Abstract
Background and purpose
The Treat Stroke to Target trial has confirmed the benefit of targeting low-density lipoprotein cholesterol (LDL-C) of <1.8 mmol/L in patients who had an ischaemic stroke (IS). However, haemorrhagic risk brought by this target level (<1.8 mmol/L) or even lower level (<1.4 mmol/L) of LDL-C should also be concerned. In this study, we aimed to demonstrate whether low LDL-C could increase the intracranial haemorrhage risk following IS.
Methods
Patients who had an IS from China Stroke Center Alliance programme with complete baseline information were prospectively enrolled. 793 572 patients who had an IS were categorised into 6 groups according to LDL-C level (<1.40 mmol/L, 1.40–1.79 mmol/L, 1.80–2.59 mmol/L, 2.60–2.99 mmol/L, 3.00–4.89 mmol/L, ≥4.90 mmol/L). The study outcome was defined as intracranial haemorrhage identified during hospitalisation. Logistic regression model was used to examine the association between different LDL-C levels and risk of intracranial haemorrhage.
Results
Compared with patients of LDL-C=1.80–2.59 mmol/L, both subgroups of LDL-C<1.40 mmol/L and LDL-C=1.40–1.79 mmol/L showed significantly higher risk of intracranial haemorrhage (OR=1.26, 95% CI=1.18 to 1.35; OR=1.22, 95% CI=1.14 to 1.30, respectively). Interaction effect was found to exist between the subgroups of intravenous thrombolytic therapy (p=0.04), rather than the subgroups of age, sex and body mass index. Moreover, the sensitivity analyses indicated that even patients who had an IS with minor stroke still suffered from the increased intracranial haemorrhage risk related to low LDL-C level.
Conclusions
Among patients who had an IS, the low LDL-C level (<1.4 mmol/L or <1.8 mmol/L) at baseline is associated with increased risk of intracranial haemorrhage during acute stage. While actively lowering LDL-C level for patients who had an IS, clinicians should also concern about the haemorrhagic risk associated with low LDL-C level.
Keywords: Stroke, Hemorrhage, Risk Factors
Introduction
For secondary prevention, patients who had an ischaemic stroke (IS) have been recommended to receive lipid-lowering therapy to reduce the recurrence risk, with evidence from the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) study.1 Previously, it remained controversial about the target level of low-density lipoprotein cholesterol (LDL-C) control in secondary prevention for IS of atherosclerotic origin.2 Recently, data from Treat Stroke to Target trial demonstrated that to stipulate patients who had an IS with a target LDL-C level of less than 1.8 mmol/L (70 mg/dL) can significantly reduce risk of subsequent cardiovascular events than those with a target range of 2.4–2.9 mmol/L (90–110 mg/L).3 Furthermore, guidelines for lipid management also updated new target value for LDL-C lowering therapy of 1.4 mmol/L (55 mg/dL) for patients with high cardiovascular risk.4 5
Therefore, it is plausible to speculate a more strict target level than 1.8 mmol/L for secondary prevention of patients who had an IS. However, conflicting opinions still exist and several studies have indicated that the aggressive cholesterol reduction may cause side effects, of which, intracranial haemorrhage, as the major haemorrhagic complication following acute IS, often causes clinical deterioration by aggravating brain tissue damage.6 Previously, one post-hoc analysis of the SPARCL study also demonstrated that haemorrhagic stroke was frequently observed in those treated with atorvastatin.7 Therefore, for patients who had an IS, it is necessary to elucidate the correlation between the low LDL-C level and risk of intracranial haemorrhagic events following IS. However, evidence on this clinically concerned matter remains limited.
In this study, we aimed to elucidate whether the low LDL-C level (<1.40 mmol/L, 1.40–1.79 mmol/L) may increase the risk of intracranial haemorrhage following IS, using the data from real world with nearly 800 000 participants.
