Abstract
Background
Cerebral small vessel disease (SVD) causes lacunar strokes (25% of all ischaemic strokes), physical frailty and cognitive impairment and vascular and mixed dementia. There is no specific treatment to prevent progression of SVD.
Methods
The LACunar Intervention Trial-2 is an investigator-initiated prospective randomised open-label blinded-endpoint phase II feasibility study assessing cilostazol and isosorbide mononitrate for preventing SVD progression. We aimed to recruit 400 patients with clinically evident lacunar ischaemic stroke and randomised to cilostazol, isosorbide mononitrate, both or neither, in addition to guideline secondary ischaemic stroke prevention, in a partial factorial design. The primary outcome is feasibility of recruitment and adherence to medication; key secondary outcomes include: drug tolerability; recurrent vascular events, cognition and function at 1 year after randomisation; and safety (bleeding, falls, death). Data are number (%) and median (IQR).
Results
The trial commenced on 5 February 2018 and ceased recruitment on 31 May 2021 with 363 patients randomised, with the following baseline characteristics: average age 64 (56.0, 72.0) years, female 112 (30.9%), stroke onset to randomisation 79.0 (27.0, 244.0) days, hypertension 267 (73.6%), median blood pressures 143.0 (130.0, 157.0)/83.0 (75.0, 90.0) mm Hg, current smokers 67 (18.5%), educationally achieved end of school examinations (A-level) or higher 118 (32.5%), modified Rankin scale 1.0 (0.0, 1.0), National Institutes Health stroke scale 1.0 (1.4), Montreal Cognitive Assessment 26.0 (23.0, 28.0) and total SVD score on brain imaging 1.0 (0.0, 2.0). This publication summarises the baseline data and presents the statistical analysis plan.
Summary
The trial is currently in follow-up which will complete on 31 May 2022 with results expected in October 2022.
Trial registration number
ISRCTN14911850.
Keywords: stroke, clinical trial, statistics
Introduction
lCerebral small vessel disease (SVD) is a major cause of stroke (lacunar ischaemic stroke), intracerebral haemorrhage and vascular and mixed dementias.1 It is most commonly due to an intrinsic disorder of the brain’s small perforating arterioles with endothelial dysfunction manifesting as impaired vasoreactivity, vascular stiffness, blood–brain barrier leakage and perivascular inflammation.1 Currently, there is no specific secondary prevention for lacunar ischaemic stroke or SVD-associated cognitive decline.2 Combined aspirin and clopidogrel increased bleeding3 and intensive antihypertensive therapy did not reduce recurrence or prevent cognitive decline4 in lacunar ischaemic stroke.
However, there are other potential interventions that might prevent SVD or reduce its development and progression, including: drugs that modulate the blood–brain barrier or lower blood lipids, immunosuppressive agents, neurotrophins, peroxisome proliferator-activated receptor-gamma agonists, rho-kinase antagonists, vitamins, anti-inflammatory agents and xanthine oxidase inhibitors.2 5 Additionally, drugs that modulate the nitric oxide (NO)-cyclic guanylate cylase-phosphodiesterase-5 and prostacyclin (PGI2)-cyclic adenylate cylase-phosphodiesterase-3 systems are attractive since they mimic key endogenous vaso-regulators.2 Both NO and PGI2 have vasodilator, antiplatelet, antileucocyte,6 antismooth muscle and proendothelial effects. NO may be administered orally as substrate (L-arginine or inorganic nitrate), organic nitrates (such as isosorbide mononitrate, ISMN) or drugs that inhibit PDE5 (such a dipyridamole7 or sildenafil) to increase and preserve levels of the NO second messenger, cyclic guanylate monophosphate. Although PGI2 has to be administered intravenously, PDE3 may be inhibited with cilostazol,8 which preserves levels of the PGI2 second messenger, cyclic adenylate monophosphate; cilostazol has showed promise in secondary prevention trials in ischaemic stroke, which included large proportions of patients with lacunar stroke subtype8; and is widely used in East Asia. In addition, in experimental models, cilostazol reversed impaired oligodendrocyte precursor cell maturation which occurs when endothelial cells are dysfunctional thus potentially reducing myelin damage and facilitating its repair,9 and increased the astrocyte-to-neuron lactate shuttle thus increasing energy supply and prolonging neuronal survival.10 Both actions are potentially relevant to reducing brain damage in SVD.
SVD can present clinically as lacunar ischaemic stroke, cognitive impairment, mobility and/or mood disorders, or be covert and detected on incidentally on brain imaging.1 These presentations are associated with increased short and long-term risk of recurrent stroke, cognitive decline, dementia and functional impairments.11–15 However, although these clinical outcomes are highly relevant to patients and clinical services,15 16 the data on their rates long term, individually or in combination, are sparse, precluding reliable sample size estimations for clinical trials in SVD.17 Furthermore, it was unclear if patients with clinically evident lacunar ischaemic stroke could be identified with key prognostic variables determined accurately in routine clinical practice, or recruited in sufficient numbers rapidly enough, to make a clinical trial in stroke presentations of SVD feasible in a practical time period. This is because lacunar ischaemic stroke may be confused clinically with mild cortical ischaemic stroke,18 MRI is not always available acutely nor positive for small subcortical stroke,19 and long-term outcomes vary with severity of SVD lesions on brain imaging,13–15 making it necessary to minimise the randomisation on SVD severity.20 Since lacunar ischaemic stroke is currently managed according to guidelines covering the whole of secondary prevention of ischaemic stroke (representing best medical care) and there is no justification currently for withholding secondary prevention, any novel drugs should be tested against a background of guideline secondary ischaemic stroke prevention.
The LACunar Intervention Trial-2 (LACI-1/2) series of trials are assessing the feasibility of recruiting patients with clinically evident lacunar ischaemic stroke and tolerability of treatment with ISMN and cilostazol,17 21–24 while also gathering data on outcome event rates, with the aim of testing these agents in a large phase III efficacy trial. In LACI-1, short-term administration of cilostazol and ISMN in addition to best medical care were well tolerated when the dose was escalated, without safety concerns, in patients with clinically evident lacunar ischaemic stroke23; additionally, these drugs appeared to reduce arterial stiffness without effects on platelet function22 and appeared to improve cerebrovascular function.24
The present trial, LACI-2, is testing the long-term feasibility and tolerability of administering cilostazol and ISMN to patients with clinically evident lacunar ischaemic stroke given on top of guideline secondary ischaemic stroke prevention while assessing safety and gathering data on clinically relevant outcome event rates.17 Here, we present the statistical analysis plan (SAP) and baseline data of LACI-2.
