Abstract
Objective
GGC repeat expansions in the human-specific NOTCH2NLC gene have been reported as the cause of neuronal intranuclear inclusion disease (NIID). Given the clinical overlap of cognitive impairment in NIID and cerebral small vessel disease (CSVD), both diseases have white matter hyperintensity on T2-fluid-attenuated inversion recovery sequences of brain MRI, and white matter hyperintensity is a primary neuroimaging marker of CSVD on MRI. Therefore, we hypothesised that the GGC repeat expansions might also contribute to CSVD. To further investigate the relationship between NOTCH2NLC GGC repeat expansions and CSVD, we performed a genetic analysis of 814 patients with the disease.
Methods
We performed a comprehensive GGC repeat expansion screening in NOTCH2NLC from 814 patients with sporadic CSVD. Their Fazekas score was greater than or equal to 3 points. Repeat-primed PCR and fluorescence amplicon length analyses were performed to identify GGC repeat expansions, and whole-exome sequencing was used to detect any pathogenic mutation in previously reported genes associated with CSVD.
Results
We identified nine (1.11%) patients with pathogenic GGC repeat expansions ranging from 41 to 98 repeats. The minor allele frequency of expanded GGC repeats in NOTCH2NLC was 0.55%.
Conclusion
Our findings suggest that intermediate-length and longer-length GGC repeat expansions in NOTCH2NLC are associated with sporadic CSVD. This provides new thinking for studying the pathogenesis of CSVD.
Keywords: cerebrovascular disorders, genetics, stroke
WHAT IS ALREADY KNOWN ON THIS TOPIC
GGC repeat expansions in the human-specific NOTCH2NLC gene have been reported as the cause of neuronal intranuclear inclusion disease (NIID). Given the clinical overlap of cognitive impairment in NIID and cerebral small vessel disease (CSVD), both have white matter hyperintensity on T2-fluid-attenuated inversion recovery of MRI. Therefore, we speculate that GGC repeat expansions also play a role in CSVD.
WHAT THIS STUDY ADDS
NOTCH2NLC GGC repeat expansions were not rare in the studied Han Chinese population with CSVD. Our findings suggest that intermediate-length and longer-length GGC repeat expansions in NOTCH2NLC are associated with sporadic CSVD. Our findings expand the scope of NIID-related disease.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our findings provide new insights into the molecular pathogenesis of CSVD, which provides clues to explore the mechanism of the disease. The mechanism of how abnormal GGC repeat expansion in the NOTCH2NLC gene increases the risk of CSVD remains unclear, and further study to establish suitable models is needed.
Introduction
Cerebral small vessel disease (CSVD) affects arterioles, arteries, capillaries and venules in the brain.1 Although the pathogenesis of CSVD remains unclear, genetics is currently recognised as one important explanatory factor.2 Genome-wide cerebral association studies have identified several susceptible genetic loci associated with CSVD in various genetic backgrounds.3–5 Some Mendelian forms of CSVD have been shown to be caused by some specific genetic mutations, such as NOTCH3 mutations in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy,6 HTRA1 mutations in cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy7 and COL4A1 & COL4A2 mutations in COL4A-related CSVD.8 9
Recently, GGC repeat expansions in the human-specific NOTCH2NLC gene were reported as a cause of neuronal intranuclear inclusion disease (NIID).10 11 It is noteworthy that a significant fraction of adult-onset NIID presents leukoencephalopathy on T2-weighted MRI scans and fluid-attenuated inversion recovery (FLAIR), implicating severe white matter hyperintensities (WMHs) as an important NIID indicator.12–14 Dementia is also a major clinical manifestation of sporadic NIID.15 16 Consider that WMH is a primary neuroimaging marker of CSVD on MRI,1 and CSVD is a major vascular dementia and cognitive dysfunction contributor.17 Therefore, we speculate that GGC repeat expansions also play a role in CSVD. We performed repeat-primed polymerase chain reaction (RP-PCR) and fluorescence amplicon length analyses to further investigate the relationship between GGC repeat expansions in NOTCH2NLC and CSVD.
Materials and methods
Enrolment
We recruited 814 sporadic Han Chinese patients with CSVD from the Neurology Department of the First Affiliated Hospital of Zhengzhou University between March 2018 and December 2021. The diagnosis was performed by neurologists and neuroimaging.18 All the patients in our study underwent a neurological examination by two experienced neurologists. The inclusion criteria were as follows: (1) age ≥18 years and (2) a Fazekas score19 ≥3 points for the degree of leukoencephalopathy. The exclusion criteria were as follows: (1) acute cerebral infarction lesions (lesions >20 mm in diameter); (2) acute cerebral haemorrhage, cerebral vascular malformation due to cerebral aneurysm, history of subarachnoid haemorrhage or untreated cerebral aneurysm (diameter >3 mm); (3) dementia due to neurodegenerative disease; (4) single-gene CSVD with known mutations; (5) with obvious non-vascular white matter lesions, such as metabolic encephalopathy, adult white matter dysplasia and multiple sclerosis; (6) a diagnosis of mental illness according to the Diagnostic and Statistical Manual of Mental Disorders V, tumours in the brain, traumatic brain injury and intracranial infection. In addition, two neurologists examined and recruited 1134 individuals matched for age and geography from the First Affiliated Hospital of Zhengzhou University as healthy control subjects. The Ethics Committee of the First Affiliated Hospital of Zhengzhou University approved this study.
