Abstract
Cytolysin A (ClyA) is a newly discovered cytolytic protein of Escherichia coli K-12 that mediates a hemolytic phenotype. We show here that highly purified ClyA and ClyA-expressing E. coli were cytotoxic and apoptogenic to fresh as well as cultured human and murine monocytes/macrophages.
Recently it was discovered that a chromosomal gene denoted clyA (also referred to as sheA and hlyE) in Escherichia coli K-12 encodes a novel hemolytic protein (5, 20, 21, 24, 25, 28; Y. Mizunoe and B. E. Uhlin, Abstr. 34th Intersci. Conf. Antimicrob. Agents Chemother., abstr. B37, 1994). However, the gene product, the 34-kDa cytolysin A (ClyA) protein, does not seem to be expressed under normal laboratory conditions. This normally latent clyA gene can be activated either by mutation in the hns locus or by overexpression of several putative regulatory genes (5, 8, 9, 19–21, 24, 25, 28; Mizunoe and Uhlin, 34th ICAAC). Both purified ClyA and ClyA-expressing E. coli are able to lyse erythrocytes from several mammalian species in both solid and liquid media, and we recently found that the protein is cytotoxic to macrophages grown in tissue culture (5, 8, 9, 19–21, 24, 25, 28; Mizunoe and Uhlin, 34th ICAAC). Those findings prompted us to further investigate the interaction of ClyA-producing bacteria and purified ClyA with mammalian cells.
Cytotoxic effects of purified ClyA protein and ClyA-expressing E. coli on fresh or cultured human and murine monocytes/macrophages.
Cell morphology changes and detachment from culture plates are conventional and useful indicators to monitor bacterial cytotoxicity. We recently described that highly purified ClyA can detach J774 macrophages from culture plates and change their cell morphology (24). In the present study, we extended our cytotoxicity measurements to other types of host cells by using a neutral red uptake assay (2) and a quantitative lactate dehydrogenase (LDH) release assay based on the fact that LDH is a strictly cytoplasmic enzyme and its presence in the culture medium reflects the disruption of the host cell plasma membrane (16).
The macrophage cell lines J774 (murine) and U937 (human) were maintained and treated as described previously (17, 18, 24, 26, 27). Human polymorphonuclear leukocytes and monocytes were isolated following a standardized procedure as described previously (11, 13). Highly purified ClyA preparations were obtained from E. coli K-12 cells carrying clone pYMZ80 (24; S. N. Wai and B. E. Uhlin, unpublished data). Proteins were diluted in complete medium and sterilized by filtration through a 0.22-μm-pore-size membrane (Schleicher & Schuell FD 030/3). J774 cells, polymorphonuclear leukocytes, or monocytes were treated with purified and filtrated ClyA in 200 μl (total volume) of cell medium. In some experiments, cells were pretreated with phorbol 12-myristate 13-acetate (PMA; Sigma) as described before (24). For testing the effect of cytochalasin D (4) on cytotoxicity, cells were pretreated with 1 μg of cytochalasin D (Sigma) ml−1 for 30 min before bacterial infection, and cytochalasin D was maintained throughout the experiment. Treatment of the bacteria and eukaryotic cells with cytochalasin D at the above concentration did not significantly reduce cell or bacterial viability (data not shown). E. coli strains MC1061/pUC18 and MC1061/pYMZ80 were used as described elsewhere (24). J774 cells were infected as described elsewhere (17, 18, 24) with bacteria at a multiplicity of infection (MOI) of 100, unless otherwise indicated.