Methods
Study cohort and participants
The China Stroke Center Alliance (CSCA) programme is a national-wide, hospital-based, multicenter, multifaceted, continuous, prospective registry and quality improvement initiative for stroke, the rational and design of which have been described in detail previously.8 The CSCA programme recruited a total of 1 006 798 patients diagnosed as IS, transient ischaemic attack (TIA), intracerebral haemorrhage or subarachnoid haemorrhage from 1955 hospitals across 31 provinces, autonomous regions or municipalities in mainland China. All participating hospitals have received healthcare quality assessments and research approval to collect data in CSCA, without requiring individual patient informed consent under the common rule or a waiver of authorisation and exemption from their institutional review board.
Eligibility criteria included age 18 years or older, primary diagnosis of acute stroke/TIA confirmed by CT or MRI of the brain, within 7 days of symptom onset and admitting to wards directly or through the emergency department. Potential participants with diagnosed cerebral venous sinus thrombosis or non-cerebrovascular diseases were excluded.8 In current study, we further excluded participants diagnosed with TIA (n=68 433), intracranial haemorrhage (n=85 499), subarachnoid haemorrhage (n=11 122), stroke not otherwise specified (n=13 015), patients with incomplete medical records (patients with missing LDL-C level (n=7530), patients with missing baseline information including blood pressure, fasting blood glucose, height and bodyweight (n=19 982), patients with the value of body mass index (BMI) exceeding the highest and lowest 0.5% scope (n=7568)). Online supplemental figure 1 shows details of inclusion and exclusion criteria for study patients in CSCA study.
svn-2022-001612supp001.pdf (747.2KB, pdf)
Clinical information and study endpoint
Data were collected via the web-based patient data collection and management tool (Medicine Innovation Research Center, Beijing, China). The information was chart reviewed, coded, deidentified and transported in a secure manner to ensure patient confidentiality in a national privacy standards way.
The following data were collected on admission: patient demographics, history of disease and medication, smoking status (current smokers, previous or non-smokers), BMI (calculated as weight in kilograms divided by height in metres squared), National Institutes of Health Stroke Scale (NIHSS) Score, history of medications and interventions (administered any of antiplatelets or anticoagulations), reperfusion strategy (with recombinant tissue plasminogen activator or urokinase), systolic blood pressure (mm Hg) and laboratory tests such as LDL-C. In-hospital outcomes and complications were also prospectively recorded. The detailed content of each category has been reported.8 Definitions of these medical complications were, in general, in accordance with definitions used in other studies.9 10 The study outcome was defined as intracranial haemorrhage during hospitalisation diagnosed by CT/MRI, including any bleeding within the intracranial vault,11 composed of haemorrhagic transformation (according to the European Cooperative Acute Stroke Study radiological classification),12 intracerebral haemorrhage and subarachnoid haemorrhage.
Statistical analysis
Patients who had an IS of CSCA were categorised into six groups according to the LDL-C levels at baseline (<1.40 mmol/L, 1.40–1.79 mmol/L, 1.80–2.59 mmol/L, 2.60–2.99 mmol/L, 3.00–4.89 mmol/L, ≥4.90 mmol/L), where the subgroup of LDL-C=1.80–2.59 mmol/L was defined as the reference group.4
Baseline characteristics were compared between groups categorised by LDL-C levels. For normally distributed data, continuous variables were analysed by the t-test, and results were presented as means with SD. For skewed data, continuous variables were analysed with the Wilcoxon rank-sum test, and results were presented as medians with IQR. Categorical variables were presented as proportions (n%) and tested by χ2 test or Fisher exact test. Logistic regression model was used to examine the association between different LDL-C levels and risk of intracranial haemorrhage. Patients who lacked of information on NIHSS Score at admission were defined as a separate subgroup with missing data of NIHSS in the multivariable adjustment. ORs with 95% CIs were calculated to measure the strength of the associations.
In addition, we performed subgroup analysis to test the interaction effect between age (<65 years vs ≥65 years at baseline), sex, BMI (<25 kg/m2 vs ≥25 kg/m2), intravenous thrombolytic therapy (yes vs no) and LDL-C, respectively, on intracranial haemorrhage risk, by logistic regression interaction models. We also conducted sensitivity analysis to elucidate whether low LDL-C level may still bring increased risk of intracranial haemorrhage in specific population, such as patients who had an IS without intravenous thrombolytic therapy and patients who had an IS with minor stroke (defined as NIHSS<4). All analyses were performed with SAS V.9.4.
