Abstract
Simple Summary
Gastroesophageal adenocarcinoma (GOA) is a cancer that has poor survival. Most cases are diagnosed when a cure is not possible, and treatment often has many side effects. It occurs less often and is associated with better outcomes in females. The reason for this is not known. We sought to use samples from a clinical trial in older adults with GOA to investigate whether this observation could be related to oestrogen and its action through oestrogen receptors. We found no clear link between outcome and oestrogen receptor expression but did note improved survival with older age and female sex.
Abstract
Gastroesophageal adenocarcinoma is a disease of older adults that is associated with a very poor prognosis. It is less common and has better outcomes in females. The reason for this is unknown but may relate to signalling via the main oestrogen receptors (ER) α and β. In this study, we sought to investigate this using the GO2 clinical trial patient cohort. GO2 recruited older and/or frail patients with advanced gastroesophageal cancer. Immunohistochemistry was performed on tumour samples from 194 patients. The median age of the population was 76 years (range 52–90), and 25.3% were female. Only one (0.5%) tumour sample was positive for ERα, compared to 70.6% for ERβ expression. There was no survival impact according to ERβ expression level. Female sex and younger age were associated with lower ERβ expression. Female sex was also associated with improved overall survival. To our knowledge, this is the largest study worldwide of ER expression in a cohort of patients with advanced gastroesophageal adenocarcinoma. It is also unique, given the age of the population. We have demonstrated that female sex is associated with better survival outcomes with palliative chemotherapy but that this does not appear to be related to ER IHC expression. The differing ER expression according to age supports the concept of a different disease biology with age.
Keywords: gastroesophageal cancer, oestrogen receptor, older adults, prognosis, biomarker
1. Introduction
Gastroesophageal adenocarcinoma (GOA) is increasing in incidence in the Western world, and the prognosis remains poor, with a 5-year survival of approximately 20% [1,2]. Despite recent advances in systemic therapy, prognosis in the advanced setting remains less than a year in biomarker-negative patients [3]. This figure is lower in older and/or frail patients [4]. There is an urgent need to identify biomarkers to accurately assess disease biology and prognostic outcomes.
It is well documented epidemiologically that in GOA, there is a male predilection, which narrows post-menopausally (~11:1 at age 50–54, ~4:1 at age 75–79) [5,6]. The reason for this has not been established; however, it does not appear to be related to female reproductive [7] or traditional risk factors [8]. It has been proposed that the endocrine milieu that occurs in pre- and peri-menopausal females may be protective.
The epidemiological observation of a protective role for oestrogen in the development of gastroesophageal cancer is supported by studies in both oesophageal and gastric cancer investigating the use of hormone replacement therapy (HRT) and tamoxifen [9,10,11], as well as the protective effect of breastfeeding [12]. This effect is not specific to females. In men, higher levels of circulating dehydroepiandrosterone, oestradiol and free oestradiol also appear protective for the development of both oesophageal and gastric adenocarcinoma [13]. Together, these observations have led to the suggestion that oestrogen may confer an anti-tumour effect, which warrants further investigation. This is supported by the observation of improved survival for females with gastroesophageal adenocarcinoma [14,15,16].
The exact mechanisms underlying this effect remain unclear; however, they may be mediated through oestrogen receptor (ER) signalling. The main ERs are ERα (ESR1) and ERβ (ESR2) [17], which are differentially expressed according to the organ; ERα is predominantly expressed in female sex organs, while ERβ is widely expressed in other tissues, including the oesophagus and stomach [18].
To date, most studies investigating the immunohistochemical expression of ERα and ERβ in GOA have focused on tumour samples from younger patients in the curative setting. There is, therefore, limited data in the advanced setting or in an older population. This is important as GOA is a disease of older age, and there is increasing evidence of differing biomarker expression and disease biology with age in other tumour groups [19]. ERs can be targeted with existing therapies, and immunohistochemistry (IHC) is easy to perform; therefore, their prognostic role also warrants investigation in this setting.
To address this knowledge gap, we utilised stored tumour samples from the GO2 trial. The GO2 trial recruited older and/or frailer patients with advanced gastroesophageal cancer, felt to better represent real-world patients encountered in clinical practice [4]. The trial sought to investigate the role of chemotherapy dose de-escalation in this population. In this post hoc study, we investigated tumour immunohistochemical expression and the prognostic role of ERα and ERβ in an older, advanced GOA population treated with palliative chemotherapy.
2. Materials and Methods
2.1. Study Cohort
The GO2 trial recruited 559 patients, including both squamous and non-squamous histology [4]. Patients were randomised to either a ‘likely to benefit’ (n = 514) or ‘uncertain to benefit’ (n = 45) arm. In the ‘likely to benefit’ arm, patients were randomised to either 100% (Level A), 80% (Level B) or 60% (Level C) doublet chemotherapy regimen of oxaliplatin/capecitabine. 100% dose was oxaliplatin 130 mg/m2 on day 1 and capecitabine 625 mg/m2 twice daily on days 1–21, on a 21-day cycle. In the ‘uncertain to benefit’ arm, patients were randomised to either Level C or supportive care alone. This study focuses on the adenocarcinoma population only.
The GO2 trial clinical database is held at the Clinical Trials Research Unit at Leeds Institute of Clinical Trials Research, University of Leeds, Leeds. All data analysis in this manuscript uses this anonymised dataset.
