Abstract
Objectives
Pharmacy automation is increasing in hospitals. The aim of this systematic review was to identify and evaluate the literature on automated unit dose dispensing systems (UDDS) producing individually packaged and labelled drugs for inpatients.
Methods
The search was conducted on eight electronic databases, including Scopus, Medline Ovid, and Cinahl, and limited to peer reviewed articles with English abstracts published 2000–2020. Studies were included in the review if drug dispensing was performed by an automated UDDS where individually packaged and labelled unit doses were subsequently assembled patient specifically for inpatients. All outcomes related to UDDS functionality were included with specific interest in medication safety, cost-efficiency and stock management. Outcomes were categorised and results synthesised qualitatively.
Results
664 publications were screened, one article identified manually, resulting in eight included articles. Outcomes of the studies were categorised as medication administration errors (MAEs), dispensing errors, costs and cost-effectiveness. Studies showed that automated UDDS reduced significantly MAEs of inpatients compared with traditional ward stock system (WSS), especially when UDs were dispensed patient specifically by unit dose dispensing robot. Patient specific drug dispensing with automated UDDS was very accurate. Of three different automated medication systems (AMSs), patient specific AMS (psAMS) was the most cost-effective and complex AMS (cAMS) the most expensive system across all error types due to the higher additional investments and operation costs of automated dispensing cabinets (ADCs). None of the studies investigated the impact on the medication management process such as efficiency, costs and stock management as primary outcome.
Conclusions
UDDS improved patient safety. However, automation is a costly investment and the implementation process is complex and time consuming. Further controlled studies are needed on the clinical and economical outcomes of automated UDDS to produce reliable knowledge for hospital decision makers on the cost-benefit of the investment and to support decision making.
Keywords: automated unit dose dispensing system, automated medication system, unit dose dispensing robot, inpatient, medication administration errors, cost-effectiveness, medication stock management
Introduction
Medication errors (MEs) have received widespread attention in recent decades and are a major concern for healthcare organisations worldwide.1–3 MEs are one of the most common causes of adverse events in healthcare, which occur in about 6% of hospital patients.4 Medication-related adverse events may cause serious patient harm and, at worst, lead to death. They also impose significant additional costs on the healthcare system. More harmful MEs are reported in intensive care units (ICUs) than in general medical wards.5 MEs occur at all stages of the medication process from prescribing to administration.1–7
In closed loop medication administration (CLMA), the whole medication process is recorded into the hospital’s electronic health record (EHR) in real-time. Automated systems and smart devices must be integrated to EHR to support the safety of the patient’s medication process. CLMA promotes cross-checking of the correct patient, drug products and prescription utilising digital technologies. The process of uninterrupted and automated medication has been shown to improve patient and medication safety through the reduction of MEs and high-quality, documented treatment.8 9
One critical phase in the medication process is the patient specific drug dispensing.10 Traditionally, dispensing has been performed in hospitals manually by nurses by picking drugs from ward original package stocks. In recent years the use of automated pharmacy dispensing systems has been widely advocated to improve efficiency and minimise dispensing errors in the medication process. Several systematic reviews have been published on the automation of the inpatient medication process.11–14 Reviews consider mainly various semi-automated dose dispensing technologies such as automated dispensing cabinets (ADCs) and carousels and fully automated multi-dose dispensing systems in which patient specific drug products are dispensed into the same pouch or container for each administration time. There is no systematic review in the literature that focuses on automated unit dose dispensing systems (UDDS).
Unit dose dispensing robots initially pack and label each drug product into individual unit packs (unit doses), which are anonymous with regards to patient information, and thereafter the unit doses are assembled patient specifically for dispensing, based on prescriptions. Individually packed and uniquely barcoded unit doses (UDs) enable electronic identification of each individual drug product in the drug dispensing and administration process, which provides complete traceability and is one of the prerequisites for an uninterrupted medication process. Unit dose dispensing robotics allow, in addition to oral drug forms, also vials, ampoules and syringes to be individually packaged and dispensed. This makes possible a broader application of the system, particularly considering patients admitted to the ICU where most medications are given intravenously. Moreover, anonymous UDs can be delivered to ward stocks non-patient specifically from which they can be picked for patients, as needed, using electronic identification of UDs and, thus, enable the continuity of the medication process.
Objectives
The aim of this systematic review was to identify and evaluate studies focusing on automated robotic UDDS producing individually packaged and labelled drugs for inpatients and the contribution of automation when used with other technologies and interventions in the medication process. The search strategy was intentionally made very strict in order to capture only literature that focused on robotic UDDS, its functionality and impact on the medication process. All outcomes related to UDDS functionality were included with specific interest in medication safety, cost-efficiency and stock management. The systematic review was performed according to the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines.15
Methods
Literature search
The initial literature search was conducted on 19 November 2019 on the following electronic databases: Scopus, Medline Ovid, Cinahl, EBM Reviews Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Health Technology Assessment Database (HTA), NHS Economic Evaluation Database, and Database of Abstracts of Reviews of Effects. A combination of search terms describing automated unit dose dispensing of drugs with inpatients was based on previous related literature and the same search strategy was used in all databases (table 1). The search was updated until 30 April 2021 by the automated search alert services of databases (excluding Cochrane Database of Systematic Reviews and HTA). The search was limited to articles with English abstracts published between 2000 and 2020 in order to capture all relevant publications. Furthermore, a manual literature search was performed to identify additional studies for systematic review.
Table 1.
