Abstract
The protozoan parasite Cryptosporidium parvum is a significant cause of diarrheal disease worldwide. Attachment to and invasion of host intestinal epithelial cells by C. parvum sporozoites are crucial steps in the pathogenesis of cryptosporidiosis. The molecular basis of these initial interactions is unknown. In order to identify putative C. parvum adhesion- and invasion-specific proteins, we raised monoclonal antibodies (MAbs) to sporozoites and evaluated them for inhibition of attachment and invasion in vitro. Using this approach, we identified two glycoproteins recognized by 4E9, a MAb which neutralized C. parvum infection and inhibited sporozoite attachment to intestinal epithelial cells in vitro. 4E9 recognized a 40-kDa glycoprotein named gp40 and a second, >220-kDa protein which was identified as GP900, a previously described mucin-like glycoprotein. Glycoproteins recognized by 4E9 are localized to the surface and apical region of invasive stages and are shed in trails from the parasite during gliding motility. The epitope recognized by 4E9 contains α-N-acetylgalactosamine residues, which are present in a mucin-type O-glycosidic linkage. Lectins specific for these glycans bind to the surface and apical region of sporozoites and block attachment to host cells. The surface and apical localization of these glycoproteins and the neutralizing effect of the MAb and α-N-acetylgalactosamine-specific lectins strongly implicate these proteins and their glycotopes as playing a role in C. parvum-host cell interactions.
Cryptosporidium parvum, an intestinal Apicomplexan parasite, is a significant cause of diarrheal disease worldwide (15, 17). In immunocompetent individuals, the disease is usually self-limiting, but it may be chronic and life threatening in immunocompromised patients such as those with AIDS. Recently, the parasite has gained notoriety as the causative agent of numerous outbreaks of waterborne diarrheal disease. There is currently no effective specific therapy approved for disease caused by this parasite.
Infection is initiated by ingestion of oocysts, which undergo excystation to release sporozoites. Attachment of sporozoites to epithelial cells and subsequent invasion of the host cell membrane are crucial primary steps in the pathogenesis of cryptosporidiosis. The ultrastructural aspects of attachment and invasion have been characterized in detail (10, 24, 33, 34). Sporozoites attach to host cells by their anterior pole. Attachment is followed by invagination of the host cell plasma membrane, which extends along the surface of the sporozoite and eventually completely surrounds it, leading to formation of a parasitophorus vacuole where the parasite undergoes further development in a unique intracellular but extracytoplasmic location.
Using in vitro models of sporozoite attachment to epithelial cells, we previously showed that attachment was dose and time dependent and was influenced by pH, divalent cations, and the degree of differentiation of host cells (20, 22). Further, attachment could be inhibited by polyclonal antibodies to C. parvum proteins as well as by glycoconjugates specific for a sporozoite surface Gal/GalNAc-binding lectin which we had previously described (20–22). A recent study confirmed the role of Gal/GalNAc-specific lectin-carbohydrate interactions in attachment (4). Previous studies have also reported that C. parvum infection in vitro can be inhibited by polyclonal or monoclonal antibodies to C. parvum proteins (5, 7, 9, 11, 23). In addition, sporozoite motility and invasion have been shown to be dependent on parasite and host cell cytoskeletal elements (4, 12, 13).
Although ultrastructural details and various factors affecting attachment and invasion have been characterized, little is known about the molecular basis of these initial host-parasite interactions or of specific parasite and host molecules which mediate them (38). Knowledge of such molecules is crucial for understanding the pathogenic mechanisms involved in the host-parasite interaction and for designing preventive and interventional strategies to combat cryptosporidiosis. The aim of this study was to identify and characterize specific parasite proteins that may be involved in the initial C. parvum-host cell interactions of attachment and invasion. In order to do this, we raised monoclonal antibodies (MAbs) to sporozoite surface proteins and screened them for inhibition of attachment and infection in vitro. In this study, we describe two C. parvum glycoproteins identified by 4E9, a MAb to a carbohydrate epitope present in multiple developmental stages of the parasite, which inhibits attachment and infection in vitro.
MATERIALS AND METHODS
Parasites.
C. parvum oocytes of the GCH1 isolate (36) were treated with 1.75% (vol/vol) sodium hypochlorite for 10 min on ice; washed with Dulbecco modified Eagle medium (Life Technologies, Grand Island, N.Y.) containing 25 mM HEPES, 100 U of penicillin per ml, and 100 μg of streptomycin per ml, and excysted for 2 h at 37°C or for 1 h in the presence of 0.25% trypsin and/or 0.75% taurocholic acid. Sporozoites were purified by isopycnic Percoll gradient centrifugation (1) or by filtration through a 2.0-μm-pore-size Nucleopore polycarbonate filter (Costar Scientific Corporation, Cambridge, Mass.).
Shed proteins (SP) were obtained by excystation of oocysts in Dulbecco modified Eagle medium for 2 h at 37°C, followed by centrifugation at 5000 × g at 4°C for 10 min. Protease inhibitors (final concentrations of 2 mM phenylmethylsulfonyl fluoride, 20 μM leupeptin, 10 μM E64, and 2 mM EDTA) were added to the supernatant, which was concentrated 10-fold by ultrafiltration. The excystation rate using this protocol ranged from 40 to 60% (depending on the age of the oocysts), compared to 60 to 80% when excystation was performed in the presence of trypsin and/or taurocholic acid. This method was used to obtain SP in order to avoid inclusion of proteins that may be released from the surface of the parasite by trypsin and/or taurocholic acid.
Other protozoan parasites were provided by A. Kane, Center for Gastroenterology Research in Absorptive and Secretory Processes, New England Medical Center, Boston, Mass. (Giardia lamblia trophozoites and Entameba histolytica trophozoites); M. E. A. Pereira, Tufts University School of Medicine, Boston, Mass. (Trypanosoma cruzi trypomastigotes and Leishmania major promastigotes); and K. Kim, Albert Einstein School of Medicine, New York, N.Y. (Toxoplasma gondii tachyzoites).
Cell culture.
Caco-2A (human intestinal epithelial) cells were obtained from the cell culture core of the Center for Gastroenterology Research in Absorptive and Secretory Processes at New England Medical Center and grown as described previously (22).
MAbs.
In order to obtain MAbs to surface epitopes, sporozoites were fixed with 1% glutaraldehyde for 30 min on ice, residual aldehyde groups were blocked with 0.1 M glycine, and sporozoites were washed with phosphate-buffered saline (PBS). BALB/c mice were immunized intraperitoneally with fixed sporozoites in complete Freund's adjuvant, followed by three intraperitoneal boosts with the same preparation using incomplete Freund's adjuvant. Spleen cells were fused with P3 × 63/Ag mouse myeloma cells and cloned in liquid medium. Clones were screened for reactivity with the surface and/or apical region of sporozoites by immunofluorescence (IF).
Clone 4E9, which showed the strongest reactivity by IF, was chosen for further study. This clone was further subcloned and determined to be of the immunoglobulin M (IgM) (kappa) isotype using an enzyme-linked immunosorbent assay (ELISA)-based isotyping kit (Southern Biotechnology Associates, Inc., Birmingham, Ala.). Ascites fluid was obtained following injection of 4E9 hybridoma cells into Pristane-primed BALB/c mice. B9A4, an IgM control MAb against Brugia malayi phosphorylcholine, was generously provided by C. Carlow, New England Biolabs, Beverly, Mass. 7B3, an IgG1 MAb to GP900 (26), was obtained as a kind gift from C. Petersen, University of California, San Francisco. 4E9 and B9A4 IgM were purified from ascites fluid using an E-Z-SEP purification kit (Pharmacia Biotech Inc., Piscataway, N.J.).
