Abstract
Background
The benefit of stroke thrombectomy for large infarct core still lacks robust randomised controlled studies.
Aim
To demonstrate the design of a clinical trial on endovascular therapy for acute anterior circulation large vessel occlusion (LVO) patients with large infarct core volume.
Design
ANGEL-ASPECT is a multicentre, prospective, randomised, open-label, blinded End-point trial to evaluate whether best medical management (BMM) combined with endovascular therapy improves neurological functional outcomes as compared with BMM alone in acute LVO patients with Alberta Stroke Program Early CT Score (ASPECTS) of 3–5 on non-contrast CT or infarct core volume range of 70–100 mL (defined as rCBF <30% on CT perfusion or ADC <620 on MRI) up to 24 hours from symptom onset or last seen well.
Study outcomes
The primary efficacy outcome is 90 (±7) days modified Rankin Scale. Symptomatic intracranial haemorrhage within 48 hours from randomisation is the primary safety outcome.
Discussion
The ANGEL-ASPECT trial will screen patients with large infarct core (ASPECTS 3–5 or 70–100 mL) through image evaluation criteria within 24 hours and explore the efficacy and safety of endovascular therapy compared with BMM.
Keywords: Thrombectomy, Stroke
What is already known on this topic
Whether thrombectomy is benefit for patients with large infarct core is still controversial.
What this study adds
This protocol demonstrated the rationale and design of ANGEL-ASPECT.
How this study might affect research, practice or policy
ANGEL-ASPECT trial will produce objective data on whether best medical management combined with endovascular therapy improves neurological outcome for acute large vessel occlusion patients with large infarct core compared with best medical management alone.
Introduction and rationale
Endovascular therapy (EVT) has now become a standard strategy for patients with acute large vascular occlusion. However, large infarct core volume is excluded from the therapy guideline of patient with acute stroke.1 Currently, the approved imaging inclusion criteria for the selection of patient is limited to Alberta Stroke Program Early CT Scores (ASPECTS) score ≥6 within 6 hours or the criteria set by the studies of diffusion-weighted imaging (DWI) or CT perfusion (CTP) Assessment With Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention (DAWN) and The Endovascular Therapy Following Imaging Evaluation for Ischaemic Stroke (DEFUSE 3) criteria in 6–16 or 24 hours.1 2 However, whether thrombectomy is of benefit for patients out of the guidelines is still controversial.3 4
HERMES collaboration found that EVT showed benefit compared with control group for patients with ASPECTS 0–45 and core volume ≥70 mL defined by CTP, or DWI MRI.6 The 90 day modified Rankin Scale (mRS) of 0–2 was 25% in EVT group vs 14% in control for patients with an ASPECT score of 0–4.5
Many studies showed benefit of thrombectomy for large infarct core volume defined by low ASPECTS or large CTP or ADC volume.3 7–10 Recently, the Recovery by Endovascular Salvage for Cerebral Ultra-Acute Embolism–Japan Large Ischemic Core Trial (RESCUE-Japan LIMIT)11 showed patients with large infarct core (ASPECTS 3–5) benefits form EVT compared with medical management alone but EVT is associated with more intracranial haemorrhages (ICH).
The benefit is obvious when narrowed the scope to low ASPECTS 3–5 or large infarct core volume 70–100 mL. The Optimising Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) trial showed that large infarct core patients may benefit from endovascular treatment, especially when treated in early time window and with a infarct core volume within 100 mL. HERMES study only had small percent of patients with a infarct core volumes for more than 100 mL.7 Based on these evidences, we designed the combination imaging selection criteria for ANGEL-ASPECT and defined large infarct core volume using low ASPECTS or large CTP or ADC volume. ANGEL-ASPECT trial will include patients with non-contrast CT (NCCT) ASPECTS 3–5 and perfusion core volume 70–100 mL when ASPECTS <3 / > 5 (6–24 hours). This combination imaging inclusion criteria allows us to include the maximum of patients with a potential benefit large infarct core for whom EVT is not recommended with level 1 evidence under current guidelines.
Methods
Hypothesis
Best medical management (BMM) combined with EVT might be superior to BMM alone in acute anterior circulation large vessel occlusive (LVO) patients with a large infarct core volume.