Results
Overall, a total of 793 572 patients who had an IS with valid information were included in this study. Among them, a total of 12 349 patients who had an IS were identified to get intracranial haemorrhage during hospitalisation.
Table 1 summarises the baseline characteristics of the six subgroups defined by LDL-C levels (<1.40 mmol/L, 1.40–1.79 mmol/L, 1.80–2.59 mmol/L (reference group), 2.60–2.99 mmol/L, 3.00–4.89 mmol/L, ≥4.90 mmol/L). Generally, patients with lower LDL-C levels were older and more likely to be women. In addition, patients who had an IS with lower LDL-C were more likely to have history of atrial fibrillation/flutter. The online supplemental table 1 describes the baseline characteristics of the patients who had an IS included in and excluded from the current study.
Table 1.
Characteristics of patients who had an IS at baseline grouped by LDL-C levels
| Variable | LDL-C categories | P value | |||||
| 1.80–2.59 (Reference) | <1.40 | 1.40–1.79 | 2.60–2.99 | 3.00–4.89 | ≥4.90 | ||
| N | 249 333 | 50 991 | 67 587 | 129 870 | 270 793 | 29 886 | – |
| Age, years | 66.60 (±12.01) | 67.38 (±12.23) | 67.48 (±12.07) | 65.99 (±11.89) | 65.30 (±11.84) | 65.20 (±12.02) | |
| Women (%) | 85 778 (34.40%) | 16 889 (33.12%) | 22 133 (32.75%) | 47 631 (36.68%) | 112 794 (41.65%) | 13 435 (44.95%) | <0.01 |
| NIHSS | 3 (2.6) | 3 (2.7) | 3 (2.6) | 3 (2.6) | 3 (2.6) | 4 (2.8) | <0.01 |
| Current Smoking, n (%) | 60 647 (24.32%) | 10 668 (20.92%) | 15 322 (22.67%) | 32 772 (25.23%) | 65 923 (24.11%) | 5500 (18.40%) | <0.01 |
| Alcohol intake, % | 59 613 (23.91%) | 11 745 (23.03%) | 15 922 (23.56%) | 31 268 (24.08%) | 62 197 (22.97%) | 6529 (21.85%) | <0.01 |
| Hypertension, % | 158 739 (63.67%) | 33 117 (64.95%) | 44 174 (65.36%) | 83 492 (64.29%) | 176 779 (65.28%) | 18 670 (62.47%) | <0.01 |
| Diabetes mellitus, % | 50 629 (20.31%) | 12 628 (24.77%) | 15 261 (22.58%) | 26 347 (20.29%) | 59 802 (22.08%) | 7171 (23.99%) | <0.01 |
| Dislipidemia, % | 15 600 (6.26%) | 3709 (7.27%) | 4753 (7.03%) | 8304 (6.39%) | 25 550 (9.44%) | 3897 (13.04%) | <0.01 |
| Previous atrial fib/flutter, % | 14 923 (5.99%) | 4371 (8.57%) | 5449 (8.06%) | 6031 (4.64%) | 10 086 (3.72%) | 1137 (3.80%) | <0.01 |
| Dual antiplatelet therapy, % | 97 860 (39.25%) | 17 697 (34.71%) | 25 393 (37.57%) | 53 069 (40.86%) | 109 214 (40.33%) | 9658 (32.32%) | <0.01 |
| Intravenous thrombolytic therapy, % | 15 135 (6.07%) | 3099 (6.08%) | 3861 (5.71%) | 8134 (6.26%) | 16 494 (6.09%) | 1452 (4.86%) | <0.01 |
| SBP, mm Hg | 148.76 (±22.55) | 144.93 (±22.59) | 146.56 (±22.24) | 150.73 (±22.84) | 152.52 (±23.22) | 151.28 (±24.14) | <0.01 |
| Body mass index, kg/m2 | 23.70 (±3.18) | 23.55 (±3.17) | 23.58 (±3.17) | 23.87 (±3.19) | 24.04 (±3.24) | 24.01 (±3.64) | <0.01 |
LDL-C, low-density lipoprotein cholesterol; NIHSS, National Institutes of Health Stroke Scale; SBP, systolic blood pressure.
