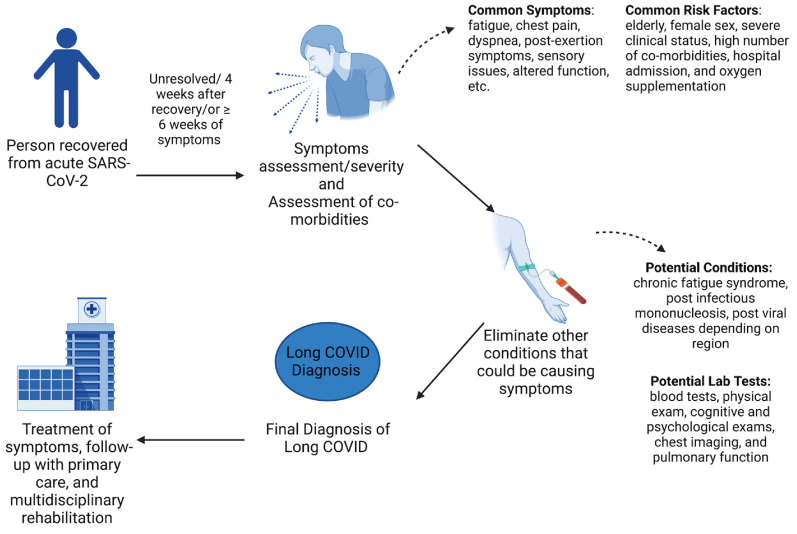
Based on our literature search, we found 11 papers that guided the diagnosis of LC-19, with Fig. 4 representing a summary of the data from these articles. This process begins with a patient who recovered from COVID-19 and is currently SARS-CoV-2 negative. Symptoms can persist, i.e., unresolved from acute infection, or appear at varying time points, such as four weeks after recovery or more than six weeks of symptoms. Patients usually follow up with healthcare providers for symptom assessment and severity, as well as assessment of other pre-existing conditions. These patients are treated for the symptoms suspecting other diseases, but if sickness continues unresolved, LC-19 may be suspected. Confirmation of LC-19 involves the exclusion of other post-viral conditions. Based on the patients’ geographical location, tests are performed to exclude conditions such as post-infectious mononucleosis, post-Ebola, post-West Nile virus, post-dengue, post-chikungunya, etc. Meeting all the inclusion and exclusion criteria ultimately results in a diagnosis of LC-19. It is recommended that patients follow up with primary care and multidisciplinary rehabilitation services if further treatment is required (Sisó-Almirall et al., 2021). According to National Health Service in the UK, multidisciplinary rehabilitation should include services for physical, cognitive, and psychological assessments, diagnostic tests, and management or appropriate onward referral to post-COVID-rehabilitation, treatment, or other support services. (Shah et al., 2021).
Fig. 4.
Schematic of LC-19 diagnosis based on the 11 papers that provided guidelines for the diagnosis pathway. Created with BioRender.com.
4. Discussion
This scoping review highlights the need for a consensus regarding the definition and, thereby, the LC-19 diagnosis processes. We found only 11 original papers detailing the process specifically. The lack of a universal definition begins with the nomenclature associated with the condition (Munblit, O'Hara et al., 2022). Most definitions of LC-19 detail the occurrence of persistent symptoms following acute COVID-19 infection with wide variability in the appearance and timing. The ambiguity of the definition translates to imprecise diagnosis, and this lack of consistency at both levels could result in delayed and ineffective interventions. An exhaustive literature search was conducted to summarize the evidence for LC-19 within observational studies and put together the most prevalent symptoms (Martimbianco et al., 2021). These symptoms included chest pain, fatigue, dyspnea, post-exertion symptoms, sensory issues, altered work/occupational functioning, and conditions for each cardiovascular, respiratory, nervous system, cognitive, mental health, and physical outcome. Furthermore, common risk factors in these studies included old age (>65 yrs), female sex, comorbidities, and factors of the severity of acute infection, such as hospital admission and oxygen supplementation. These symptoms and risks are essential in defining the LC-19 clinical diagnosis process.