Statistical analyses
Our study recruited a total of 814 patients with CSVD and collected the demographic data, clinical features and imaging features of these patients, grouped them according to patients with GGC repeat expansion and without GGC repeat expansion in NOTCH2NLC, and performed a statistical analysis using SPSS V.19.0 software. Count data are expressed as frequencies. For the measurement data that conformed to the normal distribution, we used the independent sample t-test; for the measurement data that did not conform to the normal distribution, we used the Mann-Whitney U test; for qualitative dichotomous variables, when T≥5, we used Pearson’s χ2 test; when T<5 but T≥1, we used the continuity-adjusted χ2 test; when T<1, we used Fisher’s exact test. P<0.05 was regarded for statistical significance.
Magnetic resonance imaging
The Magnetic resonance imaging (MRI) assessment of the CSVD was independently assessed by two radiologists. When there was disagreement, two radiologists consulted and decided. Finally, we selected patients with CSVD who met the inclusion and exclusion criteria. Evaluation of T1WI, T2WI, T2-FLAIR, susceptibility-weighted imaging (SWI) and diffusion-weighted imaging (DWI) scans of patients with CSVD were performed using Siemens magnetom Vida 3.0 T and PHILIPS Ingenia CX 3.0 T MRI. The parameters of Siemens magnetom Vida 3.0 T are T1WI: repetition time (TR) 200.0 ms, echo time (TE) 2.46 ms, slice interval 1.5 mm, slice thickness 5.0 mm, scanning time 28 s; T2WI: TR 4000.0 ms, TE 107 ms, slice interval 1.5 mm, slice thickness 5.0 mm, scanning time 48 s; T2-FLAIR: TR 6500.0 ms, TE 82 ms, slice interval 1.5 mm, slice thickness 5.0 mm, scanning time 1 min 18 s; SWI: TR 25.0 ms, TE 18.0 ms, slice interval 0.3 mm, slice thickness 1.5 mm, scanning time 2 min 43 s; DWI: TR 2800.0 ms, TE 68.0 ms, layer interval 1.5 mm, slice thickness 5.0 mm, scanning time 53 s. The parameters of PHILIPS Ingenia CX 3.0 T are T1WI: TR 250 ms, TE 2.3 ms, slice interval 6 mm, slice thickness 6 mm, scanning time 42 s; T2WI: TR 1873 ms, TE 70 ms, slice interval 6 mm, slice thickness 6 mm, scanning time 49 s; T2-FLAIR: TR 6500 ms, TE 130 ms, slice interval 1.5 mm, slice thickness 6 mm, scanning time 1 min 38 s; SWI: TR 31 ms, TE 7.2 ms, slice interval 0.5 mm, slice thickness 2 mm, scanning time 2 min 21 s; DWI: TR 1999 ms, TE 67 ms, slice interval 0.5 mm, slice thickness 6 mm, scanning time 24 s. WMH was assessed with brain MRI T2- FLAIR sequence, and cerebral microbleeds was assessed with brain MRI SWI sequence. The severity of WMH was assessed using the Fazekas scale.19
DNA extraction and genetic analysis
We collected peripheral blood samples from the patients and extracted genomic DNA using DNA extraction kits (#DP1101, Bio Teke, Beijing, China) according to standard protocols. We performed RP-PCR and fluorescence amplicon length analyses.10 20 In the patients screened for GGC repeat expansions, we performed whole-exome sequencing (WES) to detect any pathogenic mutations in previously reported genes associated with CSVD.20
Results
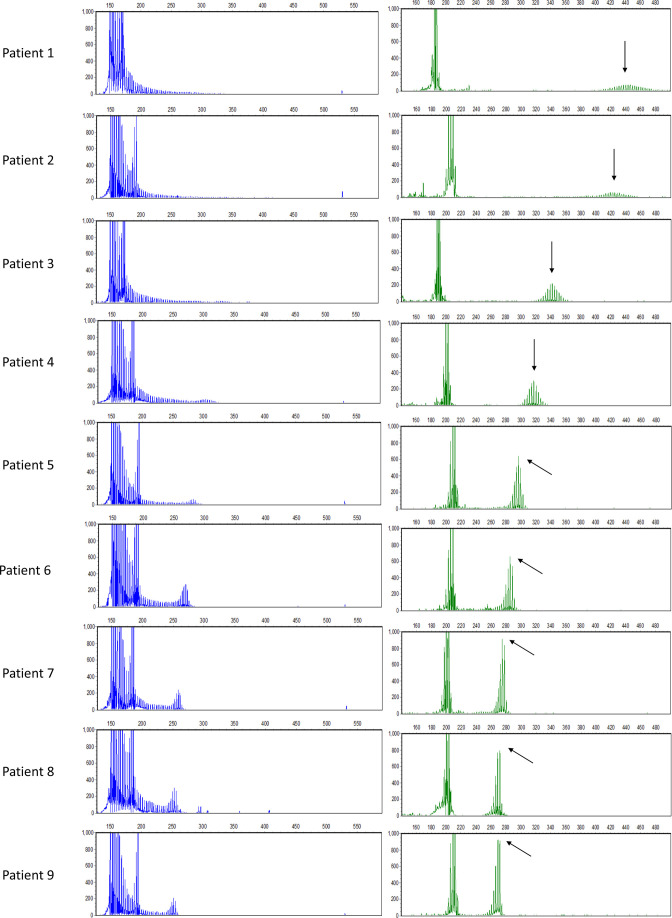