IF and FITC-lectin-binding assays.
Purified oocysts were placed on poly-l-lysine (30 μg/ml in water)-coated slides. Purified sporozoites were allowed to glide on poly-l-lysine-coated slides for 30 min at room temperature (RT). For intracellular stages, Caco-2A cells grown to confluence in 16-well chamber slides were infected with oocysts for 24 h (37). Slides with oocysts and sporozoites were fixed with 4% paraformaldehyde for 10 min at RT. Slides with intracellular stages were fixed and permeabilized with methanol. IF was performed as described previously (20). Controls included culture medium and the irrelevant IgM MAb B9A4. Binding of the following fluorescein isothiocyanate (FITC)-conjugated lectins (Sigma, St. Louis, Mo.) (2 μg/ml in PBS) was performed as described earlier (39): Helix pomatia agglutinin (HPA), Maclura pomifera agglutinin (MPA), Artocarpus integrifolia agglutinin (AIA) (Jacalin), and Sambucus nigra agglutinin (SNA). Specificity of binding was determined by preincubation of the lectin with its cognate sugar inhibitor (as described for lectin blotting below).
Immunoelectron microscopy (IEM).
Piglets and gamma interferon (IFN-γ)-knockout mice were infected with C. parvum oocysts as described previously (16, 35). Intestinal tissue, oocysts, and sporozoites were fixed in 0.25% glutaraldehyde–4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.2) (PB), at 4°C overnight. Oocysts and sporozoites were pelleted by centrifugation, and 2% agarose was added and allowed to solidify. Samples were washed with PB, dehydrated, and embedded in L. R. White Resin (Electron Microscopy Sciences, Fort Washington, Pa.). Silver-gold sections from polymerized blocks were placed on Formvar-coated 300-mesh nickel grids. Grids with sections were floated on drops of 0.05 M glycine–0.5 M NaCl–1% Tween 20–0.05 M Tris (pH 7.5) (TBST) for 30 min, on 1% bovine serum albumin (BSA) in TBST for 10 min, and on 10% normal goat serum (NGS) in TBST for 10 min. Grids were then placed on 4E9 IgM at 4°C for 1 h, followed by 1% BSA in TBST for 4 min and lastly on 10-nm-diameter colloidal gold conjugated to goat anti-mouse Ig diluted in 5% BSA–1% NGS in TBST for 30 min. Grids were washed, stained with 3% aqueous uranyl acetate, and viewed by transmission electron microscopy.
Immunoblotting and lectin blotting.
Parasite proteins were separated by gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (5 to 15% polyacrylamide) and transferred to nitrocellulose for 18 h at 395 mA at 4°C. Bound proteins were probed with MAb 4E9 and detected by chemiluminescence or colormetric methods. For the chemiluminescence method, nonspecific binding was blocked with 10% NGS in 10 mM Tris-HCl–150 mM sodium chloride (pH 8.0) (TBS) for 1 h before incubation with MAb 4E9 in 5% NGS in 0.1% Tween 20 in TBS (0.1% T-TBS) for 90 min at RT. After being washed three times with 0.1% T-TBS, strips were incubated with horseradish peroxidase-conjugated goat anti-mouse antibody (Immunopure; Pierce) diluted in 5% NGS–0.1% T-TBS for 1 h at RT. The strips were washed, incubated in SuperSignal substrate (Pierce), exposed to film, and developed. For the colormetric method, nonspecific binding was blocked with 5% nonfat milk (NFM) in TBS for 1 h before incubation with MAb 4E9 in 1% NFM in TBS for 1 h at RT. After being washed with 0.05% T-TBS, strips were incubated with alkaline phosphatase-conjugated goat anti-mouse antibody (Promega, Madison, Wis.) in 1% NFM in TBS for 1 h at RT. The strips were washed and developed with nitroblue tetrazolium (NBT)–5-bromo-4-chloro-3-indolylphosphate (BCIP) substrate. Competitive inhibition of 4E9 binding by HPA was evaluated by incubating the strips with HPA (50 μg/ml) for 10 min at RT before incubation with 4E9.
For lectin blotting, nonspecific binding was blocked with 0.1% Tween 20 in PBS (0.1% T-PBS) for 1 h at RT. Blots were incubated with the following biotinylated lectins (EY Laboratories, Inc., San Mateo, Calif.) diluted in 0.1% T-PBS (10 μg/ml) for 1 h at RT: HPA, AIA, MPA, SNA, Glycine max agglutinin (soybean agglutinin [SBA]), Dolichos biflorus agglutinin (DBA), Arachis hypogaea agglutinin (peanut agglutinin [PNA]), Sophora japonica agglutinin (SJA), Ulex europaeus agglutinin-1 (UEA-1), Maackia amurensis agglutinin (MAA), Canavalia ensiformis agglutinin (concanavalin A agglutinin [ConA]), and Triticum vulgare (wheat germ agglutinin [WGA]). To assess specificity of binding, lectins were preincubated with the following specific sugars (250 mM): N-acetylgalactosamine (GalNAc) (for SBA, HPA, DBA, and SJA), β-lactose (for PNA, SNA, and MAA), N-acetylglucosamine (for WGA), melibiose (for AIA and MPA), methyl-α-mannopyranoside (for ConA), and fucose (for UEA-1). Blots were washed with 0.1% T-PBS, incubated with an avidin-biotin-alkaline phosphatase complex (ABC reagent; Vector, San Mateo, Calif.) for 1 h at RT, washed, and developed with NBT-BCIP substrate.
Immunoprecipitation.
A mixture of sporozoites and oocysts (derived from excystation of 108 oocysts) in PBS containing protease inhibitors (described above) was lysed by five freeze-thaw cycles and detergent extraction with 1% Triton X-100. The lysate was centrifuged at 10,000 × g for 30 min. Detergent-soluble material was incubated with MAb 7B3 overnight, followed by incubation with protein G-Sepharose (Pharmacia Biotech Inc.) for 2 h at 4°C. After extensive washing with 20 mM sodium phosphate–0.5 M NaCl–0.5% Triton X-100–0.1% SDS–0.1% deoxycholate, immunoprecipitated proteins were analyzed by SDS-PAGE and immunoblotting with MAb 4E9.
Infection and attachment assays.
The effect of MAb 4E9 on C. parvum infection of Caco-2A cells was studied by a modification of an in vitro assay described earlier (37). Briefly, oocysts (1 × 104/well) preincubated with 4E9 or B9A4 IgM for 30 min at RT were incubated with Caco-2A cells (2 × 104/well) grown in 96-well tissue culture plates for 24 h at 37°C with 5% CO2. Cells were fixed, permeabilized with methanol for 10 min at RT, and washed three times with TBS. Infection was quantified by ELISA (37) using a polyclonal rabbit anti-C. parvum antibody (20) that recognizes >30 proteins, ranging from 14 to >200 kDa, present on sporozoites, merozoites, and intracellular stages but not oocysts.