Methods
The LACunar Intervention Trial-2 (LACI-2) is a UK-based investigator-initiated, prospective, randomised, partial factorial, open-label, blinded-endpoint phase II feasibility trial of cilostazol and/or ISMN. Patients with clinically evident lacunar ischaemic stroke, with no limit on the time interval since the stroke, with capacity to consent and who were independent in activities of daily living, were randomised to cilostazol, ISMN, both or neither in a partial factorial design. Patients with contraindications to one of the trial drugs could be randomised to the other drug alone. Randomised treatment was given in all patients in addition to guideline stroke prevention therapies, typically clopidogrel or aspirin, antihypertensive drugs and statins. Patients requiring oral anticoagulation were excluded.
Full details of regulatory approvals, patient assessment, inclusion and exclusion criteria, randomisation, minimisation criteria (age, baseline modified Rankin scale (mRS), National Institutes Health stroke scale (NIHSS), time from stroke to randomisation, highest educational attainment, smoking, systolic BP), dose-escalation scheme, short-term and long-term assessments are given in the protocol paper.17
The presence of an infarct relevant to the clinical presentation and a simplified estimate of total SVD lesion severity score, adapted for use on CT or MRI and by people not expert in brain imaging of SVD,17 21 were determined at the recruiting site. All diagnostic CT or MRI, scans obtained at any stroke recurrence and 1-year follow-up MRI, were collected by the trial office for central adjudication.
The primary outcome is feasibility of recruitment and adherence to medication. Key secondary outcomes include recruitment of hospital sites and participants, drug tolerability, symptoms (such as headache, nausea, palpitations) which might deter drug adherence, safety (bleeding, falls, death), recurrent vascular events (including stroke and myocardial infarction), cognition and function over 1 year of follow-up. Imaging outcomes (white matter hyperintensity (WMH) severity, new cortical or subcortical infarcts, lacunes, haemorrhage, microbleeds) are assessed with MRI at 1 year. Details of central CT and MRI adjudication are given in the LACI-2 protocol paper.17
LACI-2 will provide outcome event rates and hence information to estimate sample size, recruitment and trial procedures for a phase III trial based in clinical outcomes. Further information is given in online supplemental file and published protocol.17
svn-2022-001816supp001.pdf (1.9MB, pdf)
We present here a complete listing of baseline data; data are number (%) and median (IQR), unless otherwise stated.
The full details of the SAP25 26 are given in the accompanying online supplemental file, Appendix S1 and is presented prior to locking of the study database so that analyses are not data driven or reported selectively.27 The SAP also lists planned secondary analyses and substudies, and follows the layout suggested in guidelines.25 26
Results
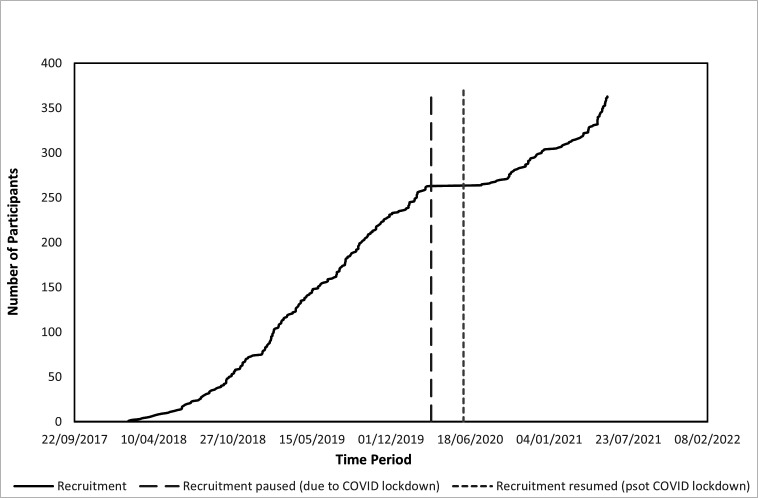
The trial commenced in February 2018; recruitment was suspended due to COVID-19 restrictions on 17 March 2020 by the Sponsor and reopened on 10 June 2020; however, the additional delays in reinstating site approvals meant that there was a total of 4 months without recruitment. Following a 6-month extension, recruitment stopped on 31 May 2021 to allow a year of follow-up prior to close, a total of 40 months (36 months excluding COVID-19 suspension, figure 1).
Figure 1.
Recruitment by time.
Of a planned 400 participants, 363 (91%) were recruited from 26 UK hospitals. At baseline, the average age was 64 (range 31–87) years (table 1). There were 112 (31%) female patients, the median NIHSS was 0 (0, 0), the mRS was 2 in 85 (23%) patients, and the time from stroke onset to randomisation was 79.0 (27.0, 244.0) days (table 1). The median age for completing education was 16 years, with 118 (33%) participants having one or more A-level equivalent or above qualifications. Vascular risk factor rates included median blood pressure of 143.0 (130.0, 157.0)/83.0 (75.0, 90.0) mm Hg, current smoking in 67 (19%), drug-treated hypertension in 258 (71%), drug-treated hyperlipidaemia in 278 (77%), diabetes mellitus in 80 (22%), atrial fibrillation in 5 (1%), carotid stenosis of >50% left 3 (0.8%), right 6 (1.7%) and history of previous stroke in 25 (7%) (table 2).
Table 1.