In our study, we screened for GGC repeat expansions in 814 patients with CSVD and found 9 patients with repeat expansions ranging from 41 to 98, which means 9 out of 814*2 (0.55%) NOTCH2NLC alleles in patients with CSVD were found harbouring the expanded NOTCH2NLC GGC repeats; the minor allele frequency of expanded GGC repeats was 0.55%. The exact repeat numbers in the nine patients were 98, 92, 65, 57, 50, 46, 43, 42 and 41 units, as revealed by the fluorescence amplicon length analysis (figure 1). Previously, we screened for the GGC repeat expansion in the NOTCH2NLC gene in the 1134 healthy controls matched for age and geography.20 All 1134 healthy controls were found harbouring fewer than 40 GGC repeats. After comparison of demographic, clinical and neuroradiological characteristics between the two groups, only speech impairment was found to be significant between the two groups in table 1. The nine patients’ demographic and clinical characteristics with expanded GGC repeats are shown in table 2. The nine patients’ mean age at symptom onset was 59.33±10.07 years. The main complaints of all nine patients were mild neurological symptoms, such as dizziness, headache and limb weakness. Two of the patients had decreased muscle strength, and one patient had decreased limb sensation. Five had a history of stroke, for which cerebral infarction predominated. Patient 3 and patient 5 had a family history of stroke. The most prominent risk factors for cerebrovascular accidents were hypertension and hyperhomocysteinaemia.
Figure 1.
Repeat-primed polymerase chain reaction and fluorescence repeat length analysis of patients harbouring GGC repeat expansions in NOTCH2NLC. The right graphs are the electropherograms of the product of fluorescence repeat length analysis. The highest signal peak of the expanded allele is indicated by a black arrow.
Table 1.
Comparison of demographic, clinical and neuroradiological characteristics between the patients with cerebral small vessel disease with GGC repeat expansion in NOTCH2NLC and those without GGC repeat expansion
| Variables | Patients without GGC repeat expansion (n=805) | Patients with GGC repeat expansion (n=9) | P value |
| Age of onset (year) | 60.86±10.43 | 59.33±10.07 | 0.664 |
| Sex (male) | 427 (53.0%) | 5 (55.6%) | 0.881 |
| Muscle weakness | 175 (21.7%) | 3 (33.3%) | 0.666 |
| Sensory disturbance | 87 (10.8%) | 1 (11.1%) | 1.000 |
| Headache | 123 (15.3%) | 1 (11.1%) | 1.000 |
| Dizzy | 342 (42.5%) | 5 (55.6%) | 0.430 |
| Vision blurred | 50 (6.2%) | 0 (0.0%) | 1.000 |
| Speech impairment | 60 (7.5%) | 3 (33.3%) | 0.024* |
| Stroke | 288 (35.8%) | 5 (55.6%) | 0.219 |
| Hypertension | 542 (67.3%) | 6 (66.7%) | 0.966 |
| Diabetes mellitus | 157 (19.5%) | 1 (11.1%) | 0.834 |
| Hyperlipaemia | 267 (33.2%) | 1 (11.1%) | 0.297 |
| Hyperhomocysteinaemia | 230 (28.6%) | 4 (44.4%) | 0.499 |
| Coronary artery disease | 85 (10.6%) | 1 (11.1%) | 1.000 |
| Family history/stroke | 137 (17.1%) | 2 (22.2%) | 0.655 |
| White matter hyperintensity | 805 (100.0%) | 9 (100.0%) | NA |
| Lacunar infarcts | 582 (72.3%) | 7 (77.8%) | 0.715 |
| Enlarged perivascular spaces | 506 (62.9%) | 5 (55.6%) | 0.652 |
| Brain atrophy | 75 (9.3%) | 2 (22.2%) | 0.458 |
Data represent the mean±SD or the number (%) of subjects. For the measurement data that conform to the normal distribution, use the independent sample t test; for the measurement data that does not conform to the normal distribution, use the Mann-Whitney U test; for qualitative dichotomous variables, when T ≥ 5, use Pearson's χ2 test; T < 5 but T ≥ 1 use continuity-adjusted χ2 test; and T < 1 use Fisher's exact test. P<0.05 was considered statistically significant,
*Represents significant.