The effect of MAb 4E9 or lectins on attachment of sporozoites to glutaraldehyde-fixed Caco-2A cells was studied using an in vitro assay as described previously (22). Briefly, Caco-2A cells grown to confluence in 96-well plates were fixed with glutaraldehyde to prevent invasion. Sporozoites were preincubated with test or control antibodies or lectins for 30 min at 4°C. For attachment assays using lectins, sporozoites were washed twice with assay buffer after preincubation with the lectins. Controls (sporozoites in assay buffer) were treated in the same way. The sporozoites were then incubated with the glutaraldehyde-fixed Caco-2A monolayers for 1 h at 37°C. Unbound parasites were washed off, and attached sporozoites were quantified by ELISA using the same antibody as for the infection assay.
Cytotoxicity assay.
Caco-2A cells grown in 96-well plates were incubated with or without purified MAb IgM (100 μg/ml) for 24 h at 37°C with 5% CO2. Possible cytotoxicity of the MAbs for the cells was assessed by measuring viability using a CellTiter96 AQueous kit (Promega).
Periodate oxidation and glycosidase digestion.
SP were separated by SDS-PAGE and transferred to nitrocellulose. Strips were incubated with 50 mM sodium acetate buffer (pH 4.5) (SAB) alone (as a control) or with 10 mM periodic acid in SAB in the dark for 1 h at RT. The strips were rinsed with SAB and incubated with 50 mM sodium borohydride in PBS for 30 min at RT and then probed with 4E9 as described above.
SP were treated with the following glycosidases (Oxford Glycosciences, Bedford, Mass.) at 37°C overnight, according to the manufacturer's recommendations: peptide-N-glycosidase F (recombinant) (50 U/ml), endo-α-N-acetylgalactosaminidase (from Streptococcus pneumoniae) (111 mU/ml), and α-N-acetylgalactosaminidase (from chicken liver) (300 mU/ml). As controls for the various glycosidases, SP were incubated with buffer alone under identical conditions. Specificity of α-N-acetylgalactosaminidase activity was assessed by addition of paranitrophenyl α-N-acetylgalactosaminide to a final concentration of 2.5 mM.
Statistical methods.
Attachment, infection, antigen-binding, and cytotoxicity assays were performed in three to six replicates, and the mean and standard error (SE) of the mean were determined. All assays were repeated at least three times. Results from representative assays are shown. Comparison of means was performed using a nonpaired Student t test. A P value of <0.05 was considered statistically significant.
RESULTS
MAb 4E9 neutralizes C. parvum infection of intestinal epithelial cells and inhibits sporozoite attachment to these cells in vitro.
In order to identify putative C. parvum adhesion- and invasion-specific proteins, we raised MAbs to sporozoites and screened them by IF to identify clones reactive with the surface or anterior portion of sporozoites (suggestive of an apical complex localization). Using this approach, we identified 4E9, an IgM MAb, which showed the strongest reactivity of all the clones by IF. To determine whether proteins recognized by 4E9 were involved in initial host-parasite interactions, we evaluated the effect of this MAb on C. parvum infection of intestinal epithelial cells using an in vitro assay (37). Compared to an irrelevant isotype-matched control MAb, 4E9 IgM neutralized infection of Caco-2A cells in a dose-dependent manner, with almost complete inhibition occurring at a concentration of 100 μg/ml (Fig. 1A). The results obtained with this assay were similar to those obtained using a previously described IF-based assay (37) in which intracellular stages are directly visualized by phase-contrast and epifluorescence microscopy (R. Verdon and H. Ward, unpublished data). The inhibition of infection was not due to toxicity of 4E9 for host cells, since the MAb had no effect on viability (data not shown). 4E9 IgM did not agglutinate sporozoites or oocysts at the concentrations used, indicating that the inhibitory effect was not due to agglutination of the parasite.
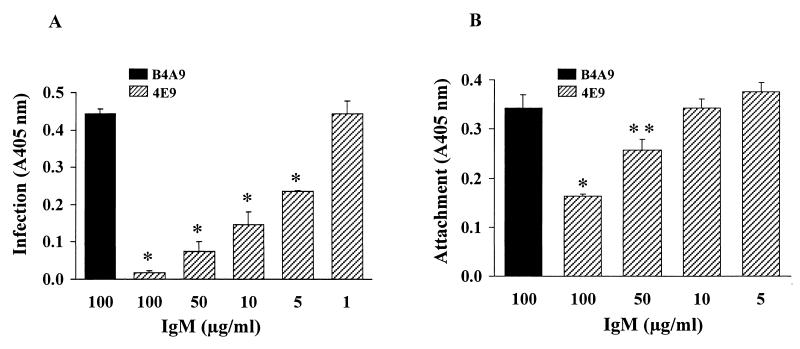
FIG. 1.
Effect of MAb 4E9 on C. parvum infection of and attachment to Caco-2A cells. Oocysts (A) or sporozoites (B) were preincubated with increasing concentrations of 4E9 or control IgM and allowed to infect (A) or attach to (B) Caco-2A cells. Infection and attachment were quantified by ELISA. Results are expressed as the mean of absorbance values at 405 nm ± SE. ∗, P < 0.001 compared to control IgM, ∗∗, P < 0.05 compared to control IgM.
In order to determine if the antibody inhibited infection by blocking adherence of sporozoites to intestinal cells, we studied its effect on attachment using an in vitro assay in which cells are fixed to prevent invasion (22). As seen in Fig. 1B, 4E9 inhibited sporozoite attachment to fixed Caco-2A cells in a dose-dependent manner, with 50% inhibition occurring at 100 μg/ml. The decrease in attachment measured by ELISA was not due to reduced binding of the detecting polyclonal antibody to proteins recognized by 4E9, since similar results were obtained using an IF-based assay (20) in which attached sporozoites are directly visualized by phase-contrast and epifluorescence microscopy (D. Hamer and H. Ward, unpublished data). These results suggested that the neutralizing effect of the MAb is mediated at least in part by blocking of sporozoite attachment to host cells, resulting in subsequent inhibition of infection.
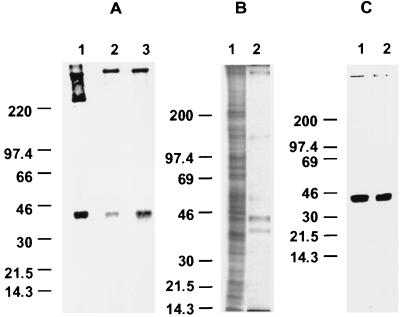
FIG. 4.
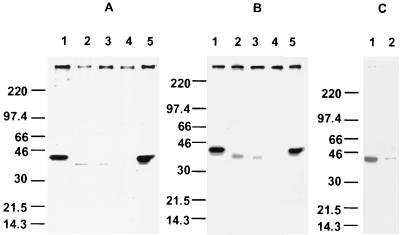
Identification of proteins recognized by 4E9. Oocysts, sporozoites, and SP were analyzed for reactivity with MAb 4E9 by immunoblot analysis. (A) Reactivity of MAb 4E9 with oocysts (lane 1), sporozoites (lane 2), and shed proteins (lane 3) by immunoblotting. (B and C) Protein profiles of oocyst and sporozoite proteins (lane 1) and SP (lane 2) as determined by silver staining (B) and immunoblotting with 4E9 (C). Numbers are molecular weights in thousands.