Baseline characteristics by small vessel disease score as determined by recruiting site
| Variable | N | Participants | cSVD | Score* |
| All | None/mild | Moderate to severe | ||
| No of patients, N | 363 | 99 | 264 | |
| Demographics | ||||
| Age, years† | 363 | 64.0(56.0, 72.0) | 62.0(54.0, 69.0) | 65.0(57.5, 73.0) |
| ≤70 years (%) | 363 | 251 (69.1) | 78 (78.8) | 173 (65.5) |
| Sex, female (%) | 363 | 112 (30.9) | 28 (28.3) | 84 (31.8) |
| modified Rankin Scale (/6) | 363 | 1.0(0.0, 1.0) | 1.0(0.0, 1.0) | 1.0(0.0, 1.0) |
| >1 (%)† | 363 | 85 (23.4) | 23 (23.2) | 62 (23.5) |
| Onset to randomisation (days)† | 363 | 79.0(27.0, 244.0) | 73.0(27.0, 244.0) | 84.5(28.5, 246.5) |
| ≤100 (%) | 363 | 206 (56.7) | 64 (64.6) | 142 (53.8) |
| Age completing education (years) | 363 | 16.0(15.0, 18.0) | 16.0(15.0, 17.0) | 16.0(15.0, 18.0) |
| Highest education level (%)† test | ||||
| Primary school | 363 | 2 (0.6) | 0 (0.0) | 2 (0.8) |
| Secondary school | 363 | 129 (35.5) | 36 (36.4) | 93 (35.2) |
| O-level/GCSE or equivalent‡ | 363 | 114 (31.4) | 34 (34.3) | 80 (30.3) |
| A-level or equivalent‡ | 363 | 54 (14.9) | 17 (17.2) | 37 (14.0) |
| College or university undergraduate | 363 | 37 (10.2) | 7 (7.1) | 30 (11.4) |
| College or university postgraduate | 363 | 27 (7.4) | 5 (5.1) | 22 (8.3) |
| Lifestyle | ||||
| Smoking (%)† | ||||
| Current | 363 | 67 (18.5) | 17 (17.2) | 50 (18.9) |
| Past | 363 | 154 (42.4) | 36 (36.4) | 118 (44.7) |
| Never | 363 | 142 (39.1) | 46 (46.5) | 96 (36.4) |
| Medical history (%) | ||||
| Hypertension | 363 | 267 (73.6) | 58 (58.6) | 209 (79.2) |
| Hypertension, drug treated | 363 | 258 (71.1) | 55 (55.6) | 203 (76.9) |
| Hyperlipidaemia | 363 | 281 (77.4) | 75 (75.8) | 206 (78.0) |
| Hyperlipidaemia, drug treated | 363 | 278 (76.6) | 75 (75.8) | 203 (76.9) |
| Diabetes mellitus | 363 | 80 (22.0) | 20 (20.2) | 60 (22.7) |
| Insulin | 363 | 18 (5.0) | 5 (5.1) | 13 (4.9) |
| Oral agents | 363 | 58 (16.0) | 18 (18.2) | 40 (15.2) |
| Lifestyle | 363 | 24 (6.6) | 3 (3.0) | 21 (8.0) |
| Atrial fibrillation | 363 | 5 (1.4) | 1 (1.0) | 4 (1.5) |
| Heart failure | 363 | 4 (1.1) | 0 (0.0) | 4 (1.5) |
| Previous stroke | 363 | 25 (6.9) | 2 (2.0) | 23 (8.7) |
| Prior TIA | 363 | 29 (8.0) | 5 (5.1) | 24 (9.1) |
| Family history, young stroke | 363 | 55 (15.2) | 16 (16.2) | 39 (14.8) |
| Current medications (%) | ||||
| Anticoagulants | 363 | 5 (1.4) | 1 (1.0) | 4 (1.5) |
| Antibiotics | 363 | 2 (0.6) | 0 (0.0) | 2 (0.8) |
| Antihypertensive | 363 | 277 (76.3) | 67 (67.7) | 210 (79.5) |
| ACE-inhibitors | 132 (36.4) | 32 (32.3) | 100 (37.9) | |
| Angiotensin-II RA | 54 (14.9) | 14 (14.1) | 40 (15.2) | |
| Renin inhibitor | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Beta-RA | 41 (11.3) | 10 (10.1) | 31 (11.7) | |
| CCB | 164 (45.2) | 33 (33.3) | 131 (49.6) | |
| Diuretic | 51 (14.0) | 13 (13.1) | 38 (14.4) | |
| Alpha-RA | 17 (4.7) | 3 (3.0) | 14 (5.3) | |
| Centrally active | 2 (0.6) | 1 (1.0) | 1 (0.4) | |
| Antiplatelet | 363 | 352 (97.0) | 97 (98.0) | 255 (96.6) |
| Lipid lowering | 363 | 341 (93.9) | 91 (91.9) | 250 (94.7) |
| Proton pump inhibitor | 363 | 128 (35.3) | 30 (30.3) | 98 (37.1) |
| PDE5 inhibitor | 363 | 7 (1.9) | 3 (3.0) | 4 (1.5) |
| Grapefruit juice more than once a week | 363 | 15 (0.04) | 3 (0.03) | 12 (0.04) |
| Other drugs | 363 | 236 (65.0) | 67 (67.7) | 169 (64.0) |
| Nor of drugs taken/day | 363 | 4.0 (3.0, 4.0) | 3.0 (3.0, 4.0) | 4.0 (3.0, 5.0) |
Data are number (%), median (IQR).
*According to scan read at site.
†Minimisation variable.
‡School exams at age 16 (O-level/GCSE or equivalent) and 18 (A-level or equivalent).
CCB, calcium channel blocker; F/T, full time; P/T, part time; RA, receptor antagonist; SVD, small vessel disease; TIA, transient ischaemic attack.
Table 2.
