Abstract
Study Design
Review article.
Objectives
A review of literature on the treatment of pyogenic spondylodiscitis in geriatric patients was performed with the aim to give an overview about these special patients and a recommendation on necessary diagnostics as well as conservative and operative treatment options.
Methods
A systematic computerized literature search was done by the spondylodiscitis working group of the German Society for Orthopedics and Trauma Surgery.
Results
Spondylodiscitis has an increasing incidence by age with a peak at 75 years or older. The 1-year mortality without an appropriate treatment is with 15 to 20% extremely high. Pathogen detection is the essential diagnostic step and the basis for a sufficient antibiotic treatment. Geriatric patients have initially less elevated inflammatory parameters. Compared to younger patients. They have a longer length of hospital stay and take longer for CRP normalization. Even the outcome between conservative and operative treatment is comparable after one year. Patients with spinal instability, immobilizing pain, epidural abscess, and newly emerged neurological deficits should be considered for operative treatment.
Conclusions
The treatment of geriatric patients with pyogenic spondylodiscitis must take into account that these patients usually have multiple comorbidities. The main goals are resistance-based antibiotics and the shortest possible time of immobilization of the patient.
Keywords: spondylodiscitis, geriatric patients, conservative, operative, comorbidities, resistance-based antibiotics
Spondylodiscitis Study Group, Spine Section of the German Society of Orthopaedic and Trauma Surgeons
Introduction
With increasing age in Western countries, the likelihood of secondary diseases such as neoplasia, metabolic diseases and susceptibility to infections increases. A significant influence seems to be the decline in the efficiency of the own immune system, the so-called immunosenescence.1,2 With regard to all age groups, pyogenic spondylodiscitis occurs most frequently in 70-79 years old patients. Moreover, the incidence of spondylodiscitis is estimated .4 – 2.4 per 100 000 per year. 3 Several studies point to an increasing incidence due to an aging population with up to 11.3/100 000.4,5 Besides reports on a bimodal age-distribution 40-50 years ago,6-9 today there seems to be a rising evidence for a peak mainly in the population aged 75 years and older. 5 Without an appropriate treatment, the overall mortality rate is reported in literature to be as high as 15-20%. However, the reason for this is the often long period between the onset of the disease and the final diagnosis with initiation of the adequate therapy. 10 Unspecific symptoms and the absence of clinical presentations such as fever at diagnosis and initiation of therapy are among the reasons for the delay. With the growing population of multimorbid and immunocompromised patients in particular, spondylodiscitis should be included in differential diagnostic considerations at an early stage, even in the presence of nonspecific symptoms. For this reason, we included key points on the diagnosis and treatment of pyogenic spondylodiscitis in this review as part of a comprehensive systematic review also considering the current guidelines of the Infectious Disease Society of America (IDSA) and the German guidelines.
Material and Methods
Study Design
We conducted a comprehensive systematic review of the literature according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) checklist and algorithm. 11
Study Characteristics
Investigations between 2000 and 2020 were included. For analyses, prospective and retrospective observational studies that dealt with spondylodiscitis in geriatric patients were considered. Furthermore, only articles in German or English language were included.
Information Sources
The authors performed an initial search of PubMed, Google scholar and Cochrane databases for investigations for possible inclusion in the review.
Search
The keywords used in the research were ((“elderly”) OR (“older age”) OR (“geriatric”) OR (“aged”)) AND ((“spondylodiscitis”) OR (“discitis”) OR (“Spinal infection”) OR (“Spinal Osteomyelitis”) OR (“Pyogenic spinal infection”)
Study Selection
The authors limited the research to observational studies, while systematic reviews, meta-analyses, case series and case reports were excluded. Titles and abstracts were reviewed. Duplicates were removed and full texts were checked for suitability. In cases where a decision could not be taken based on information from the title and abstract, the full text was evaluated. The final decision was made based on the analysis of the full text. The study selection process was carried out independently by three authors (NHvdH, CH, MJS) (Figure 1).
Figure 1.
Prisma Flow chart.
Data Items
The main prerequisite was that included studies dealt with spondylodiscitis in elderly >65 years. The authors performed an initial search of the above-mentioned databases for investigations for possible inclusion in the review. First, title and abstracts were screened. In cases where a decision could not be taken due to the information from the title and abstract the full text was evaluated. In the end, the final decision was made based on the analysis of the full text. Studies were selected according to the following inclusion criteria: (a) Incidence, (b) diagnostic workup, (c) non-surgical therapy, (d) surgical therapy.
Synthesis of Results
We extracted data concerning study characteristics including author’s name, title, year of publication and journal published. Outcome parameters were analysed according to the abovementioned inclusion criteria. For all included studies, we used the Oxford Centre for Evidence-Based Medicine (2011) guidelines for defining the level of evidence. The strength of recommendation was defined using the GRADE approach (Grading of Recommendations Assessment, Development and Evaluation). Regarding the risk of bias every included investigation was graded with respect to the Newcastle Ottawa scale (Table 1).
