Abstract
A model of cutaneous leishmaniasis using 102 Leishmania major metacyclic promastigotes inoculated into the footpads of genetically resistant C57BL/6 mice was studied in order to more accurately reproduce the evolution of lesion formation and the kinetics of parasite growth and immune response as they might occur in naturally exposed reservoirs and in human hosts. In contrast to the more conventional experimental model employing 106 metacyclic promastigotes, in which the rapid development of footpad lesions was associated with an increasing number of amastigotes in the site, the low-dose model revealed a remarkably “silent” phase of parasite growth, lasting approximately 6 weeks, during which peak parasitic loads were established in the absence of any overt pathology. Footpad swelling was observed after 6 weeks, coincident with the onset of parasite clearance and with production of high levels of interleukin-12 (IL-12) and gamma interferon (IFN-γ) in draining lymph nodes. Low-dose challenge of IL-12- and IFN-γ-depleted or -deficient mice provided strong evidence that the induction or expression of cellular immunity is essentially absent during the first 6 to 8 weeks of intracellular growth, since the concentration of amastigotes in the site was not enhanced compared to that for wild-type animals during this time. By monitoring the ability of infected mice to transmit parasites to vector sand flies, it was observed that following low-dose challenge, footpads without apparent lesions provided an efficient source of parasites for exposed flies and that the low-dose challenge actually extended the duration of parasite transmissibility during the course of infection.
Leishmania major infection in different inbred mouse strains is a widely used model for the study of Th1 or Th2 responses that control resistance or susceptibility, respectively, to this intracellular parasite (15, 30). The resistant C57BL/6 mouse, in particular, is believed to be a relevant model of L. major infections in humans, which are characterized by the development of localized cutaneous lesions that spontaneously heal. The model has been extremely successful in defining the cells, cytokines, and effector molecules involved in acquired resistance, which to date has been shown to require the interleukin-12 (IL-12)-driven activation of Th1 cells for production of high levels of gamma interferon (IFN-γ) which in turn activates leishmanicidal mechanisms in infected macrophages (3, 12, 16, 32, 33). In the mouse model, which has typically employed high doses (105 to 107) of promastigotes inoculated subcutaneously into the footpad, the onset of lesion formation, high tissue parasite burden, and immunity is relatively rapid compared to that with natural infection. As a consequence, the model has not been as informative in clarifying the relationships between lesion formation, parasite numbers, and immune response.
A model of cutaneous leishmaniasis resulting from low-dose challenge of genetically resistant mice would more closely mimic the evolution of self-limiting L. major infections that occur in natural rodent reservoirs and in human hosts. In the present studies, 102 metacyclic promastigotes of L. major were inoculated into the footpads of C57BL/6 mice and into IL-12- and IFN-γ-deficient or -depleted mice, and the outcome of infection was evaluated by measurement of footpad swelling, by quantitation of the parasite concentration in the site, and by monitoring the capacity of infected footpads to transmit L. major to vector sand flies. This natural infection model revealed a “silent” phase of infection, lasting 6 to 8 weeks, that favored the establishment of peak and transmissible numbers of parasites in the site in the absence of pathology, followed by lesion formation that was associated with high levels of IFN-γ and the onset of parasite clearance. Parasite growth was not enhanced in the immunodeficient mice over the first 6 to 8 weeks, providing strong evidence that the induction of Th1 responses is effectively avoided during a remarkably sustained period of intracellular growth in vivo.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from the Division of Cancer Treatment, National Cancer Institute (Frederick, Md.). C57BL/6 mice deficient in IL-12p40 were purchased from Taconic (Germantown, N.Y.).
Inoculum preparation and quantification of amastigote concentration in infected tissue.
L. major clone V1 (MHOM/IL/80/Friedlin) was cultured in 199 medium with 20% heat-inactivated fetal calf serum (HyClone Laboratories Inc., Logan, Utah), 100 U of penicillin/ml, 100 μg of streptomycin/ml, 2 mM l-glutamine, 40 mM HEPES, 0.1 mM adenine (in 50 mM HEPES), 5 mg of hemin/ml (in 50% triethanolamine), and 1 mg of 6-biotin (M199/S)/ml. Infective-stage metacyclic promastigotes of L. major were isolated from stationary culture (5 to 6 days old) by negative selection using peanut agglutinin (24) (Vector Laboratories Inc., Burlingame, Calif.). Either 106 or 102 metacyclic promastigotes were inoculated subcutaneously into the left hind footpad using a 27.5-gauge needle in a volume of 50 μl. The evolution of the lesion was monitored by measuring footpad width using a metric caliper. The parasite concentration in the inoculated footpad was determined by homogenizing a weighed amount of tissue using a Teflon-coated microtissue grinder in a microcentrifuge tube containing 200 μl of M199/S. The tissue homogenates and cell suspensions of draining lymph node cells were serially diluted in a 96-well flat-bottom microtiter plate containing biphasic medium prepared using 50 μl of NNN medium with 30% defibrinated rabbit blood and overlaid with 50 μl of M199/S. The number of viable parasites was determined from the reciprocal of the highest dilution at which promastigotes could be detected after 7 days of incubation at 26°C and was expressed as parasites per milligram of tissue.