NA, not available.
Table 2.
Clinical characteristics of the patients harbouring GGC repeat expansion in NOTCH2NLC
| Patient | Sex | Age at onset | Repeat number | Initial symptoms | Duration | Cerebrovascular accident | Muscle weakness | Sensory disturbance | ||
| Stroke | Risk factors | Family history/stroke | ||||||||
| 1 | M | 62 | 98 | Dizziness | 1 Year | – | HHcy | – | – | – |
| 2 | F | 66 | 92 | Dizziness | 1 Year | CI, ICH | HTN | – | – | – |
| 3 | F | 64 | 65 | Dizziness | 10 Years | – | HTN, HL | + | – | – |
| 4 | M | 62 | 57 | Weakness of the limb | 2 Months | CI | HL, DM | – | + | + |
| 5 | M | 37 | 50 | Speech impairment | 2 Months | CI | HTN, HHcy | + | – | – |
| 6 | F | 48 | 46 | Dizziness, Headache | 10 Days | – | HTN | – | – | – |
| 7 | M | 63 | 43 | Speech impairment | 4 Months | CI | – | – | + | – |
| 8 | F | 67 | 42 | Speech impairment, Weakness of the limb |
5 Months | CI | HTN, HHcy, CAD | – | + | – |
| 9 | M | 65 | 41 | Dizziness | 6 Days | – | HTN, HHcy | – | ||
‘–’ indicates absent and ‘+’ indicates present.
CAD, coronary artery disease; CI, cerebral infarction; DM, diabetes mellitus; F, female; HHcy, hyperhomocysteinaemia; HL, hyperlipaemia; HTN, hypertension; ICH, intracerebral haemorrhage; M, male.;
Comparing the clinical features between the patients with CSVD with Fazekas score ≥5 (n=189) and Fazekas score ≤4 (n=616) in patients without GGC repeat expansion (online supplemental table 1), we found significant differences between the two groups in demographic characteristics, clinical manifestations and imaging findings. The patients in the Fazekas score ≥5 group were older, and there were relatively more male patients. Regarding clinical manifestations, the patients in the Fazekas score ≥5 group had limb weakness and stroke more frequently than the Fazekas score ≤4 group, but the patients had fewer headache and dizziness than the Fazekas score ≤4 group. As for risk factors, the patients in the Fazekas score ≥5 group had more diabetes and hyperlipidaemia than the Fazekas score ≤4 group. Finally, imaging findings showed that the patients in the Fazekas score ≥5 group had more severe lacunar infarction. These differences are strongly associated with a high white matter burden in CSVD.
svn-2022-001631supp002.pdf (81.3KB, pdf)
In patients 1, 2 and 3, who had the three most expanded GGC repeat numbers, moderate-to-severe deep and periventricular WMH were identified on T2-weighted FLAIR images of brain MRI (online supplemental figure 1). These three patients were also found to have much longer disease duration than those patients with CSVD with fewer than 60 GGC repeats in the NOTCH2NLC gene (table 2); their Fazekas score was the highest, > 4 points, and the only two cases of patients with brain atrophy were among them (table 3 and online supplemental figure 1); all three patients had moderate cognitive impairment, and patient 2 also had problems with anxiety and depression (table 4). The majority of patients had a Fazekas score of 3 points with intermediate-length repeat expansions (table 3 and online supplemental figure 1); among the other six patients, five patients had mild to moderate cognitive impairment, three patients had depression and anxiety problems and one patient had anxiety problems (table 4). Other typical neuroimaging markers of CSVD detected, including multiple lacunar infarcts, cerebral microbleeds and enlarged perivascular spaces, were not significant features in these nine patients (table 3).
Table 3.
Neuroradiological features of the patients harbouring GGC repeat expansion in NOTCH2NLC
| Patient | The Fazekas score of WMH | LI | CMB | EPS | BA | |
| Periventricular | Deep | |||||
| 1 | 2 | 2 | + | NA | + | + |
| 2 | 3 | 1 | – | + | – | + |
| 3 | 3 | 2 | + | + | + | – |
| 4 | 2 | 1 | + | – | + | – |
| 5 | 2 | 2 | + | + | – | – |
| 6 | 2 | 1 | – | NA | – | – |
| 7 | 2 | 1 | + | NA | + | – |
| 8 | 2 | 1 | + | + | + | – |
| 9 | 2 | 1 | + | NA | – | – |
‘+’ indicates present and ‘−’ indicates absent.