Baseline clinical and investigation characteristics by small vessel disease score as determined by recruiting site
| Variable | N | Participants | cSVD | Score* | P value |
| All | None/mild | Moderate to evere | |||
| No of patients, N | 363 | 99 | 264 | ||
| Clinical data (%) | |||||
| SBP mm Hg† | 363 | 143.0 (130.0, 157.0) | 143.0 (128.0, 160.0) | 143.0 (131.0, 155.5) | 0.94 |
| DBP mm Hg | 363 | 83.0 (75.0, 90.0) | 82.0 (75.0, 90.0) | 83.0 (75.5, 90.0) | 0.89 |
| NIHSS (/42)† | 363 | 0.0 (0.0, 2.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 2.0) | 0.34 |
| Duration of symptoms (days) | 145 | 2.0 (1.0, 5.0) | 2.0 (1.0, 7.0) | 2.0 (1.0, 5.0) | 0.30 |
| Ongoing symptoms | 363 | 218 (60.1) | 58 (58.6) | 160 (60.6) | 0.73 |
| Weakness, side of weakness | 363 | 288 (79.3) | 80 (80.8) | 208 (78.8) | 0.67 |
| Left (%) | 363 | 148 (40.8) | 37 (37.4) | 111 (42.0) | |
| Right (%) | 363 | 138 (38.0) | 43 (43.4) | 95 (36.0) | |
| Both (%) | 363 | 2 (0.6) | 0 (0.0) | 2 (1.0) | |
| Sensory loss, side of loss | 363 | 148 (40.8) | 43 (43.4) | 105 (39.8) | 0.53 |
| Left (%) | 363 | 74 (20.4) | 19 (19.2) | 55 (20.8) | |
| Right (%) | 363 | 71 (19.6) | 24 (24.2) | 47 (17.8) | |
| Both (%) | 363 | 3 (0.8) | 0 (0.0) | 3 (1.1) | |
| Ataxia | 363 | 84 (23.1) | 17 (17.2) | 67 (25.4) | 0.010 |
| Left (%) | 363 | 41 (11.3) | 5 (5.1) | 36 (13.6) | |
| Right (%) | 363 | 39 (10.7) | 12 (12.1) | 27 (10.2) | |
| Neglect/inattention | 363 | 6 (1.7) | 1 (1.0) | 5 (1.9) | 0.56 |
| Dysphasia | 363 | 19 (5.2) | 6 (6.1) | 13 (4.9) | 0.67 |
| Dysarthria | 363 | 111 (30.6) | 28 (28.3) | 83 (31.4) | 0.56 |
| Visual loss | 363 | 11 (3.0) | 6 (6.1) | 5 (1.9) | 0.039 |
| Cognition | |||||
| MOCA total (/30) | 363 | 26.0 (23.0, 28.0) | 26.0 (23.0, 28.0) | 26.0 (24.0, 28.0) | 0.098 |
| ≤24 (%) | 363 | 119 (32.8) | 39 (39.4) | 80 (30.3) | 0.10 |
| Trail making test part B | |||||
| Time (seconds) | 359 | 110.0 (75.0, 170.0) | 112.0 (74.0, 168.0) | 108.0 (76.0, 171.0) | 0.73 |
| Points | 359 | 25.0 (23.0, 25.0) | 25.0 (23.0, 25.0) | 25.0 (23.0, 25.0) | 0.39 |
| Investigations (%) | |||||
| Type of scan after index stroke (%) | |||||
| CT only | 363 | 101 (27.8) | 40 (40.4) | 61 (23.1) | 0.001 |
| MRI only | 363 | 43 (11.8) | 9 (9.1) | 34 (12.9) | 0.32 |
| Both | 363 | 219 (60.3) | 50 (50.5) | 169 (64.0) | 0.019 |
| Stroke-CT scan (days) | 320 | 1.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 1.0 (0.0, 1.0) | 0.26 |
| Stroke-MRI scan (days) | 262 | 3.0 (1.0, 9.0) | 2.0 (1.0, 5.0) | 3.0 (1.0, 11.0) | 0.13 |
| Acute stroke lesion present* | 363 | 296 (81.5) | 68 (68.7) | 228 (86.4) | <0.001 |
| Lesion side of brain, left (%)* | 363 | 164 (45.2) | 45 (45.5) | 119 (45.1) | 0.95 |
| SVD score 1 (%)* | 363 | 206 (56.7) | 0 (0.0) | 206 (78.0) | – |
| WMH/hypoattenuations, yes (%)* | 363 | 179 (49.3) | 0 (0.0) | 179 (67.8) | – |
| Total SVD score*† | 363 | 1.0 (0.0, 2.0) | 0.0 (0.0, 0.0) | 1.0 (1.0, 2.0) | – |
| SVD moderate/severe (%)* | 363 | 264 (72.7) | 0 (0.0) | 264 (100.0) | – |
| Carotid stenosis (%) | 363 | 315 (86.8) | 90 (90.9) | 225 (85.2) | 0.16 |
| Degree of stenosis left <50% | 363 | 312 (86.0) | 90 (90.9) | 222 (84.1) | |
| Degree of stenosis right <50% | 363 | 309 (85.1) | 90 (90.9) | 219 (83.0) | |
| ECG (%) | 0.93 | ||||
| Sinus | 360 | 347 (96.4) | 94 (95.9) | 253 (96.6) | |
| AF | 360 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Other | 360 | 13 (3.6) | 4 (4.1) | 9 (3.4) | |
| Haemoglobin, g/L | 361 | 14.2 (13.3, 15.2) | 14.3 (13.2, 15.3) | 14.2 (13.3, 15.2) | 0.55 |
| Creatinine (µmol/l) | 363 | 77.0 (66.0, 90.0) | 82.0 (69.0, 91.0) | 76.0 (66.0, 90.0) | 0.21 |
| eGFR (mL/min) | 362 | 75.0 (75.0, 78.0) | 75.0 (75.0, 81.0) | 75.0 (75.0, 75.0) | 0.10 |
Data are number (%), median (IQR).
*According to scan read at site.
†Minimisation variable.
F/T, full time; NIHSS, National Institutes Health stroke scale; P/T, part time; SVD, small vessel disease; WMH, white matter hyperintensity.
Baseline clinical findings included persistent unilateral weakness in 288 (79%), sensory change in 148 (41%), neglect/inattention in 6 (2%), dysphasia in 19 (5%) and visual disturbance in 11 (3%) (table 2). On cognitive testing, the median Montreal Cognitive Assessment score was 26.0 (23.0, 28.0) with 119 (33%) having a score below 25 signifying cognitive impairment. The median score on the trail making test, part B, a test of executive function, was 25.0 (23.0, 25.0) points.
A majority of participants, 219 (60%), had both an admission CT scan and MRI brain imaging (320, 88%, had CT and 229, 65%, had MRI) (table 2). Imaging findings as reported by the recruiting site included an acute ischaemic stroke lesion thought to be consistent with the lacunar stroke symptoms in 296 (82%), WMHs present in 179 (49%) and median modified total SVD score of 1.0 (0.0, 2.0).
On central adjudication, a visible index infarct was present in 319 (88%) and averaged 10×8×10 mm in size (table 3). Only one scan was considered to be normal, that is, to have neither an acute infarct responsible for the recent stroke symptoms or any background changes of SVD or atrophy. Microbleeds were present in 20% of patients in mainly lobar (28%), deep (41%) or mixed lobar/deep (31%) locations (table 4). Atrophy was present in 75% of participants. WMHs were present in 95% of participants: 76% had up to Fazekas 2 periventricular and 82% had up to Fazekas 2 deep WMH scores. Sixty-two per cent of participants had one or more old vascular lesions including lacunes present in 96% (table 5).