The epitope recognized by 4E9 is present in multiple developmental stages.
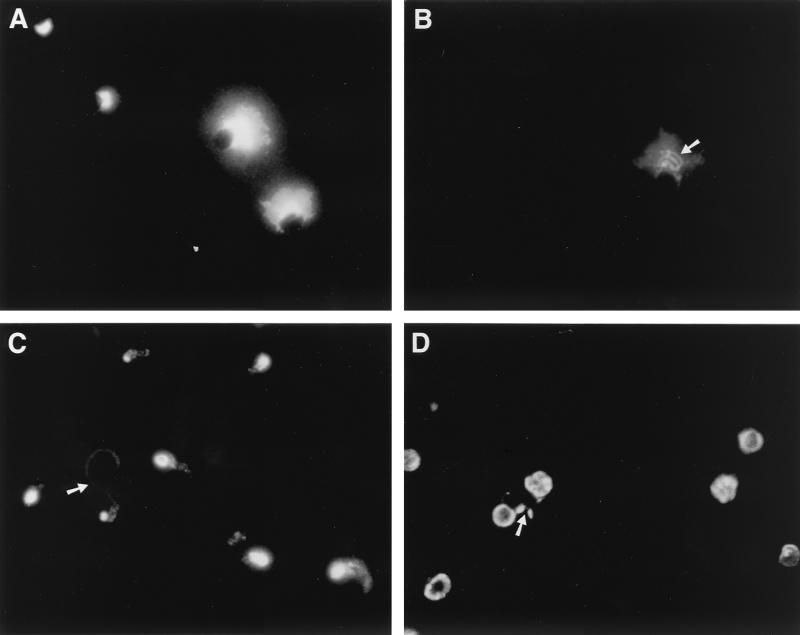
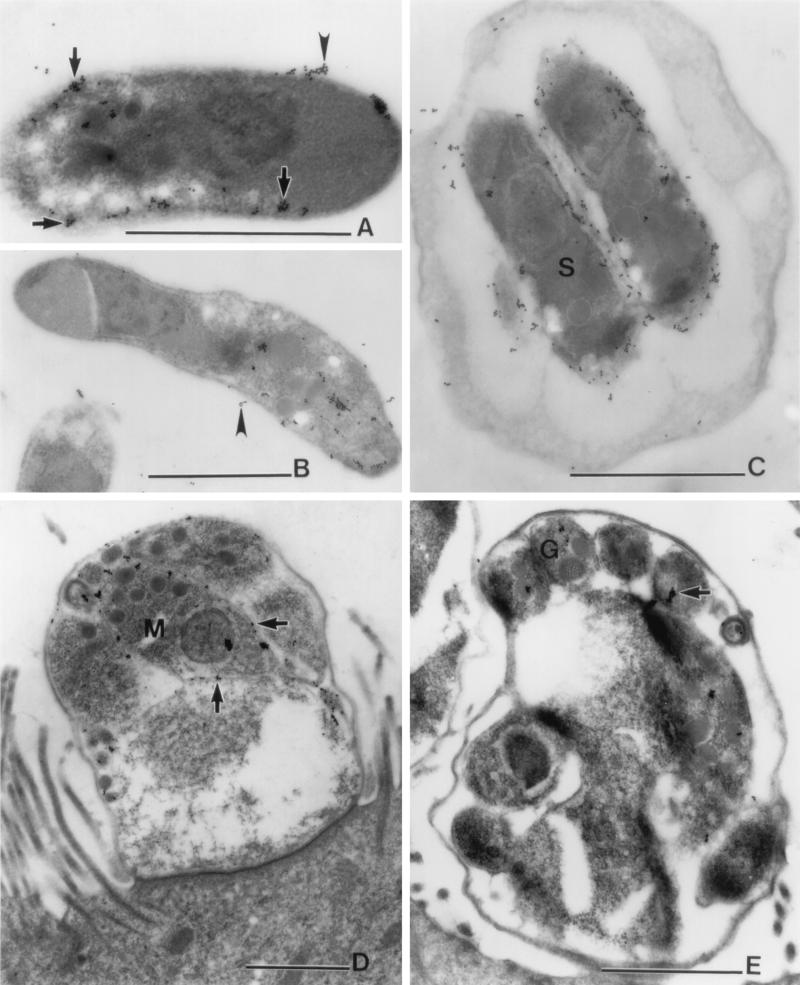
We used an IF assay to localize the epitope recognized by 4E9 in various developmental stages (Fig. 2). This MAb reacted with material shed from oocysts that were undergoing excystation (Fig. 2A) as well as with the surface of newly excysted sporozoites (Fig. 2B). In addition, 4E9 reacted with the surface and apical region of purified sporozoites as well as with material shed in trails from sporozoites during gliding motility (Fig. 2C). In infected Caco-2A cells, 4E9 reacted with the surface of merozoites within meronts or newly released from them (Fig. 2D). These findings were confirmed and extended by IEM (Fig. 3). Label was detected at the surface of sporozoites and in locations surrounding the sporozoite (Fig. 3A and B), consistent with shedding of the protein from the surface. Apical complex organelles were not visualized in this preparation. In oocysts (Fig. 3C), label was localized on the surface of sporozoites. Examination of meronts in infected pig ileum (Fig. 3D) revealed labeling on the surface of merozoites. In addition, there was reactivity with microgametes in IFN-γ-knockout mouse infected intestine (Fig. 3E). Taken together, these results showed that proteins containing the epitope recognized by MAb 4E9 are shed in trails during gliding motility and suggested a surface as well as possible apical complex organelle localization in sporozoites and merozoites.
FIG. 2.
Reactivity of MAb 4E9 with C. parvum stages as determined by IF. (A and B) Oocysts. Material shed from excysting oocysts (A) and the surface of newly excysted sporozoites (arrow) (B) is labeled. (C) Sporozoites. The surface and apical region of sporozoites and of material shed in trails (arrow) from sporozoites allowed to glide on poly-l-lysine coated slides are labeled. (D) Intracellular stages. Meronts and merozoites newly emerged from them (arrow) in infected Caco-2A cells are labeled.
FIG. 3.
Localization of the epitope recognized by MAb 4E9 on C. parvum stages as determined by immunoelectron microscopy. (A and B) Sporozoites. Label is present on the surface (arrows) and in locations surrounding the sporozoites (arrowheads). (C) Oocysts. Label is present on the surface of sporozoites (S) within the oocyst. (D) Meront from ileum of infected piglet intestinal tissue. Label (arrows) is on the surface of merozoites (M). (E) Microgamont from intestine of infected IFN-γ-knockout mouse. Label (arrow) is present on microgametes (G). Bars, 1 μm.
MAb 4E9 recognizes two major C. parvum peptides of 40 and >900 kDa.