Between November 2014 and January 2018, formalin-fixed paraffin-embedded (FFPE) tumour blocks were collected from 395/559 (70.7%) patients in the GO2 trial. Biospecimen collection was an optional part of the GO2 trial design from the outset and included in the ethical trial approval (REC Number 13/YH/0229). These samples were initially collected and resided within the NHS Grampian Biorepository (REC Number 16/NS/0055). No more samples were collected as part of this study. The samples were also registered with the NHS Tayside Biorepository (REC approval 17/ES/0130). Following the transfer of tissue, all analysis was performed in NHS Tayside.
2.2. Immunohistochemistry
All sectioning and IHC were performed by the NHS Tayside Biorepository, and slides were provided for scoring. Antigen retrieval and de-paraffinisation were performed using DAKO EnVision™ FLEX Target Retrieval solution (high pH) buffer in a DAKO PT Link. Sections were blocked in Flex Peroxidase-Blocking Reagent and incubated overnight at 4 °C with anti-Estrogen Receptor Beta 14C8 (ab288 Abcam, Cambridge, UK) at a dilution of 1 in 500 and anti-Estrogen Receptor Alpha (SP1 Ventana, Tucson, AZ, USA) at a dilution of 1 in 50. Immunostaining using the DAKO EnVision™ FLEX system (Agilent Technologies, Santa Clara, CA, USA) on a DAKO Autostainer Link48 was carried out according to the manufacturer’s protocol. DAKO substrate working solution was used as a chromogenic agent for 2 × 5 min, and sections were counterstained with EnVision™ FLEX haematoxylin. Sections known to stain positively were included in each batch, and negative controls were prepared by replacing the primary antibody with the DAKO antibody diluent.
Individual biomarker expression was assessed by two independent observers (SW and MAB), one of whom was a trained gastrointestinal pathologist. Both observers were blinded to clinical data. Nuclear, cytoplasmic or cell membrane staining was considered, and ERα and ERβ receptor expression was recorded by calculating H-scores. The H-score incorporated staining intensity and frequency, with consensus agreement of discordant results. Scoring was based on intensity (0 = no staining, 1 = weak, 2 = moderate and 3 = strong staining observed) and percentage of tumour cells staining positive. These two values were multiplied to give an H-score between 0 and 300 for the section. A dichotomous classification was initially used to categorise H-scores into high (score 201–300), moderate (score 101–200), low (score 1–100) or negative expression. In the ERβ (ESR2) IHC population, the upper quartile H-score cut-off value was 100; therefore, for subsequent analysis, the moderate and high expression groups were combined. There was a good correlation between IHC H-score and Allred Score (Pearson R = 0.868 (95% CI; 0.828–0.899), p < 0.0001). An H-score of 100 equates to an Allred Score of 6.
Differences between clinicopathological characteristics according to ERα and ERβ expression were calculated using chi-squared tests with correction for multiple testing. Associations between sex hormone receptor expression groups and progression-free (PFS) and overall survival (OS) were investigated using Cox proportional hazards regression, producing unadjusted and adjusted hazard ratios (HR) and 95% confidence intervals (CIs). All analyses were adjusted for the GO2 study stratification factors; age group at randomisation, sex, stage, primary site, and dose level administered. All statistical analysis was performed using R statistical software (version 4.0.2; R Core Team 2021, Vienna, Austria. Available online: https://www.R-project.org/ accessed on 27 April 2023).
3. Results
3.1. Patient Cohort
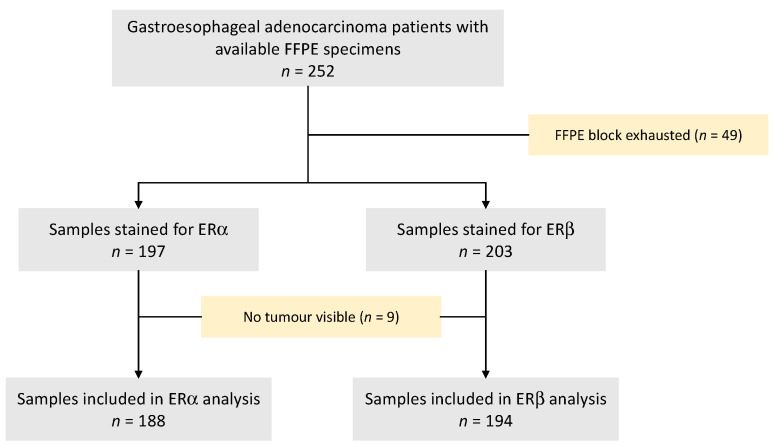
FFPE blocks from 252 patients with advanced GOA were available. From these, 49 had already been exhausted, and therefore 203 FFPE blocks were suitable for immunohistochemical staining. ERβ was performed first, followed by ERα with tumours visible in 194 (95.6%) and 188 (92.6%) samples, respectively (Figure 1).
Figure 1.
CONSORT diagram of GO2 patient selection for ERα and ERβ immunohistochemistry. ER—oestrogen receptor; FFPE—formalin-fixed paraffin-embedded.
3.1.1. ERα and ERβ Expression
For ERα, 187 (99.5%) of the 188 samples had no visible expression. One (0.5%) sample had low-intensity staining in 50% of visible tumour cells. Subsequent analysis relating to demographics and survival was not performed.