Treatment of mice with anti-cytokine antibodies.
Anti-mouse IL-12p40 (c17.8; rat immunoglobulin G1 [IgG1]), anti-mouse IFN-γ (XMG-6; rat IgG1), and anti-β-galactosidase isotype control (GL113; rat IgG1) were purified from ascites by ammonium sulfate precipitation, and 1 mg was injected intraperitoneally (i.p.) into mice at the time of challenge and then on a weekly basis until 10 weeks postinfection.
Cell stimulation and cytokine production.
Popliteal lymph nodes draining the inoculated footpad were removed, a single-cell suspension was made by maceration through a fine-mesh stainless steel sieve, and 8 × 105 cells/well (on a 96-well plate) were cultured in 200 μl of complete RPMI consisting of RPMI 1640 medium (Biofluids, Rockville, Md.) supplemented with 10% fetal bovine serum (Gibco BRL), 1 mM sodium pyruvate, 2 mM l-glutamine, 0.05 mM 2-mercaptoethanol, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. For antigen-specific responses, the lymph node cells from individual mice were cultured in the presence of 2.5 μg of concanavalin A/ml or 25 μg of soluble leishmania antigen (SLA)/ml prepared from Friedlin strain promastigotes. Culture supernatants were removed at 72 h and tested for the presence of IL-4 and IFN-γ by a two-site sandwich enzyme-linked immunosorbent assay.
Measurement of cytokine expression by reverse transcription (RT)-PCR.
Relative levels of cytokine mRNA were determined by RT-PCR as previously described (8). Briefly, cells were harvested after stimulation and thoroughly washed. Cytokine mRNA levels were analyzed at 24 h after stimulation. Total RNA was prepared by lysis in RNA-STAT-60 (TEL-Test B Inc., Friendswood, Tex.) followed by precipitation from the aqueous phase as recommended by the manufacturer. Recovered RNA was resuspended in diethyl pyrocarbonate-treated deionized water, and 1 μg of RNA was used for reverse transcription. PCR were performed on serial dilutions of cDNA, and a sample (10 μl) of each reaction was electrophoresed through a 1.0% agarose gel and visualized with ethidium bromide. The number of cycles was chosen based on the generation of a readily detectable product while remaining on the linear part of the amplification curve. For each sample, PCR was also performed on hypoxanthine phosphoribosyltransferase (HPRT), and cDNA was adjusted to equivalent levels. Ethidium bromide-stained gels were photographed with an Eagle Eye II Still Video System (Stratagene, La Jolla, Calif.), and the intensity of fluorescence was determined using the Eaglesight software. Each pair of primers spanned at least one intron, allowing mRNA to be distinguished from any contaminating genomic DNA. The following cycle numbers and sequences were used: HPRT (30 cycles), sense, GTT GGA TAC AGG CCA GAC TTT GTT G, and HPRT antisense, GAG GGT AGG CTG GCC TAT AGG CT; IFN-γ (29 cycles), sense, TGG AGG AAC TGG CAA AAG GAT GGT, and IFN-γ antisense, TTG GGA CAA TCT CTT CCC CAC; IL-4 (34 cycles), sense, ACG AGG TCA CAG GAG AAG GGA CGC CAT GCA, and IL-4 antisense, TCA TTC ATG GAG CAG CTT ATC GAT GAA TCC; IL-12p40 (30 cycles), sense, GAC CCT GCC CAT TGA ACT GGC, and p40 antisense, CAA CGT TGC ATC CTA GGA TCG.
Transmissibility of parasites from infected footpads to sand flies.
Two- to four-day-old Phlebotomus papatasi females were obtained from a colony initiated by field-caught specimens from the Jordan Valley and reared at the Department of Entomology, Walter Reed Army Institute of Research. Sand flies (15 to 20 flies/vial) were exposed to infected footpads (up to 2 h) in order to examine the transmissibility to sand flies of parasites contained in the lesions. The vials were sealed at one end with flexible rubber material with a small slit that permitted insertion of the footpad into the vial while maintaining the enclosure. Prior to their exposure to flies, the mice were anaesthetized i.p. with 200 μl of 20 mg of ketamine-HCl/ml. Blood-fed females from each vial were separated and maintained in individual pots lined with plaster of Paris, given a 50% sucrose–5% albumin solution and water, and dissected 48 to 72 h later for the presence of Leishmania promastigotes.