The epitope recognized by MAb 4E9 was present on two major bands as determined by immunoblotting: a 40-kDa peptide and a very-high-molecular-mass peptide of >220 kDa (Fig. 4A). The latter peptide comigrated with a previously described >900-kDa glycoprotein (GP900) recognized by MAb 7B3 (26) by immunoblotting (data not shown). Oocyst and sporozoite proteins immunoprecipitated by MAb 7B3 were recognized by 4E9 by immunoblotting, confirming that this peptide was GP900 (data not shown). The 40-kDa protein (designated gp40) and GP900 were present in oocysts and sporozoites. An additional 250-kDa band was also detected only in oocysts (Fig. 4A). There was no reactivity of the control IgM MAb B4A9 (not shown) with any of these proteins, confirming the specificity of 4E9. In addition, 4E9 did not react with proteins from other protozoa (Giardia lamblia, E. histolytica, Toxoplasma gondii, Trypanosoma cruzi, and L. major) in immunoblotting (not shown), indicating that the epitope recognized by MAb 4E9 is specific for Cryptosporidium.
Analysis of SP by SDS-PAGE and silver staining revealed the presence of ∼5 major bands of >900, 120, 55, 40, and 35 kDa (Fig. 4B). Two of these, the >900- and 40-kDa bands, were recognized by MAb 4E9 by immunoblotting, confirming the IF finding that these proteins are shed from the parasite (Fig. 4C). Since this preparation (SP) was enriched in these two proteins, it was used for further characterization of the epitope recognized by 4E9.
The epitope recognized by 4E9 is glycosylated and contains α-linked GalNAc residues.
Reactivity of 4E9 with GP900 and gp40 was abolished by mild periodate treatment, suggesting that the epitope is glycosylated (not shown). In order to determine the nature of the carbohydrates present, SP were probed with a panel of biotinylated lectins with various sugar specificities (Table 1). Of these, HPA, SBA, AIA, MPA, UEA-1, and WGA bound to both GP900 and gp40, whereas ConA and DBA bound to GP900 but not gp40. Binding was specific, since it could be inhibited by the appropriate cognate sugar hapten (not shown). Of note was the binding of a number of αGalNAc-specific lectins. The patterns of reactivity of αGalNAc-specific lectins such as HPA were identical to that of 4E9 (Fig. 5A and B). In addition, reactivity of 4E9 could be competitively inhibited by HPA (Fig. 5C). These results suggested that the epitope recognized by 4E9 contains the same carbohydrate residues as that bound by the αGalNAc-specific lectins.
TABLE 1.
Lectin binding profiles of GP900 and gp40
| Lectin | Monosaccharide specificity | Oligosaccharide specificitya | Binding with:
|
|
|---|---|---|---|---|
| GP900 | gp40 | |||
| HPA | αGalNAc | Tn, T, F, A | + | + |
| DBA | αGalNAc | F, A | + | − |
| AIA | αGal, αGalNAc | Tn, T | + | + |
| SBA | α (β) GalNAc | Tn, T | + | + |
| MPA | αGalNAc, αGal | Tn, T | + | + |
| PNA | βGal | T | − | − |
| SJA | βGalNAc, Gal | T | − | − |
| UEA-1 | αl-Fuc | Fuc α1-2Galβ1-4GlcNAc | + | + |
| ConA | Glc, Man | Branched Man | + | − |
| WGA | GlcNAc | GlcNAcβ1-4GlcNAc | + | + |
| SNA | Neu5NAc, βGal | Neu5NAc α2-6 Gal | − | − |
| MAA | Neu5NAc | Neu5NAc α2-3 Gal | − | − |
Tn, GalNAc α1-Ser/Thr; T, Galβ1-3GalNAc; F, GalNAcα1-3GalNAc; A, GalNAcα1-3Gal.
FIG. 5.
Glycosylation of proteins recognized by 4E9. (A and B) SP were treated with increasing concentration of α-N-acetylgalactosaminidase (lane 1, no enzyme; lane 2, 100 mU/ml; lane 3, 200 mU/ml; lane 4, 300 mU/ml; lane 5, 300 mU/ml with 2.5 mM para-nitrophenyl-α-N-acetylgalactosaminide) and analyzed by immunoblotting with 4E9 (A) and lectin blotting with HPA (B). (C) Blots of SP were pre-incubated with HPA (lane 2) or buffer alone (lane 1) and then analyzed by immunoblotting with 4E9. Numbers are molecular weights in thousands.
In order to determine the nature of the glycosidic linkage of the saccharides in the epitope recognized by 4E9, SP were treated with specific glycosidases and probed with 4E9 by immunoblotting. Treatment with peptide-N-glycosidase F (which cleaves N-linked glycans) had no effect on 4E9 reactivity (data not shown). Treatment with endo-α-N-acetylgalactosaminidase, which is specific for Galβ1-3GalNAc linked to Ser or Thr, also had no effect (not shown), suggesting that (i) this disaccharide is not part of the epitope recognized by 4E9, or (ii) if it is present, it is substituted with sialic acid, fucose, or GalNAc residues. Treatment with increasing concentrations of α-N-acetylgalactosaminidase, which cleaves terminal α1- 3GalNAc or GalNAcα 1-Ser/Thr from O-linked glycoproteins, resulted in decreasing reactivity with both 4E9 and the lectin HPA and a shift to 36 kDa for gp40 (Fig. 5A and B). This effect was specific since it could be inhibited by the substrate pNP α-N-acetyl-galactosaminide. These results suggest that GP900 and gp40 contain αGalNAc residues and that these residues are terminal in gp40.
αGalNAc-specific lectins bind to sporozoites and inhibit attachment to host cells.
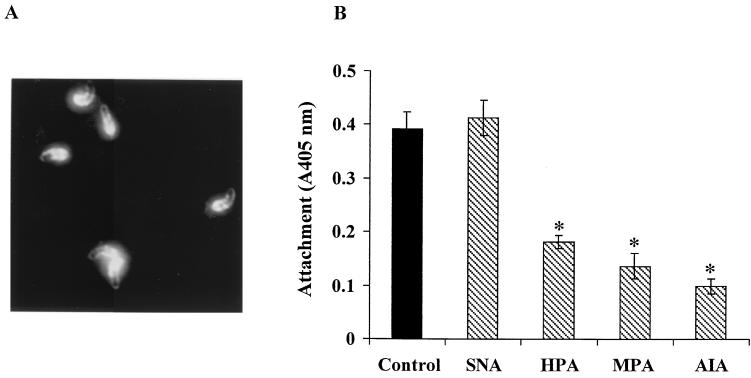
To investigate the possibility that αGalNAc residues present in the epitope recognized by 4E9 are involved in attachment, we first determined whether lectins specific for these residues bound to sporozoites. The results showed that the lectins HPA (Fig. 6A) and MPA and AIA (data not shown) all bound to the surface and apical region of sporozoites in a pattern similar to that of MAb 4E9. The binding was specific, since it could be inhibited by preincubation with the cognate sugar hapten (not shown). We next determined the effect of these lectins on sporozoite attachment to host cells. The results (Fig. 6B) showed that all three αGalNAc-specific lectins significantly inhibited attachment at a concentration of 10 μg/ml, whereas SNA, which did not bind to sporozoites (data not shown) or to GP900 and gp40 (Table 1) had no significant effect. Since the lectins were washed off after preincubation with the sporozoites, the inhibitory effect was not due to binding of the lectins to the host cells. These results strongly implicate the αGalNAc residues of GP900 and/or gp40 in mediating sporozoite attachment to host cells.
FIG. 6.