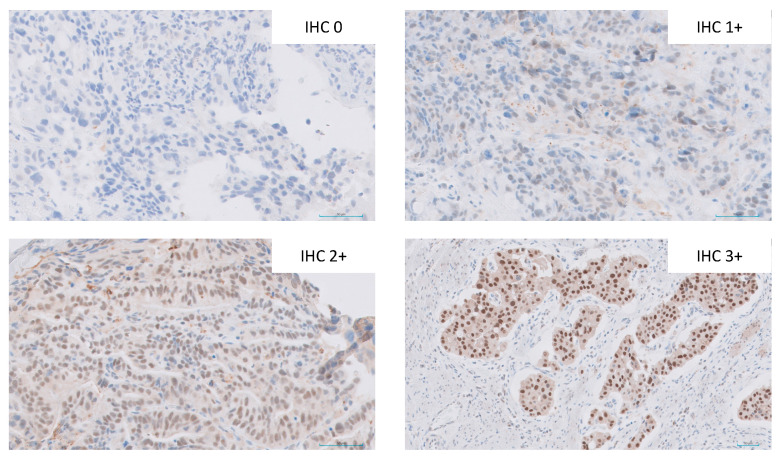
Examples of ERβ staining intensity are shown in Figure 2. Of the 194 samples available for ERβ analysis, the ERβ positivity rate was 70.6%; 57 (29.4%) had no expression, 98 (50.5%) had low expression (H-score 1–100), 35 (18.0%) had moderate expression (H-score 101–200) and 4 (2.1%) had high expression (H-score 201–300).
Figure 2.
Examples of immunohistochemical staining intensity (×40 magnification) of ERβ from samples included in the study.
Demographics according to expression level are shown in Table 1. The median age of the population was 76 (range 52–90), and 25.3% were female. There were no differences in age, sex distribution, ECOG PS, site of primary or the presence/absence of metastasis according to expression level group. Despite the lack of significant difference in sex distribution between expression groups, female patients had lower rates of any ERβ expression than males, although this did not reach significance (61.2% vs 73.8%, p = 0.137). ERβ was expressed in significantly fewer patients aged younger than 65 (46.7%) than in the 65–75 cohort (78.3%) and the older than 75 cohort (69.1%) (p = 0.045) (Table 2). Expression was similar irrespective of the site of the primary tumour.
Table 1.
Baseline demographics according to ERβ immunohistochemical expression group. OX—oxaliplatin/capecitabine. * False discovery rate correction for multiple testing.
| ESR2 IHC Expression Group | ||||
|---|---|---|---|---|
| No Expression | Low Expression | Moderate/High Expression | p-Value | |
| (N = 57) | (N = 98) | (N = 39) | (q-Value) * | |
| Age Group | ||||
| <65 | 8 (14.0%) | 4 (4.1%) | 3 (7.7%) | 0.151 |
| 65–75 | 15 (26.3%) | 39 (39.8%) | 15 (38.5%) | (0.483) |
| >75 | 34 (59.6%) | 55 (56.1%) | 21 (53.8%) | |
| Sex | ||||
| Male | 38 (66.7%) | 76 (77.6%) | 31 (79.5%) | 0.241 |
| Female | 19 (33.3%) | 22 (22.4%) | 8 (20.5%) | (0.483) |
| ECOG PS | ||||
| 0 | 8 (14.0%) | 10 (10.2%) | 8 (20.5%) | 0.434 |
| 1 | 31 (54.4%) | 62 (63.3%) | 19 (48.7%) | (0.652) |
| 2+ | 18 (31.6%) | 26 (26.5%) | 12 (30.8%) | |
| Dose Level | ||||
| 100% OX | 20 (35.1%) | 37 (37.8%) | 9 (23.1%) | 0.2 |
| 80% OX | 16 (28.1%) | 32 (32.7%) | 10 (25.6%) | (0.483) |
| 60% OX | 21 (36.8%) | 29 (29.6%) | 20 (51.3%) | |
| Primary Site | ||||
| Oesophagus | 19 (33.3%) | 34 (34.7%) | 10 (25.6%) | 0.779 |
| GOJ | 18 (31.6%) | 29 (29.6%) | 11 (28.2%) | (0.827) |
| Gastric | 20 (35.1%) | 35 (35.7%) | 18 (46.2%) | |
| Metastasis present | ||||
| Metastasis | 36 (63.2%) | 64 (65.3%) | 27 (69.2%) | 0.827 |
| No metastasis | 21 (36.8%) | 34 (34.7%) | 12 (30.8%) | (0.827) |
| GO2 Frailty Group | ||||
| No/mild frailty | 7 (12.3%) | 15 (15.3%) | 11 (28.2%) | 0.109 |
| Moderate frailty | 19 (33.3%) | 22 (22.4%) | 6 (15.4%) | (0.422) |
| Severe frailty | 31 (54.4%) | 61 (62.2%) | 22 (56.4%) | |
Table 2.