Statistical analysis.
Student's unpaired t test was used to determine the statistical significance of the values obtained.
RESULTS
Evolution of footpad lesions following high- and low-dose inoculation of L. major.
Inoculation of 106 metacyclic promastigotes into the footpad led to the rapid development of lesions that peaked at approximately 3 weeks and were resolved slowly over the subsequent 10 weeks. Inoculation of 102 metacyclic promastigotes also consistently induced footpad swelling in C57BL/6 mice, though in this case the onset of lesion formation was delayed until 6 to 8 weeks, peaked at around week 10, and was resolved by week 16 (Fig. 1A). The relationship between lesion progression and parasite numbers in the site was examined by quantifying the number of viable amastigotes per milligram of tissue excised from the inoculation site. For the high-dose inoculations, there appeared to be a direct correlation between the numbers of parasites in the site and the size of the lesion, at least during the early stages of lesion development (Fig. 1B). The peak concentration of parasites was observed at around 3 weeks (2 × 104); by week 7 the parasite load had been reduced by more than 98%, and by week 11 the excised tissue from the majority of footpads was negative for parasites. Following low-dose inoculation, the tissue was negative for parasites at 1 week and then again at 3 weeks, but by 7 weeks, the concentration of parasites per milligram of tissue had increased to a level that was comparable to the peak concentration detected at week 3 following high-dose inoculation (1.2 × 105). Nonetheless, the total number of tissue amastigotes established following the high-dose footpad infections was considerably higher, since the peak concentrations were associated with a greater amount of inflamed tissue. By week 11, the concentration of parasites in the low-dose inoculation site had been reduced by more than 99%. Thus, in contrast to the mice inoculated with 106 metacyclic promastigotes, lesion formation following inoculation of 102 promastigotes did not correlate with parasite amplification in the site but did correlate with parasite clearance from the site. Interestingly, following the resolution of the lesion, the persistent presence of a low number of parasites was noted in the site for up to 20 weeks postinfection.
FIG. 1.
Lesion development (A) and parasite concentration in footpads (B) in C57BL/6 mice inoculated in the left hind footpad with 102 (●) or 106 (□) L. major metacyclic promastigotes. (A) Mean footpad width is expressed in millimeters ± 1 standard deviation; 12 to 16 mice per group were used. (B) The geometric mean number of viable amastigotes per milligram of tissue ± 1 standard deviation is shown; 4 to 5 mice per group were used.
Immune response in draining lymph node following high- and low-dose inoculation.
Lymph node cells draining the inoculation site produced high levels of IFN-γ in response to SLA in vitro 3 weeks after high-dose challenge, and this response remained high throughout the healing phase (weeks 5 to 7). In contrast, following low-dose inoculation, IFN-γ production in response to SLA in vitro remained undetectable or low until week 7, correlating with the reduction in the parasite concentration in the site (Table 1). The enzyme-linked immunosorbent assay data were confirmed by RT-PCR analysis of cytokine mRNA obtained from antigen-stimulated cells. Following high-dose inoculation, there was a 17-fold increase in IFN-γ transcripts and an 11-fold increase in mRNA for IL-12p40 as early as week 3 (Fig. 2A). Transcript levels for both cytokines remained high at each subsequent time point examined, up to week 15. A small and transient increase in IL-4 mRNA was observed at week 3. Following low-dose inoculation, no enhancement in IFN-γ mRNA levels was observed until week 7, at which time point a sixfold increase was seen (Fig. 2B). This continued to increase to 14-fold and 17-fold at weeks 11 and 15, respectively. A small induction of IL-12p40 mRNA was observed at week 3, and despite the strong production of IFN-γ during the later stage of infection, IL-12p40 transcripts increased only slightly during this time (approximately fivefold). Thus, in contrast to the high-dose model, the evolution of immunity following low-dose inoculation was delayed and coincided more closely with parasite killing and lesion development.
TABLE 1.
Antigen-specific IFN-γ production by lymph node cells from mice infected with a high or low dose of L. major
| Week postinoculation | Production of IFN-γ (pg/ml) witha:
|
|
|---|---|---|
| Low dose (102) | High dose (106) | |
| 3 | 0 | 1,541 ± 544 |
| 5 | 189 ± 156 | >2,000 |
| 7 | 1,970 ± 585 | 2,153 ± 1,468 |
Values are means ± the standard deviations of IFN-γ levels in supernatants from antigen-stimulated cultures minus results for medium controls. Six mice per group were used, and results were pooled from two separate experiments.
FIG. 2.
RT-PCR analysis of cytokine mRNA obtained from antigen-stimulated popliteal lymph node cells from mice infected with 106 (A) or 102 (B) L. major metacyclic promastigotes. Results are expressed as the fold increase in IFN-γ (black bars), IL-12 (hatched bars), and IL-4 (gray bars) mRNA in antigen-stimulated cultures compared to that in unstimulated controls.