Table 3.
Adjudicated baseline imaging characteristics by small vessel disease score as determined by recruiting site
| Variable | N | All | SVD | Score | P value |
| Low | Moderate-severe | ||||
| Patients randomised | 363 | 99 | 264 | ||
| Scan | |||||
| Scan type (%) | |||||
| CT | 363 | 320 (88.2) | 90 (90.9) | 230 (87.1) | 0.32 |
| Time to CT (days) | 320 | 1.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 1.0 (0.0, 1.0) | 0.19 |
| MRI | 362 | 263 (72.5) | 60 (60.6) | 203 (76.9) | 0.0020 |
| Time to MRI (days) | 263 | 3.0 (1.0, 9.0) | 2.0 (1.0, 5.0) | 3.0 (1.0, 11.0) | 0.097 |
| Scan quality (%) | 0.28 | ||||
| Good | 237 (65.3) | 66 (66.7) | 171 (64.8) | ||
| Moderate | 118 (32.5) | 29 (29.3) | 89 (33.7) | ||
| Poor | 8 (2.2) | 4 (4.0) | 4 (1.5) | ||
| Index lesion (%) | |||||
| Normal scan | 1 (0.3) | 1 (1.0) | 0 (0.0) | 1.00 | |
| Lesion present (type) | 0.0006 | ||||
| Primary infarct | 319 (87.9) | 77 (77.8) | 242 (91.7) | ||
| Primary haemorrhage | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Mimic | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| No acute lesion visible | 43 (11.8) | 21 (21.2) | 22 (8.3) | ||
| Infarct side of brain | 320 | 0.22 | |||
| Right | 142 (44.4) | 29 (37.7) | 113 (46.5) | ||
| Left | 175 (54.7) | 48 (62.3) | 127 (52.3) | ||
| Both | 3 (0.9) | 0 (0.0) | 3 (1.2) | ||
| Location (%) | 320 | ||||
| Small subcortical infarct | 311 (97.2) | 72 (93.5) | 239 (98.4) | 0.33 | |
| Internal capsule | 110 (34.4) | 21 (27.3) | 89 (36.6) | ||
| External capsule | 4 (1.3) | 1 (1.3) | 3 (1.2) | ||
| Lentiform nucleus | 31 (9.7) | 7 (9.1) | 24 (9.9) | ||
| Internal border zone | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Centrum semiovale | 83 (25.9) | 23 (29.9) | 60 (24.7) | ||
| Thalamus | 64 (20.0) | 17 (22.1) | 47 (19.3) | ||
| Cerebellum | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Brainstem lesion (pons) | 18 (5.6) | 3 (3.9) | 15 (6.2) | ||
| Medulla | 1 (0.3) | 0 (0.0) | 1 (0.4) | ||
| Non-small subcortical infarct | |||||
| MCA territory | 320 | 9 (2.8) | 4 (5.2) | 5 (2.1) | 0.23 |
| PCA territory | 320 | 2 (0.6) | 1 (1.3) | 1 (0.4) | 0.42 |
| Infarct size (mm) | 320 | ||||
| A/P | 10.0 (8.0, 12.0) | 10.0 (8.0, 12.0) | 10.0 (8.0, 12.0) | 0.97 | |
| R/L | 9.0 (6.0, 11.0) | 9.0 (6.0, 10.0) | 9.0 (6.0, 11.0) | 0.91 | |
| Cranio-caudal | 10.0 (8.0, 15.0) | 10.0 (8.0, 12.0) | 10.0 (8.0, 15.0) | 0.15 |
Data are number (%), median (IQR) or mean (SD). The number of participants with data is 363 unless stated.
Index lesion=main cause of stroke symptoms.
Index small subcortical infarct=acute lacunar.
Non-small subcortical infarct=large artery cortical or large subcortical or posterior circulation.
SVD, small vessel disease.
Table 4.
Adjudicated baseline imaging characteristics (microhaemorrhages, atrophy, white matter hyperintensities and perivascular spaces) by small vessel disease score as determined by recruiting site
| Variable | N | All | SVD | Score | P value |
| Low | Moderate- severe | ||||
| Patients randomised | 363 | 99 | 264 | ||
| Microhaemorrhages | 196 | 39 (19.9) | 9 (20.9) | 30 (19.6) | 0.85 |
| No microhaemorrhages (%) | 39 | 0.18 | |||
| 1 | 9 (23.1) | 5 (55.6) | 4 (13.3) | ||
| 2 | 7 (17.9) | 3 (33.3) | 4 (13.3) | ||
| 3 | 5 (12.8) | 0 (0.0) | 5 (16.7) | ||
| 4 | 2 (5.1) | 0 (0.0) | 2 (6.7) | ||
| ≥5 | 15 (38.5) | 1 (11.1) | 14 (46.7) | ||
| Type of microhaemorrhages (%) | 39 | 0.61 | |||
| Lobar | 11 (28.2) | 2 (22.2) | 9 (30.0) | ||
| Deep | 16 (41.0) | 5 (55.6) | 11 (36.7) | ||
| Both | 12 (30.8) | 2 (22.2) | 10 (33.3) | ||
| Superficial siderosis present | 41 | 2 (4.9) | 0 (0.0) | 2 (6.3) | 1.00 |
| Siderosis focal | 2 (100.0) | 0 (0.0) | 2 (100.0) | 1.00 | |
| Siderosis disseminated | 0 (0.0) | 0 (0.0) | 0 (0.0) | – | |
| Siderosis location | 1.00 | ||||
| Left | 1 (50.0) | 0 (0.0) | 1 (50.0) | ||
| Right | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Both | 1 (50.0) | 0 (0.0) | 1 (50.0) | ||
| Atrophy (%) | |||||
| Brain volume reduction | 362 | 272 (75.1) | 63 (64.3) | 209 (79.2) | 0.0036 |
| Central brain tissue volume | 272 | 0.42 | |||
| Moderate | 199 (73.2) | 50 (79.4) | 149 (71.3) | ||
| Severe | 64 (23.5) | 11 (17.5) | 53 (25.4) | ||
| Cortical brain tissue volume | 272 | 0.12 | |||
| Moderate | 203 (74.6) | 43 (68.3) | 160 (76.6) | ||