Design and patient population
ANGEL-ASPECT study is a multicentred, prospective, randomised, open-label, blinded End-point (PROBE) trial. Patients with acute LVO of middle cerebral artery (M1 segment), and/or distal internal carotid artery (ICA) (intracranial segment), determined by CT angiography (CTA) or MR angiography (MRA), and who meet eligibility criteria and do not meet exclusion criteria will be considered for enrolment at 46 sites in China (online supplemental table 1). Box 1 lists the inclusion criteria and exclusion criteria.
Box 1. Summary of inclusion and exclusion criteria.
Inclusion criteria
Centre inclusion criteria
(1) Equipped with emergency department and neurology department for patients who had a stroke.
(2) Equipped with stroke team operating on 24/7.
(3) Capable of endovascular treatment and intravenous thrombolysis for acute ischaemic stroke patients.
Clinical inclusion criteria:
(1) Age 18–80 years.
(2) Presenting with symptoms consistent with acute ischaemic stroke.
(3) Prestroke modified Rankin Scale score 0–1.
(4) National Institute of Health Stroke Scale (NIHSS) score 6–30 at the time of randomisation.
(5) Randomisation can be finished within 24 hours of stroke onset (stroke onset time is defined as last known well time).
(6) Informed consent signed.
Neuroimaging inclusion criteria:
(1) CT angiography or MR angiography proved occlusion of internal carotid artery terminal or M1 segment of middle cerebral artery.
(2) Combination of non-contrast CT (NCCT) ASPECTS and perfusion core volume when ASPECTS <3 or > 5 (6–24 hours). Imaging evidence of low ASPECTS (based on NCCT) or large infarct Core (defined as rCBF <30% on CT perfusion or ADC <620 on MRI) filling one of the following criteria:
(1) ASPECTS 3–5.
(2) ASPECTS>5 (6–24 hours) with infarct core volume 70–100 mL, to catch the patients who may have true large infarct core but missed by misinterpreted upper limit ASPECTS.
(3) ASPECTS <3 with infarct core volume 70–100 mL, to catch the patients who may have true large volume core with range of ASPECTS 3–5 but missed by misinterpreted lower limit ASPECTS.
Exclusion criteria
Centre exclusion criteria
(1) Centres in which the number of acute ischaemic stroke cases treated with endovascular procedures are less than 20 per year.
(2) Incapable of complying with the protocol to proceed with the research.
Clinical exclusion criteria
(1) Females who are pregnant, or those of childbearing, potential with positive urine or serum beta Human Chorionic Gonadotropin test.
(2) Known severe allergy (more severe than skin rash) to contrast agents uncontrolled by medications.
(3) Refractory hypertension that is difficult to be controlled by drugs (defined as persistent systolic blood pressure >185 mm Hg or diastolic blood pressure >110 mm Hg).
(4) Known haemorrhagic tendency (including but not limited to): baseline platelet count <100×109/L /L; heparin was administered within 48 hours with APTT ≥35 s; on anticoagulant therapy with warfarin and international normalised ratio (INR) >1.7 (patients with no history or suspected coagulopathy do not need to wait for laboratory results of INR or APTT prior to enrolment).
(5) Parenchymal organ surgery and biopsy were performed in the past 1 month.
(6) Any active bleeding or recent bleeding (gastrointestinal bleeding, urinary bleeding, etc) in the past 1 month.
(7) Undergoing haemodialysis or peritoneal dialysis; known severe renal insufficiency with glomerular filtration rate <30 mL/min or serum creatinine >220 mmol/L (2.5 mg/dL mg/dL).
(8) Brain tumour (with mass effect).
(9) The expected survival time is less than 1 year year (such as complicated with malignant tumour, serious heart and lung diseases).
(10) Participation in other interventional randomised clinical trials that may confound outcome assessment of the trial.
(11) Other circumstances that the investigator considers inappropriate for participation in the trial or that may pose significant risks to patients (such as inability to understand and/or follow the study procedures and/or follow-up due to mental disorders, cognitive or emotional disorders).
Specific neuroimaging exclusion criteria
(1) Midline shift or herniation, mass effect with effacement of the ventricles.