Evolution of footpad lesions in cytokine-depleted and IL-12p40 knockout mice.
The role of IL-12 in immunity following high- and low-dose infection was investigated in both anti- IL-12-treated and genetically deficient IL-12p40 knockout C57BL/6 mice. As reported by others, footpad lesions in anti-IL-12-treated mice failed to heal following conventional high-dose inoculation; footpad swelling was already greater than in the control-treated mice by week 3, and the swelling increased rapidly up to 6 weeks postinfection, at which time ulcers began to appear and the experiment was terminated for these mice (Fig. 3A). Lesion development following high-dose inoculation was again correlated with the number of parasites in the site. By 3 weeks, there was already a fourfold increase in the number of amastigotes per milligram of tissue in the anti-IL-12-treated mice, and by 5 weeks this difference was more than 100-fold, as the organisms were killed in the control mice and continued to grow in the immunodeficient mice (Fig. 4A). The anti-IL-12 treatment in mice infected with 102 metacyclic promastigotes also prevented them from healing their primary footpad lesions. Notably, there was no reduction in the time of lesion appearance, which still took up to 8 weeks following parasite delivery to become obvious (Fig. 3B). The difference in footpad swelling was not apparent until week 10, at which time the lesions in the anti-IL-12p40-treated mice continued to progress while the lesions in the control mice began to resolve. A quantitative comparison of the parasite loads failed to reveal any increase in the anti-IL-12-treated mice at weeks 3, 5, and 7 (Fig. 4B). The peak concentration of parasites established in the control mice, observed around week 7, was again comparable to the numbers established following high-dose inoculation and was again achieved in the absence of any overt pathological changes in the site. It was not until week 10 that the numbers of parasites in the site diverged, with a 10-fold increase in the anti-IL-12p40-deficient mice and a greater than 99% reduction in the controls (Fig. 4B).
FIG. 3.
Effects of anti-IL-12 treatment on development of footpad lesions in C57BL/6 mice infected with 106 (A) or 102 (B) L. major metacyclic promastigotes. Starting at the time of challenge and then on a weekly basis, each mouse was injected i.p. with 1 mg of anti-mouse IL-12p40 (c17.8; rat IgG1) (◊) or with anti-β-galactosidase (GL113) as a control (□) until 10 weeks postinfection. Mean footpad width is expressed in millimeters ± 1 standard deviation; 8 to 12 mice per group were used.
FIG. 4.
Effects of anti-IL-12 treatment on parasite loads in footpads of C57BL/6 mice infected with 106 (A) or 102 (B) L. major metacyclic promastigotes. Mice were injected with anti-IL-12p40 (c17.8; rat IgG1) (◊) or with anti-β-galactosidase control (GL113) (□). Results are expressed as the geometric mean number of viable amastigotes per milligram of tissue ± 1 standard deviation; 4 mice per group were used. The asterisk indicates the significant difference between values at the indicated time point (P < 0.05).
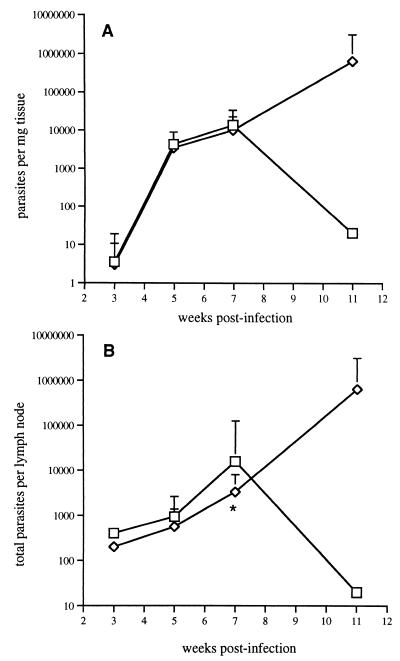
The outcome of infection in IL-12p40-depleted mice was confirmed in IL-12p40 knockout mice. Following delivery of 102 metacyclic promastigotes, the growth of amastigotes in the footpad remained identical for the wild-type and deficient mice for at least the first 7 weeks, at which time both strains harbored approximately 104 parasites in the site of inoculation (Fig. 5A). However, by week 11, few viable organisms remained in the footpads of the wild-type mice, while the IL-12p40-deficient mice harbored more than 5 × 105 amastigotes per mg of tissue. An analysis of the parasite load in the lymph node draining the footpad revealed a similar delay in the effects of the IL-12 deficiency on parasite amplification and/or dissemination (Fig. 5B). In fact, a slight reduction in the total number of amastigotes per lymph node was observed in the deficient mice at weeks 3, 5, and 7. Week 11 again revealed a huge disparity in tissue parasite burden, with a reduction in the lymph nodes of wild-type mice and a massive increase to approximately 2 × 106 per node in the deficient mice.