Search strategy of the systematic literature review
| # | Search term and Boolean operator |
| 1 | unit-dose OR ‘unit dose’ ADJ2 dispens* OR distribut* OR deliver* (mp) |
| 2 | automated ADJ2 medication OR dispens* OR distribution* OR deliver* ADJ2 system* (mp) |
| 3 | pharmac* ADJ2 robot* OR automation (mp) |
| 4 | 1 OR 2 OR 3 |
| 5 | hospital* OR ward* OR inpatient* OR ‘in-patient’ (mp) |
| 6 | 4 AND 5 |
| 7 | limit 6 to yr=2000–2020 |
Inclusion and exclusion criteria
Peer-reviewed original articles and review articles were included only if the studies examined drug dispensing for inpatients and dispensing was performed by an automated UDDS where drugs were initially packed and labelled into individual unit doses (anonymous with regards to patient information) and subsequently collected patient specifically (table 2). The combined use of automated UDDS with other technologies or interventions in the medication process were also included.
Table 2.
Inclusion and exclusion criteria of articles for the systematic literature review
| Inclusion criteria | Exclusion criteria | |
| Population (P) | Studies performed with inpatients or in hospital settings | Studies performed with outpatients in community settings |
| Focus of the study, Intervention (I) |
Studies utilising automated unit dose dispensing system (UDDS) for drug dispensing in which drugs are first individually packaged and labelled as unit doses without patient information (anonymous) and then assembled patient specifically by robot or manually at the ward Automated UDDS combined with other technologies or interventions in the medication process |
Studies using solely original pack dispensing systems, other drug dose dispensing technologies such as automated multi-dose dispensing system (one or more drugs are packed into a pouch or container containing patient information, for each time of administration), various semi-automated dose dispensing technologies (carousels, automatic dispensing cabinets) or manual drug dispensing system Studies only describing the implementation or development of the UDDS Inadequate description of automated drug dispensing process Studies not concerning drug dispensing |
| Comparison (C) | Control group is not required | – |
| Outcomes (O) | Outcomes related UDDS functionality such as inpatient’s medication process and medication stock management | No outcomes from the functionality of UDDS |
| Study design (S) | Peer reviewed journal articles; original articles and systematic review articles | Not peer reviewed publications; letters, editorials, news, commentaries, conference proceedings and non-scientific publications |
| Time (T) | Articles published between 2000 and 2020 | Articles published before 2000 and after 2020 |
| Language | English language articles or foreign language articles with an English abstract | – |
All outcomes related UDDS functionality such as effect on the inpatient’s medication process and medication stock management were included. The inclusion and exclusion criteria were defined using PICOS (Patient, Intervention, Comparison, Outcome and Study design and type) (table 2).
Study selection, data extraction and quality assessment
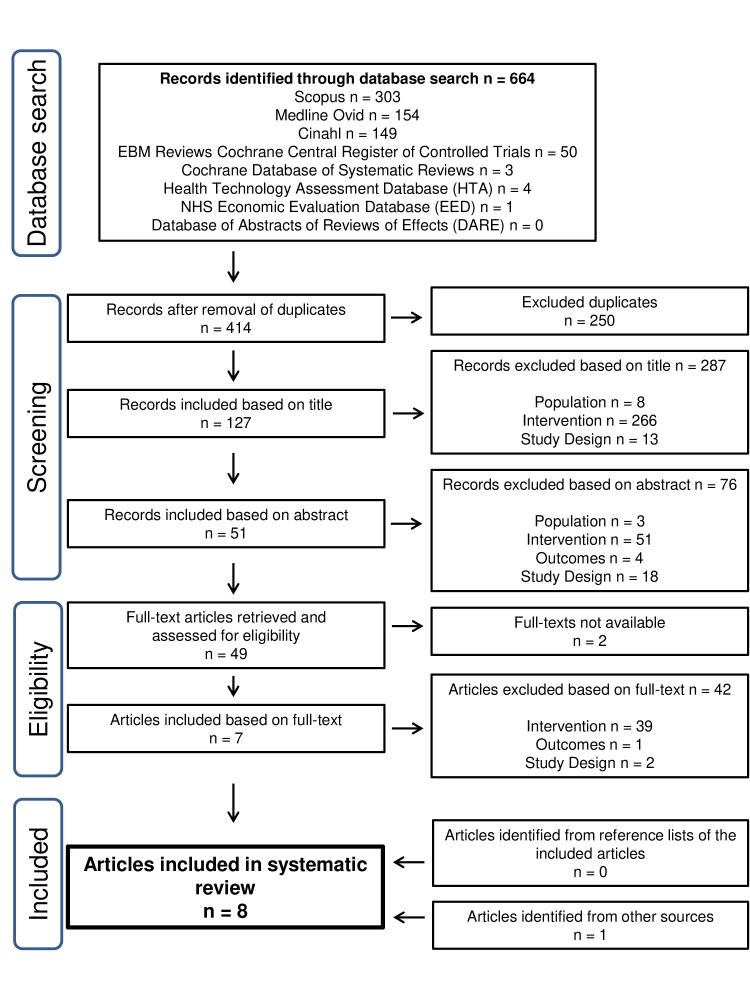
The references were screened independently by two researchers (KH, HA): first by title, then by abstract, and finally by full-text using a standardised form (figure 1). Inclusion disagreements were resolved by consensus discussion with a third researcher (ES-P). Articles were included in the systematic review only if the articles clearly met the inclusion criteria for the study (table 2). The reference lists of included studies and relevant pre-reviewed articles were manually searched for additional eligible articles. Excluded publications were categorised according to PICOS (figure 1).
Figure 1.
Flow chart of database search and article screening in the study.
The data were extracted from each full-text article included in the review and categorised according to primary outcomes by one reviewer (KH) and checked against the original publications by a second reviewer (AMT) (table 3). The quality of included articles was assessed using the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) system independently by two reviewers (KH, HA).16
Table 3.