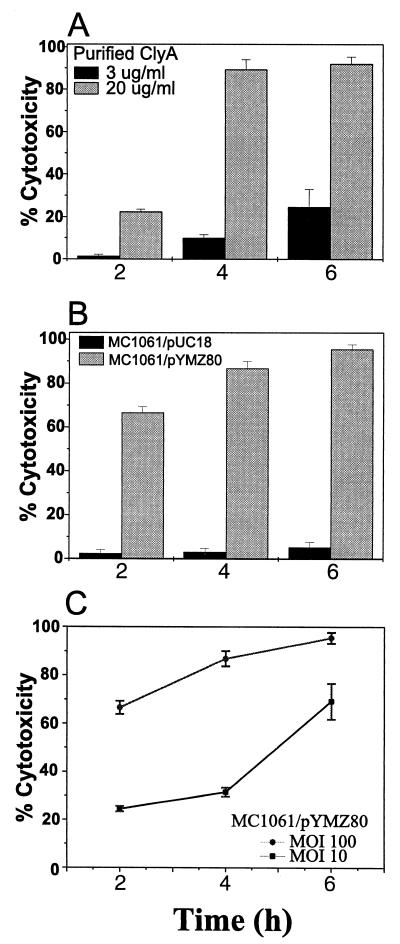
As shown in Fig. 1, LDH release was both ClyA concentration and bacterial infection dose (i.e., MOI) dependent. ClyA at 20 μg ml−1 caused more than 20% LDH release after 2 h of treatment, while no detectable LDH release came from cells treated with one-sixth as much ClyA (Fig. 1A). LDH release remained at baseline level for the vector control MC1061/pUC18, whereas the ClyA-expressing strain MC1061/pYMZ80 was cytotoxic to J774 macrophages at each time interval tested (Fig. 1B and C). MC1061/pYMZ80 showed approximately one-third as much cytotoxicity at an MOI of 10 compared with that at an MOI of 100, at both 2 and 4 h postinfection (p.i.). The cytotoxicity was even greater at 6 h p.i. and approached a level similar to that at the higher MOI (Fig. 1C). The kinetics of cytotoxicity within 6 h of treatment over three time intervals are shown in Fig. 1.
FIG. 1.
Cytotoxicity as manifested by LDH release from murine macrophages J774. About 2 × 104 cells were seeded each well in 96-well plates and treated with highly purified ClyA or infected at different MOIs with ClyA-expressing or vector control E. coli as described elsewhere (17, 18, 24). Killing was assayed at several time intervals and expressed as percent cytotoxicity. Data are means ± standard deviations (n = 4) from one representative experiment of three. The E. coli strains used here do not have endogenous LDH activity when grown aerobically. (A) Dose-dependent toxicity of purified ClyA. Cells were pretreated with PMA as described elsewhere (24). (B) Cytotoxicity of ClyA-expressing E. coli MC1061/pYMZ80 compared with its plasmid vector control MC1061/pUC18 at an MOI of 100. (C) Infection dose-dependent toxicity of ClyA-expressing E. coli (MOI of 100 versus 10).
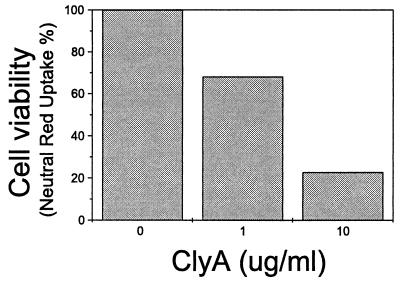
The effect of ClyA on viability of human primary monocytes was monitored using a neutral red uptake assay as described before (2, 11, 13). The viability of freshly isolated human peripheral monocytes showed a dose-related decline after treatment with ClyA for 20 h (Fig. 2), and the effect was similar to that seen with cultured J774 macrophages. Exposure of human peripheral blood lymphocytes or monocytes to 10 μg of ClyA ml−1 for 1 h caused about 20% LDH release, which is comparable to the cytotoxicity level of ClyA on cultured macrophages (Fig. 1A).
FIG. 2.
Viability results of neutral red uptake by human peripheral monocytes after 20 h of ClyA treatment. Cells (about 2 × 105 cells per well in 96-well plates) were prepared, treated, and assayed as described elsewhere (2, 11, 13). Viability value (neutral red uptake) of untreated cells was taken as 100%. Note the ClyA dose-dependent tendency.
Apoptosis judged by DNA fragmentation of macrophages infected with ClyA-expressing E. coli or treated with purified ClyA.
The biochemical hallmark of apoptosis is the cleavage of chromatin into nucleosomal fragments, resulting in multimers of 180 to 200 bp (15, 30). However, it has been reported that necrotic cells may have irregular DNA fragmentation and generate higher-molecular-weight DNA fragments (23). We used three complementary assays to determine whether the predominant macrophage cell death induced by these treatments was due to apoptosis.