(2) Evidence of acute intracranial haemorrhage.
(3) Acute bilateral strokes or multiple intracranial vessels occlusions.
svn-2022-001865supp001.pdf (182.4KB, pdf)
Randomisation
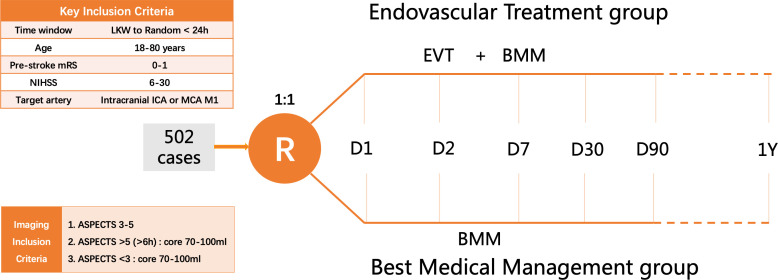
The random code will be generated through a central network randomisation system with 24 hours real-time randomisation online based on the simple randomisation method. The researcher in each centre will obtain the random code from the central network randomisation system according to the enrolment order, and patients who meet the inclusion criteria and obtain informed consent will be randomly assigned to the following treatment groups in a 1:1 ratio (figure 1):
Figure 1.
Study design: randomisation algorithm. ASPECTS, Alberta Stroke Program Early CT Score; EVT, endovascular therapy; ICA, internal carotid artery; mRS, modified Rankin Scale; NIHSS, National Institute of Health Stroke Scale; MCA, middle cerebral artery.
BMM plus EVT group: patients will receive EVT on the basis of BMM, with stent retriever thrombectomy or contact aspiration thrombectomy preferred for EVT.
BMM group: patients will receive the BMM alone.
Intervention
Endovascular therapy
When the patient’s condition permits, local anaesthesia is the first choice for rapid initiation of arterial puncture and EVT. If the condition requires, sedation can be used, and intubation can be considered for patients at high risk of airway collapse. If the patient is expected to have poor intraoperative cooperation even with sedation or is at high risk of using sedation or airway conditions due to the patient’s illness, general anaesthesia should be used. Return to the neurointensive care unit with intubation or not should be determined according to the surgical results.
Systemic heparinisation is not recommended for preoperative and intraoperative treatment. Femoral artery is suggested for arterial puncture, and long sheath, guiding catheter or balloon guiding catheter can be used. Stent retriever (Solitaire, EMBOTRAP, Reco, Captor and other first-line stent retriever systems) and contact aspiration (Penumbra aspiration system and other first-line aspiration system) are recommended as the first choice for thrombectomy.
All the operations should be performed using devices approved by the National Medical Products Administration and should be performed in accordance with the approved intended use and operating instructions.
Best medical management
All enrolled patients should receive BMM in accordance with the recommendation of Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders.12 This includes intravenous thrombolysis therapy for patients meeting the guidelines. Patients who meet the criteria should receive intravenous thrombolysis therapy according to the guidelines, while informed consent and screening are still available for patients who have completed intravenous thrombolysis. Patients who plan to undergo or are undergoing intravenous thrombolysis therapy will continue or terminate intravenous thrombolysis therapy after enrolment according to the investigator’s judgement. Patients who had completed intravenous thrombolysis prior to randomisation were also eligible for enrolment in this trial. All patients are recorded with the name, dosage, and time of intravenous thrombolysis drugs in detail. Antiplatelet agents are not recommended within 24 hours after intravenous thrombolysis unless the patient has undergone balloon dilatation or stent implantation, at which time the antithrombotic strategy is determined by the onsite investigator. Non-intravenous thrombolysis patients will be treated with aspirin, unless an indication for early anticoagulation is present.