Characteristics and outcomes of the studies (n=8) included in the systematic review
| Reference; country; setting |
Study design; grade |
Objective | Methods | Intervention; unit dose dispensing robot used |
Outcomes |
| Medication administration error assessment | |||||
| Cousein et al
17; France; short stay geriatric unit (40 beds) within a general hospital |
Before-after observational study; moderate |
To assess the effect of automated UDDS on MAEs | Observation periods of medication administration rounds were 3 months for WSS, 2 months for UUDS1 and 1 month for USSD2. Within the rounds administered drugs to prescribed drugs were compared. Outwith the operating hours of the UD robot, drugs were available for the nurses via ADC. MAEs were calculated, classified and compared between the study periods. Additionally, error gravity and risk reduction for the patients were defined. Statistical analysis: Student’s t-test (quantitative variables) and χ2 test or Fisher’s exact (qualitative variables); 95% CI and two-tailed α level of 0.05. |
Implementation of patient specific UDDS including UD dispensing robot, CPOE, pharmaceutical prescription control and ADC in medication process. UDDS had two variants; UDDS1 contained CPOE without eMAR and UDDS2 contained both CPOE and eMAR. versus Traditional WSS with manual drug dispensing by nurses. No control group. UD dispensing robot: PillPick, Swisslog |
MAEs
Patient risk for being exposed to one or more MAEs
Medication error gravity
|
| Risør et al
18; Denmark; two haematological wards (21 and 22 beds, respectively) within a university hospital |
Prospective, controlled before-after study; moderate |
To evaluate the impact of psAMS on MAEs and MAE subtypes | Study was conducted in two wards having comparable workflow, medication profile and flow of patients. The observation periods were performed 3 weeks before and after implementation in both intervention and control wards. MAEs were identified and calculated by direct observation of the medication administration process. MAEs were further divided into clinical errors (ie, patient did not receive the medication as prescribed) and procedural errors (ie, deviations from written procedures or guidelines that may lead to a clinical error). Statistical analysis: logistic regression; p≤0.05, OR and 95% CI. Sub-analyses and sensitivity analyses |
Implementation of psAMS including ADD, CPOE, pharmaceutical prescription control, eMAR and BCMA in medication process. versus Traditional WSS with manual drug dispensing by nurses, CPOE and eMAR in control group. UD dispensing robot: PillPick, Swisslog |
MAEs
|
| Risør et al
23; Denmark; two acute medical units (17 and 14 beds, respectively) within a university hospital |
Prospective, controlled before-after study; moderate |
To evaluate the effectiveness of cAMS and npsAMS on MAEs and MAE subtypes | Study was performed in two units having similar medication handling process and comparable medication profile. Measurement of MAEs was performed by direct observations of the medication administration process in intervention and control units during a 3 week period as initial baseline and two subsequent follow-up periods after implementation. Any discrepancy between administered and prescribed medication or deviations from written procedures or guidelines was considered as an error and categorised by error types (clinical errors or procedural errors). Statistical analysis: logistic regression; p≤0.05, OR and 95% CI. Sub-analyses |
Implementation of cAMS and npsAMS sequentially: first cAMS consisting of ADD, CPOE, pharmaceutical prescription control, eMAR, BCMA and ADC was implemented and subsequently npsAMS consisting of ADD, CPOE, eMAR and BCMA. versus Traditional WSS with manual drug dispensing by nurses, CPOE and eMAR in control group. UD dispensing robot: PillPick, Swisslog |
MAEs
|
| Dispensing error assessment | |||||
| Le Gonidec et al
20; France; medical ward of a jailhouse (350 patients) |
Observational study; very low |
To evaluate the performance of ADD – work flow and drug dispensing errors | Drugs were dispensed as UDs patient specifically for 7 days at the time (average 3 UDs per prescription) by ADD robot. UD packing and dispensing rates, dispensing errors and security of medication circuit were observed for 3 months. Study was conducted 2.5 years after implementation of the ADD robot. No statistical analysis |
Implementation of ADD integrated with CPOE and dispensing software. No comparison with previous drug dispensing system. UD dispensing robot: PillPick, Swisslog |
UD packing and dispensing rates
Dispensing errors
Secondary outcome
|
| Sutra et al
21; France; multiple types of geriatric wards (total 307 beds) within university hospital |
Observational study; very low |
To assess the impact of automated UDDS on drug dispensing errors – for appropriate corrective operations before implementing the UDDS broader | Drug dispensing errors were observed systematically by nurses in wards supplied by automated UDDS over 13 month period and classified into four categories: errors related to the UDDS, software related errors, errors in the declarations or prescriptions and errors related to pharmacy (non-automation errors). Results were presented as errors over the 13 month period, with monthly monitoring of error rates. Study was conducted 1 year after the implementation of the automated UDDS. No statistical analysis |
Implementation of automated UDDS with integrated prescription, warehouse management and automation management software and pharmaceutical analysis of the prescription. No comparison with previous drug dispensing system. UD dispensing robot: Athena, Sinteco |
Dispensing errors (including errors not related to UDDS)
Dispensing errors (related to UDDS)
|
| Economic evaluation | |||||
| Risør et al
19; Denmark; two haematological wards (21 and 22 beds, respectively) within a university hospital |
Original effectiveness data obtained from prospective, controlled before-after study by Risør et al
18; moderate |
To evaluate the costs and cost-effectiveness of psAMS and to identify the cost factors related to psAMS | The economic evaluation compared psAMS with the traditional WSS in which medicines were delivered in their original packages to wards and dispensed by nurses. The cost analysis was performed in the hospital setting using two 6 month study periods (baseline and follow-up). The analysis used a short-term incremental costing approach and the assumption that only the costs related to medicine delivery and handling would change. Total costs were divided into costs of handling, waste, pharmaceutical services, PDAs and unit dose bags. Costs included labour and annual running costs, purchase costs of automated dispensing robot, development costs of interfaces between eMAR and PDAs, and costs of facilities. In cost-effectiveness evaluation the cost analysis and effects of psAMS to MAE rates were used. Calculation and statistical analysis: calculation of the total running and implementation costs, the annual amount of MAEs, the number of avoided MAEs and costs per avoided errors. Sensitivity analyses |