ERβ IHC expression according to age group in the GO2 gastroesophageal adenocarcinoma population. * False discovery rate correction for multiple testing.
| Age Group | ||||
|---|---|---|---|---|
| <65 (N = 15) |
65–75 (N = 69) |
>75 (N = 110) |
p-Value (q-Value) * |
|
| ERβ expression | ||||
| No expression | 8 (53.3%) | 15 (21.7%) | 34 (30.9%) | 0.045 |
| Positive expression | 7 (46.7%) | 54 (78.3%) | 76 (69.1%) | −0.045 |
3.1.2. ERβ Expression and Survival
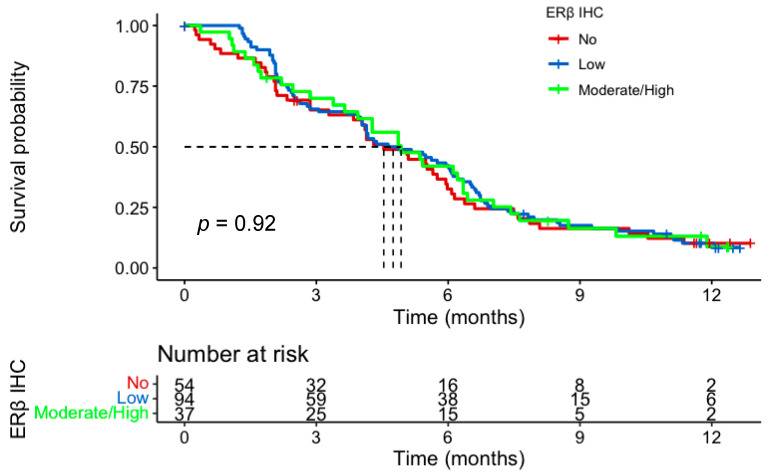
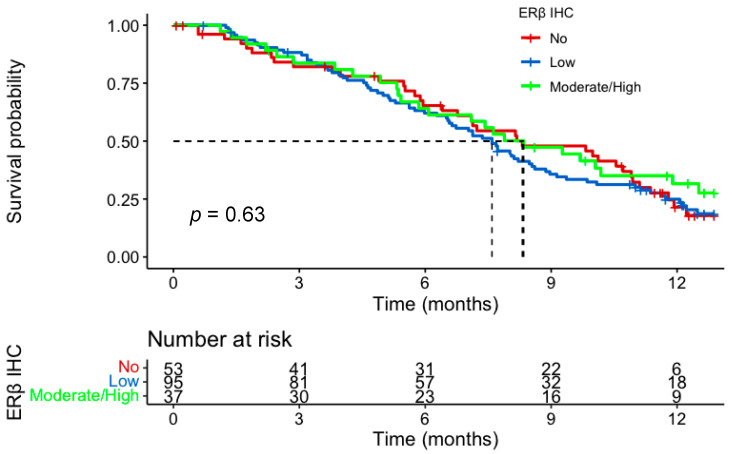
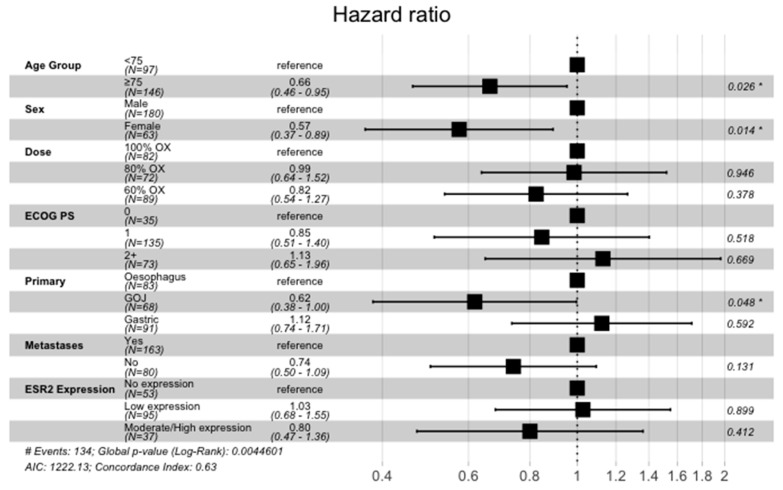
In the IHC cohort, 185 of the 194 patients received at least one cycle of chemotherapy and were included in the survival analysis. There was no evidence of a prognostic role for ERβ IHC expression for either PFS or OS. Median PFS for no, low, and moderate/high expression groups was 4.5 months (m) (95% CI; 3.8–6.0), 4.8 m (95% CI; 4.0–6.11) and 4.9 m (95% CI; 3.9–6.4), respectively. Median OS for the three groups was 8.3 m (95% CI; 6.8–10.9) vs 7.6 m (95% CI; 6.5–8.6) vs 8.3 m (95% CI; 6.1–12.5) (Figure 3 and Figure 4). There was no impact of chemotherapy dose level on survival in either test of interaction with IHC expression or on Cox-regression analysis (Figure 5). Of note, in the tested population, female sex (HR, 0.57; 95% CI 0.37–0.89; p = 0.014) and older age (HR, 0.66; 95% CI 0.46–0.95; p = 0.026) were good prognostic factors. This was also the case in the intention to treat GO2 adenocarcinoma population (HR, 0.73; p = 0.027).
Figure 3.
Progression-free survival according to ERβ IHC expression in the advanced gastroesophageal adenocarcinoma GO2 population who received at least one cycle of chemotherapy. Groups based on histoscore: no, 0; low, 0–100; moderate/high, 101–300.
Figure 4.