Imaging protocol
Baseline imaging
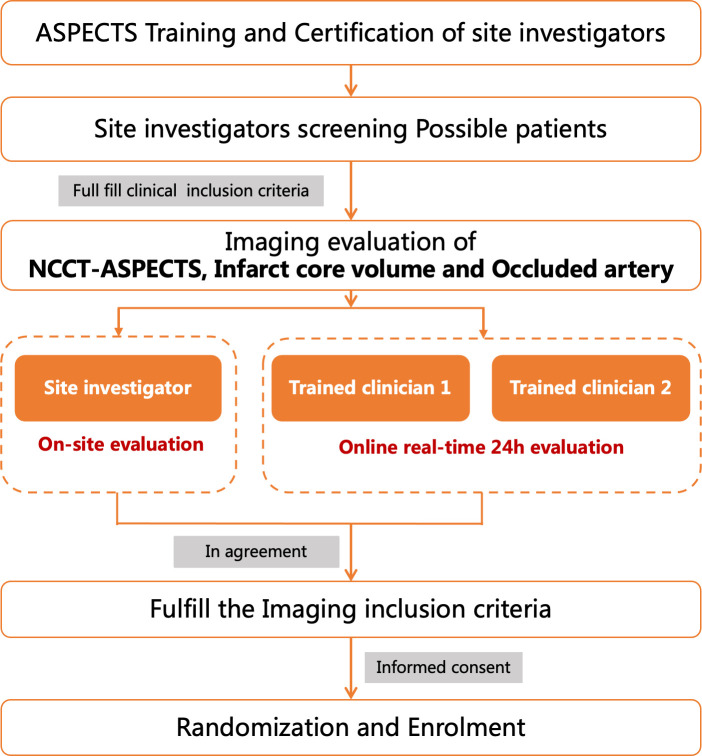
All investigators were trained on the imaging protocol and use of RAPID software, and participated in the network training, simulation test and examination of NCCT-ASPECTS before enrolment. ASPECTS training and test is conducted through the online training system of the trial website (http://angel-aspect.org). Those who pass the exam (>80 points) will obtain the ASPECTS assessment qualification certificate and be qualified for imaging assessment. During imaging screening, researchers in the subcentre with imaging evaluation qualifications and two trained neuroradiologists from the trial team will conduct real-time online image evaluation of ASPECTS, occlusion site, infarct core volume to ensure the accuracy of imaging evaluation (figure 2). The infarct core volume was automatically evaluated by iSchemaView automated RAPID software (V.5.0.4, iSchemaView, California, USA), and the infarction core volume is defined as relative cerebral blood volume (rCBF) <30% based on CTP or apparent diffusion coefficient (ADC) <620 based on MRI. Occlusion site was evaluated by CTA or MRA. Collateral status was evaluated by CTA or MRA and also by HIR in Tmax of CTP.
Figure 2.
Imaging evaluation working flow. ASPECTS, Alberta Stroke Program Early CT Score.
Primary outcomes
Ninety days (±7 days) mRS.
Secondary outcomes
Ninety days (±7 days) mRS 0–2.
Ninety days (±7 days) mRS 0–3.
Thirty-six hours (±12 hours) NIHSS 0–1 or decrease ≥10 from baseline.
Infarct core volume change from baseline, at 7 days (±1 day) or at discharge assessed with NCCT or at 36 hours (±12 hours) assessed with MRI.
Thirty-six hours (±12 hours) target artery recanalisation rate assessed with CTA or MRA.
Safety outcomes
Symptomatic ICH (sICH) within 48 hours from randomisation (Heidelberg Bleeding Classification) is the primary safety endpoint of this trial.13 Secondary safety outcomes include the following events: All-cause mortality within 90 days (±7 days); any ICH within 48 hours from randomisation (Heidelberg Bleeding Classification); decompressive hemicraniectomy during hospitalisation.
Data safety and monitoring board
The data safety and monitoring board (DSMB) will have meetings within scheduled time and monitor the progress of trial to guarantee the trial in accordance with the standards of ethics and ensure the patient safety. The DSMB is constituted by committee members of academic areas and independent statistician. All the DSMB members should not be involved in the implementation of the trial. Before the enrolment of the trial, a DSMB charter should be confirmed by all the DSMB members and executive committee members. This DSMB charter should include the membership, role and DSMB responsibilities. During the DSMB meeting, the DSMB members will generate recommendations and the DSMB chair will hand over to steering committee right away after the meeting.