FIG. 5.
Parasite loads in footpads (A) and in a draining lymph node (B) following infection of C57BL/6 (□) and IL-12p40(−/−) (◊) mice with 102 L. major metacyclic promastigotes. Results are expressed as the geometric mean number of viable amastigotes ± 1 standard deviation; 4 to 5 mice per group were used. The asterisk indicates the significant difference between values at the indicated time point (P < 0.05).
To investigate whether the delayed effects of the IL-12 deficiency might be due to an IL-12-independent pathway of IFN-γ production early in the course of infection, the C57BL/6 mice were depleted of IFN-γ by treatment with anti-IFN-γ antibodies once a week for the first 10 weeks following delivery of 106 or 102 metacyclic promastigotes. By 2.5 weeks after high-dose challenge, the IFN-γ-depleted mice already harbored 4 times more parasites per milligram of tissue than the mice treated with control antibodies (data not shown). In contrast, the concentration of amastigotes present in the footpads of IFN-γ-depleted mice at 4, 5, and 7 weeks after low-dose challenge was lower than in the control-treated mice (Fig. 6). By week 8, the onset of parasite clearance in the control mice was apparent, whereas parasites continued to grow in the IFN-γ-depleted mice, which maintained extremely high tissue parasite burdens to week 11.
FIG. 6.
Effects of anti-IFN-γ treatment on parasite loads in footpads of C57BL/6 mice infected with 102 L. major metacyclic promastigotes. Mice were treated at the time of challenge and then on a weekly basis with 1 mg of XMG-6/mouse (◊) or with anti-β-galactosidase (GL113) (□). Results are expressed as the geometric mean number of viable amastigotes ± 1 standard deviation; 4 to 5 mice per group were used. The asterisk indicates the significant difference between values at the indicated time point (P < 0.05).
Ability of footpads harboring L. major to transmit infection to P. papatasi.
The high- and low-dose challenge models revealed clear differences in the kinetics of and relationship between parasite growth and lesion formation. The models also revealed differences in the degree to which parasites were able to persist in the inoculation site following resolution of the lesion. The consequences of these differences in terms of the ability of the infected footpads to transmit L. major to P. papatasi (i.e., to maintain the host as a potential reservoir of infection) were investigated. Two weeks after high-dose inoculation, the footpads provided a highly efficient source of parasites for infection of sand flies (47% of fed flies). This rate declined to 26% of fed flies at 5 weeks and to 21% by week 6, and by 8 weeks, the transmissibility of the site had been almost completely lost (1% of fed flies) (Fig. 7). Following exposure of flies to footpads inoculated with 102 metacyclic promastigotes, the efficiency of transmission was never as high as that seen early following high-dose challenge; however, the duration of transmissibility was significantly extended. Successful transmission of parasites was first observed at 6 weeks, peaked in efficiency at 7 weeks (19% of fed flies), and continued at a low frequency up to 19 weeks following footpad challenge (Fig. 7). Thus, the ability of the inoculation site to provide a source of L. major to exposed sand flies following low-dose challenge was unrelated to lesion size and was extended by at least 6 weeks relative to that for footpads inoculated with 106 metacyclic promastigotes.
FIG. 7.
Ability of C57BL/6 mice challenged with 102 (●) or 106 (□) L. major metacyclic promastigotes to transmit parasites to P. papatasi. Individual footpads of infected mice (4 to 6 mice per group at each time point) were exposed to 15 to 20 sand flies for 2 h. Blood-fed females from each vial were separated and dissected after 48 to 72 h and examined microscopically for the presence of promastigotes. Results are pooled from two separate experiments and are expressed as the percentages of the total numbers of blood-fed flies that were positive for promastigotes at each time point.
DISCUSSION
L. major infection in genetically resistant C57BL/6 mice, typically employing high doses of promastigotes inoculated into the footpad, remains a widely used model to reproduce the pathogenesis and immunity associated with self-limiting cutaneous leishmaniasis in humans. In the present studies, the relationship between parasite growth, lesion formation, and immunity has been reexamined in an infection model that takes into account a key aspect of natural transmission: low-dose metacyclic promastigote challenge. In conjunction with a careful monitoring of the parasite concentration in the inoculation site over time, the model has revealed two discrete stages in the evolution of cutaneous lesions: the first, lasting approximately 6 weeks, favors the growth of the parasite in the site in the absence of any overt histopathological changes, and the second drives the killing of the parasite that is coincident with lesion formation.