Overall Survival according to ERβ IHC expression in the advanced gastroesophageal adenocarcinoma GO2 population who received at least one cycle of chemotherapy. Groups based on histoscore: no—0; low, 0–100; moderate/high, 101–300.
Figure 5.
Cox regression analysis incorporating ERβ (labelled ESR2) IHC expression and stratification factors in the advanced gastroesophageal adenocarcinoma GO2 population who received at least one cycle of chemotherapy. * p-value < 0.05.
4. Discussion
Gastroesophageal cancer is more common in males, and female sex is associated with improved outcomes. It has been proposed that this observation could be related to the effect of oestrogen on signalling via oestrogen receptors. This has been investigated previously, but mainly in younger populations treated with curative intent. In this study, we investigated tumour IHC expression of the oestrogen receptors α and β in a population of older adults with advanced GOA. We also investigated the prognostic role of this expression in patients treated with palliative chemotherapy. This is the largest report to date on this topic in an advanced setting and also provides valuable data on an older patient population.
We found that only one of the 188 (0.5%) patients expressed ERα. In contrast, 70.4% of samples had ERβ expression. There were no clear demographic associations with expression level; however, females had a lower proportion of tumours with ERβ expression. Survival analysis according to ERα expression was not possible, but there was no evident relationship between ERβ expression and either PFS or OS with palliative chemotherapy. Of note, female sex and older age were favourable prognostic markers in Cox regression analysis, and ERβ expression appeared to differ according to age.
Our ER IHC findings are in keeping with previous studies in both oesophageal and gastric adenocarcinoma (Table 3). Expression of ERα in published literature ranges from 0–40%, while ERβ expression ranges from 31–100% [20,21,22,23,24,25,26,27,28,29,30,31,32,33]. One of the main challenges in comparing studies is that a cut-off for ER IHC positivity is not yet defined in gastric cancer. There is also a range of scoring methods used, including the Allred scoring system used in breast cancer [34]. We attempted to address this challenge by using histoscore, which enabled any positivity, and strength of positivity to be explored separately.
Table 3.
Studies of ERα and ERβ IHC expression and outcome in gastroesophageal cancer. ER—oestrogen receptor, OAC—oesophageal adenocarcinoma.
| Author | Year | Site | Age | Setting | Number | ERα | ERβ | Survival |
|---|---|---|---|---|---|---|---|---|
| Oesophageal Cancer | ||||||||
| McMenamin [20] | 2018 | EAC | Mean 63 | Radical | 139 | 4% | 31% | ERα—no impact on survival ERβ—non-significant improvements |
| Kalayarasan [21] | 2008 | GOA | Mean 57.6 | All stages | 15 | 0% | 100% | - |
| Al-Khyatt [22] | 2018 | OAC | Median 65 (range 30–79) | Radical | 28 | 2.9% | 41.2% | ERα and ERβ—poorer survival |
| Liu [23] | 2004 | OAC | Not available | Radical | 27 | - | ERβ1 85% ERβ2 81% ERβ3 100% ERβ5 100% |
- |
| Akgun [24] | 2002 | OAC | Not available | Radical | 23 | - | 100% | - |
| Tiffan [25] | 2003 | OAC | Range 29–90 | Radical | 20 | 40% | - | - |
| Gastric Cancer | ||||||||
| Tang [26] | 2017 | Gastric | Median 58 | All stages | 150/153 | 6% | 93.5% | ERα—poorer survival ERβ—non-significant poorer survival |
| Gan [27] | 2012 | Gastric | Not available | All stages | 848/823 | 12% | 91.9% | ERα—improved survival ERβ—no impact on survival |
| Xu [28] | 2010 | Gastric | Mean 57 Range 31–79 |
Radical | 211 | 25.6% | 49.3% | ERα—poorer survival ERβ—better survival |
| Da Silva [29] | 2022 | Gastric | Mean 62.4 | All stages | 345 | 1.8% | 98.2% | ERα—no impact on survival ERβ—no impact on survival |
| Wang [30] | 2007 | Gastric | Median 60 (range 32–87) | All stages | 39 | 18.2% | 43.6% | - |
| Zhou [31] | 2016 | Gastric | All under 40 (mean 33.8) | Radical | 139 | 49.6% | 87.8% | ERα—no impact on survival ERβ—no impact on survival |
| Ryu [32] | 2012 | Gastric | Unknown | Radical | 148 | - | 45.3% | ERβ—no impact on survival |
| Jukic [33] | 2017 | Gastric | Mean 69 Range 35–90 |
Radical | 60 | 20% | - | - |
For ERβ, we observed numerically lower expression in females in our cohort. This has been reported previously in other studies of gastric cancer. Ryu et al., in a study of 148 gastric cancers, reported a positivity rate of 61.2% in males compared to 38.8% in females, p = 0.931 [32]. In a larger study of 823 patients by Gan et al., the positivity rate was 92.7% in males vs 89.8% in females, p = 0.166. The higher rates of ERβ expression in males may suggest a potential biological role for oestrogen and/or ERβ in the development and progression of GOA.
In contrast, differences in expression according to sex have not been seen in oesophageal adenocarcinoma, but published studies are of smaller size. In the largest to date, McMenamin et al. explored expression in 138 tumours, the majority of which were gastroesophageal junctional (84.1%). Although the majority of positive expression in the cohort was seen in males, the rates of positivity were 31.5% in males compared to 30% in females [20].