BA, brain atrophy; CMB, cerebral microbleeds; EPS, enlarged perivascular spaces; LI, lacunar infarcts; NA, not available; WMH, white matter hyperintensity.
Table 4.
Cognitive and psychological characteristics of the patients harbouring GGC repeat expansion in NOTCH2NLC
| Patient | MMSE* | MoCA† | TMT‡ | HADS§ | AD8¶ | BNT** | ||
| A | B | A | D | |||||
| 1 | 25 | 15 | 74 | 140 | 8 | 4 | 3 | 16 |
| 2 | 17 | 11 | 236 | NA | 18 | 11 | 2 | 12 |
| 3 | 25 | 17 | 87 | 156 | 5 | 4 | 3 | 15 |
| 4 | 26 | 18 | 61 | NA | 9 | 13 | 4 | 19 |
| 5 | 28 | 26 | 90 | 168 | 14 | 8 | 1 | 19 |
| 6 | 27 | 24 | 30 | NA | 1 | 6 | 1 | 20 |
| 7 | 18 | 11 | 152 | NA | 3 | 4 | 4 | 18 |
| 8 | 27 | 25 | 156 | 330 | 10 | 8 | 7 | 16 |
| 9 | 27 | 24 | 42 | 72 | 8 | 4 | 0 | 19 |
*MMSE: a score of 27–30 indicates normal; 21–26 indicates mildly impaired cognitive function; 10–20 indicates moderately impaired cognitive function; 0–9 indicates severely impaired cognitive function.
†MoCA: a score below 26 suggests impaired cognitive function.
‡TMT: the results of both TMT A and B were recorded as the number of seconds required to complete the test, a TMT A score higher than 78 reveals deficient cognitive function, a TMT B score higher than 273 reveals deficient cognitive function.
§HADS: HADS-A, evaluating anxiety; HADS-D, evaluating depression. A score of 0–7 indicates asymptomatic (normal and mild); 8–10 indicates anxiety or depression probably present (moderate); 11–21 indicates anxiety or depression definitely present (severe).
¶AD8: a score higher than or equal to 2 suggests possible cognitive impairment.
**BNT: 20-items version.
AD8, Ascertain Dementia 8-item Questionnaire; BNT, Boston naming test; HADS, Hospital Anxiety and Depression Scale; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; NA, not available; TMT A&B, Trial Making Test A and B.
svn-2022-001631supp001.pdf (323KB, pdf)
Discussion
Our study set out to assess the relationship between expanded GGC repeats in NOTCH2NLC and CSVD. We identified nine patients with pathogenic expanded GGC repeats in NOTCH2NLC, from 41 to 98 repeats. WES failed to identify any pathogenic mutations in previously reported CSVD-related genes in any of these patients.21 Moreover, eight of them had definite risk factors for cerebrovascular accidents, and none presented any of the typical symptoms of adult-onset NIID. Brain MRI scans of these patients did not reveal a high symmetrical signal in the corticomedullary junctions on DWI.19
The minor allele frequency of expanded GGC repeats in NOTCH2NLC was 0.55%, considerably lower than in patients with NIID, but closer to that in a cohort of patients with dementia.10 11 Given that CSVD is a major factor leading to vascular dementia,17 both NIID and CSVD have WMH on T2-FLAIR sequence, and we speculate that NOTCH2NLC GGC repeat expansions contribute to CSVD. It is worth mentioning that Jiao et al showed that the mean repeat size in patients with vascular dementia was 19.76±5.33 units, with 16 units (4/37, 10.8%) being the most frequent repeat size,21 which might be explained by the small sample size and absence of healthy controls. In our previous study,20 GGC repeats in 1134 healthy individuals ranged from 6 to 39 units, with a mean repeat size of 18.80±5.07. We defined the number of GGC repeat expansions ranging from 41 to 60 as intermediate-length expansions; besides, the number of GGC repeats in the control group was fewer than 40 times in the vast majority of studies on NOTCH2NLC expanded GGC repeats reported.10 11 22 23 Therefore, we considered 41 as a critical value for pathogenic repeat number in patients with CSVD.
In our study, brain MRI of all patients with CSVD carrying pathogenic expanded GGC repeats showed moderate to severe WMH based on the Fazekas scale. Although the typical symmetrical high signal in corticomedullary junctions on DWI is an indicator for NIID diagnosis,19 severe WMH on T2-FLAIR brain MRI scans could be seen in almost all patients who had dementia-dominant NIID. Furthermore, a recent study found that expanded NOTCH2NLC GGC repeats are a leading cause of adult leukoencephalopathy. This disease was characterised by WMH on T2-FLAIR images with no known genetic aetiology in a Japanese cohort.14 Significant WMH on T2-FLAIR images were also observed in three expanded GGC repeat carriers after screening for such GGC repeat expansions in a Chinese AD cohort.21 Furthermore, our study found that patients 1, 2, 3, 4 and 5, who had the five most expanded GGC repeat numbers among the RP-PCR-positive patients, presented with relatively severe WMH on T2-FLAIR brain MRI scans. All these findings agree with the hypothesis that WMH on T2-FLAIR images might be a neuroradiologic marker of NOTCH2NLC GGC repeat expansions, which is expected to become a breakthrough point in exploring the relationship between NOTCH2NLC GGC repeat expansions and CSVD.