| Severe | 5 (1.8) | 0 (0.0) | 5 (2.4) | ||
| WMH (%) | |||||
| WMH present | 362 | 342 (94.5) | 87 (88.8) | 255 (96.6) | 0.0038 |
| Anterior white matter lucency | 342 | ||||
| Adjoining ventricles | 116 (33.9) | 32 (36.8) | 84 (32.9) | 0.51 | |
| Covering ventricle to cortex | 56 (16.4) | 6 (6.9) | 50 (19.6) | 0.0057 | |
| Posterior white matter lucency | 342 | ||||
| Adjoining ventricles | 114 (33.3) | 27 (31.0) | 87 (34.1) | 0.60 | |
| Covering ventricle to cortex | 55 (16.1) | 10 (11.5) | 45 (17.6) | 0.18 | |
| Anterior and/or Posterior white matter lucency | 342 | ||||
| Adjoining ventricles | 136 (39.8) | 36 (41.4) | 100 (39.2) | 0.72 | |
| Covering ventricle to cortex | 68 (19.9) | 11 (12.6) | 57 (22.4) | 0.050 | |
| Periventricular WMH Fazekas score | 342 | <0.0001 | |||
| 1 | 131 (38.3) | 48 (55.2) | 83 (32.5) | ||
| 2 | 129 (37.7) | 32 (36.8) | 97 (38.0) | ||
| 3 | 80 (23.4) | 7 (8.0) | 73 (28.6) | ||
| Deep WMH Fazekas score | 342 | 0.0002 | |||
| 1 | 178 (52.0) | 59 (67.8) | 119 (46.7) | ||
| 2 | 102 (29.8) | 14 (16.1) | 88 (34.5) | ||
| 3 | 42 (12.3) | 5 (5.7) | 37 (14.5) | ||
| Periventricular and/or Deep WMH Fazekas score |
342 | <0.0001 | |||
| 1 | 123 (36.0) | 46 (52.9) | 77 (30.2) | ||
| 2 | 137 (40.1) | 34 (39.1) | 103 (40.4) | ||
| 3 | 82 (24.0) | 7 (8.0) | 75 (29.4) | ||
| Enlarged PVS (%), MRI only | 194 | ||||
| Enlarged PVS | 194 (100.0) | 43 (100.0) | 151 (100.0) | 1.00 | |
| Basal ganglia score | |||||
| ≤10 | 62 (32.0) | 23 (53.5) | 39 (25.8) | 0.0008 | |
| 11–20 | 74 (38.1) | 16 (37.2) | 58 (38.4) | ||
| 20–40 | 36 (18.6) | 4 (9.3) | 32 (21.2) | ||
| >40 | 22 (11.3) | 0 (0.0) | 22 (14.6) | ||
| Centrum semiovale score | |||||
| ≤10 | 30 (15.5) | 10 (23.3) | 20 (13.2) | 0.30 | |
| 11–20 | 52 (26.8) | 13 (30.2) | 39 (25.8) | ||
| 20–40 | 82 (42.3) | 15 (34.9) | 67 (44.4) | ||
| >40 | 30 (15.5) | 5 (11.6) | 25 (16.6) |
Data are number (%), median (IQR) or mean (SD). The number of participants with data is 363 unless stated.
PVS, perivascular spaces; WMH, white matter hyperintensities.
Table 5.
Adjudicated baseline imaging characteristics (old vascular and non-stroke lesions) by small vessel disease score as determined by recruiting site
| Variable | N | All | SVD | Score | P value |
| Low | Moderate- severe | ||||
| Patients randomised | 363 | 99 | 264 | ||
| Old vascular lesions (%) | 362 | 224 (61.9) | 38 (38.8) | 186 (70.5) | <0.0001 |
| Old cortical infarct | 224 | 11 (4.9) | 1 (2.6) | 10 (5.4) | 0.70 |
| Old striatocapsular infarct | 224 | 2 (0.9) | 1 (2.6) | 1 (0.5) | 0.31 |
| Old borderzone infarct | 224 | 2 (0.9) | 1 (2.6) | 1 (0.5) | 0.31 |
| Old lacunar infarct | 224 | 216 (96.4) | 36 (94.7) | 180 (96.8) | 0.54 |
| No of lacunes (%) | 224 | 0.11 | |||
| 1 | 40 (17.9) | 10 (26.3) | 30 (16.1) | ||
| 2 | 46 (20.5) | 8 (21.1) | 38 (20.4) | ||
| 3 | 42 (18.8) | 8 (21.1) | 34 (18.3) | ||
| 4 | 26 (11.6) | 6 (15.8) | 20 (10.8) | ||
| ≥5 | 62 (27.7) | 4 (10.5) | 58 (31.2) | ||
| Old brainstem/cerebellar non-lacunar infarcts | 224 | 35 (15.6) | 2 (5.3) | 33 (17.7) | 0.083 |
| Probable old haemorrhage | 224 | 4 (1.8) | 0 (0.0) | 4 (2.2) | 1.00 |
| Non-stroke lesions (%) | |||||
| Non-stroke lesion present | 362 | 13 (3.6) | 3 (3.1) | 10 (3.8) | 1.00 |
| Classification of non-stroke (%) | 13 | ||||
| Cerebral tumour | 2 (15.4) | 0 (0.0) | 2 (20.0) | 1.00 | |
| Aneurysm | 1 (7.7) | 0 (0.0) | 1 (10.0) | 1.00 | |
| Vascular malformation | 1 (7.7) | 1 (33.3) | 0 (0.0) | 1.00 | |
| Other non-stroke classification | 8 (61.5) | 2 (66.7) | 6 (60.0) | 1.00 |
Data are number (%), median (IQR), or mean (SD). The number of participants with data is 363 unless stated.
In univariate analyses and in comparison with participants with lower SVD scores (table 1), those with moderate or severe SVD scores were older and more likely to have had a previous stroke, be taking antihypertensive drugs and have ataxia, and less likely to have visual loss. Additionally, participants with more severe SVD scores were more likely to have had MRI at diagnosis and to have a relevant acute ischaemic stroke lesion present on imaging (table 3). Similarly, in comparison with participants with a low SVD score, those with a moderate/high score had more atrophy, WMHs and their severity (table 4), and the presence of old vascular lesions (table 5).