svn-2022-001612supp002.pdf (82.2KB, pdf)
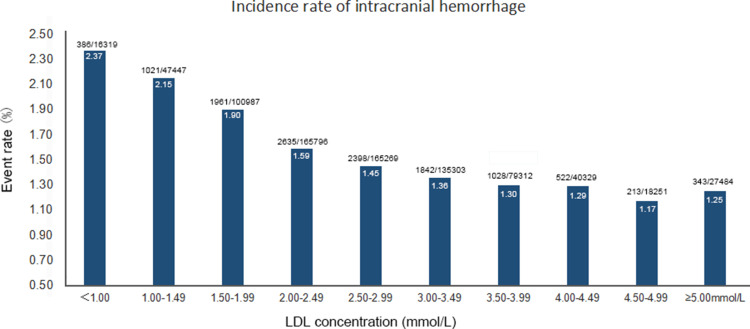
Figure 1 shows the event rates of intracranial haemorrhage across different ranges of LDL-C level (a 0.50 mmol/L reduction of each subgroup from ≥5.00 mmol/L to <1.00 mmol/L). In general, there was a continuous trend of increased intracranial haemorrhage with the reduction of LDL-C level, with the p for trend<0.01. Online supplemental figure 2 shows similar trends for event rates of haemorrhagic transformation, intracerebral haemorrhage and subarachnoid haemorrhage, across different ranges of LDL-C level.
Figure 1.
Event rates of intracranial haemorrhage across per 0.5 mmol/L of LDL-C. LDL-C, low-density lipoprotein cholesterol.
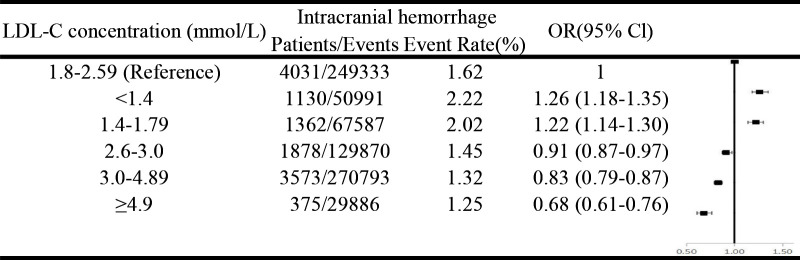
Figure 2 shows that after multivariable adjustment, compared with the patients of LDL-C=1.80–2.59 mmol/L, both subgroups of LDL-C<1.40 and LDL-C=1.40–1.79 showed significantly higher risk of intracranial haemorrhage (OR=1.26, 95% CI=1.18 to 1.35; OR=1.22, 95% CI=1.14 to 1.30, respectively). Online supplemental figure 3 shows similar results for haemorrhagic transformation, intracerebral haemorrhage and subarachnoid haemorrhage.
Figure 2.
ORs and 95% CIs of intracranial haemorrhage according to different low-density lipoprotein cholesterol (LDL-C) levels. Adjusted for age, sex, smoking, alcohol intake, National Institutes of Health Stroke Scale (NIHSS) (0–4; 5–15; 16–20; 21–42; missing NIHSS); medical history (hypertension; diabetes mellitus); history of anticoagulation; dual antiplatelet therapy; systolic blood pressure (per 20 mm Hg); fasting glucose; intravenous thrombolytic therapy; body mass index, kg/m2 (<18.5, 18.5–25, 25–30, ≥30). LDL-C, low-density lipoprotein cholesterol.