In the sporadic adult form of NIID, dementia is the most prominent symptom.13 16 Several previous studies reported that NOTCH2NLC expanded GGC repeats were found in AD-affected families and patients with sporadic AD. Affected patients harbouring a relatively large number of repeats usually had older age of onset and presented with typical and severe dementia symptoms.11 21 Consistent with this, the nine patients developed cognitive dysfunctions, anxiety and depression problems with different degrees of severity. In fact, growing research has revealed the potential role of vascular pathology in AD progression, especially in older individuals.24 As we know, CSVD is a major vascular dementia and cognitive dysfunction contributor,17 and our study found that expanded GGC repeats in NOTCH2NLC could partly explain either neurodegenerative or vascular dementia. Of course, further research is required to investigate the specific pathogenicity of NOTCH2NLC GGC repeat expansions in patients with CSVD.
Liu et al reported that NIID accounts for one-fifth of patients with adult-onset genetically undiagnosed non-vascular leukoencephalopathies in Taiwan.25 The patients with NIID they identified were found harbouring 73–323 GGC repeats in the NOTCH2NLC gene. However, our study investigated the relationship between CSVD and GGC repeat expansion, and the patients who were NOTCH2NLC-positive CSVD we identified were found to be harbouring relatively smaller NOTCH2NLC GGC repeats (41–100), especially the 41–60 GGC repeats, which we termed the ‘intermediate-length’ repeats.20 Thus, our study mainly demonstrated the relationship between CSVD and fewer than 100 GGC repeats.
In our study, the GGC repeat expansion in the NOTCH2NLC gene might act as a risk factor for CSVD, a cluster of vascular disorders. The mechanisms by which the GGC repeats in the NOTCH2NLC gene lead or are associated with vascular injury remain elusive. However, ubiquitin-positive intranuclear inclusions and p62-positive have been identified in the blood vessels of patients with NIID.26 Moreover, fragile X-associated tremor/ataxia syndrome (FXTAS), which is attributed to a CGG repeat expansion in another gene (FMR1), has highly overlapping clinical and radiological features with NIID.27 Intranuclear inclusions have been identified in the endothelial cells of capillaries in patients with FXTAS, which were thought to lead to cerebrovascular dysfunction.28 Taking all these factors together, we speculate that the GGC repeat expansion may lead to cerebrovascular injury via the deposition of p62-positive and ubiquitin-positive intranuclear inclusions and the ensuing dysfunction in the endothelial cells of capillaries.
Skin or other tissue biopsy is the only useful tool for the antemortem diagnosis of NIID.29 In clinical practice, there are currently no unified diagnostic criteria in NIID. The diagnosis is mainly based on typical clinical manifestations combined with the high symmetrical signal in the corticomedullary junctions on DWI to determine a suspicious diagnosis. The clinical features of NIID are variable, with the most common involvement of the central, peripheral and autonomic nerves. Unfortunately, none of these nine patients with CSVD with the GGC repeat expansion agreed to accept further examination, including a skin biopsy. The nine patients with repeat expansions only had symptoms in the central nervous system. Besides, there were no typical manifestations of NIID on imaging; that is, brain MRI scans of these nine patients with CSVD did not reveal a high symmetrical signal in the corticomedullary junctions on DWI.19 In addition, by asking the parents and children of these patients about their physical conditions, and investigating their family history of disease, we found no suspected medical history.
GGC repeat expansions have been found in NIID, adult-onset leukoencephalopathy, essential tremor disease and other diseases.11 14 20 30–33 Therefore, a study proposes to define the term NIID-related disease (NIIDRD) as referring to a series of diseases, such as NIID and other diseases caused by GGC repeat expansion in the NOTCH2NLC gene. Our findings suggest that GGC repeat expansion is also associated with CSVD, which expands the scope of NIIDRD. Screening for the GGC repeat expansion in the NOTCH2NLC gene needs to be conducted in more patients with CSVD to clarify the relationship between GGC repeats and CSVD. Moreover, the blood vessel organoids of CSVD patients who had NOTCH2NLC-positive are an excellent model to explore the mechanisms underlying NOTCH2NLC GGC repeats-induced vascular dysfunction.34
Conclusion
NOTCH2NLC GGC repeat expansions were not rare in the studied Han Chinese population with CSVD. Our findings suggest that the intermediate-length and longer-length GGC repeat expansions in NOTCH2NLC are associated with sporadic CSVD. Our findings provide new insights into the molecular pathogenesis of CSVD, which provides clues to explore the mechanism of the disease. This study has certain limitations: the patients came from a single recruitment centre, and this study only recruited patients with CVSD with a Fazekas score of WMH ≥3 points on neuroimaging. Unfortunately, the nine patients refused to accept further clinical re-evaluation. The mechanism of how abnormal GGC repeat expansion in the NOTCH2NLC gene increases the risk of CSVD remains unclear, and further study to establish suitable models is needed.