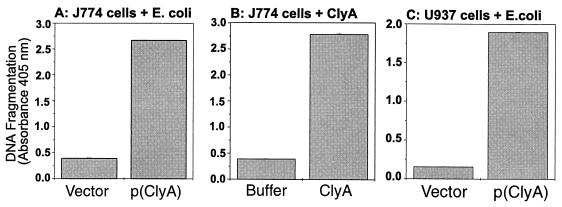
Photometric determination of the histone-associated DNA fragments released by the treated cells was performed with the sensitive cell death detection enzyme-linked immunosorbent assay (ELISA; Boehringer Mannheim GmbH) according to the manufacturer's instructions and as described elsewhere (18). Substrate reaction time was 15 min. Both ClyA-expressing E. coli MC1061/pYMZ80 (Fig. 3A and C) and purified ClyA (Fig. 3B) induced strong signals representing small DNA fragments released to the cell supernatant.
FIG. 3.
Fragmentation of DNA in human and murine macrophages (about 2 × 104 cells per well in 96-well plates) as assayed by ELISA. Data are means ± standard deviations of duplicate samples from one representative experiment of two. Shown are results for infected murine macrophages J774 at 12 h p.i. at an MOI of 100 (A), J774 cells treated with purified ClyA for 12 h (B), and infected human macrophages U937 at 6 h p.i. at an MOI of 100 (C). Bars: 1, vector strain MC1061/pUC18; 2, ClyA-expressing strain MC1061/pYMZ80; 3, Tris-HCl buffer (20 mM, pH 7.5); 4, purified ClyA protein (20 μg ml−1); 5, MC1061/pUC18; 6, MC1061/pYMZ80.
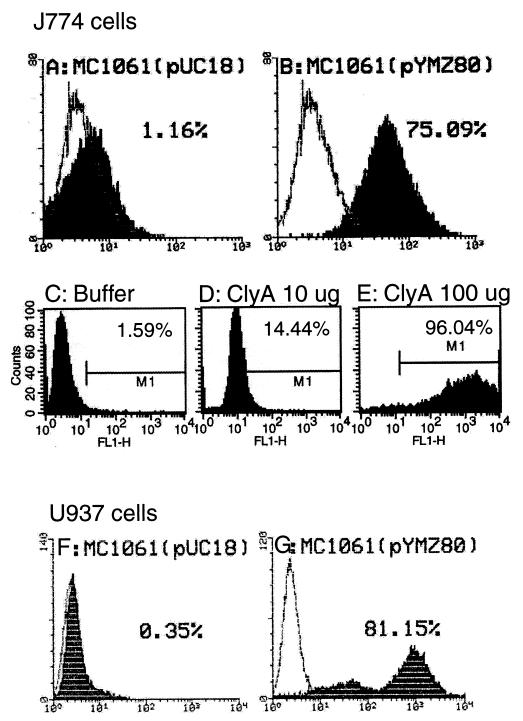
Macrophage apoptosis was further quantified by TUNEL (terminal deoxynucleotidyltransferase [TdT]-mediated dUTP nick end labeling [7])-FACS (fluorescence-activated cell sorting) analysis of the treated cells exactly as described before (18). A ClyA-expressing strain caused about the same percentage of apoptosis in both murine and human macrophages (Fig. 4B and G), while the levels with the vector control remained at baseline (Fig. 4A and F). The dose- and time-dependent effect of purified ClyA shown in the cytotoxicity measurements was also evident in this TUNEL-FACS analysis. J774 cells had an apoptosis percentage of about 15% when exposed to 10 μg of ClyA for 36 h (Fig. 4D), and there were 96% apoptotic cells when 10 times more ClyA was added (Fig. 4E); in contrast, values for cells in the control wells (containing Tris-HCl buffer [20 mM, pH 7.5] instead of ClyA protein) remained at the background level (Fig. 4C). The ClyA-treated cells did not show values above the baseline level during the first 12 h (data not shown). It should be noted that more cells were needed in the TUNEL-FACS experiment (about 5 × 106 cells) than in the cytotoxicity and ELISA experiments (about 2 × 104 cells) described above.