Table 2 shows the results of subgroup analyses. After adjusting for potential confounders, we observed that significant interaction effect exists between the subgroups of intravenous thrombolytic therapy (yes vs no) (p=0.04). However, age (<65 years, ≥65 years), sex and BMI (<25 kg/m2, ≥25 kg/m2) did not significantly modify the correlation between LDL-C level and risk of intracranial haemorrhage.
Table 2.
Subgroup analyses
| Reference 1.80–2.59 | <1.40 | 1.40–1.79 | 2.60–2.99 | 3.00–4.89 | ≥4.90 | P interaction | |
| (1) Age | |||||||
| Cases/population, event rate (%) | 1445/103 217, 1.40 | 365/19 540, 1.87 | 450/25 925, 1.74 | 678/56 717, 1.20 | 1413/125 079, 1.13 | 169/13 789, 1.23 | 0.20 |
| <65 years, OR (95% CI) | 1 | 1.21 (1.08 to 1.36) | 1.21 (1.09 to 1.35) | 0.87 (0.79 to 0.95) | 0.81 (0.75 to 0.87) | 0.74 (0.63 to 0.87) | |
| Cases/population, event rate (%) | 2586/146 116, 1.77 | 765/31 451, 2.43 | 912/41 662, 2.19 | 1200/73 153, 1.64 | 2160/145 714, 1.48 | 206/16 097, 1.28 | |
| ≥65 years OR (95% CI) | 1 | 1.28 (1.18 to 1.39) | 1.22 (1.13 to 1.32) | 0.95 (0.88 to 1.02) | 0.85 (0.80 to 0.90) | 0.64 (0.56 to 0.74) | |
| (2) Sex | |||||||
| Cases/population, event rate (%) | 2548/163 555, 1.56 | 736/34 102, 2.16 | 883/45 454, 1.94 | 1126/82 239, 1.37 | 1980/157 999, 1.25 | 215/16 451, 1.31 | 0.70 |
| Men, OR (95% CI) | 1 | 1.26 (1.16 to 1.37) | 1.21 (1.12 to 1.31) | 0.90 (0.84 to 0.96) | 0.82 (0.77 to 0.87) | 0.72 (0.63 to 0.83) | |
| Cases/population, event rate (%) | 1483/85 778, 1.73 | 394/16 889, 2.33 | 479/22 133, 2.16 | 752/47 631, 1.58 | 1593/112 794, 1.41 | 160/13 435, 1.19 | |
| Women, OR (95% CI) | 1 | 1.25 (1.11 to 1.40) | 1.23 (1.11 to 1.37) | 0.94 (0.86 to 1.03) | 0.85 (0.78 to 0.91) | 0.64 (0.54 to 0.75) | |
| (3) Body mass index | |||||||
| Cases/population, event rate (%) | 2977/176 545, 1.69 | 833/36 815, 2.26 | 1036/48 736, 2.13 | 1334/89 252, 1.49 | 2456/181 432, 1.35 | 262/20 396, 1.28 | 0.40 |
| <25 kg/m2, OR (95% CI) | 1 | 1.23 (1.14 to 1.33) | 1.23 (1.15 to 1.33) | 0.91 (0.85 to 0.97) | 0.82 (0.77 to 0.86) | 0.67 (0.59 to 0.76) | |
| Cases/population, event rate (%) | 1054/72 788, 1.45 | 297/14 176, 2.10 | 326/18 851, 1.73 | 544/40 618, 1.34 | 1117/89 361, 1.25 | 113/9490, 1.19 | |
| ≥25 kg/m2, OR (95% CI) | 1 | 1.34 (1.17 to 1.52) | 1.17 (1.03 to 1.32) | 0.93 (0.84 to 1.04) | 0.86 (0.79 to 0.94) | 0.72 (0.59 to 0.87) | |
| (4) Thrombolysis treatment | |||||||
| Cases/population, event rate (%) | 675/15 139 | 160/3099 | 177/3861 | 284/8134 | 541/16 494 | 46/1452 | 0.04 |
| With thrombolysis treatment, OR (95% CI) | 1 | 1.01 (0.84 to 1.21) | 0.96 (0.81 to 1.14) | 0.81 (0.70 to 0.94) | 0.79 (0.70 to 0.89) | 0.63 (0.46 to 0.85) | |
| Cases/population, event rate (%) | 3356/234 198 | 970/47 892 | 1185/63 726 | 1594/121 736 | 3032/254 299 | 329/28 434 | |
| Without thrombolysis treatment, OR (95% CI) | 1 | 1.30 (1.21 to 1.40) | 1.26 (1.18 to 1.35) | 0.94 (0.88 to 0.99) | 0.84 (0.80 to 0.88) | 0.69 (0.62 to 0.78) | |
Adjusted for age, sex, smoking, alcohol intake, National Institutes of Health Stroke Scale (NIHSS) (0–4; 5–15; 16–20; 21–42; missing data of NIHSS); medical history (hypertension; diabetes mellitus); history of anticoagulation; dual antiplatelet therapy; systolic blood pressure (per 20 mm Hg); fasting glucose; intravenous thrombolytic therapy; body mass index, kg/m2 (<18.5, 18.5–25, 25–30, ≥30).