Interventions are presented by Risør et al
18
UD dispensing robot: PillPick, Swisslog |
Running and implementation costs
Cost-effectiveness
|
| Risør et al
24; Denmark; two haematological wards (21 and 22 beds, respectively) and two acute medical units (17 and 14 beds, respectively) within a university hospital |
Original effectiveness data obtained from two prospective, controlled before-after studies by Risør et al
18
23; moderate |
To evaluate the costs and cost-effectiveness of psAMS, cAMS and npsAMS by using a model-based indirect cost-effectiveness comparison of three different, real world AMSs | The economic evaluation compared psAMS, cAMS and npsAMS with traditional WSS, respectively. The cost analysis was performed and contained the same cost factors as described previously by Risør et al
19. In cost-effectiveness analysis the costs of AMSs defined in the cost analysis and effects of AMSs to MAE rates were used. Calculation and statistical analysis: Calculation of the total running costs and costs per dose. The effect of consumption and costs were standardised for an indirect comparative analysis. Calculation of the estimated number of MAEs and the number of avoided MAEs for each consumption scenario. ICERs for different drug consumption scenarios. Non-parametric simulations based on the mean/OR and SE of the mean on the observed parameters. The probability of cost-effectiveness related to different assumed values per avoided error interpreted as CEACs. |
Interventions for psAMS are presented by Risør et al
18
Interventions for cAMS and npsAMS are presented by Risør et al 23 UD dispensing robot: PillPick, Swisslog |
Running costs
Cost-effectiveness
|
| Lappalainen et al
22; Finland; 21 somatic adult wards within university hospital |
BIA; low |
To assess the net cost impact of psAMS implementation | BIA included theoretical salary costs, investments (purchase of automated dispensing robot, constitution costs of cleanroom, development costs of software interphases) as well as space and overhead costs related to AMS over a 10 year period. The hospital’s own databases and experts, data provided by the robot supplier and publicly available reliable statistics and databases were used as data sources for costs. In addition, the effect of increasing the number of hospital wards using the AMS was evaluated. Statistical analysis: one-way sensitivity analyses to assess the robustness of the results over a 10 year period |
Implementation of an imaginary psAMS including ADD, CPOE, pharmaceutical prescription verification, eMAR and BCMA in medication process. versus Traditional WSS with manual drug dispensing. UD dispensing robot not specified |
Running costs
|
Grade: high, moderate, low, very low.
ADC, automated dispensing cabinet; ADD, automated drug dispensing; AMS, automated medication system; BCMA, barcode-assisted medication administration; BIA, budget impact analysis; cAMS, complex automated medication system; CEAC, cost-effectiveness acceptability curve; CPOE, computerised physician order entry; eMAR, electronic medication administration record; ICER, incremental cost per avoided error; MAE, medication administration error; npsAMS, non-patient specific automated medication system; PDA, personal digital assistant; psAMS, patient specific automated medication system; UD, unit dose; UDDS, unit dose dispensing system; WSS, ward stock system.
Data synthesis and analysis
Results of each study were grouped based on the primary outcomes and, furthermore, subtitled to clarify the results. Qualitative summary of results was performed by two researchers (KH, AMT). Due to the varying study settings and diverse outcome reporting of the few included studies, formal quantitative synthesis of evidence from the results was not possible.
Results
Included studies and study quality
A total of 664 references were found from the initial literature search and seven articles met the inclusion criteria (figure 1). One article was identified through manual literature search. No additional articles were identified by alert services. Thus, a total of eight articles were included for result synthesis: economic evaluations (n=3), controlled before-after-studies (n=2), observational studies (n=2), and before-after observational study (n=1) (table 3). Studies were conducted in France (n=3), Denmark (n=4), and Finland (n=1) and the quality of the studies was graded as moderate (n=5), low (n=1), and very low (n=2). None of the studies were excluded based on quality. A few conference abstracts were also captured, which were excluded from the review, but used to support discussion of results.
In all studies, automated UDDS was used for drug dispensing to serve inpatients in multiple types of wards and units. Some studies used the terminology of automated medication system (AMS) to describe automated UDDS (table 4). Furthermore, subtypes of AMS have been specified in some studies such as psAMS (patient specific AMS), cAMS (complex AMS), and npsAMS (non-patient specific AMS). Where possible, the abbreviations are used in the review as presented in the original studies in question.
Table 4.
Abbreviations used in included studies to describe the automated unit dose dispensing systems
| Abbreviation | Description of the system | Reference |
| UDDS Unit dose dispensing system |
Individually packaged and labelled, anonymous unit doses assembled into patient specific co-packs using unit dose dispensing robot, based on physician orders | Cousein et al 17 |
| AMS Automated medication system |
Individually packaged and labelled, anonymous unit doses produced by unit dose dispensing robot, assembled patient specifically in an AMS subtype-dependent manner | Risør et al
18
Risør et al 19 Risør et al 23 Risør et al 24 Lappalainen et al 22 |
| psAMS Patient specific automated medication system |
Individually packaged and labelled, anonymous unit doses assembled into patient specific co-packs using unit dose dispensing robot, based on physician orders | Risør et al 24 |
| cAMS Complex automated medication system |
Individually packaged and labelled, anonymous unit doses produced by unit dose dispensing robot and delivered into automated dispensing cabinets of the wards, assembled patient specifically from the cabinets | Risør et al
23
Risør et al 24 |
| npsAMS Non-patient specific automated medication system |
Individually packaged and labelled, anonymous unit doses produced by unit dose dispensing robot and delivered into manual ward stocks, assembled patient specifically from the manual stocks | Risør et al
23
Risør et al 24 |
No English abbreviations were used in the French literature.20 21
Seven out of eight studies reported the brand of the used UD dispensing robots, which were PillPick, Swisslog (n=6) and Athena, Sinteco (n=1).