As seen in Fig. 4, one of the consistent issues is the lack of agreement on the timing of the condition resulting in an arbitrary division of LC-19 into sub-groups. One group created a proposed classification system based on hospitalization data and timing of symptoms: acute post-COVID symptoms [week 5 to week 12], long post-COVID symptoms [week 12 to week 24], and persistent post-COVID symptoms [lasting more than 24 weeks] (Fernández-De-las-peñas et al., 2021). There is also a considerable difference of opinion regarding the necessity of an active acute phase (qPCR positivity) before an LC-19 diagnosis. Earlier guidelines stressed the need for a positive laboratory COVID-19 diagnosis as a requirement for subsequent LC-19 determination. However, current guidelines state that laboratory tests are not required to establish exposure to the virus, and even asymptomatic exposure is sufficient to induce a response to the virus, ultimately leading to LC-19 (Centers for Disease Control and Prevention, 2022). Considering that most countries are pushing for more accessible at-home antigen-based testing or self-quarantining based on symptoms, many patients experiencing possible LC-19 may not have an initial qPCR or lab-based COVID-19 diagnosis record.
Retrospective studies of electronic health record data exemplify the complexity of LC-19 diagnosis and challenges for care due to clinical variability and resultant care fragmentation. Pfaff et al. designed machine learning models based on health records from National COVID Cohort Collaborators (NC3) using artificial intelligence (AI) to identify patients with LC-19 and those who might warrant special care (Pfaff et al., 2022). According to their model, the most critical factors were outpatient clinic utilization after acute COVID-19, patient age, dyspnea, and other concurrent diagnosis and medication.
The number of records and their publication location is extremely important as these are points that play a role in the decision and policy-making arena. Our findings show the paucity of papers from Africa and South America, which is worrisome as some of the highly contagious and immune response-evading variants first appeared in those areas of the world (Tegally et al., 2022). Other than the WHO-led Delphi study that used an international panel to create a standard definition for LC-19 for diagnosis purposes, few other studies involved a wide range of countries, expertise, health systems, and stakeholders across different domains (Soriano et al., 2022).
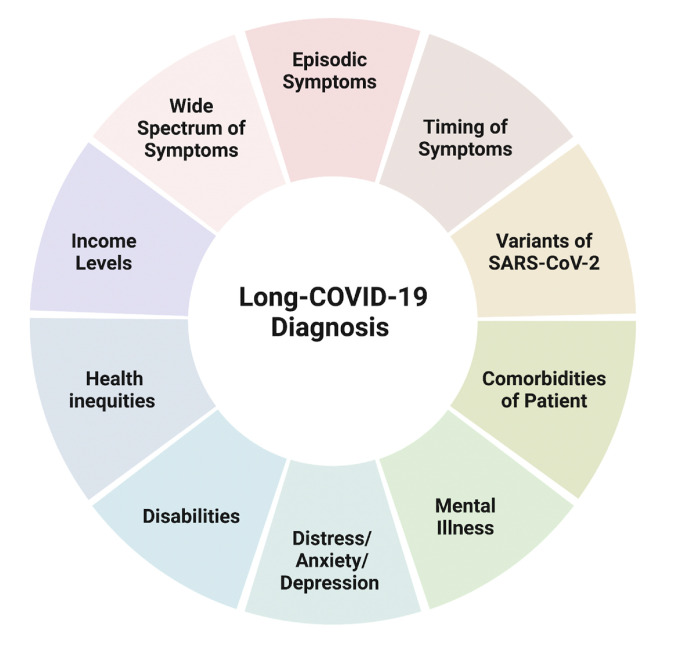
The diagnostic criteria being subjective rather than objective leads to underestimation in certain groups in the population. In the US, the Black and Hispanic community was affected more than others in the acute phase of COVID, but their LC-19 rates are similar to those of the Caucasian community (Farrah, 2022; Hill and Artiga, 2022). The reason for this discrepancy is not understood, but it is concerning as these groups tend to have lesser access to healthcare (Farrah, 2022), which may cause underdiagnosis. Retrospective Cohort studies indicate higher rates in women and people with co-morbidities. Elderly or patients with co-morbidities are hard to diagnose as LC-19 symptoms sometimes are an aggravation of pre-existing conditions. As symptoms fluctuate unpredictably over days, weeks, or even months affecting well-being and ability to perform daily activities, LC-19 is deemed episodic (Brown and O’Brien, 2021). In addition, the numbers affected are dependent on the initial SARS-COV2 disease variant, with the Delta variant causing more cases of LC-19 than the Omicron [10.8% vs. 4.5%] (Antonelli et al., 2022). Clinicians indicate a wariness to give an LC-19 diagnosis due to the lack of treatment and potential use of the diagnosis for social benefits (e.g., unemployment, work accommodation, or disability). Fig. 5 represents potential confounding factors that delay or impact LC-19 diagnosis. These factors are essential to consider in the need for standardizing the LC-19 diagnosis methods for earlier and more accurate detection of this condition and thus minimize the negative impact on personal, community, national, and international levels.