Discussion
SVD is common worldwide and, so far, whether presenting covertly or with stroke, cognitive or physical impairment, or neuropsychiatric symptoms,28 has no demonstrated interventions that prevent or limit its development and progression.2–4 16 The LACI trials are assessing the tolerability and feasibility of recruiting patients, the outcome event rates, and tolerability and safety of treatment with ISMN and cilostazol17 21–24 in addition to guideline secondary stroke prevention, with the aim of testing these agents in a large phase III efficacy trial.
The baseline characteristics presented here show that LACI-2 is representative of patients with clinically evident lacunar ischaemic stroke, a marker of SVD. In particular, the average age was younger than for other types of ischaemic stroke at 64 years,29 there was a preponderance of males (69%)30 and most participants had relatively few physical signs or impairments resulting from their stroke.15 A majority of participants were diagnosed with hypertension and taking antihypertensive medication, and most were taking antiplatelet or lipid lowering drugs as guideline secondary prevention of ischaemic stroke. Many were ex- smokers or current smokers. Very few had atrial fibrillation or carotid stenosis (about 1%) reflecting that, while emboli can enter and intracranial large artery atheroma may obstruct perforating cerebral arterioles, these are rare in, and uncommon causes of, lacunar ischaemic stroke.29 31
A minority of participants had clinical cortical features such as dysphasia (19, 5.2%), neglect (6, 2%) or hemianopia (11, 3%), reflecting the overlap in symptoms between cortical and lacunar ischaemic stroke,18 and potential difficulty of recruiting pure lacunar syndrome populations on the basis of clinical syndrome and CT scanning in busy regional hospital stroke services. Nonetheless, it is reassuring that very few patients were found to have a primary cortical infarct on central expert scan read (3%), the remaining 97% having either a recent small subcortical (ie, acute lacunar) infarct as the primary cause of symptoms, or no definite visible relevant lacunar infarct but no alternative cause for their symptoms.
On adjudicated brain imaging, most participants (264, 73%) were judged to have a moderate to severe SVD score by the recruiting hospital, consistent with patients with lacunar ischaemic stroke, and demonstrating that a simplified version of the SVD score, suitable for use on CT or MRI, can be applied in busy stroke services.32
The trial is in follow-up and once this is completed, the database will be cleaned and locked. Therefore there may be minor changes in the baseline data between that provided here and in subsequent publications. Analysis will follow the SAP given here as a online supplemental file. If reasonable drug adherence and safety are confirmed, then a phase III trial will be designed and submitted for funding using the vascular and cognitive outcome event rates to power the next phase of the trial.
svn-2022-001816supp002.pdf (132.6KB, pdf)
Acknowledgments
We thank the patients and their relatives for their time and effort to participate in LACI-2; and the Trial Steering Committee, Sponsor, Data Monitoring Committee, Edinburgh Clinical Trials Unit staff, UK Clinical Research Network, Scottish Stroke Research Network, International Advisory Panel, and all staff at participating sites (listed in the protocol paper (Ref 17)), for their support. For further details on LACI 2 Investigator Group, refer to online supplemental file 2 in this article.
Footnotes
Contributors: JMW is the chief investigator, obtained ethics and regulatory approvals; PMB is Director of the Nottingham Stroke Trials Unit. KO is the current trial manager, responsible for daily running of the trial including regulatory compliance; FD is a Principle Investigator; AAM provides statistical expertise; IM and LJW are the trial statisticians; PMB drafted the manuscript; all other authors commented and edited it; all authors approved the final version for submission. JMW is the guarantor for the trial.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by British Heart Foundation (CS/15/5/31475), the Alzheimer’s Society (AS-PG-14–033), EU Horizon 2020 SVDs@Target (666881), MRC UK DRI, Fondation Leducq (16/05 CVD), NHS Research Scotland, The Stroke Association and Garfield-Weston Foundation, Chief Scientist Office (UC), and National Institute of Health Research. PMB is Stroke Association Professor of Stroke Medicine and an Emeritus NIHR Senior Investigator.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. Not applicable since main paper/results yet to be analysed and published.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by East Midlands Nottingham 2 Research Ethics Committee of the Health Research Authority number 17/EM/0077 on 10/05/2017. Participants gave informed consent to participate in the study before taking part.
References
- 1. Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol 2019. 10.1016/S1474-4422(19)30079-1 [DOI] [PubMed] [Google Scholar]
- 2. Bath PM, Wardlaw JM. Pharmacological treatment and prevention of cerebral small vessel disease: a review of potential interventions. Int J Stroke 2015;10:469–78. 10.1111/ijs.12466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3., Benavente OR, Hart RG, et al. , SPS3 Investigators . Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med 2012;367:817–25. 10.1056/NEJMoa1204133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4., Benavente OR, Coffey CS, et al. , SPS3 Study Group . Blood-Pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013;382:507–15. 10.1016/S0140-6736(13)60852-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Appleton JP, Scutt P, Sprigg N, et al. Hypercholesterolaemia and vascular dementia. Clin Sci 2017;131:1561–78. 10.1042/CS20160382 [DOI] [PubMed] [Google Scholar]
- 6. Bath PM, Hassall DG, Gladwin AM, et al. Nitric oxide and prostacyclin. divergence of inhibitory effects on monocyte chemotaxis and adhesion to endothelium in vitro. Arterioscler Thromb 1991;11:254–60. 10.1161/01.ATV.11.2.254 [DOI] [PubMed] [Google Scholar]
- 7. Leonardi-Bee J, Bath PMW, Bousser M-G, et al. Dipyridamole for preventing recurrent ischemic stroke and other vascular events: a meta-analysis of individual patient data from randomized controlled trials. Stroke 2005;36:162–8. 10.1161/01.STR.0000149621.95215.ea [DOI] [PubMed] [Google Scholar]