Footnotes
Y-cW and YF contributed equally.
Contributors: YW, YF, YX and C-HS contributed to the conception and design of the study. W-KY, SS, J-DL, L-LY, Z-CZ, S-SL and YD contributed to collecting clinical data of the patients. YW and YF contributed to the DNA extraction and genetic analysis. YW, YF, YG, YJ and Y-SL contributed to the analysis of data. YW contributed to drafting and critical revision of the manuscript. YX is responsible for the overall content as guarantor. All authors critically reviewed the manuscript and approved it as submitted.
Funding: This work was supported by the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences [grant number 2020-PT310-01], the National Natural Science Foundation of China to Dr. Chang-he Shi [grant number 82171247, 81974211], the National Natural Science Foundation of China to Dr. Yu-ming Xu [grant numbers U1904207].
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University. Reference number: 2021-KY-1059-002. Participants gave informed consent to participate in the study before taking part.
References
- 1. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9:689–701. 10.1016/S1474-4422(10)70104-6 [DOI] [PubMed] [Google Scholar]
- 2. Choi JC. Genetics of cerebral small vessel disease. J Stroke 2015;17:7–16. 10.5853/jos.2015.17.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kubo M, Hata J, Ninomiya T, et al. A nonsynonymous SNP in PRKCH (protein kinase C eta) increases the risk of cerebral infarction. Nat Genet 2007;39:212–7. 10.1038/ng1945 [DOI] [PubMed] [Google Scholar]
- 4. Fornage M, Debette S, Bis JC, et al. Genome-Wide association studies of cerebral white matter lesion burden: the charge Consortium. Ann Neurol 2011;69:928–39. 10.1002/ana.22403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Woo D, Falcone GJ, Devan WJ, et al. Meta-Analysis of genome-wide association studies identifies 1q22 as a susceptibility locus for intracerebral hemorrhage. Am J Hum Genet 2014;94:511–21. 10.1016/j.ajhg.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joutel A, Vahedi K, Corpechot C, et al. Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients. Lancet 1997;350:1511–5. 10.1016/S0140-6736(97)08083-5 [DOI] [PubMed] [Google Scholar]
- 7. Hara K, Shiga A, Fukutake T, et al. Association of HTRA1 mutations and familial ischemic cerebral small-vessel disease. N Engl J Med 2009;360:1729–39. 10.1056/NEJMoa0801560 [DOI] [PubMed] [Google Scholar]
- 8. Vahedi K, Boukobza M, Massin P, et al. Clinical and brain MRI follow-up study of a family with COL4A1 mutation. Neurology 2007;69:1564–8. 10.1212/01.wnl.0000295994.46586.e7 [DOI] [PubMed] [Google Scholar]
- 9. Gunda B, Mine M, Kovács T, et al. Col4A2 mutation causing adult onset recurrent intracerebral hemorrhage and leukoencephalopathy. J Neurol 2014;261:500–3. 10.1007/s00415-013-7224-4 [DOI] [PubMed] [Google Scholar]
- 10. Sone J, Mitsuhashi S, Fujita A, et al. Long-Read sequencing identifies GGC repeat expansions in NOTCH2NLC associated with neuronal intranuclear inclusion disease. Nat Genet 2019;51:1215–21. 10.1038/s41588-019-0459-y [DOI] [PubMed] [Google Scholar]
- 11. Tian Y, Wang J-L, Huang W, et al. Expansion of human-specific GGC repeat in neuronal intranuclear inclusion disease-related disorders. Am J Hum Genet 2019;105:166–76. 10.1016/j.ajhg.2019.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sone J, Kitagawa N, Sugawara E, et al. Neuronal intranuclear inclusion disease cases with leukoencephalopathy diagnosed via skin biopsy. J Neurol Neurosurg Psychiatry 2014;85:354–6. 10.1136/jnnp-2013-306084 [DOI] [PubMed] [Google Scholar]
- 13. Sone J, Mori K, Inagaki T, et al. Clinicopathological features of adult-onset neuronal intranuclear inclusion disease. Brain 2016;139:3170–86. 10.1093/brain/aww249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okubo M, Doi H, Fukai R, et al. GGC Repeat Expansion of NOTCH2NLC in Adult Patients with Leukoencephalopathy. Ann Neurol 2019;86:962–8. 10.1002/ana.25586 [DOI] [PubMed] [Google Scholar]
- 15. Munoz-Garcia D, Ludwin SK. Adult-Onset neuronal intranuclear hyaline inclusion disease. Neurology 1986;36:785–90. 10.1212/WNL.36.6.785 [DOI] [PubMed] [Google Scholar]