Fig. 5.
Schematic representing potential confounding factors that delay or impact Long-COVID-19 diagnosis. Created with BioRender.com.
Based on the evidence found in our literature search, only 11 original articles specifically discuss aspects of diagnosing LC-19. Due to the variability in the international clinical definition and diagnostic parameters, it is challenging to determine the gold standard for LC-19 diagnosis and, thus, a consistent standard of care and treatment plans for LC-19 patients. Therefore, it is imperative to clearly define the disease and the important time points within this definition. Following these, more studies are warranted evaluating risks and biomarkers to create a standardized protocol for early LC-19 diagnosis and treatment. The establishment of international consensus guidelines of LC-19 is vital due to the continuous increase in the number of patients and its subsequent burden on the healthcare system.
5. Conclusion
Currently, there is no standardized test to diagnose LC-19. When a probable patient presents, doctors observe the symptoms and arrive at the final disease determination by inclusion and exclusion (e.g., differential diagnosis). It is important to note that there is no one or set of symptoms typical of LC-19; other diseases and conditions can present similar symptoms making diagnosis challenging. The current differential diagnosis process is based on the framework put forward by WHO Delphi recommendations and parent countries’ directives. In most cases, this process involves a lengthy process of an additional battery of tests, especially to rule out other conditions/diseases. Therefore, LC-19 diagnosis is very subjective because of the variability in time of onset, lack of clinical symptoms exclusive to the condition, concurrent reactivation of other diseases, pre-existing conditions, risk factors, and complex and disparate symptoms. Features of LC-19 are also dependent on the SARS-COV2 variant causing the acute disease (Antonelli et al., 2022). Studies focusing on identifying LC-19-specific biomarkers may help to disambiguate the LC-19 diagnostic process. Another area of informational vacuum is the correlation between COVID-19 variants and the type and severity of LC-19 that ensues. Our study deals with only adults, as not enough information about LC-19 in children and how it affects them is available.
A clear set of standardized directions to reach the diagnosis is thus a first and necessary step to optimize the recognition and care of persons experiencing the condition in community and healthcare settings. Due to the lack of a clear directive and considering the many unknowns surrounding the natural history of the disease and further recovery/sequelae from COVID-19, continued discussion and agreement on a definition/diagnosis will help future research and management of these patients. Better identification would result in better management of this condition, in turn affecting the lives of millions of people in the world.
6. Limitations of study
Limitations of this study are that these results and conclusions were determined from the limited number of current records about LC-19. Clinicians' reluctance to give an LC-19 diagnosis could be due to the lack of specific therapy for treatment, the patient's health insurance status (private vs. state-funded), and implications of economic impact, such as a disability diagnosis. Another limitation of this study is the paucity of papers from Africa, South America, and Australia across all categories. More research and publications from these geographic areas could provide greater insight into LC-19, as many COVID-19 variants have originated within these populations. Africa and South America are also the two continents where vaccination rates have been lower, impacting the number and symptoms of LC-19. Despite these limitations, this scoping review sheds light on the categories lacking literature within LC-19 diagnosis records and will hopefully provide a baseline for future work.
Declaration of competing interest
The authors declare that they have no competing financial interests or personal relationships that could be perceived to have influenced the work reported in this paper.
Acknowledgments
The authors would like to acknowledge the Clemson University Creative Inquiry program and the National Institute Of General Medical Sciences of the National Institutes of Health under Award Number P20GM121342 (SC-TRIMH) for funding. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data availability
No data was used for the research described in the article.