Data from forced-feeding experiments suggest that as few as 102 parasites may approximate the number of metacyclic promastigotes that are delivered by an infected sand fly (38). The ability of 102 promastigotes to initiate self-curing lesions in a resistant mouse strain may not have been predicted based on reports that 102 parasites failed to establish footpad lesions in resistant mice (7, 17) or even in “susceptible” BALB/c mice (4). It should be noted that in these studies purified metacyclic inocula were not used, so that the size of the actual infectious challenge is not known. The ability of a challenge inoculum of as low as 102 L. major promastigotes to initiate the development of small, healing footpad lesions in genetically resistant mice has been described in at least three prior studies (6, 22, 35). In these studies, however, the number of parasites present in the inoculation site was not monitored over time, so that the relationship between parasite growth and lesion development could not be evaluated. In the present studies, 102 metacyclic promastigotes consistently initiated an impressive growth of amastigotes in the footpad that eventually equaled or exceeded the peak parasite densities achieved following inoculation of 106 metacyclic promastigotes. And while in each case the infections produced lesions that eventually healed, the largest footpad swelling that was observed following high-dose injection roughly coincided with the peak numbers of amastigotes in the site, whereas the peak parasite concentration following low-dose challenge occurred prior to lesion formation. A similar dissociation between parasitic load and cutaneous pathology has recently been observed following low-dose infection in the ear dermis (Y. Belkaid et al., unpublished data).
When footpad swelling first appeared between 8 and 10 weeks after a low-dose challenge, it was associated with a reduced tissue parasite burden. Furthermore, the peak inflammatory response was observed at the time when most of the organisms had already been killed or cleared from the site. Prior studies using high-dose challenge have also noted that footpad swelling persists well after parasite clearance (14, 34). Other studies have described the simultaneous development of pathology and immune response (21, 25), and the delayed appearance of footpad lesions in major histocompatibility complex class II-deficient C57BL/6 mice (9) and in SCID mice (28) indicated T-cell involvement in the inflammatory process. Still, in these and other studies (7, 13), as in our own experience with high-dose infections reported here, the early stages of lesion formation and progression have generally been associated with increasing numbers of parasites in the site. The low-dose challenge reveals that under more natural conditions, L. major is capable of sustained growth that is remarkably dissociated from any overt cutaneous pathology and that lesion formation clearly coincides with the onset of host immunity and parasite killing.
The continuous growth of amastigotes in the inoculation site for up to 6 to 8 weeks in the absence of lesion formation suggests that the immune responses that mediate parasite killing and pathology are effectively avoided throughout this period of time. While antigen-specific IFN-γ and IL-12 responses in the draining node were at relatively low levels for at least the first 5 weeks following low-dose challenge, these assays do not necessarily reflect responses that are localized to the inoculation site, and even low levels of these cytokines might be expected to moderate the early course of infection. Therefore, the results for the IL-12-deficient mice and for the IL-12- and IFN-γ-depleted mice offer the strongest evidence that the induction or expression of cellular immunity is essentially absent during the first 6 to 8 weeks following parasite delivery, since even in the complete absence of these cytokines, parasite growth during this time was not enhanced compared to that for wild-type or control-treated C57BL/6 mice. In contrast, following high-dose challenge, the effects of the anti-IL-12 and anti-IFN-γ treatments in terms of footpad swelling and parasite numbers became apparent within the first 3 weeks, consistent with prior reports regarding these treatments (11, 33) and with the relatively rapid onset of IL-12 and IFN-γ responses (1 to 2 weeks) that has been repeatedly described for C57BL/6 mice following inoculation with high numbers of parasites (7, 11, 23, 26, 29). It seems likely that the ability of the parasite to establish such a discrete and prolonged silent phase of intracellular growth may be undermined by an excessive inoculum that makes parasites and released antigens rapidly available to dendritic cells, which in contrast to infected macrophages have been shown to produce IL-12 (10, 37) and to drive Th1 responses in vivo (18, 19). The low-dose infection model, in which parasites are more likely to be selectively targeted to and confined to macrophages early on, supports the biologic significance of a large number of in vitro studies indicating that the immune response initiation functions of Leishmania-infected macrophages, and IL-12 production in particular, are severely compromised (2, 5, 20, 23, 27, 36).