Some studies (n=5) compared the post-automation outcomes against manual drug dispensing (either pre-automation or non-automated ward). One of the studies used a theoretical automation process in budget impact analysis. Some studies (n=2) had no comparator or control group.
All reported outcomes were quantitative and categorised according to their primary outcomes as medication administration errors (MAEs), dispensing errors, running and implementation costs, and cost-effectiveness (table 3).
Automated unit dose dispensing systems
The medication process, into which the UD dispensing robot was implemented, varied between the studies. In most of the studies (n=6) UDs were dispensed by the robotic system as patient specific co-packs (psAMS).17–22 Second, anonymous UDs (n=1) were dispensed to wards and collected for patients manually by nurses either from ADC (cAMS) or ward stock (npsAMS).23 One study compared three different collection methods of UDs for patients.24 Computerised physician order entry (CPOE) was used in all studies. Furthermore, in some studies pharmaceutical prescription control, electronic medication administration record (eMAR) and barcode-assisted medication administration (BCMA) were included in the medication process (table 3).
Medication administration errors
The impact of automated UDDS on the number of MAEs was investigated (n=3) and reduction was observed in all studies (table 3).17 18 23 Cousein et al 17 and Risør et al 18 showed that patient specific UDDS (psAMS) reduced MAE rates significantly by 53% and 57%, respectively, compared with WSS, where medicines were delivered to the wards in their original packaging and dispensed to patients manually by nurses. All MAE types (dose omission, wrong dose, wrong drug product, wrong administration time) were reduced with UDDS.17 Furthermore, corresponding analysis of MAE gravity showed lower prevalence of errors requiring monitoring, therapy or intervention.
When MAEs were divided into clinical errors and procedural errors, a 94% reduction of clinical errors was observed with psAMS compared with WSS.18 Moreover, clinical errors did not occur at all with psAMS when personal digital assistant (PDA) scanning was used correctly during BCMA. Procedural errors were also decreased (30%) but not significantly. The most frequently observed error type was lack of patient identification control.
Compared with traditional WSS, AMS reduced MAE rates by 47% when anonymous UDs were delivered to wards and collected in a patient specific way from ADC (cAMS).23 MAEs were reduced as well when anonymous UDs were collected from manual ward stock (npsAMS), although non-significantly (27%). The most frequently observed procedural errors were related to lack of documentation such as drug substitution or deviating strength of drug product. Omission of dose was the most frequently observed clinical error.
Dispensing errors
Dispensing errors of the robotic system were studied (n=2) during the patient specific UD dispensing process and the dispensing error rates observed were 0.5% at maximum (table 3).20 21 However, the studies did not provide any comparator or control to dispensing error rates observed for manual drug dispensing processes.
Running and implementation costs
Running and implementation costs of AMSs were studied (n=3) in the hospital setting (table 3).19 22 24 Total running costs of the AMSs consisted primarily of the acquisition costs of the dispensing robot and setting up of the facilities, running costs of staff, maintenance, and UD packaging materials. Implementation costs of psAMS included planning, development and implementation.19
Comparative analysis of different AMSs showed psAMS being a slightly more costly system than npsAMS.24 The total incremental costs were clearly the highest with cAMS due to the higher additional investments and operation costs of ADCs.
Cost-effectiveness
Cost-effectiveness was evaluated (n=2) by comparing the incremental costs and effects achieved by each AMS compared with traditional WSS, respectively (table 3).19 24 The comparative incremental cost-effectiveness model showed that psAMS was the most cost-effective and cAMS was the most expensive across all error types.24 Costs of psAMS were in fact only marginally higher than with WSS, while resulting in clearly avoided errors. The cost-effectiveness acceptability curves showed also that the psAMS was the most cost-effective of the three systems.
None of the included studies investigated effects on the medication management process, such as efficiency, costs and stock management. One study showed that the automated dispensing system saved working time of technical staff previously required for manual collection of drugs while not reducing the work of pharmacists.20 However, the study did not investigate the cost effects of the saved working time.
Discussion
Effects on medication administration and dispensing errors
The studies showed that automated UDDS reduced significantly MAEs compared with traditional WSS, especially when UDs were dispensed patient specifically.17 18 Corresponding results were reported in conference proceedings.25 26 Automated UDDS revealed very high correctness of patient specific drug dispensing (minimum 99.5%).20 21 Reduction of dispensing errors by UDDS have been ascribed to less human error factors and the fact that the automatically collected co-packs are traceable to the patient, fully barcoded and checked automatically. Nevertheless, new risks have been identified, such as fading ink on the packaging, device outages and other information technology issues.27 Furthermore, late changes in the medication, carried out after automated patient specific drug dispensing, may expose the patient to medication errors.17 23 Therefore, patient specific drug dispensing should be done daily or even more often for hospital ward patients, based on an up-to-date medication list.