The observation of lower ERβ expression in samples from younger patients has also been reported by Ryu et al.; however, an age cut-off of 50 years was used; 26.9% vs. 73.1%, p = 0.027 [32]. Gan et al. reported a positivity rate of 93.9% in patients aged 65 and older compared to 87.7% in those younger than 40. There was no difference in ERβ expression with age in McMenamin et al., with a positivity of 31.3% in those aged 70 and older compared to 28.6% in those younger than 50 [20].
When considering expression, it is important to consider that the findings presented in this study are in a population which is different in terms of age, fitness and stage of disease to previous literature. It is increasingly recognised that cancer disease biology differs with age [19], and it is possible, therefore, that expression of IHC biomarkers may differ in the GO2 population.
In our study, there was no impact of ERβ IHC expression on either PFS or OS within the trial population. This finding is in keeping with most previously published studies. The only exception is a study by Xu et al. that observed a survival benefit for ERβ expression in 211 patients with gastric cancer treated with curative intent [28]. Importantly the study population differed from ours; the mean age of the ERβ-positive and negative populations was 56.4 and 57.5 years, respectively, and the ERβ-negative cohort had higher nodal positivity (64.5% vs. 54.8%).
Our observation of improved survival with female sex in both the IHC population and the GO2 intention to treat population agrees with previously published data in both the curative and palliative settings [15,16,35]. The improved survival with older age, when controlled for other factors, supports the concept of differing disease biology with age in GOA.
The strengths of this study are that the samples were obtained from a clinical trial cohort. As such, the demographic and outcome data are reliable. In addition, this is a large sample size which is unique given the patient profile, stage of disease and standardised chemotherapy agents administered. An added strength is the ability to investigate the impact of chemotherapy dose level on outcome. The main limitation is that only one section of tumour was analysed for each receptor, and, as such, we were unable to mitigate the challenge of tumour heterogeneity.
In summary, this is the largest study of oestrogen receptor expression in an advanced gastroesophageal population. It also provides the first data on this topic, specifically in an older population. We demonstrate that ERα receptor expression is rare, while ERβ receptor expression occurs in most samples. IHC expression does not appear to impact survival; however, it may be influenced by patient age and/or sex. Female sex and older age were good prognostic factors in our population.
5. Conclusions
In this post hoc biomarker analysis of a completed clinical trial in older adults with advanced gastroesophageal cancer, IHC expression of oestrogen receptor-α was rare, while expression of oestrogen receptor-β was common. Oestrogen receptor expression was not prognostic; however, female sex and older age were associated with improved outcomes.
Acknowledgments
We thank the participating patients, clinicians, research nurses, and other support staff in participating centres, as well as GO2 trial investigators and the chairs and members of the Independent Trial Steering and Data Monitoring and Ethics Committees (listed in Supplement 4 of the primary analysis publication which can be found at: https://doi.org/10.1001/jamaoncol.2021.0848, accessed on 1 April 2023).
Author Contributions
Conception and Design: M.A.B., L.C.S. and R.D.P. Data Collection: R.D.P., P.S.H., M.J.S. and the GO2 investigators. Samples processing: S.B., G.S. and S.K. Analysis and Interpretation of Data: M.A.B. and S.W. Manuscript Writing: M.A.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The GO2 trial (ISRCTN44687907) was conducted in accordance with the Declaration of Helsinki, approved by the UK National Research Ethics Service and overseen by independent Trial Steering and Data Monitoring & Ethics Committees.
Informed Consent Statement
Informed consent for the use of tumour specimens for future biomarker investigation was obtained from all subjects involved in the GO2 study.
Data Availability Statement
Data and script can be obtained on request from the corresponding author.
Conflicts of Interest
MAB reported personal fees from Ipsen and Servier. RDP reported personal fees from Eli Lilly, Bristol Myers Squib, and Servier and grants from AstraZeneca, Roche, Sanofi, Merck Sharp & Dohme, Five Prime Therapeutics, and Jansen outside the submitted work. MJS reported grants from Cancer Research UK during the conduct of the GO2 study. PSH reported grants from Cancer Research UK during the conduct of the study and institutional research funding from Novartis, Pfizer, Eli Lilly, Daiichi-Sanchyo, and Eisai outside the submitted work. No other disclosures were reported.