FIG. 4.
Quantitative TUNEL-FACS measurements of apoptosis of infected (MOI of 100) J774 (A and B) and U937 (F and G) macrophages at 12 h (data from one of two repeated tests which gave similar results) or ClyA-treated J774 cells at 36 h (C to E), using FACScan LYSIS II or CellQuest. The quantified percentages of apoptosis are shown. The y axis represents the number of cells (10,000); the x axis represents fluorescence intensity. Shaded areas are macrophages with TdT and overlaid by cells without TdT. The initial cell number in each well of a six-well plate was about 5 × 106. (A) Vector control strain MC1061/pUC18; (B) ClyA-expressing strain MC1061/pYMZ80; (C) Tris-HCl buffer (20 mM, pH 7.5); (D) ClyA (10 μg); (E) ClyA (100 μg); (F) vector control strain MC1061/pUC18; (G) ClyA-expressing strain MC1061/pYMZ80.
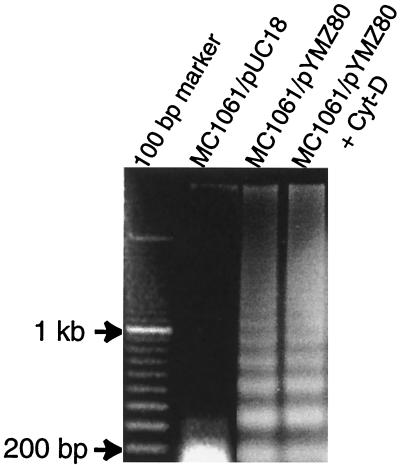
DNA of the infected macrophages was extracted as follows. Cells were harvested and treated with lysis buffer (1% NP-40 in 20 mM EDTA–50 mM Tris-HCl [pH 7.5]) (10) at 37°C. The lysates were extracted once with an equal volume of phenol and once with an equal volume of chloroform-isoamyl alcohol (24:1, vol/vol) before precipitation with ethanol. The precipitates were dried and solubilized in 10 mM Tris (pH 8.0)–1 mM EDTA. Electrophoresis was performed with a 1.5% agarose gel containing 0.5 μg of ethidium bromide ml−1 in Tris-acetate-EDTA buffer (pH 8.2). DNA was visualized by UV light and photographed. As evidenced by electrophoresis of genomic DNA, a nucleosome ladder pattern of DNA degradation was observed in J774 cells infected with MC1061/pYMZ80 but not in J774 cells infected with the vector control MC1061/pUC18 (Fig. 5). It was also evident that cytochalasin D did not inhibit the apoptogenic property of MC1061/pYMZ80 on J774 cells (Fig. 5, lane 4) under the conditions used here.
FIG. 5.
Fragmentation of genomic DNA from about 5 × 106 to 1 × 107 J774 macrophages infected with MC1061/pYMZ80 at an MOI of 100 with or without cytochalasin D (Cyt-D) at 12 h p.i. DNA purification and gel electrophoresis were carried out as described in the text. MC1061/pYMZ80-infected cells generated ladders of multimers of 180 bp characteristic of apoptosis. Lanes: 1, 100-bp DNA ladder; 2, vector control strain MC1061/pUC18; 3, ClyA-expressing strain MC1061/pYMZ80; 4, ClyA-expressing strain MC1061/pYMZ80 with 1 μg of Cyt-D ml−1.
It is roughly estimated in recent studies that pores generated by ClyA (about 2.5 to 3 nm) appear to be somewhat larger than those generated by HlyA (20, 21, 24, 29). The damaged host cell membrane might allow the influx and/or efflux of certain ions which could trigger apoptosis directly or indirectly (1, 12, 22, 23). Thus, the pore-forming activity of ClyA might be responsible for induction of apoptosis as described for other pore-forming toxins (6, 14). Ca2+ is generally regarded as a common signal for initiation of apoptosis. Increase in calcium concentration has been shown to activate degradative processes in programmed cell death directly by stimulating endonucleases or indirectly by promoting activation of calcium-dependent proteases and phosphatases (1, 3, 12, 22, 23). One may speculate that the pore-forming property of ClyA could cause the modification of the intracellular level of Ca2+, which may in turn trigger the apoptosis cascade. The Ca2+- and Mg2+-dependent endonuclease activated during the apoptotic process cleaves the genomic DNA at the internucleosomal regions, thereby generating mono- and oligonucleosomes.