Sample size
In this study, a multicentre, open, randomised, parallel control design method was used. The primary measure of efficacy was mRS score at 90±7 days after randomisation (considered as ordered variable). According to the literature data and clinical experts’ opinions, the parameters were set as follows: (1) The proportion of mRS score 0–6 in control group was 3%, 4%, 10%, 17%, 16%, 12% and 38%, respectively; (2) The average treatment effect of EVT improved the outcome with the common OR value for improvement of mRS reached 1.73; (3) Two interim analyses were planned. Adjusted level α=0.046 (two sided) and power 1−β=0.90 and (4) The sample size was allocated to the EVT group and the control group in a 1:1 ratio. Based on these parameters, the total sample size was 452. Considering 10% attrition rate, the final total sample size was 502 cases, 251 cases in each group.
Interim analyses will take place when 1/3 (168 cases) and 2/3 (336 cases) have completed a 3-month follow-up. O’Brien-Fleming boundaries will be used at the interim analysis with alpha of two-sided 0.0002 (stage 1), 0.0123 (stage 2) and 0.046 (stage 3, final analysis). PASS software (NCSS, V.11) was used to calculate the sample size.
Statistical analyses
Data will be analysed both based on intention-to-treat principle in main analysis and in per-protocol set for sensitivity analysis. T-test or Wilcoxon rank-sum test will be used for comparison between continuous variables, and χ2 tests, Fisher’s exact test or Wilcoxon sum-rank test will be used for comparison between categorical variables. For primary efficacy endpoint, an ordinal logistic regression model will be used to calculate the common OR as well as their 95% CIs. A two-sided with p<0.046 was considered significant for primary outcome. For secondary efficacy endpoints like 90-day mRS 0–2 will be analysed using a binary logistic regression model. The infarct core volume change from baseline will be analysed by using Student’s t-test or Wilcoxon rank-sum test as appropriate. χ2 test and Fisher’s exact test will be used to compare the differences in the incidence of adverse events and serious adverse events between the two groups. Ahead of the lockdown of data and the breaking of code, a final SAP will be issued. All analyses will be performed using SAS software, V.9.4 (SAS Institute) and two sided with p<0.05 will be considered significant.
Study organisation
The steering committee will have meeting twice a year to oversight the trial and give strategic input. Safety outcomes, adverse events and serious adverse events are adjusted by clinical events adjudication committee. Imaging are adjudicated by an independent imaging core lab (Tiantan Neuroimaging Center of Excellence). Trained assessors will adjudicate the effective outcomes and all the data are masked to treatment assignment. The DSMB will have meetings within scheduled time and monitor the progress of trial to guarantee the trial in accordance with the standards of ethics and ensure the patient safety. The committees are provided in online supplemental table 2.
Discussion
Thrombectomy for acute large vascular occlusion patient with small infarct core is highly recommended by most guidelines. Mounting evidence indicates that thrombectomy has potential benefits for patients with low ASPECTS of 3–5 or large infarct core volumes from 70 mL to 100 mL. Currently, many randomised controlled trials are exploring the efficacy and safety of EVT for acute LVO patients with large infarct core volume.4
The first completed trial RESCUE-JAPAN LIMIT (NCT03702413) showed that thrombectomy is benefit in 90 days mRS 0–3 for patients with low ASPECT score.11 However, its population was significantly biased by DWI-ASPECTS and patients with good mismatch defined by DWI/ fluid-attenuated inversion recovery (FLAIR) signal (no early FLAIR signal change) beyond 6 hours, and more than 70% of the cases were treated within 6 hours. Trials with different criteria and sample size with more cases are needed to identify the efficacy and safety of thrombectomy for LVO patients with a large infarct core based on NCCT ASPECTS or volume within the early and extended time window. Many ongoing trials are trying to address the benefit of EVT with large infarct core: Efficacy and Safety of Thrombectomy in Stroke With Extended Lesion and Extended Time Window (TENSION; NCT03094715),14 In Extremis Large Stroke Therapy Evaluation–ASPECT 0–5 (NCT03811769), Thrombectomy for Emergent Salvage of Large Anterior Circulation Ischemic Stroke (NCT03805308), A Randomized Controlled Trial to Optimize Patient’s SELECT 2 (NCT03876457),15 ANGEL-ASPECT (NCT04551664) and Stroke in patients with large Ischaemic Core: Assessment of Reperfusion therapy Impact on Outcome (SICARIO). The information of registered trials was showed in online supplemental table 3.