The ability of the parasite to establish such a sustained interval of growth in the mammalian host might underlie the maintenance of its transmission cycle in nature, since this improves the chance that vector sand flies will encounter infected tissue. This point was directly addressed in an experiment that is the first, so far as we are aware, to monitor the ability of an experimental host to transmit Leishmania back to sand flies during the course of infection. While the footpads that were infected using a high-dose challenge efficiently transmitted L. major to P. papatasi for at least the first 6 weeks, by 8 weeks the ability of these exposed sites to transmit parasites had been almost completely lost. In contrast, footpads infected using 102 metacyclic promastigotes provided a source of infective blood meals for a span of at least 12 weeks. Of note is the fact that footpads without demonstrable lesions transmitted parasites to flies during the acute stage of infection, as did footpads harboring low-level, persistent infections following healing. The persistence of L. major in the footpad and draining node following healing in resistant mice has been noted previously (1, 18, 31, 34). We consistently observed that this chronic state was more efficiently established following a low-dose challenge. Our results indicate that the failure of immune animals to completely eliminate parasites from the site is not an artifact of a high-dose challenge and, more importantly, that the persistent presence of low numbers of parasites in the skin can maintain the reservoir potential of the mammalian host.
REFERENCES
- 1.Aebischer T, Moody S F, Handman E. Persistence of virulent Leishmania major in murine cutaneous leishmaniasis: a possible hazard for the host. Infect Immun. 1993;61:220–226. doi: 10.1128/iai.61.1.220-226.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belkaid Y, Butcher B, Sacks D L. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: selective impairment of IL-12 induction in Leishmania-infected cells. Eur J Immunol. 1998;28:1389–1400. doi: 10.1002/(SICI)1521-4141(199804)28:04<1389::AID-IMMU1389>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Belosevic M, Finbloom D S, Van Der Meide P H, Slayter M V, Nacy C A. Administration of monoclonal anti-IFN-gamma antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989;143:266–274. [PubMed] [Google Scholar]
- 4.Bretscher P A, Wei G, Menon J N, Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science. 1992;257:539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 5.Carrera L, Gazzinelli R T, Badolato R, Hieny S, Muller W, Kuhn R, Sacks D L. Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J Exp Med. 1996;183:515–526. doi: 10.1084/jem.183.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diefenbach A, Schindler H, Donhauser N, Lorenz E, Laskay T, MacMicking J, Rollinghoff M, Gresser I, Bogdan C. Type 1 interferon (IFNalpha/beta) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity. 1998;8:77–87. doi: 10.1016/s1074-7613(00)80460-4. [DOI] [PubMed] [Google Scholar]
- 7.Doherty T M, Coffman R L. Leishmania major: effect of infectious dose on T cell subset development in BALB/c mice. Exp Parasitol. 1996;84:124–135. doi: 10.1006/expr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 8.Doherty T M, Seder R A, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J Immunol. 1996;156:735–741. [PubMed] [Google Scholar]
- 9.Erb K, Blank C, Ritter U, Bluethmann H, Moll H. Leishmania major infection in major histocompatibility complex class II-deficient mice: CD8+ T cells do not mediate a protective immune response. Immunobiology. 1996;195:243–260. doi: 10.1016/S0171-2985(96)80043-X. [DOI] [PubMed] [Google Scholar]
- 10.Gorak P M, Engwerda C R, Kaye P M. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur J Immunol. 1998;28:687–695. doi: 10.1002/(SICI)1521-4141(199802)28:02<687::AID-IMMU687>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 11.Heinzel F P, Rerko R M, Ahmed F, Pearlman E. Endogenous IL-12 is required for control of Th2 cytokine responses capable of exacerbating leishmaniasis in normally resistant mice. J Immunol. 1995;155:730–739. [PubMed] [Google Scholar]
- 12.Heinzel F P, Schoenhaut D S, Rerko R M, Rosser L E, Gately M K. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill J O. Resistance to cutaneous leishmaniasis: acquired ability of the host to kill parasites at the site of infection. Infect Immun. 1984;45:127–132. doi: 10.1128/iai.45.1.127-132.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill J O, North R J, Collins F M. Advantages of measuring changes in the number of viable parasites in murine models of experimental cutaneous leishmaniasis. Infect Immun. 1983;39:1087–1094. doi: 10.1128/iai.39.3.1087-1094.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Launois P, Tacchini-Cottier F, Parra-Lopez C, Louis J A. Cytokines in parasitic diseases: the example of cutaneous leishmaniasis. Int Rev Immunol. 1998;17:157–180. doi: 10.3109/08830189809084491. [DOI] [PubMed] [Google Scholar]