Conclusions.
Taken together, our data demonstrate that purified ClyA and a ClyA-expressing E. coli strain were cytotoxic to both human and murine macrophages in a dose- and time-dependent way and induced a massive amount of apoptosis as determined by several assays showing host cell DNA fragmentation. Further studies will hopefully elucidate the precise mechanisms of ClyA-induced apoptosis of host cells. Our findings that this protein, in addition to being merely a hemolysin, is more widely cytocidal and has the capacity to induce macrophage apoptosis should prompt studies of how ClyA might contribute to pathogenicity of certain E. coli strains.
Acknowledgments
We are grateful to Guangqian Zhou for supplying a sample of PMA.
This work was supported by grants from the Swedish Natural Science Research Council, the Swedish Medical Research Council, the Swedish Institute, the Wenner-Gren Foundations, and the Göran Gustafsson Foundation for Research in Natural Sciences and Medicine.
REFERENCES
- 1.Anderson P. Kinase cascades regulating entry into apoptosis. Microbiol Mol Biol Rev. 1997;61:33–46. doi: 10.1128/mmbr.61.1.33-46.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borenfreud E, Puerener J A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett. 1985;24:119–124. doi: 10.1016/0378-4274(85)90046-3. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y J, Zychlinsky A. Apoptosis induced by bacterial pathogens. Microb Pathog. 1994;17:203–212. doi: 10.1006/mpat.1994.1066. [DOI] [PubMed] [Google Scholar]
- 4.Cooper J A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del Castillo I, Leal S C, Moreno F, del Castillo I. The Escherichia coli K-12 sheA gene encodes a 34-kDa secreted haemolysin. Mol Microbiol. 1997;25:107–115. doi: 10.1046/j.1365-2958.1997.4391813.x. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Prada C, Tall B D, Elliott S E, Hoover D L, Nataro J P, Venkatesan M M. Hemolysin-positive enteroaggregative and cell-detaching Escherichia coli strains cause oncosis of human monocyte-derived macrophages and apoptosis of murine J774 cells. Infect Immun. 1998;66:3918–3924. doi: 10.1128/iai.66.8.3918-3924.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gavrieli Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gómez-Gómez J M, Blázquez J, Baquero F, Martínez J L. hns mutant unveils the presence of a latent haemolytic activity in Escherichia coli K-12. Mol Microbiol. 1996;19:909–910. doi: 10.1046/j.1365-2958.1996.443955.x. [DOI] [PubMed] [Google Scholar]
- 9.Green J, Baldwin M L. The molecular basis for the differential regulation of the hlyE-encoded haemolysin of Escherichia coli by FNR and HlyX lies in the improved activating region 1 contact of HlyX. Microbiology. 1997;143:3785–3793. doi: 10.1099/00221287-143-12-3785. [DOI] [PubMed] [Google Scholar]
- 10.Herrmann M, Lorenz H-M, Voll R, Woith W, Kalden J R. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res. 1994;22:5506–5507. doi: 10.1093/nar/22.24.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holm A, Kalfas S, Holm S E. Killing of Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus by human polymorphonuclear leukocytes in serum and saliva. Oral Microbiol Immunol. 1993;8:134–140. doi: 10.1111/j.1399-302x.1993.tb00655.x. [DOI] [PubMed] [Google Scholar]
- 12.Iwase M, Lally E T, Berthold P, Korchak H M, Taichman N S. Effects of cations and osmotic protectants on cytolytic activity of Actinobacillus actinomycetemcomitans. Infect Immun. 1990;58:1782–1788. doi: 10.1128/iai.58.6.1782-1788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansson, A., L. Hänström, and S. Kalfas. Inhibition of Actinobacillus actinomycetemcomitans leukotoxicity by bacterial proteases. Oral Microbiol. Immunol., in press. [DOI] [PubMed]