- 16. Araki K, Sone J, Fujioka Y, et al. Memory loss and frontal cognitive dysfunction in a patient with adult-onset neuronal intranuclear inclusion disease. Intern Med 2016;55:2281–4. 10.2169/internalmedicine.55.5544 [DOI] [PubMed] [Google Scholar]
- 17. Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013;12:483–97. 10.1016/S1474-4422(13)70060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–38. 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sugiyama A, Sato N, Kimura Y, et al. Mr imaging features of the cerebellum in adult-onset neuronal intranuclear inclusion disease: 8 cases. AJNR Am J Neuroradiol 2017;38:2100–4. 10.3174/ajnr.A5336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shi C-H, Fan Y, Yang J, et al. NOTCH2NLC intermediate-length repeat expansions are associated with Parkinson disease. Ann Neurol 2021;89:182–7. 10.1002/ana.25925 [DOI] [PubMed] [Google Scholar]
- 21. Jiao B, Zhou L, Zhou Y, et al. Identification of expanded repeats in NOTCH2NLC in neurodegenerative dementias. Neurobiol Aging 2020;89:142.e1–142.e7. 10.1016/j.neurobiolaging.2020.01.010 [DOI] [PubMed] [Google Scholar]
- 22. Ishiura H, Shibata S, Yoshimura J, et al. Noncoding CGG repeat expansions in neuronal intranuclear inclusion disease, oculopharyngodistal myopathy and an overlapping disease. Nat Genet 2019;51:1222–32. 10.1038/s41588-019-0458-z [DOI] [PubMed] [Google Scholar]
- 23. Deng J, Gu M, Miao Y, et al. Long-read sequencing identified repeat expansions in the 5'UTR of the NOTCH2NLC gene from Chinese patients with neuronal intranuclear inclusion disease. J Med Genet 2019;56:758–64. 10.1136/jmedgenet-2019-106268 [DOI] [PubMed] [Google Scholar]
- 24. Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol 2015;11:157–65. 10.1038/nrneurol.2015.10 [DOI] [PubMed] [Google Scholar]
- 25. Liu YH, Chou YT, Chang FP, et al. Neuronal intranuclear inclusion disease in patients with adult-onset non-vascular leukoencephalopathy. Brain 2022;145:3010–21. 10.1093/brain/awac135 [DOI] [PubMed] [Google Scholar]
- 26. Chen H, Lu L, Wang B, et al. Re-defining the clinicopathological spectrum of neuronal intranuclear inclusion disease. Ann Clin Transl Neurol 2020;7:1930–41. 10.1002/acn3.51189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ng ASL, Xu Z, Chen Z, et al. NOTCH2NLC-linked neuronal intranuclear inclusion body disease and fragile X-associated tremor/ataxia syndrome. Brain 2020;143:e69. 10.1093/brain/awaa210 [DOI] [PubMed] [Google Scholar]
- 28. Salcedo-Arellano MJ, Wang JY, McLennan YA, et al. Cerebral microbleeds in fragile X-associated tremor/ataxia syndrome. Mov Disord 2021;36:1935–43. 10.1002/mds.28559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sone J, Tanaka F, Koike H, et al. Skin biopsy is useful for the antemortem diagnosis of neuronal intranuclear inclusion disease. Neurology 2011;76:1372–6. 10.1212/WNL.0b013e3182166e13 [DOI] [PubMed] [Google Scholar]
- 30. Sun Q-Y, Xu Q, Tian Y, et al. Expansion of GGC repeat in the human-specific NOTCH2NLC gene is associated with essential tremor. Brain 2020;143:222–33. 10.1093/brain/awz372 [DOI] [PubMed] [Google Scholar]
- 31. Hayashi T, Katagiri S, Mizobuchi K, et al. Heterozygous GGC repeat expansion of NOTCH2NLC in a patient with neuronal intranuclear inclusion disease and progressive retinal dystrophy. Ophthalmic Genet 2020;41:93–5. 10.1080/13816810.2020.1723119 [DOI] [PubMed] [Google Scholar]
- 32. Fang P, Yu Y, Yao S, et al. Repeat expansion scanning of the NOTCH2NLC gene in patients with multiple system atrophy. Ann Clin Transl Neurol 2020;7:517–26. 10.1002/acn3.51021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jih K-Y, Chou Y-T, Tsai P-C, et al. Analysis of NOTCH2NLC GGC repeat expansion in Taiwanese patients with amyotrophic lateral sclerosis. Neurobiol Aging 2021;108:210–2. 10.1016/j.neurobiolaging.2021.07.011 [DOI] [PubMed] [Google Scholar]
- 34. Wimmer RA, Leopoldi A, Aichinger M, et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 2019;565:505–10. 10.1038/s41586-018-0858-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2022-001631supp002.pdf (81.3KB, pdf)
svn-2022-001631supp001.pdf (323KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.