Binding of HPA to sporozoites and effect of lectins on attachment to host cells. (A) Binding of FITC-conjugated HPA to the surface and apical region of sporozoites. (B) Sporozoites were preincubated with lectins, washed, and allowed to attach to Caco-2A cells. Attachment was quantified by ELISA. Results are expressed as the mean of absorbance values at 405 nm ± SE. ∗, P < 0.001 compared to the control.
DISCUSSION
In this study, we have shown that 4E9, a MAb to a carbohydrate epitope present on two glycoproteins in multiple developmental stages of C. parvum, blocks attachment to and consequent infection of host cells in vitro. This suggested that the epitope recognized by the antibody and/or the glycoprotein(s) on which it is present is involved in mediating initial host-parasite interactions and could be a potential target for intervention. We therefore proceeded to characterize the epitope recognized by 4E9 and the glycoproteins on which it is present.
The epitope recognized by 4E9 was carbohydrate in nature, suggesting that the proteins on which it was present were glycosylated. This was confirmed by lectin binding to proteins recognized by 4E9. A striking finding was the binding of αGalNAc-specific lectins such as HPA, AIA, and MPA. Competitive inhibition of 4E9 binding by the lectin HPA and a similar pattern of binding of the FITC-conjugated lectins as determined by IF and immunoblotting confirmed that the epitope recognized by the MAb contains αGalNAc residues and suggested that these glycans may be involved in mediating attachment. This possibility was strengthened by the finding that the αGalNAc-specific lectins inhibited sporozoite attachment to host cells. The inhibitory effect of the lectins on attachment is consistent with a recent study which reported irreversible inhibition of C. parvum infection of epithelial cells in vitro by αGalNAc-specific lectins (19). The αGalNAc residues may mediate attachment by direct interaction with host carbohydrate-binding proteins. Alternatively, these glycans may function as a bridge between host and parasite carbohydrate-binding proteins. The latter possibility is consistent with our earlier finding of a Gal- and GalNAc-specific sporozoite lectin which is implicated in C. parvum attachment (21, 22) and with a recent study confirming the involvement of Gal/GalNAc-specific lectin-carbohydrate interactions in attachment (4). Whether the protein moieties of these glycoproteins are also involved in attachment and, if so, the relative roles of the carbohydrate and protein components remain to be determined.
The major protein recognized by 4E9 by immunoblotting is a 40-kDa glycoprotein (gp40), which is present in oocysts and sporozoites and is shed from the parasite. The pattern of reactivity of αGalNAc-specific lectins with gp40 is suggestive of the presence of the Tn determinant, which consists of terminal αGalNAc residues linked to Ser/Thr residues of the protein backbone (40). The presence of terminal αGalNAc residues in gp40 was further confirmed by the decrease in reactivity and shift in Mr caused by α-N-acetylgalactosaminidase treatment. We have recently cloned and sequenced the gene encoding gp40 (3). Analysis of the deduced amino acid sequence of this gene revealed the presence of multiple predicted mucin-type O-glycosylation sites (many of which are present in a polyserine domain), which is consistent with the biochemical findings of O-linked GalNAc residues in gp40 in the present study.
The other protein recognized by 4E9 is a previously described glycoprotein named GP900, which is localized to micronemes of invasive stages and is shed from the surface of sporozoites (2, 26). GP900 has previously been shown to be N glycosylated (26). The pattern of lectin binding to GP900 was consistent with the presence of internal or terminal αGalNAc residues (40). α-N-Acetylgalactosaminidase treatment had no discernible effect on 4E9 reactivity with GP900, suggesting that the αGalNAc residues may be internal or that if they are terminal, as in the case of gp40, a small shift in molecular weight may not be resolved due to the very high Mr of the protein. The presence of αGalNAc residues suggests that GP900 contains mucin-type O-linked oligosaccharides. This possibility is strengthened by the presence of multiple threonine residues in two mucin-like domains of GP900, which could provide sites for O-glycosylation (2).
Mucins are a group of heterogeneous high-molecular-weight glycoproteins which are heavily O glycosylated (14). The lectin binding profiles of gp40 and GP900 are suggestive of the presence of mucin-type O glycosylation and in particular of the Tn (GalNAc α1-Ser/Thr) and T (Galβ1-3GalNAc) carbohydrate determinants. These determinants are commonly present in core structures of the carbohydrate side chains of mucins. They are usually cryptic in O-linked oligosaccharides of mucins in normal tissue, but they may be terminally exposed in mucins on tumor cells (40). In addition to GalNAc, N-acetylglucosamine (GlcNAc) and galactose (Gal) O-glycans of human intestinal and other mucins contain peripheral sugars, including sialic acid, which mask the core structures. However, unlike those of other mucins, the O-glycans of gp40 and GP900 do not appear to contain sialic acid (based on lack of reactivity with the sialic acid-specific lectins SNA and MAA), which is consistent with the presence of core structures such as the T and Tn determinants being terminally exposed. Binding of the lectin WGA (and lack of binding of the sialic-acid-specific lectins SNA and MAA) to both gp40 and GP900 is suggestive of the presence of GlcNAc residues. These residues may be present on N-linked oligosaccharides, since there are potential N-glycosylation sites in the deduced amino acid sequences of both GP900 and gp40 (2, 3). Alternatively, GlcNAc may be present in an O-glycosidic linkage to serine and threonine residues. Interestingly, this type of glycosylation is present in SREHP, a serine-rich surface membrane protein of the intestinal protozoan parasite Entameba histolytica, which is also implicated in host-parasite interactions (31).
The O-glycosylated state of mucins contributes greatly to their adhesive as well as protective functional properties. The mucin-type O-glycosylation profiles of both gp40 and GP900 and the finding of mucin-like domains in the deduced amino acid sequences of GP900 (2) and gp40 may therefore be consistent with a functional role for these proteins in mediating adherence to host cells. Mucin-like proteins of other protozoa have also been implicated as playing a role in host-parasite interactions. Thus, Trypanosoma cruzi expresses an abundance of mucin-like proteins on the surface, encoded by a large gene family (6, 30). These proteins are shed from the surface and are believed to mediate invasion of host cells by the parasite.
Other investigators have described the presence of high-molecular-weight glycoproteins with features similar to GP900 in C. parvum (reviewed in reference 38). Riggs et al. described a 1,300-kDa glycoprotein, CSL, which was identified using a MAb to a carbohydrate epitope (28). This MAb neutralized infection in vitro, suggesting that the protein may be involved in attachment and/or invasion. A recent study by that group confirmed that CSL contains a sporozoite ligand for attachment to intestinal epithelial cells (23). However, as those authors point out, CSL and GP900 appear to be different, since the former but not the latter is localized to dense granules (28). In addition, posterior translocation of CSL (but not GP900) occurs along the sporozoite pellicle by a cytoskeletal-dependent process (28). A high-molecular-weight glycoprotein identified in association with 190- and 40-kDa bands by an IgM MAb, which reacted with sporozoites and intracellular stages as determined by IF, was described by McDonald et al. (25). Robert et al. also described a high-molecular-weight glycoprotein recognized by MAbs to a carbohydrate epitope, which reacted with multiple developmental stages, as determined by IEM (29). The relationship, if any, between GP900, CSL, and the other high-molecular-weight glycoproteins remains to be determined. However, as suggested by Langer and Riggs, the presence of multiple distinct high-Mr glycoproteins in C. parvum is consistent with their finding of several high-molecular-weight species of varying pI in silver-stained two-dimensional gels (23).