- 8. McHutchison C, Blair GW, Appleton JP, et al. Cilostazol for secondary prevention of stroke and cognitive decline: systematic review and meta-analysis. Stroke 2020;51:2374–85. 10.1161/STROKEAHA.120.029454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rajani RM, Quick S, Ruigrok SR, et al. Reversal of endothelial dysfunction reduces white matter vulnerability in cerebral small vessel disease in rats. Sci Transl Med 2018;10. doi: 10.1126/scitranslmed.aam9507. [Epub ahead of print: 04 07 2018]. [DOI] [PubMed] [Google Scholar]
- 10. Hasel P, Dando O, Jiwaji Z, et al. Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nat Commun 2017;8:15132. 10.1038/ncomms15132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sprigg N, Gray LJ, Bath PMW, et al. Stroke severity, early recovery and outcome are each related with clinical classification of stroke: data from the 'Tinzaparin in Acute Ischaemic Stroke Trial' (TAIST). J Neurol Sci 2007;254:54–9. 10.1016/j.jns.2006.12.016 [DOI] [PubMed] [Google Scholar]
- 12. Sprigg N, Gray LJ, Bath PMW, et al. Early recovery and functional outcome are related with causal stroke subtype: data from the tinzaparin in acute ischemic stroke trial. J Stroke Cerebrovasc Dis 2007;16:180–4. 10.1016/j.jstrokecerebrovasdis.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 13. Georgakis MK, Duering M, Wardlaw JM, et al. WMH and long-term outcomes in ischemic stroke: a systematic review and meta-analysis. Neurology 2019;92:e1298-e308. 10.1212/WNL.0000000000007142 [DOI] [PubMed] [Google Scholar]
- 14. Debette S, Schilling S, Duperron M-G, et al. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA Neurol 2019;76:81–94. 10.1001/jamaneurol.2018.3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McHutchison CA, Cvoro V, Makin S, et al. Functional, cognitive and physical outcomes 3 years after minor lacunar or cortical ischaemic stroke. J Neurol Neurosurg Psychiatry 2019;90:436–43. 10.1136/jnnp-2018-319134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wardlaw JM, Debette S, Jokinen H, et al. ESO guideline on covert cerebral small vessel disease. Eur Stroke J 2021;6:CXI–CLXII. 10.1177/23969873211012132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wardlaw J, Bath PMW, Doubal F, et al. Protocol: the lacunar intervention trial 2 (LACI-2). A trial of two repurposed licenced drugs to prevent progression of cerebral small vessel disease. Eur Stroke J 2020;5:297–308. 10.1177/2396987320920110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Potter G, Doubal F, Jackson C, et al. Associations of clinical stroke misclassification ('clinical-imaging dissociation') in acute ischemic stroke. Cerebrovasc Dis 2010;29:395–402. 10.1159/000286342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Makin SDJ, Doubal FN, Dennis MS, et al. Clinically confirmed stroke with negative diffusion-weighted imaging magnetic resonance imaging: longitudinal study of clinical outcomes, stroke recurrence, and systematic review. Stroke 2015;46:3142–8. 10.1161/STROKEAHA.115.010665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Appleton JP, Woodhouse LJ, Adami A, et al. Imaging markers of small vessel disease and brain frailty, and outcomes in acute stroke. Neurology 2020;94:e439–52. 10.1212/WNL.0000000000008881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blair GW, Appleton JP, Law ZK, et al. Preventing cognitive decline and dementia from cerebral small vessel disease: the LACI-1 trial. protocol and statistical analysis plan of a phase IIa dose escalation trial testing tolerability, safety and effect on intermediary endpoints of isosorbide mononitrate and cilostazol, separately and in combination. Int J Stroke 2018;13:530–8. 10.1177/1747493017731947 [DOI] [PubMed] [Google Scholar]
- 22. Appleton JP, Blair GW, Flaherty K, et al. Effects of isosorbide mononitrate and/or cilostazol on hematological markers, platelet function, and hemodynamics in patients with lacunar ischaemic stroke: safety data from the lacunar Intervention-1 (LACI-1) trial. Front Neurol 2019;10:723. 10.3389/fneur.2019.00723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blair GW, Appleton JP, Flaherty K, et al. Tolerability, safety and intermediary pharmacological effects of cilostazol and isosorbide mononitrate, alone and combined, in patients with lacunar ischaemic stroke: the lacunar Intervention-1 (LACI-1) trial, a randomised clinical trial. EClinicalMedicine 2019;11:34–43. 10.1016/j.eclinm.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blair GW, Janssen E, Stringer MS, et al. Effects of cilostazol and isosorbide mononitrate on cerebral hemodynamics in the LACI-1 randomized controlled trial. Stroke 2022;53:29–33. 10.1161/STROKEAHA.121.034866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gamble C, Krishan A, Stocken D, et al. Guidelines for the content of statistical analysis plans in clinical trials. JAMA 2017;318:2337–43. 10.1001/jama.2017.18556 [DOI] [PubMed] [Google Scholar]
- 26. Hemming K, Kearney A, Gamble C, et al. Prospective reporting of statistical analysis plans for randomised controlled trials. Trials 2020;21:898. 10.1186/s13063-020-04828-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. MacMahon S, Collins R. Reliable assessment of the effects of treatment on mortality and major morbidity, II: observational studies. The Lancet 2001;357:455–62. 10.1016/S0140-6736(00)04017-4 [DOI] [PubMed] [Google Scholar]
- 28. Clancy U, Gilmartin D, Jochems ACC, et al. Neuropsychiatric symptoms associated with cerebral small vessel disease: a systematic review and meta-analysis. Lancet Psychiatry 2021;8:225–36. 10.1016/S2215-0366(20)30431-4 [DOI] [PubMed] [Google Scholar]
- 29. Jackson CA, Hutchison A, Dennis MS, et al. Differing risk factor profiles of ischemic stroke subtypes: evidence for a distinct lacunar arteriopathy? Stroke 2010;41:624–9. 10.1161/STROKEAHA.109.558809 [DOI] [PubMed] [Google Scholar]
- 30. Jiménez-Sánchez L, Hamilton OKL, Clancy U, et al. Sex differences in cerebral small vessel disease: a systematic review and meta-analysis. Front Neurol 2021;12:756887. 10.3389/fneur.2021.756887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Del Bene A, Makin SDJ, Doubal FN, et al. Variation in risk factors for recent small subcortical infarcts with infarct size, shape, and location. Stroke 2013;44:3000–6. 10.1161/STROKEAHA.113.002227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Staals J, Makin SDJ, Doubal FN, et al. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014;83:1228–34. 10.1212/WNL.0000000000000837 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2022-001816supp001.pdf (1.9MB, pdf)
svn-2022-001816supp002.pdf (132.6KB, pdf)
Data Availability Statement
No data are available. Not applicable since main paper/results yet to be analysed and published.