UDDS combining the use of anonymous UDs and decentralised ADCs or WSS was also shown to be more patient safe than traditional WSS.23 More effective reduction of MAEs was observed with cAMS than npsAMS, largely driven by the reduction in the procedural errors, where ADC controlled access to the correct UDs. Use of barcode product verification of UDs from ADC can be expected to decrease dispensing errors even more.13 28 29 Stocking of anonymous UDs in ADC could be a proper system to improve patient medication safety in hospital acute care units such as emergency departments and ICUs. They are associated with a high risk of medication errors as patients and their medications change frequently, making patient specific UDDS inappropriate.
Unlike traditional WSS, the UDDS delivers drug products (UDs) labelled and barcoded and, thus, enables BCMA at the bedside. The combination of eMAR and BCMA has been shown to be an important intervention to improve medication safety by reducing administration errors.9 30 The system verifies electronically that the correct drug products are being administered to the right patient, allowing confirmation of dose, timing and route so that an alert will be raised at any discrepancy between prescription and dosing. No clinical errors occurred when PDA scanning of UDs was used correctly during BCMA.18 It should be kept in mind that individually packaged UDs are ‘look alike’ drug products, making scanning a priority in all stages of the medication process.
UDDS increase the adaptability of the medication process to computerised procedures and electronic information flow. As discussed above, it is not possible to isolate the impact on MAEs to the dispensing of UDs only.17 18 In all included studies, UDDS contained e-prescribing and automated transfer of prescriptions for the dispensing robot through information system integrations. Furthermore, all but one study20 integrated ADD with other technologies and interventions including pharmaceutical prescription control, ADCs, eMAR and BCMA. It is very likely that all technologies and interventions before and during the drug dispensing reduced the errors at dispensing stage, whereas eMAR and BCMA functioned as an extra control in the administration stage.
Further reduction in MEs can be achieved by clinical verification of medication before the automated UD dispensing.31 32
Effects on costs
Cost analyses of the psAMSs compared with traditional WSS were partly inconsistent between the studies.19 22 24 In the Finnish modelling study, the salary costs of pharmacy staff and nurses was assessed lower with psAMS than with WSS, which compensated for the increased running costs of psAMS, resulting in similar initial costs of the systems and, moreover, after return on investments lower costs for psAMS.22 Instead, in the Danish study psAMS increased the workload of pharmaceutical staff due to additional technical prescription control, resulting in higher total costs of psAMS.19 24 Similar outcomes with the Danish study was reported in conference proceedings.25 31 The costs of AMSs per dose or ward were observed to decrease as the number of dispensed unit doses or wards increased, particularly for the cAMS.
By applying the cost estimates for adverse drug events (ADEs) from previous literature, Risør et al 19 concluded that psAMS was cost-neutral, as the potential cost savings from avoided ADEs may outbalance the incremental costs of psAMS. Further studies on the cost-effectiveness are required as preventable ADEs may vary according to the patient care environment, and the true economic impact of MEs has not been accurately estimated to date.33 However, the comparative assessment between the AMSs was relevant as the systems used the same primary outcomes and standardised estimates. The most cost-effective system was psAMS, and cAMS was clearly the most expensive system as ADCs increased the costs significantly. Nevertheless, cAMS may turn out to be cost-effective in high-risk departments. The needs and requirements of different patient care environments differ and, thus, customised UDDS should be tailored for particular care areas.
Effects on medication stock management
No direct outcomes related to medication stock management with UDDS were reported. However, some conference proceedings suggest that automated UDDS could be effective in stock management. UDDS clearly shortened the time needed for patient specific dispensing and reduced drug expenditure by 30% compared with traditional WSS.26 31 Additionally, expired drugs were not detected when UDDS was utilised,34 decreasing drug wastage. The lack of studies on stock management highlights the need for future economic impact studies.
One study pointed out that utilisation of ADD freed up working time of pharmacy technicians.20 In a recent systematic review, centralised and hybrid dispensing systems improved the quality of patient care by increasing nursing time as drug logistics were handed over to pharmacy technicians and pharmacist activities concentrated on clinical aspects.12
Advantages of unit dose versus multi-dose dispensing systems
Compared with the fully automated multi-dose dispensing system, the UD technology provides supposedly several advantages for dispensing medicines to hospital patients. Each UD contains the information of the drug product allowing easy identification of drugs by nurses to implement medication changes, if needed.25 34 Patients are usually hospitalised for relatively short time periods and their medications are often changed. Second, unlike multi-dose dispensing robots, UD robots can also dispense intravenous medications such as drug ampoules, vials and syringes, which increases the range of products dispensed by robot to inpatients. Dispensing intravenous medicines by UDDS is assumed to reduce MAEs and make the medication process safer,18 as intravenous therapy has been associated with a higher prevalence of medication errors compared with oral.35 36 Additionally, the ability of the robot to individually package medicinal products in their primary blisters reduces the risk of drug cross-contamination during dispensing, enables dispensing of harmful drug products, and facilitates medication stock management as the original shelf life of the drug products can be utilised and unopened unit doses returned to stock, minimising the waste.
Implementation of unit dose dispensing systems
Implementation of UDDS into the hospital medication process is a complex and time-consuming procedure.27 37 It changes substantially the workflow, daily routines and workload distribution. An unoptimised process and poor reliability of the robotic system may result in inefficient work and dissatisfied staff as observed by Veyrier et al.37 Implementation should be based on pre-operational risk assessment, considering facilities, new skills and work practices of staff, and adequate technical support, especially in information technology.27 37 To achieve a successful process, comprehensive analysis and planning of the whole medication process is essential throughout the implementation leading to re-scheduled and re-distributed workload.
Limitations of the systematic review
This systematic review has several limitations. The search was limited to articles with English abstracts and all studies were performed in Europe, which may have produced a bias to the results. In line with study objectives, the search strategy was intentionally made very strict in order to capture only papers that focused on the automated robotic unit dose dispensing systems, their functionality and effects. The limited number of studies, varying study designs, and methods and flaws of control groups and/or statistical analyses of included studies increased the risk of bias and may have affected the results.