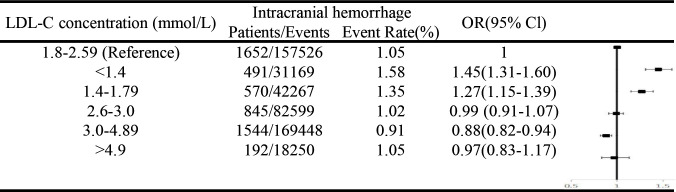
Figure 3 shows the results of sensitivity analyses. Compared with LDL-C=1.80–2.60 mmol/L, LDL-C=1.40–1.79 mmol/L and LDL-C<1.40 mmol/L may still increase the risk of intracranial haemorrhage among patients who had an IS who have minor stroke (NIHSS≤3).
Figure 3.
Sensitivity analysis. Adjusted for age, sex, smoking, alcohol intake; medical history (hypertension; diabetes mellitus); history of anticoagulation; dual antiplatelet therapy; systolic blood pressure (per 20 mm Hg); fasting glucose; intravenous thrombolytic therapy; body mass index, kg/m2 (<18.5, 18.5–25, 25–30, ≥30). LDL-C, low-density lipoprotein cholesterol; IS, ischaemic stroke.
Discussion
In this large cohort with real-world data, we demonstrated the correlation between low LDL-C level and risk of intracranial haemorrhage following acute IS during hospitalisation. Compared with the patients of LDL-C=1.80–2.59 mmol/L (70–100 mg/L), patients of low LDL-C level (1.40–1.79 mmol/L (50–70 mg/L) or <1.4 mmol/L (<50 mg/L)) exhibited significantly higher risk of new intracranial haemorrhage occurrence.
Our study with large sample size confers certain statistical power to elucidate the issue of intracranial haemorrhage in patients who had an IS across various LDL-C ranges, which also adds evidence from real world. Moreover, our study targets at the patients who had an IS, in whom the low LDL-C level has been verified to generate benefits in long term but the evidence remains unknown during the acute stage. From our study, it is indicated that the haemorrhagic risk related to low LDL-C level may exist during the acute stage of IS. Thus, it is necessary to fully evaluate the haemorrhagic risk before prescribing the intensive lipid-lowering therapy to patients with acute IS. Notably, the risk-benefit relationship of low LDL-C level was not demonstrated in our study. Therefore, our conclusions should not be interpreted to contradict the use of lipid-lowering therapy, such as statins, in all patients during the acute stage of IS.