Gut and Nelson described a 15-kDa glycoprotein which also had a lectin-binding profile suggestive of Tn (GalNAc α1-Ser/Thr) or T (Galβ1-3GalNAc) carbohydrate determinants (18). This protein is also localized to the surface of sporozoites and is shed from them during gliding motility. Since the Tn and T determinants have been implicated in adhesion in other systems, those authors suggested that these determinants present on C. parvum glycoproteins might be involved in attachment and invasion and showed that C. parvum infection could be irreversibly inhibited by T- and Tn-specific lectins in vitro (19), a result consistent with our findings in the present study. Recently, the gene encoding this protein has been cloned and sequenced (3, 27, 32). Interestingly, we (3) and Strong et al. (32) have shown that this protein (named gp15) is encoded by the same gene encoding gp40 and that both proteins are most likely proteolytic fragments of a precursor protein expressed in intracellular stages of the parasite. Although gp15 has also been shown to display O-linked glycosylation, this protein apparently does not contain the epitope recognized by 4E9, since it is not recognized by this MAb on immunoblots.
Attachment and invasion of other Apicomplexans such as Toxoplasma and Plasmodium have been shown to be mediated by proteins exocytosed or shed from apical complex organelles (8). The proteins recognized by 4E9 have many features in common with these other Apicomplexan proteins. They are present in the invasive stages of the parasite, have a surface and/or apical location, are shed from the parasite, and are deposited in trails during gliding movement. Neutralization of attachment and infection by MAb 4E9 and by lectins specific for the epitope recognized by it implicates these proteins and/or their glycotopes in these interactions. A previous study showed that native GP900, a cysteine-rich domain of the recombinant protein, and antibodies to it competitively inhibited C. parvum infection of MDCK cells in vitro, suggesting that GP900 mediates attachment and invasion. We have recently shown that antibodies to gp40 block infection in vitro and that native gp40 binds specifically to intestinal epithelial cells, suggesting that this protein is also involved in attachment and/or invasion (3). The relative contributions of GP900 and gp40 to attachment and invasion are currently under investigation. However, it is very likely, as is the case in other Apicomplexans where multiple parasite and host molecules are involved, that both may have a role in these processes. The involvement of these glycoproteins in the initial host-parasite interaction provides a basis for devising strategies designed to inhibit this interaction and raises the possibility that they may serve as targets of potential specific preventive or therapeutic modalities.
ACKNOWLEDGMENTS
This work was supported by Public Health Service (PHS) grants AI33384 and AI40344 and by the cell culture core of the GRASP Digestive Disease Center (PHS grant DK34928P30). Davidson Hamer was supported by PHS training grant AI07389. Renaud Verdon was supported by a fellowship from the Programme Lavoisier (Ministère des Affaires Etrangères, France).
We gratefully acknowledge Smitha Jaison for technical assistance, Carolyn Petersen and Clothilde Carlow for MAbs, Kami Kim and Anne Kane for parasites, and Carolyn Petersen, Ajit Varki, and Shiv Pillai for helpful discussions.
REFERENCES
- 1.Arrowood M J, Sterling C R. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J Parasitol. 1987;73:314–319. [PubMed] [Google Scholar]
- 2.Barnes D A, Bonnin A, Huang J X, Gousset L, Wu J, Gut J, Doyle P, Dubremetz J F, Ward H, Petersen C. A novel multi-domain mucin-like glycoprotein of Cryptosporidium parvum mediates invasion. Mol Biochem Parasitol. 1998;96:93–110. doi: 10.1016/s0166-6851(98)00119-4. [DOI] [PubMed] [Google Scholar]
- 3.Cevallos A M, Zhang X, Waldor M K, Jaison S, Zhou X, Tzipori S, Neutra M R, Ward H D. Molecular cloning and expression of a gene encoding Cryptosporidium parvum glycoproteins gp40 and gp15. Infect Immun. 2000;68:4108–4116. doi: 10.1128/iai.68.7.4108-4116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X M, LaRusso N F. Mechanisms of attachment and internalization of Cryptosporidium parvum to biliary and intestinal epithelial cells. Gastroenterology. 2000;118:368–379. doi: 10.1016/s0016-5085(00)70219-8. [DOI] [PubMed] [Google Scholar]
- 5.Crabb J H. Antibody-based immunotherapy of cryptosporidiosis. Adv Parasitol. 1998;40:121–149. doi: 10.1016/s0065-308x(08)60119-0. [DOI] [PubMed] [Google Scholar]
- 6.Di Noia J M, D'Orso I, Aslund L, Sanchez D O, Frasch A C. The Trypanosoma cruzi mucin family is transcribed from hundreds of genes having hypervariable regions. J Biol Chem. 1998;273:10843–10850. doi: 10.1074/jbc.273.18.10843. [DOI] [PubMed] [Google Scholar]
- 7.Doyle P S, Crabb J, Petersen C. Anti-Cryptosporidium parvum antibodies inhibit infectivity in vitro and in vivo. Infect Immun. 1993;61:4079–4084. doi: 10.1128/iai.61.10.4079-4084.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubremetz J F, Garcia-Reguet N, Conseil V, Fourmaux M N. Apical organelles and host-cell invasion by Apicomplexa. Int J Parasitol. 1998;28:1007–1013. doi: 10.1016/s0020-7519(98)00076-9. [DOI] [PubMed] [Google Scholar]
- 9.Elliot B C, Wisnewski A V, Johnson J, Fenwick-Smith D, Wiest P, Hamer D, Kresina T, Flanigan T P. In vitro inhibition of Cryptosporidium parvum infection by human monoclonal antibodies. Infect Immun. 1997;65:3933–3935. doi: 10.1128/iai.65.9.3933-3935.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fayer R, Speer C A, Dubey J P. General biology of Cryptosporidium. In: Fayer R, editor. Cryptosporidiosis of man and animals. Boca Raton, Fla: CRC Press Inc; 1990. pp. 1–42. [Google Scholar]
- 11.Flanigan T, Marshall R, Redman D, Kaetzel C, Ungar B. In vitro screening of therapeutic agents against Cryptosporidium: hyperimmune cow colostrum is highly inhibitory. J Protozool. 1991;38:225S–227S. [PubMed] [Google Scholar]
- 12.Forney J R, DeWald D B, Yang S, Speer C A, Healey M C. A role for host phosphoinositide 3-kinase and cytoskeletal remodeling during Cryptosporidium parvum infection. Infect Immun. 1999;67:844–852. doi: 10.1128/iai.67.2.844-852.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forney J R, Vaughan D K, Yang S, Healey M C. Actin-dependent motility in Cryptosporidium parvum sporozoites. J Parasitol. 1998;84:908–913. [PubMed] [Google Scholar]
- 14.Forstner J F, Oliver M G, Sylvester F A. Production, structure and biologic relevance of gastrointestinal mucins. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press; 1995. pp. 71–88. [Google Scholar]