Conclusions
In general, the studies concluded that implementation of UDDS in the medication process improved patient safety. Cost-efficiency of the UDDS varied according to the setting, with patient specific UDDS being the most cost-effective system. Avoided ADEs may outbalance the potential incremental costs of UDDS, which could contribute to overall cost-efficiency. Studies reporting the impact of UDDS on medication stock management was not detected.
The current review reveals that studies focusing on automated robotic dispensing systems, which package drug products in individual unit dose packs, are rare. Further controlled studies are required using different UDDS settings in hospitals. Implementation of UD dispensing robots in hospitals appears to be increasing, also in Europe. Automation is a costly investment and the implementation process is complex and time consuming. Therefore, hospital decision makers and budget managers need better knowledge on the cost-benefit balance of the investment to support decision making.
Acknowledgments
Eric Stilgenbauer is acknowledged for reviewing the French literature. The study was conducted as part of the Community and Hospital Pharmacy Specialisation Programme.
Footnotes
Contributors: All authors made substantial contributions to the current manuscript. KH and HA designed the study protocol and conducted the systematic review. KH and AMT extracted, synthesised and analysed the data. All authors provided input into the initial manuscript draft, commented and revised the subsequent drafts, and approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Not applicable.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Kohn LT, Corrigan JM, Donaldson MS. To err is human: building a safer health system. Washington, DC: National Academy Press, 2000. [PubMed] [Google Scholar]
- 2. Aspden P, Wolcott JA, Bootman JL, et al . Preventing medication errors: quality chasm series. Washington, DC: The National Academies Press, 2007. [Google Scholar]
- 3. WHO . Medication without harm – WHO global patient safety challenge on medication safety. Geneve, 2017. Available: http://apps.who.int/iris/bitstream/10665/255263/1/WHO-HIS-SDS-2017.6 [DOI] [PubMed]
- 4. Krähenbühl-Melcher A, Schlienger R, Lampert M, et al. Drug-related problems in hospitals: a review of the recent literature. Drug Saf 2007;30:379–407. 10.2165/00002018-200730050-00003 [DOI] [PubMed] [Google Scholar]
- 5. Latif A, Rawat N, Pustavoitau A, et al. National study on the distribution, causes, and consequences of voluntarily reported medication errors between the ICU and non-ICU settings. Crit Care Med 2013;41:389–98. 10.1097/CCM.0b013e318274156a [DOI] [PubMed] [Google Scholar]
- 6. Allard J, Carthey J, Cope J, et al. Medication errors: causes, prevention and reduction. Br J Haematol 2002;116:255–65. 10.1046/j.1365-2141.2002.03272.x [DOI] [PubMed] [Google Scholar]
- 7. Lisby M, Nielsen LP, Mainz J. Errors in the medication process: frequency, type, and potential clinical consequences. Int J Qual Health Care 2005;17:15–22. 10.1093/intqhc/mzi015 [DOI] [PubMed] [Google Scholar]
- 8. Franklin BD, O'Grady K, Donyai P, et al. The impact of a closed-loop electronic prescribing and administration system on prescribing errors, administration errors and staff time: a before-and-after study. Qual Saf Health Care 2007;16:279–84. 10.1136/qshc.2006.019497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burkoski V, Yoon J, Solomon S, et al. Closed-loop medication system: leveraging technology to elevate safety. Nurs Leadersh 2019;32:16–28. 10.12927/cjnl.2019.25817 [DOI] [PubMed] [Google Scholar]
- 10. James KL, Barlow D, McArtney R, et al. Incidence, type and causes of dispensing errors: a review of the literature. Int J Pharm Pract 2009;17:9–30. 10.1211/ijpp.17.1.0004 [DOI] [PubMed] [Google Scholar]
- 11. Tsao NW, Lo C, Babich M, et al. Decentralized automated dispensing devices: systematic review of clinical and economic impacts in hospitals. Can J Hosp Pharm 2014;67:138–48. 10.4212/cjhp.v67i2.1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahtiainen HK, Kallio MM, Airaksinen M, et al. Safety, time and cost evaluation of automated and semi-automated drug distribution systems in hospitals: a systematic review. Eur J Hosp Pharm 2020;27:253–62. 10.1136/ejhpharm-2018-001791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carvalho MF, Marques JM, Marta CB, et al. Effectiveness of the automated drug dispensing system: systematic review and meta-analysis. Rev Bras Enferm 2020;73:e20180942. 10.1590/0034-7167-2018-0942 [DOI] [PubMed] [Google Scholar]
- 14. Batson S, Herranz A, Rohrbach N, et al. Automation of in-hospital pharmacy dispensing: a systematic review. Eur J Hosp Pharm 2021;28:58–64. 10.1136/ejhpharm-2019-002081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 16. Guyatt GH, Oxman AD, Kunz R, et al. What is ’quality of evidence’ and why is it important to clinicians? BMJ 2008;336:995–8. 10.1136/bmj.39490.551019.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cousein E, Mareville J, Lerooy A, et al. Effect of automated drug distribution systems on medication error rates in a short-stay geriatric unit. J Eval Clin Pract 2014;20:678–84. 10.1111/jep.12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Risør BW, Lisby M, Sørensen J. An automated medication system reduces errors in the medication administration process: results from a Danish hospital study. Eur J Hosp Pharm 2016;23:189–96. 10.1136/ejhpharm-2015-000749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Risør BW, Lisby M, Sørensen J. Cost-effectiveness analysis of an automated medication system implemented in a Danish hospital setting. Value Health 2017;20:886–93. 10.1016/j.jval.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 20. Le Gonidec P, Diallo ML, Djoussa-Kambou S, et al . Performances d’une solution associant l’automate de délivrance Pillpick® au logiciel de prescription Pharma® utilisée pour une activité de dispensation délivrance nominative dans une unite de consultation et de soins ambulatoire. Performances of an automated dispensing system combined with a computerized prescription order entry. Ann Pharm Fr 2009;67:84–90. 10.1016/j.pharma.2008.12.004 [DOI] [PubMed] [Google Scholar]