Funding Statement
This study was funded by the Scottish Chief Scientist Office (CAF/20/01). The GO2 trial was funded by Cancer Research UK (trial number: CRUK/12/022) and ran within the UK National Health Service. Funders had no role in designing, undertaking or reporting the study, and the views expressed in this publication are those of the author(s) and not necessarily those of the funders, NHS or the UK Department of Health and Social Care.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Smyth E.C., Lagergren J., Fitzgerald R.C., Lordick F., Shah M.A., Lagergren P., Cunningham D. Oesophageal cancer. Nat. Rev. Dis. Prim. 2017;3:17048. doi: 10.1038/nrdp.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smyth E.C., Nilsson M., Grabsch H.I., van Grieken N.C., Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D., Starling N., Rao S., Iveson T., Nicolson M., Coxon F., Middleton G., Daniel F., Oates J., Norman A.R., et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N. Engl. J. Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 4.Hall P.S., Swinson D., Cairns D.A., Waters J.S., Petty R., Allmark C., Ruddock S., Falk S., Wadsley J., Roy R., et al. Efficacy of Reduced-Intensity Chemotherapy With Oxaliplatin and Capecitabine on Quality of Life and Cancer Control Among Older and Frail Patients With Advanced Gastroesophageal Cancer: The GO2 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021;7:869–877. doi: 10.1001/jamaoncol.2021.0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathieu L.N., Kanarek N.F., Tsai H.L., Rudin C.M., Brock M.V. Age and sex differences in the incidence of esophageal adenocarcinoma: Results from the Surveillance, Epidemiology, and End Results (SEER) Registry (1973–2008) Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus. 2014;27:757–763. doi: 10.1111/dote.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sipponen P., Correa P. Delayed rise in incidence of gastric cancer in females results in unique sex ratio (M/F) pattern: Etiologic hypothesis. Gastric Cancer. 2002;5:213–219. doi: 10.1007/s101200200037. [DOI] [PubMed] [Google Scholar]
- 7.Persson C., Inoue M., Sasazuki S., Kurahashi N., Iwasaki M., Ye W., Tsugane S. Female reproductive factors and the risk of gastric cancer in a large-scale population-based cohort study in Japan (JPHC study) Eur. J. Cancer Prev. 2008;17:345–353. doi: 10.1097/CEJ.0b013e3282f521e4. [DOI] [PubMed] [Google Scholar]
- 8.Löfdahl H.E., Lu Y., Lagergren J. Sex-specific risk factor profile in oesophageal adenocarcinoma. Br. J. Cancer. 2008;99:1506–1510. doi: 10.1038/sj.bjc.6604701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brusselaers N., Maret-Ouda J., Konings P., El-Serag H.B., Lagergren J. Menopausal hormone therapy and the risk of esophageal and gastric cancer. Int. J. Cancer. 2017;140:1693–1699. doi: 10.1002/ijc.30588. [DOI] [PubMed] [Google Scholar]
- 10.Lindblad M., Rodríguez L.A.G., Chandanos E., Lagergren J. Hormone replacement therapy and risks of oesophageal and gastric adenocarcinomas. Br. J. Cancer. 2005;94:136–141. doi: 10.1038/sj.bjc.6602906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandanos E., Lindblad M., Jia C., Rubio C.A., Ye W., Lagergren J. Tamoxifen exposure and risk of oesophageal and gastric adenocarcinoma: A population-based cohort study of breast cancer patients in Sweden. Br. J. Cancer. 2006;95:118–122. doi: 10.1038/sj.bjc.6603214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B.J., Zhang B., Yan S.S., Li Z.C., Jiang T., Hua C.J., Lu L., Liu X.Z., Zhang D.H., Zhang R.S., et al. Hormonal and reproductive factors and risk of esophageal cancer in women: A meta-analysis. Dis. Esophagus. 2015;29:448–454. doi: 10.1111/dote.12349. [DOI] [PubMed] [Google Scholar]
- 13.Petrick J.L., Hyland P.L., Caron P., Falk R.T., Pfeiffer R.M., Dawsey S.M., Abnet C., Taylor P.R., Weinstein S.J., Albanes D., et al. Associations Between Prediagnostic Concentrations of Circulating Sex Steroid Hormones and Esophageal/Gastric Cardia Adenocarcinoma Among Men. Gynecol. Oncol. 2018;111:34–41. doi: 10.1093/jnci/djy082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold M., Morgan E., Bardot A., Rutherford M.J., Ferlay J., Little A., Møller B., Bucher O., De P., Woods R.R., et al. International variation in oesophageal and gastric cancer survival 2012–2014: Differences by histological subtype and stage at diagnosis (an ICBP SURVMARK-2 population-based study) Gut. 2021;71:1532–1543. doi: 10.1136/gutjnl-2021-325266. [DOI] [PubMed] [Google Scholar]
- 15.Sugimachi K., Matsuoka H., Matsufuji H., Maekawa S., Kai H., Okudaira Y. Survival rates of women with carcinoma of the esophagus exceed those of men. Surg. Gynecol. Obstet. 1987;164:541–544. [PubMed] [Google Scholar]
- 16.Morita M., Otsu H., Kawano H., Kasagi Y., Kimura Y., Saeki H., Ando K., Ida S., Oki E., Tokunaga E., et al. Gender differences in prognosis after esophagectomy for esophageal cancer. Surg. Today. 2013;44:505–512. doi: 10.1007/s00595-013-0573-x. [DOI] [PubMed] [Google Scholar]