Recently, several randomised clinical trials have demonstrated that an addition of PCSK9 monoclonal antibodies to statin therapy can provide a further reduction in risk of atherosclerotic cardiovascular disease including IS, which is directly and positively correlated with the incrementally achieved LDL-C reduction.13–15 In addition, studies on clinical safety of the very low achieved LDL-C values have proved reassuring. However, considering the small sample size of incident cases of haemorrhagic events (incident cases of Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk: 54, incident cases of ODYSSEY OUTCOMES: 25), it is worth noting that these currently available data is still limited.14 15 A recent meta-analysis of PCSK9 trials also showed that the potential effects of PCSK9 remain inconclusive due to the lack of statistical power of rarer events such as haemorrhagic events.16 Accordingly, data from real world with abundant sample size to ensure the statistical power is desirable. Actually, there have always been warnings that ‘excessively low blood lipid levels may increase the risk of haemorrhagic events’.6 During a follow-up of 9 years, one prospective study including 96 043 participants concluded that there was a significant correlation between lower LDL-C and higher risk of intracerebral haemorrhage when LDL-C<70 mg/dL.17 Besides, a prospective cohort study among 27 937 women observed that LDL-C levels<70 mg/dL and low triglyceride levels were associated with increased risk of haemorrhagic stroke over a 19-year period.18 However, these studies have not provided evidence on risk of intracranial haemorrhage following IS. In the current study with nearly 800 000 patients, we demonstrated that the low level of baseline LDL-C was significantly associated with subsequent risk of intracranial haemorrhage during acute stage of IS.
Besides the LDL-C, a number of other risk factors have also been identified to involve in intracranial haemorrhage following IS.6 19 Among them, our study further showed that age may have a significant interaction effect with lower LDL-C level in affecting intracranial haemorrhage risk. Stratified by age category (<65 years vs ≥65 years), older patients who had an IS with lower LDL-C level suffered from higher intracranial haemorrhage risk, indicating that more care should be taken when considering a strict LDL-C control among older patients who had an IS. Moreover, our study also observed the effect of low LDL-C level on increased risk of intracranial haemorrhage in patients who did not receive thrombolytics and patients with minor stroke (NIHSS<4). Hence, it is indicated that for the certain populations who were considered to be at low risk of intracranial haemorrhage,6 20 clinicians should still concern about the risk of bleeding related to low LDL-C level.
We should also acknowledge several limitations. First, the CSCA is an administrative database, providing large sample size but limited clinical information. Thus, several clinical variables are currently lacked in most of the participants, such as the information on type of anticoagulants during hospitalisation, mechanical thrombectomy and functional outcome. Second, this cohort only recorded the outcomes during the short follow-up in hospitalisation, which prevented us from further investigating the long-term risk of intracranial haemorrhage under different LDL-C levels. Thus, the bleeding risk related to low LDL-C level needs to be further verified by radiographic follow-up for longer periods. Besides, the CSCA study only collected LDL-C level on admission, lacking the information on long term LDL-C level before the occurrence of IS. Third, clinicians do not routinely screen all patients who had an IS with repeated neuroimaging such as CT or MRI during hospitalisation, and asymptomatic haemorrhagic events might be ignored in clinical work. Thus, as a registry study based on medical records, it is possible that most of the intracranial haemorrhage recorded by CSCA programme was symptomatic. As a result, the event rate recorded by CSCA might be lower than what actually occurred. Moreover, the lack of the information on neuroimaging (MRI of the brain with T2* or SWAN) also prevented us from analysing the relationship between LDL-C level and haemorrhagic tendency, such as old cerebral haemorrhage and cerebral microbleed. Fourth, since this study included Chinese patients only, it may limit the generalisability of the conclusions to other populations.
Conclusions
In summary, our study warns that during the acute stage of IS, low LDL-C level (LDL-C<1.4 mmol/L or LDL-C<1.8 mmol/L) is accompanied with increased risk of intracranial haemorrhage. While actively lowering LDL-C level for patients who had an IS, clinicians should also concern about the haemorrhagic risk associated with low LDL-C level.
Footnotes
JX and ZC contributed equally.