References
- Antonelli M., Pujol J.C., Spector T.D., Oureselin S., Steves C. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet. 2022;399(10343):2263–2264. doi: 10.1016/j.athoracsur.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.A., O'Brien K.K. Conceptualising Long COVID as an episodic health condition. BMJ Glob. Health. 2021;6(9):4–7. doi: 10.1136/bmjgh-2021-007004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carfi A., Bernabi R., Landi F. Persistent symptoms in patients after acute COVID-19. American Med. Assoc. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . vols. 1–16. 2022. (COVID-19 Post-COVID Conditions : Information for Healthcare Providers). [Google Scholar]
- Daitch V., Yelin D., Awwad M., Guaraldi G., Milić J., Mussini C., Falcone M., Tiseo G., Carrozzi L., Pistelli F., Nehme M., Guessous I., Kaiser L., Vetter P., Bordas-Martínez J., Durà-Miralles X., Peleato-Catalan D., Gudiol C., Shapira-Lichter I., et al. Characteristics of long-COVID among older adults: a cross-sectional study. Int. J. Infect. Dis. 2022;125:287–293. doi: 10.1016/j.ijid.2022.09.035. [DOI] [PubMed] [Google Scholar]
- Davis H.E., Assaf G.S., McCorkell L., Wei H., Low R.J., Re’em Y., Redfield S., Austin J.P., Akrami A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gennaro F., Pizzol D., Marotta C., Antunes M., Racalbuto V., Veronese N., Smith L. Coronavirus diseases (COVID-19) current status and future perspectives: a narrative review. Int. J. Environ. Res. Publ. Health. 2020;17(2690):1–11. doi: 10.3390/ijerph17082690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerlund L.S., Shakar S., Nielsen H., Bodilsen J. Positive predictive value of the ICD-10 diagnosis code for long-COVID. Clin. Epidemiol. 2022;14(January):141–148. doi: 10.2147/CLEP.S344515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah T. Scientific American; 2022. People of Color with Long COVID Face Uphill Battle to Be Heard.https://www.scientificamerican.com/article/people-of-color-with-long-covid-face-uphill-battle-to-be-heard/ [Google Scholar]
- Fernández-De-las-peñas C., Palacios-Ceña D., Gómez-Mayordomo V., Cuadrado M.L., Florencio L.L. Defining post-covid symptoms (Post-acute covid, long covid, persistent post-covid): an integrative classification. Int. J. Environ. Res. Publ. Health. 2021;18(5):1–9. doi: 10.3390/ijerph18052621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh T., Knight M., A'Court C., Buxton M., Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370 doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., Ludden C., Reeve R., Rambaut A., Peacock S.J., Robertson D.L. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19(7):409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill L., Artiga S. vols. 1–9. 2022. https://www.kff.org/coronavirus-covid-19/issue-brief/covid-19-cases-and-deaths-by-race-ethnicity-current-data-and-changes-over-time/ (COVID-19 Cases and Deaths by Race/Ethnicity: Current Data and Changes over Time). Kff.Org. [Google Scholar]
- Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Monetary Fund Policy responses to COVID19. December. 2020:1–189. 2020. [Google Scholar]
- Kim Y., Kim S.E., Kim T., Yun K.W., Lee S.H., Lee E., Seo J.-W., Jung Y.H., Chong Y.P. Preliminary guidelines for the clinical evaluation and management of long COVID. Infect. Chemotherapy. 2022;54(Issue 3) doi: 10.3947/ic.2022.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J.R., Martin M.R., Martin J.D., Kuhn P., Hicks J.B. Modeling the onset of symptoms of COVID-19. Front. Public Health. 2020;8(August) doi: 10.3389/fpubh.2020.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Huang D.Q., Zou B., Yang H., Hui W.Z., Rui F., Yee N.T.S., Liu C., Nerurkar S.N., Kai J.C.Y., Teng M.L.P., Li X., Zeng H., Borghi J.A., Henry L., Cheung R., Nguyen M.H. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 2021;93(3):1449–1458. doi: 10.1002/jmv.26424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martimbianco A.L.C., Pacheco R.L., Bagattini Â.M., Riera R. Frequency, signs and symptoms, and criteria adopted for long COVID-19: a systematic review. Int. J. Clin. Pract. 