- 16.Liew F Y, Millott S, Parkinson C, Palmer R M, Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990;144:4794–4797. [PubMed] [Google Scholar]
- 17.Menon J N, Bretscher P A. Parasite dose determines the Th1/Th2 nature of the response to Leishmania major independently of infection route and strain of host or parasite. Eur J Immunol. 1998;28:4020–4028. doi: 10.1002/(SICI)1521-4141(199812)28:12<4020::AID-IMMU4020>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Moll H, Flohe S, Rollinghoff M. Dendritic cells in Leishmania major-immune mice harbor persistent parasites and mediate an antigen-specific T cell immune response. Eur J Immunol. 1995;25:693–699. doi: 10.1002/eji.1830250310. [DOI] [PubMed] [Google Scholar]
- 19.Moll H, Fuchs H, Blank C, Rollinghoff M. Langerhans cells transport Leishmania major from the infected skin to the draining lymph node for presentation to antigen-specific T cells. Eur J Immunol. 1993;23:1595–1601. doi: 10.1002/eji.1830230730. [DOI] [PubMed] [Google Scholar]
- 20.Piedrafita D, Proudfoot L, Nikolaev A V, Xu D, Sands W, Feng G J, Thomas E, Brewer J, Ferguson M A, Alexander J, Liew F Y. Regulation of macrophage IL-12 synthesis by Leishmania phosphoglycans. Eur J Immunol. 1999;29:235–244. doi: 10.1002/(SICI)1521-4141(199901)29:01<235::AID-IMMU235>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 21.Pirmez C, Cooper C, Paes-Oliveira M, Schubach A, Torigian V K, Modlin R L. Immunologic responsiveness in American cutaneous leishmaniasis lesions. J Immunol. 1990;145:3100–3104. [PubMed] [Google Scholar]
- 22.Preston P M, Dumonde D C. Experimental cutaneous leishmaniasis. V. Protective immunity in subclinical and self-healing infection in the mouse. Clin Exp Immunol. 1976;23:126–138. [PMC free article] [PubMed] [Google Scholar]
- 23.Reiner S L, Zheng S, Wang Z E, Stowring L, Locksley R M. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J Exp Med. 1994;179:447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sacks D L, Hieny S, Sher A. Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of Leishmania major promastigotes. J Immunol. 1985;135:564–569. [PubMed] [Google Scholar]
- 25.Sadick M D, Locksley R M, Raff H V. Development of cellular immunity in cutaneous leishmaniasis due to Leishmania tropica. J Infect Dis. 1984;150:135–138. doi: 10.1093/infdis/150.1.135. [DOI] [PubMed] [Google Scholar]
- 26.Sadick M D, Locksley R M, Tubbs C, Raff H V. Murine cutaneous leishmaniasis: resistance correlates with the capacity to generate interferon-gamma in response to Leishmania antigens in vitro. J Immunol. 1986;136:655–661. [PubMed] [Google Scholar]
- 27.Sartori A, Oliveira M A, Scott P, Trinchieri G. Metacyclogenesis modulates the ability of Leishmania promastigotes to induce IL-12 production in human mononuclear cells. J Immunol. 1997;159:2849–2857. [PubMed] [Google Scholar]
- 28.Satoskar A, Brombacher F, Dai W J, McInnes I, Liew F Y, Alexander J, Walker W. SCID mice reconstituted with IL-4-deficient lymphocytes, but not immunocompetent lymphocytes, are resistant to cutaneous leishmaniasis. J Immunol. 1997;159:5005–5013. [PubMed] [Google Scholar]
- 29.Scharton-Kersten T, Afonso L C, Wysocka M, Trinchieri G, Scott P. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J Immunol. 1995;154:5320–5330. [PubMed] [Google Scholar]
- 30.Scott P, Farrell J P. Experimental cutaneous leishmaniasis: induction and regulation of T cells following infection of mice with Leishmania major. Chem Immunol. 1998;70:60–80. doi: 10.1159/000058698. [DOI] [PubMed] [Google Scholar]
- 31.Stenger S, Donhauser N, Thuring H, Rollinghoff M, Bogdan C. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J Exp Med. 1996;183:1501–1514. doi: 10.1084/jem.183.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stenger S, Thuring H, Rollinghoff M, Bogdan C. Tissue expression of inducible nitric oxide synthase is closely associated with resistance to Leishmania major. J Exp Med. 1994;180:783–793. doi: 10.1084/jem.180.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sypek J P, Chung C L, Mayor S E, Subramanyam J M, Goldman S J, Sieburth D S, Wolf S F, Schaub R G. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Titus R G, Marchand M, Boon T, Louis J A. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 35.Titus R G, Ribeiro J M. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988;239:1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- 36.Vieira L Q, Hondowicz B D, Afonso L C, Wysocka M, Trinchieri G, Scott P. Infection with Leishmania major induces interleukin-12 production in vivo. Immunol Lett. 1994;40:157–161. doi: 10.1016/0165-2478(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 37.von Stebut E, Belkaid Y, Jakob T, Sacks D L, Udey M C. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J Exp Med. 1998;188:1547–1552. doi: 10.1084/jem.188.8.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warburg A, Schlein Y. The effect of post-bloodmeal nutrition of Phlebotomus papatasi on the transmission of Leishmania major. Am J Trop Med Hyg. 1986;35:926–930. doi: 10.4269/ajtmh.1986.35.926. [DOI] [PubMed] [Google Scholar]