- 21. Sutra C, Vitale G, Pagès A, et al . Délivrance nominative centralisée automatisée: recueil et analyse sur 13 mois des non-conformités déclarées par les services de gériatrie au centre hospitalier universitaire de Toulouse. Automated unit-dose dispensing system: data collections and analysis of nonconformities over a 13-month period in Toulouse hospital. Le Pharmacien Hospitalier et Clinicien 2016;51:164–71. [Google Scholar]
- 22. Lappalainen K, Knuuti K, Turpeinen M, et al . Yliopistosairaalan vuodeosastojen lääkejakoprosessin automatisaation budjettivaikutusanalyysi. DOSIS 2019;1:6–19. [Google Scholar]
- 23. Risør BW, Lisby M, Sørensen J. Complex automated medication systems reduce medication administration errors in a Danish acute medical unit. Int J Qual Health Care 2018;30:457–65. 10.1093/intqhc/mzy042 [DOI] [PubMed] [Google Scholar]
- 24. Risør BW, Lisby M, Sørensen J. Comparative cost-effectiveness analysis of three different automated medication systems implemented in a Danish hospital setting. Appl Health Econ Health Policy 2018;16:91–106. 10.1007/s40258-017-0360-8 [DOI] [PubMed] [Google Scholar]
- 25. Viprey M, Burgos Leon-Djian C, Dode X, et al. The effect of a robotic unit dose drug dispensing system on medicines administration errors and the cost of drug dispensing [abstract]. Eur J Hosp Pharm 2013;20:A85 10.1136/ejhpharm-2013-000276.238 [DOI] [Google Scholar]
- 26. Al Nemari M, Zayed E, Bin Dous A, et al . Impact of robotics on patient safety and productivity [abstract]. Eur J Hosp Pharm 2019;26:A247. 10.1136/ejhpharm-2019-eahpconf.532 [DOI] [Google Scholar]
- 27. Martinez L, Bloch V, Jacob A, et al . Sécurisation de la dispensation individuelle et nominative suite l’implantation d’un automate de dispensation nominative: cartographie des risques a priori au sein d’un pharmacie usage intérieur. Securing the distribution of patient-specific unit dose medication following the installation of a unit dose dispensing system: cartography of risks a priori within a hospital pharmacy. Ann Pharm Fr 2018;76:473–88. 10.1016/j.pharma.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 28. Ragan R, Bond J, Major K, et al. Improved control of medication use with an integrated bar-code-packaging and distribution system. Am J Health Syst Pharm 2005;62:1075–9. 10.1093/ajhp/62.10.1075 [DOI] [PubMed] [Google Scholar]
- 29. Oldland AR, Golightly LK, May SK, et al. Electronic inventory systems and barcode technology: impact on pharmacy technical accuracy and error liability. Hosp Pharm 2015;50:34–41. 10.1310/hpj5001-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poon EG, Keohane CA, Yoon CS, et al. Effect of bar-code technology on the safety of medication administration. N Engl J Med 2010;362:1698–707. 10.1056/NEJMsa0907115 [DOI] [PubMed] [Google Scholar]
- 31. Costantini A, Di Candilo C, Cinalli C, et al. Is the unit dose process a tool for patient safety and for implementing ‘lean thinking’ in the drug supply chain? [abstract] Eur J Hosp Pharm 2014;21:A65–6. 10.1136/ejhpharm-2013-000436.161 [DOI] [Google Scholar]
- 32. Corridoni S, Sorice P, Armillei L, et al . Clinical risk management through the ‘unit dose’ system [abstract]. Eur J Hosp Pharm 2021;28:A160–1. 10.1136/ejhpharm-2021-eahpconf.334 [DOI] [Google Scholar]
- 33. Walsh EK, Hansen CR, Sahm LJ, et al. Economic impact of medication error: a systematic review. Pharmacoepidemiol Drug Saf 2017;26:481–97. 10.1002/pds.4188 [DOI] [PubMed] [Google Scholar]
- 34. Monbaliu S, Beckers M, Stroo K, et al . Evaluation of the implementation of the automated medication organization [abstract]. Eur J Hosp Pharm 2017;24:A109. 10.1136/ejhpharm-2017-000640.241 [DOI] [Google Scholar]
- 35. McLeod MC, Barber N, Franklin BD. Methodological variations and their effects on reported medication administration error rates. BMJ Qual Saf 2013;22:278–89. 10.1136/bmjqs-2012-001330 [DOI] [PubMed] [Google Scholar]
- 36. Keers RN, Williams SD, Cooke J, et al. Prevalence and nature of medication administration errors in health care settings: a systematic review of direct observational evidence. Ann Pharmacother 2013;47:237–56. 10.1345/aph.1R147 [DOI] [PubMed] [Google Scholar]
- 37. Veyrier M, Nicolas L, Cavagna P, et al . Intégration d’un automate de dispensation nominative au sein d’une pharmacies usage intérieure: analyse prospective de processus et mise en place de mesures correctives. Integration of an automated personalized drug dispensing system within a hospital pharmaceutical service: prospective processes analysis and implementation of corrective measures. Le Pharmacien Hospitalier et Clinicien 2019;54:145–55. 10.1016/j.phclin.2018.10.057 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. Not applicable.