2021;75(10):1–16. doi: 10.1111/ijcp.14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munblit D., Nicholson T., Akrami A., Apfelbacher C., Chen J., De Groote W., Diaz J.V., Gorst S.L., Harman N., Kokorina A., Olliaro P., Parr C., Preller J., Schiess N., Schmitt J., Seylanova N., Simpson F., Tong A., Needham D.M., et al. A core outcome set for post-COVID-19 condition in adults for use in clinical practice and research: an international Delphi consensus study. Lancet Respir. Med. 2022;10(7):715–724. doi: 10.1016/S2213-2600(22)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff E.R., Girvin A.T., Bennett T.D., Bhatia A., Brooks I.M., Deer R.R., Dekermanjian J.P., Jolley S.E., Kahn M.G., Kostka K., McMurry J.A., Moffitt R., Walden A., Chute C.G., Haendel M.A., Bramante C., Dorr D., Morris M., Parker A.M., et al. Identifying who has long COVID in the USA: a machine learning approach using N3C data. The Lancet Digital Health. 2022;4(7):e532–e541. doi: 10.1016/S2589-7500(22)00048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveendran A.V. Long COVID-19: challenges in the diagnosis and proposed diagnostic criteria. Diabetes Metabol. Syndr.: Clin. Res. Rev. 2021;15(1):145–146. doi: 10.1016/j.dsx.2020.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah W., Hillman T., Playford E.D., Hishmeh L. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ. 2021;372:10–13. doi: 10.1136/bmj.n136. [DOI] [PubMed] [Google Scholar]
- SIGN . 2022. Managing the Long-Term Effects of COVID-19; pp. 2–3. [Google Scholar]
- Sisó-Almirall A., Brito-Zerón P., Ferrín L.C., Kostov B., Moreno A.M., Mestres J., Sellarès J., Galindo G., Morera R., Basora J., Trilla A., Ramos-Casals M. Long covid-19: proposed primary care clinical guidelines for diagnosis and disease management. Int. J. Environ. Res. Publ. Health. 2021;18(8) doi: 10.3390/ijerph18084350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.v. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022;22(4):e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegally H., Moir M., Everatt J., Giovanetti M., Scheepers C., Wilkinson E., Subramoney K., Makatini Z., Moyo S., Amoako D.G., Baxter C., Althaus C.L., Anyaneji U.J., Kekana D., Viana R., Giandhari J., Lessells R.J., Maponga T., Maruapula D., et al. Emergence of SARS-CoV-2 omicron lineages BA.4 and BA.5 in South Africa. Nat. Med. 2022;28(9):1785–1790. doi: 10.1038/s41591-022-01911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S., Chang Z., Wang Y., Wu M., Zhang W., Zhou G., Zou X., Tian H., Xiao T., Xing J., Chen J., Han J., Ning K., Wu T. Clinical characteristics and reasons for differences in duration from symptom onset to release from quarantine among patients with COVID-19 in liaocheng, China. Front. Med. 2020;7(May):1–8. doi: 10.3389/fmed.2020.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricco A.C., Lillie E., Zarin W., O'Brien K.K., Colquhoun H., Levac D., Moher D., Peters M.D.J., Horsley T., Weeks L., Hempel S., Akl E.A., Chang C., McGowan J., Stewart L., Hartling L., Aldcroft A., Wilson M.G., Garritty C., et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann. Intern. Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- WebMD . 2022. Long COVID Mimics Other Post-Viral Conditions; pp. 1–5. [Google Scholar]
- World Health Organization WHO coronavirus (COVID-19) dashboard. February. 2023:1–5. [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China. JAMA Intern. Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yale Medicine . 2020. What is Long Covid? pp. 19–21. [Google Scholar]
- Yelin D., Moschopoulos C.D., Margalit I., Gkrania-Klotsas E., Landi F., Stahl J.P., Yahav D. ESCMID rapid guidelines for assessment and management of long COVID. Clin. Microbiol. Infection. 2022;28(7):955–972. doi: 10.1016/j.cmi.2022.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.