ANGEL-ASPECT trial is a PROBE study initiated by researchers to explore the effectiveness and safety of EVT in patients with anterior circulation LVO with ASPECTS 3–5 or infarction core volume 70–100 mL.
In this trial, we limited the range of ASPECT score and infarct core volume. We expect to decrease risks while exploring the benefits of thrombectomy for patients with large core infarction. A combination of NCCT ASPECTS and perfusion core volume when ASPECTS <3 or > 5 (6–24 hours) was used as imaging inclusion criteria. The primary imaging inclusion criteria of ANGEL-ASPECT are NCCT ASPECT score 3 to 5, and the infarct core volume 70–100 mL is used as auxiliary inclusion criteria when ASPECTS <3 or > 5 (6–24 hours). There is no unified definition of large core infarction, so this complex imaging criteria allows us to expand the potential benefit to as many patients as possible. More precisely, the imaging inclusion criteria for ANGEL-ASPECT are: (1) If NCCT ASPECTS is 3–5 and presentation is within 24 hours of onset, patients are enrolled without limitation of infarct core volume for patients with NCCT ASPECTS 3–5 is the key target population. (2) The patients with NCCT ASPECTS 0–2 and core infarction volume 70–100 mL determined by CTP or DWI MRI are also included considering the potential benefit of the volume and subjective nature of ASPECTS. 3. The patients with NCCT ASPECTS >5, between 6 and 24 hours of onset, and infarct core volume 70–100 mL, which are beyond the infarct core volume of DAWN and DEFUSE 3 criteria are also enrolled.
The benefits of expanding the time window for patients with low ASPECTS are worth expecting, so the time window of ANGEL-ASPECT is 24 hours. Subgroup analysis will focus on age, wake-up stroke, last known well to random time, NIHSS score, thrombolysis, occlusion site, ipsilateral ICA occlusion, ASPECT score, infarct volume and stroke type.
There are conflicts on definition of large infarct core16 17 and on whether patients with ASPECTS 3–5 and CTP or ADC volume <70 mL can be considered as having large infarct core.4 We know that if a patient has a low ASPECT score but a favourable core volume on CTP, EVT may be benefit for him.10 This benefit has been previously evidenced by many high-quality researches18 19 although these studies included some patients with an ASPECT score less than 6. For infarct core volume, CTP could offer a more objective measurement than ASPECT score, but this has not yet been confirmed in a high-quality trial. In our trial, we may include patients with low ASPECT score and favourable CTP-defined infarct core volume, and this allows for the comparison of these two imaging criteria. When a patient’s imaging is evaluated by both ASPECT score and CTP defined infarct volume, the further subgroup analysis maybe very helpful in clarifying the mechanism if EVT is found to be benefit for large infarct core.
The goal of ANGEL-ASPECT trial is to include the maximum patients with a potential benefit large infarct core volume for whom EVT is not recommended with level 1 evidence under current guidelines.4 The combination imaging inclusion criteria with NCCT ASPECTS and perfusion core volume when ASPECTS <3 />5 aims to include more potential large core volume based on ASPECTS and uses perfusion as further screening to include maximal potential benefit patients.
Summary and conclusions
ANGEL-ASPECT trial will produce objective data on whether BMM combined with EVT improves neurological outcomes for patients with large infarct core volume in acute anterior circulation LVO compared with BMM alone.
Footnotes
Twitter: @Xiaochuan Huo, @dliebesk, @VitorMendesPer1
XH and GM contributed equally.
Contributors: ZM, ZR and VMP designed the study; XH drafted the manuscript; YW, DSL, YW, LL, XZ, XT, DS and GM provided critical comments/revisions of the manuscript.
Funding: This study was funded by Covidien Healthcare International Trading (Shanghai) Co., Ltd., Johnson & Johnson MedTech, Genesis MedTech (Shanghai) Co.,Ltd. and Shanghai HeartCare Medical Technology Co., Ltd.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The ANGEL-ASPECT trial was approved by ethics committee at Beijing Tiantan Hospital (IRB approval number: KY2020-072-02) and all participating centres. Participants gave informed consent to participate in the study before taking part.