- 15.Griffiths J K. Human cryptosporidiosis: epidemiology, transmission, clinical disease, treatment, and diagnosis. Adv Parasitol. 1998;40:37–85. doi: 10.1016/s0065-308x(08)60117-7. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths J K, Theodos C, Paris M, Tzipori S. The gamma interferon gene knockout mouse: a highly sensitive model for evaluation of therapeutic agents against Cryptosporidium parvum. J Clin Microbiol. 1998;36:2503–2508. doi: 10.1128/jcm.36.9.2503-2508.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerrant R L. Cryptosporidiosis: an emerging, highly infectious threat. Emerg Infect Dis. 1997;3:51–57. doi: 10.3201/eid0301.970106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gut J, Nelson R G. Cryptosporidium parvum sporozoites deposit trails of 11A5 antigen during gliding locomotion and shed 11A5 antigen during invasion of MDCK cells in vitro. J Eukaryot Microbiol. 1994;41:42S–43S. [PubMed] [Google Scholar]
- 19.Gut J, Nelson R G. Cryptosporidium parvum: lectins mediate irreversible inhibition of sporozoite infectivity in vitro. J Eukaryot Microbiol. 1999;46:48S–49S. [PubMed] [Google Scholar]
- 20.Hamer D H, Ward H, Tzipori S, Pereira M E, Alroy J P, Keusch G T. Attachment of Cryptosporidium parvum sporozoites to MDCK cells in vitro. Infect Immun. 1994;62:2208–2213. doi: 10.1128/iai.62.6.2208-2213.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joe A, Hamer D H, Kelley M A, Pereira M E, Keusch G T, Tzipori S, Ward H D. Role of a Gal/GalNAc-specific sporozoite surface lectin in Cryptosporidium parvum-host cell interaction. J Eukaryot Microbiol. 1994;41:44S. [PubMed] [Google Scholar]
- 22.Joe A, Verdon R, Tzipori S, Keusch G T, Ward H D. Attachment of Cryptosporidium parvum sporozoites to human intestinal epithelial cells. Infect Immun. 1998;66:3429–3432. doi: 10.1128/iai.66.7.3429-3432.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langer R, Riggs M. Cryptosporidium parvum apical complex glycoprotein CSL contains a sporozoite ligand for intestinal epithelial cells. Infect Immun. 1999;67:5282–5291. doi: 10.1128/iai.67.10.5282-5291.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lumb R, Smith K, O'Donoghue P J, Lanser J A. Ultrastructure of the attachment of Cryptosporidium sporozoites to tissue culture cells. Parasitol Res. 1988;74:531–536. doi: 10.1007/BF00531630. [DOI] [PubMed] [Google Scholar]
- 25.McDonald V, McCrossan M V, Petry F. Localization of parasite antigens in Cryptosporidium parvum-infected epithelial cells using monoclonal antibodies. Parasitology. 1995;110:259–268. doi: 10.1017/s0031182000080847. [DOI] [PubMed] [Google Scholar]
- 26.Petersen C, Gut J, Doyle P S, Crabb J H, Nelson R G, Leech J H. Characterization of a >900,000-MrCryptosporidium parvum sporozoite glycoprotein recognized by protective hyperimmune bovine colostral immunoglobulin. Infect Immun. 1992;60:5132–5138. doi: 10.1128/iai.60.12.5132-5138.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Priest J W, Kwon J P, Arrowood M J, Lammie P J. Cloning of the immunodominant 17-kDa antigen from Cryptosporidium parvum. Mol Biochem Parasitol. 2000;106:261–271. doi: 10.1016/s0166-6851(99)00223-6. [DOI] [PubMed] [Google Scholar]
- 28.Riggs M W, Stone A L, Yount P A, Langer R C, Arrowood M J, Bentley D L. Protective monoclonal antibody defines a circumsporozoite-like glycoprotein exoantigen of Cryptosporidium parvum sporozoites and merozoites. J Immunol. 1997;158:1787–1795. [PubMed] [Google Scholar]
- 29.Robert B, Antoine H, Dreze F, Coppe P, Collard A. Characterization of a high molecular weight antigen of Cryptosporidium parvum micronemes possessing epitopes that are cross-reactive with all parasitic life cycle stages. Vet Res. 1994;25:384–398. [PubMed] [Google Scholar]
- 30.Schenkman S, Ferguson M A, Heise N, de Almeida M L, Mortara R A, Yoshida N. Mucin-like glycoproteins linked to the membrane by glycosylphosphatidylinositol anchor are the major acceptors of sialic acid in a reaction catalyzed by trans-sialidase in metacyclic forms of Trypanosoma cruzi. Mol Biochem Parasitol. 1993;59:293–303. doi: 10.1016/0166-6851(93)90227-o. [DOI] [PubMed] [Google Scholar]
- 31.Stanley S L, Jr, Tian K, Koester J P, Li E. The serine-rich Entamoeba histolytica protein is a phosphorylated membrane protein containing O-linked terminal N-acetylglucosamine residues. J Biol Chem. 1995;270:4121–4126. doi: 10.1074/jbc.270.8.4121. [DOI] [PubMed] [Google Scholar]
- 32.Strong W B, Gut J, Nelson R G. Cloning and sequence analysis of a highly polymorphic Cryptosporidium parvum gene encoding a 60-kilodalton glycoprotein and characterization of its 15- and 45-kilodalton zoite surface antigen products. Infect Immun. 2000;68:4117–4134. doi: 10.1128/iai.68.7.4117-4134.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzipori S. Cryptosporidiosis in perspective. Adv Parasitol. 1988;27:63–129. doi: 10.1016/S0065-308X(08)60353-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tzipori S, Griffiths J K. Natural history and biology of Cryptosporidium parvum. Adv Parasitol. 1998;40:5–36. doi: 10.1016/s0065-308x(08)60116-5. [DOI] [PubMed] [Google Scholar]
- 35.Tzipori S, McCartney E, Lawson G H, Rowland A C, Campbell I. Experimental infection of piglets with Cryptosporidium. Res Vet Sci. 1981;31:358–368. [PubMed] [Google Scholar]
- 36.Tzipori S, Rand W, Griffiths J, Widmer G, Crabb J. Evaluation of an animal model system for cryptosporidiosis: therapeutic efficacy of paromomycin and hyperimmune bovine colostrum-immunoglobulin. Clin Diagn Lab Immunol. 1994;1:450–463. doi: 10.1128/cdli.1.4.450-463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verdon R, Keusch G T, Tzipori S, Grubman S A, Jefferson D M, Ward H D. An in vitro model of infection of human biliary epithelial cells by Cryptosporidium parvum. J Infect Dis. 1997;175:1268–1272. doi: 10.1086/593695. [DOI] [PubMed] [Google Scholar]
- 38.Ward H, Cevallos A M. Cryptosporidium: molecular basis of host-parasite interaction. Adv Parasitol. 1998;40:151–185. doi: 10.1016/s0065-308x(08)60120-7. [DOI] [PubMed] [Google Scholar]
- 39.Ward H D, Alroy J, Lev B I, Keusch G T, Pereira M E. Biology of Giardia lamblia. Detection of N-acetyl-D-glucosamine as the only surface saccharide moiety and identification of two distinct subsets of trophozoites by lectin binding. J Exp Med. 1988;167:73–88. doi: 10.1084/jem.167.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu A M, Sugii S J. Differential binding properties of Ga1NAc and/or Ga1 specific lectins. Adv Exp Med Biol. 1988;228:205–263. [PubMed] [Google Scholar]