- 17.Kuiper G.G., Enmark E., Pelto-Huikko M., Nilsson S., Gustafsson J.A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Böttner M., Thelen P., Jarry H. Estrogen receptor beta: Tissue distribution and the still largely enigmatic physiological function. J. Steroid Biochem. Mol. Biol. 2013;139:245–251. doi: 10.1016/j.jsbmb.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Van Herck Y., Feyaerts A., Alibhai S., Papamichael D., Decoster L., Lambrechts Y., Pinchuk M., Bechter O., Herrera-Caceres J., Bibeau F., et al. Is cancer biology different in older patients? Lancet Healthy Longev. 2021;2:e663–e677. doi: 10.1016/S2666-7568(21)00179-3. [DOI] [PubMed] [Google Scholar]
- 20.McMenamin C., Trainor J., Coleman H.G., McManus D.T., McQuaid S., Bingham V., James J., Salto-Tellez M., Johnston B.T., Turkington R. Sex hormone receptor expression and survival in esophageal adenocarcinoma: A prospective cohort study. Oncotarget. 2018;9:35300–35312. doi: 10.18632/oncotarget.26236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalayarasan R., Ananthakrishnan N., Kate V., Basu D. Estrogen and progesterone receptors in esophageal carcinoma. Dis. Esophagus. 2008;21:298–303. doi: 10.1111/j.1442-2050.2007.00767.x. [DOI] [PubMed] [Google Scholar]
- 22.Al-Khyatt W., Tufarelli C., Khan R., Iftikhar S.Y. Selective oestrogen receptor antagonists inhibit oesophageal cancer cell proliferation in vitro. BMC Cancer. 2018;18:121. doi: 10.1186/s12885-018-4030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L., Chirala M., Younes M. Expression of estrogen receptor-beta isoforms in Barrett’s metaplasia, dysplasia and esophageal adenocarcinoma. Anticancer Res. 2004;24:2919–2924. [PubMed] [Google Scholar]
- 24.Akgun H., Lechago J., Younes M. Estrogen receptor-beta is expressed in Barrett’s metaplasia and associated adenocarcinoma of the esophagus. Anticancer Res. 2002;22:1459–1461. [PubMed] [Google Scholar]
- 25.Tiffin N., Suvarna S.K., Trudgill N.J., Riley S.A. Sex hormone receptor immunohistochemistry staining in Barrett’s oesophagus and adenocarcinoma. Histopathology. 2003;42:95–96. doi: 10.1046/j.1365-2559.2003.01513_3.x. [DOI] [PubMed] [Google Scholar]
- 26.Tang W., Liu R., Yan Y., Pan X., Wang M., Han X., Ren H., Zhang Z. Expression of estrogen receptors and androgen receptor and their clinical significance in gastric cancer. Oncotarget. 2017;8:40765–40777. doi: 10.18632/oncotarget.16582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gan L., He J., Zhang X., Zhang Y.-J., Yu G.-Z., Chen Y., Pan J., Wang J.-J., Wang X. Expression profile and prognostic role of sex hormone receptors in gastric cancer. BMC Cancer. 2012;12:566. doi: 10.1186/1471-2407-12-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu C.Y., Guo J.L., Jiang Z.N., Xie S.D., Shen J.G., Shen J.Y., Wang L.B. Prognostic role of estrogen receptor alpha and estrogen receptor beta in gastric cancer. Ann. Surg. Oncol. 2010;17:2503–2509. doi: 10.1245/s10434-010-1031-2. [DOI] [PubMed] [Google Scholar]
- 29.DA Silva A.C.C., Pereira M.A., Ramos M.F.K.P., Cardili L., Ribeiro U., Jr., Zilberstein B., Mello E.S., Castria T.B. Gastric cancer with positive expression of estrogen receptor alpha: A case series from a single western center. Arq. Bras. Cir. Dig. 2022;34:e1635. doi: 10.1590/0102-672020210002e1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang M., Pan J.-Y., Song G.-R., Chen H.-B., An L.-J., Qu S.-X. Altered expression of estrogen receptor alpha and beta in advanced gastric adenocarcinoma: Correlation with prothymosin alpha and clinicopathological parameters. Eur. J. Surg. Oncol. (EJSO) 2007;33:195–201. doi: 10.1016/j.ejso.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Zhou F., Xu Y., Shi J., Lan X., Zou X., Wang L., Huang Q. Expression profile of E-cadherin, estrogen receptors, and P53 in early-onset gastric cancers. Cancer Med. 2016;5:3403–3411. doi: 10.1002/cam4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryu W.-S., Kim J.-H., Jang Y.-J., Park S.-S., Um J.-W., Park S.-H., Kim S.-J., Mok Y.-J., Kim C.-S. Expression of estrogen receptors in gastric cancer and their clinical significance. J. Surg. Oncol. 2012;106:456–461. doi: 10.1002/jso.23097. [DOI] [PubMed] [Google Scholar]
- 33.Jukic Z., Radulovic P., Stojković R., Mijic A., Grah J., Kruslin B., Ferencic Z., Fucic A. Gender Difference in Distribution of Estrogen and Androgen Receptors in Intestinal-type Gastric Cancer. Anticancer Res. 2017;37:197–202. doi: 10.21873/anticanres.11306. [DOI] [PubMed] [Google Scholar]
- 34.Allred D.C., Clark G.M., Elledge R., Fuqua S.A.W., Brown R.W., Chamness G.C., Osborne C.K., McGuire W.L. Association of p53 Protein Expression With Tumor Cell Proliferation Rate and Clinical Outcome in Node-Negative Breast Cancer. Gynecol. Oncol. 1993;85:200–206. doi: 10.1093/jnci/85.3.200. [DOI] [PubMed] [Google Scholar]
- 35.Yang D., Hendifar A., Lenz C., Togawa K., Lenz F., Lurje G., Pohl A., Winder T., Ning Y., Groshen S., et al. Survival of metastatic gastric cancer: Significance of age, sex and race/ethnicity. J. Gastrointest. Oncol. 2011;2:77–84. doi: 10.3978/j.issn.2078-6891.2010.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and script can be obtained on request from the corresponding author.