References
- 1. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/american stroke association. Stroke 2019;50:e344–418. 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 2. Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO)- European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg 2019;11:535–8. 10.1136/neurintsurg-2018-014568 [DOI] [PubMed] [Google Scholar]
- 3. Meyer L, Bechstein M, Bester M, et al. Thrombectomy in extensive stroke may not be beneficial and is associated with increased risk for hemorrhage. Stroke 2021;52:3109–17. 10.1161/STROKEAHA.120.033101 [DOI] [PubMed] [Google Scholar]
- 4. Ren Z, Huo X, Ma G, et al. Selection criteria for large core trials: rationale for the ANGEL-ASPECT study design. J Neurointerv Surg 2022;14:107–10. 10.1136/neurintsurg-2021-017798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Román LS, Menon BK, Blasco J, et al. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data. Lancet Neurol 2018;17:895–904. 10.1016/S1474-4422(18)30242-4 [DOI] [PubMed] [Google Scholar]
- 6. Campbell BCV, Majoie CBLM, Albers GW, et al. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol 2019;18:46–55. 10.1016/S1474-4422(18)30314-4 [DOI] [PubMed] [Google Scholar]
- 7. Sarraj A, Hassan AE, Savitz S, et al. Outcomes of endovascular thrombectomy vs medical management alone in patients with large ischemic cores: a secondary analysis of the optimizing patient's selection for endovascular treatment in acute ischemic stroke (select) study. JAMA Neurol 2019;76:1147–56. 10.1001/jamaneurol.2019.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seners P, Oppenheim C, Turc G, et al. Perfusion imaging and clinical outcome in acute ischemic stroke with large core. Ann Neurol 2021;90:417–27. 10.1002/ana.26152 [DOI] [PubMed] [Google Scholar]
- 9. Kerleroux B, Janot K, Hak JF, et al. Mechanical thrombectomy in patients with a large ischemic volume at presentation: systematic review and meta-analysis. J Stroke 2021;23:358–66. 10.5853/jos.2021.00724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bouslama M, Barreira CM, Haussen DC, et al. Endovascular reperfusion outcomes in patients with a stroke and low aspects is highly dependent on baseline infarct volumes. J Neurointerv Surg 2022;14:117–21. 10.1136/neurintsurg-2020-017184 [DOI] [PubMed] [Google Scholar]
- 11. Yoshimura S, Sakai N, Yamagami H, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med 2022;386:1303–13. 10.1056/NEJMoa2118191 [DOI] [PubMed] [Google Scholar]
- 12. Liu L, Chen W, Zhou H, et al. Chinese stroke association guidelines for clinical management of cerebrovascular disorders: Executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc Neurol 2020;5:159–76. 10.1136/svn-2020-000378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. von Kummer R, Broderick JP, Campbell BCV, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke 2015;46:2981–6. 10.1161/STROKEAHA.115.010049 [DOI] [PubMed] [Google Scholar]
- 14. Bendszus M, Bonekamp S, Berge E, et al. A randomized controlled trial to test efficacy and safety of thrombectomy in stroke with extended lesion and extended time window. Int J Stroke 2019;14:87–93. 10.1177/1747493018798558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sarraj A, Hassan AE, Abraham M, et al. A randomized controlled trial to optimize patient's selection for endovascular treatment in acute ischemic stroke (SELECT2): study protocol. Int J Stroke 2022;17:689–93. 10.1177/17474930211035032 [DOI] [PubMed] [Google Scholar]
- 16. Jadhav AP, Hacke W, Dippel DWJ, et al. Select wisely: the ethical challenge of defining large core with perfusion in the early time window. J Neurointerv Surg 2021;13:497–9. 10.1136/neurintsurg-2021-017386 [DOI] [PubMed] [Google Scholar]
- 17. Sarraj A, Campbell B, Ribo M, et al. Selection criteria for large core trials: dogma or data? J Neurointerv Surg 2021;13:500–4. 10.1136/neurintsurg-2021-017498 [DOI] [PubMed] [Google Scholar]
- 18. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–18. 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–18. 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2022-001865supp001.pdf (182.4KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.