Contributors: All authors report no disclosures relevant to the manuscript. YJW accepted full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: This work was supported by grants from the Ministry of Science and Technology of the People’s Republic of China (2016YFC0901002, 2016YFC0901001, 2017YFC1310901, 2017YFC1307905, 2018YFC1312903), grants from Beijing Municipal Administration of Hospitals’ Mission Plan (SML20150502), grants from Beijing Municipal Science & Technology Commission (D171100003017002, D151100002015003) and grants from National Science and Technology Major Project (2017ZX09304018).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:1374–59. 10.1016/j.jvs.2006.10.008 [DOI] [PubMed] [Google Scholar]
- 2. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American heart association/american stroke association. Stroke 2011;42:227–76. 10.1161/STR.0b013e3181f7d043 [DOI] [PubMed] [Google Scholar]
- 3. Amarenco P, Kim JS, Labreuche J, et al. A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med 2020;382:9–19. 10.1056/NEJMoa1910355 [DOI] [PubMed] [Google Scholar]
- 4. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–97. 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 5. Sabatine MS, Giugliano RP, Keech AC. Fourier steering committee and investigators. evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2012;376:1713–22. 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 6. Roth EM, McKenney JM, Hanotin C, et al. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med 2012;367:1891–900. 10.1056/NEJMoa1201832 [DOI] [PubMed] [Google Scholar]
- 7. Mach F, Baigent C, Catapano AL. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2019;00:1–178. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 8. Álvarez-Sabín J, Maisterra O, Santamarina E, et al. Factors influencing haemorrhagic transformation in ischaemic stroke. Lancet Neurol 2013;12:689–705. 10.1016/S1474-4422(13)70055-3 [DOI] [PubMed] [Google Scholar]
- 9. Goldstein LB, Amarenco P, Szarek M, et al. Hemorrhagic stroke in the stroke prevention by aggressive reduction in cholesterol levels study. Neurology 2008;70:2364–70. 10.1212/01.wnl.0000296277.63350.77 [DOI] [PubMed] [Google Scholar]
- 10. Wang Y, Li Z, Wang Y, et al. Chinese stroke center alliance: a national effort to improve healthcare quality for acute stroke and transient ischaemic attack: rationale, design and preliminary findings. Stroke Vasc Neurol 2018;3:256–62. 10.1136/svn-2018-000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weimar C, Roth MP, Zillessen G, et al. Complications following acute ischemic stroke. Eur Neurol 2002;48:133–40. 10.1159/000065512 [DOI] [PubMed] [Google Scholar]
- 12. Davenport RJ, Dennis MS, Wellwood I, et al. Complications after acute stroke. Stroke 1996;27:415–20. 10.1161/01.STR.27.3.415 [DOI] [PubMed] [Google Scholar]
- 13. Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European cooperative acute stroke study (ECASS). JAMA 1995;274:1017–25. [PubMed] [Google Scholar]
- 14. Ridker PM, Revkin J, Amarenco P, et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med 2017;376:1527–39. 10.1056/NEJMoa1701488 [DOI] [PubMed] [Google Scholar]
- 15. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–107. 10.1056/NEJMoa1801174 [DOI] [PubMed] [Google Scholar]
- 16. Schmidt AF, Pearce LS, Wilkins JT, et al. Pcsk9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev 2017;4:CD011748. 10.1002/14651858.CD011748.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ma C, Gurol ME, Huang Z, et al. Low-density lipoprotein cholesterol and risk of intracerebral hemorrhage: a prospective study. Neurology 2019;93:e445–57. 10.1212/WNL.0000000000007853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rist PM, Buring JE, Ridker PM, et al. Lipid levels and the risk of hemorrhagic stroke among women. Neurology 2019;92:e2286–94. 10.1212/WNL.0000000000007454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo Y, Yang Y, Zhou M, et al. Risk factors of haemorrhagic transformation for acute ischaemic stroke in Chinese patients receiving intravenous recombinant tissue plasminogen activator: a systematic review and meta-analysis. Stroke Vasc Neurol 2018;3:203–8. 10.1136/svn-2018-000141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. The NINDS t-PA Stroke Study Group . Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. The NINDS t-PA stroke study group. Stroke 1997;28:2109–18. 10.1161/01.str.28.11.2109 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2022-001612supp001.pdf (747.2KB, pdf)
svn-2022-001612supp002.pdf (82.2KB, pdf)
Data Availability Statement
Data are available in a public, open access repository.