- 14.Jonas D, Schultheis B, Klas C, Krammer P H, Bhakdi S. Cytocidal effects of E. coli hemolysin on human T lymphocytes. Infect Immun. 1993;61:1715–1721. doi: 10.1128/iai.61.5.1715-1721.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr J F R, Wyllie A H, Currie A R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korzeniewski C, Callewaert D M. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983;64:313–320. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- 17.Lai X-H, Wang S-Y, Uhlin B E. Expression of cytotoxicity by potential pathogens in the standard Escherichia coli collection of reference (ECOR) strains. Microbiology. 1999;145:3295–3303. doi: 10.1099/00221287-145-11-3295. [DOI] [PubMed] [Google Scholar]
- 18.Lai X-H, Xu J-G, Melgar S, Uhlin B E. An apoptotic response by J774 macrophage cells is common upon infection with diarrheagenic Escherichia coli. FEMS Microbiol Lett. 1999;172:29–34. doi: 10.1111/j.1574-6968.1999.tb13445.x. [DOI] [PubMed] [Google Scholar]
- 19.Libby S J, Goebel W, Ludwig A, Buchmeier N, Bowe F, Fang F C, Guiney D G, Songer J G, Heffron F. A cytolysin encoded by Salmonella is required for survival within macrophages. Proc Natl Acad Sci USA. 1994;91:489–493. doi: 10.1073/pnas.91.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludwig A, Bauer S, Benz R, Bergmann B, Goebel W. Analysis of the SlyA-controlled expression, subcellular localization and pore-forming activity of a 34 kDa haemolysin (ClyA) from Escherichia coli K-12. Mol Microbiol. 1999;31:557–567. doi: 10.1046/j.1365-2958.1999.01196.x. [DOI] [PubMed] [Google Scholar]
- 21.Ludwig A, Tengel C, Bauer S, Bubert A, Benz R, Mollenkopf H J, Goebel W. SlyA, a regulatory protein from Salmonella typhimurium, induces a haemolytic and pore-forming protein in Escherichia coli. Mol Gen Genet. 1995;249:474–484. doi: 10.1007/BF00290573. [DOI] [PubMed] [Google Scholar]
- 22.McConkey D J, Orrenius S. Signal transduction pathways to apoptosis. Trends Cell Biol. 1994;4:370–374. doi: 10.1016/0962-8924(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 23.Ojcius D M, Zychlinsky A, Zheng L M, Young J D-E. Ionophore-induced apoptosis: role of DNA fragmentation and calcium fluxes. Exp Cell Res. 1991;197:43–49. doi: 10.1016/0014-4827(91)90477-c. [DOI] [PubMed] [Google Scholar]
- 24.Oscarsson J, Mizunoe Y, Li L, Lai X-H, Wieslander Å, Uhlin B E. Molecular analysis of the cytolytic protein ClyA (SheA) from Escherichia coli. Mol Microbiol. 1999;32:1226–1238. doi: 10.1046/j.1365-2958.1999.01435.x. [DOI] [PubMed] [Google Scholar]
- 25.Oscarsson J, Mizunoe Y, Uhlin B E, Haydon D J. Induction of haemolytic activity in Escherichia coli by the slyA gene product. Mol Microbiol. 1996;20:191–199. doi: 10.1111/j.1365-2958.1996.tb02500.x. [DOI] [PubMed] [Google Scholar]
- 26.Ralph P, Nakoinz I. Phagocytosis and cytolysis by a macrophage tumor and its cloned cell line. Nature. 1975;257:393–394. doi: 10.1038/257393a0. [DOI] [PubMed] [Google Scholar]
- 27.Sundström C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U937) Int J Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 28.Uhlin B E, Mizunoe Y. Expression of a novel contact-hemolytic activity by E. coli. J Cell Biochem. 1994;18A:71. [Google Scholar]
- 29.Wallace A J, Stillman T J, Atkins A, Jamieson S J, Bullough P A, Green J, Artymiuk P J. E. coli hemolysin E (HlyE, ClyA, SheA): X-ray crystal structure of the toxin and observation of membrane pores by electron microscopy. Cell. 2000;100:265–276. doi: 10.1016/s0092-8674(00)81564-0. [DOI] [PubMed] [Google Scholar]
- 30.Wyllie A H. Glucocorticoid induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]