Table 1.
Study characteristics of the included Investigations.
| # | Puiblicationdate | Authors | Main findings | No. of patients | Type of study | EBM-Level | GRADE | NewcastleOttawa Scale |
|---|---|---|---|---|---|---|---|---|
| 1 | 2009 | Kapsalaki E. et al. | Spontaneous spondylodiscitis should be considered in every patient with back pain accompaniedby fever and laboratory markers of inflammation. | 8 | review | IV | low | n/a |
| The major predisposing risk factor seems to be uncontrolled diabetes. | ||||||||
| 2 | 2008 | Yang S.C. et al. | Perkutaneous endoscopy is superior to CT-guided biopsy for identifyen pathogen | 52 | therapeutic study | III | moderate | *** |
| 3 | 2009 | Deininger, M.H. et al. | minimally invasive percutaneous fixation is a feasible and effective technique to achieve immediate pain release,avoid long-term immobilization and overcome the disadvantages of a dorsoventral procedure. | 12 | prospective cohort study | III | moderate | *** |
| 4 | 2004 | Mann, S. et al. | Surgical treatment is the modality of choice in patients with acute spinal osteomyelitis. The decision whether an anterior or posterior approach should be used must be made on an individual basis. | 24 | prospective cohort study | III | moderate | ** |
| 5 | 2006 | Frangen, T.M. et al. | Initial surgical treatment included posterior internal fixation only. Abscesses can be drained percutaneously.Ventral debridement and stabilization is only recommended if insufficient stability can be obtained by dorsal fixation alone, as shown by the persistence of infection or pain. | 78 | retrospective study | IV | low | * |
| 6 | 2007 | Chaudhary, S.B. et al. | Early detection and aggressive treatment are paramount inmanaging postprocedural spinal infections and limiting their long-term sequelae. | n/a | review | IV | low | n/a |
| 7 | 2008 | Hutchinson, C. et al. | The mean time from symptom onset to diagnosis was 34 days.Most patients presented with back pain and elevated CRP.Differentiation between discitis and other spinal infections does not appear to be important | 41 | retrospective study | IV | low | * |
| 8 | 2007 | Butler, J.S. et al. | In the majority of cases, conservative management of pyogenic spinal infectionwith antibiotic therapy and spinal bracing is very successful. | 48 | retrospective study | IV | low | * |
| 9 | 2009 | Robinson, Y. et al. | This study reveals healing and improved function after expandable titanium cage implantation in all patients. | 25 | prospective study | III | moderate | *** |
| 10 | 2007 | Suess, O. et al. | Same-stage instrumentation allows early postoperative mobilization of the patient, which is advantageous,especially for an increasingly elderly population and in patients with comorbidities. | 24 | retrospective study | IV | low | ** |
| 11 | 2009 | Maus, U. et al. | PCT is not useful as diagnostic tool or monitoring parameter for spondylodiscitis. | 35 | prospective study | III | moderate | *** |
| 12 | 2008 | Robinson, Y. et al. | Prerequisites fur the usefulness of titanium cages are a radical debridement, correction of deformity, and additional bony fusion by bone grafting. | 22 | prospective study | III | moderate | *** |
| 13 | 2010 | Uchida, K. et al. | In case of neurological impairment an urgent MRI staging regimen should be started. | 37 | retrospective study | IV | low | * |
| 14 | 2015 | Dreimann, M. et al. | Within al follow-up time of 19 months, the the modified posterior transversectomy with 360-degree decompression and anterior wall reconstruction with titanium cages in combination with posterior instrumentationfor sagittal alignment correction is a reliable, effective, and safe treatment option. | 10 | retrospective study | V | Very low | n/a |
| 15 | Yoon, Y.K. et al. | Differentiation between TBC and Pyogenic Spondy, risk index from WBc, PCT and CrP | 117 | retrospective study | IV | Low | *** | |
| 16 | 2015 | Park, K.H. et al. | There is no difference in conservative treatment and surgical instrumentation. A prolonged antibiotic treatment > 6 weeks reduces the risk of recurrence. | 153 | retrospective study | IV | Low | ** |
| 17 | 2014 | Shiban, E. et al. | The results of our retrospective study show that surgical treatment of spondylodiscitis with a staged surgical approach (if needed) and a short1-2-week period of intravenous antibiotics followed by 3 months of oral antibiotics is appropriate for most patients in whom conservative treatment has failed or is not advisable.The choice of the used material has no impact on the healing rate. | 113 | retrospective study | IV | Low | ** |
| 18 | 2013 | Özkan, N. et al. | PMMA is an feasible alternative for bone grafts or cages in the treatment of cervical vertebral osteomyelitis. | 21 | retrospective study | IV | Low | * |
| 19 | 2010 | Hempelmann, R.G. et al. | The morbidity and mortality suggest that this surgical treatment is not the therapy of first choice in high-risk septic patients,but may be considered in patients when conservative management has failed. | 18 | retrospective study | IV | Very low | |
| 20 | 2009 | Sobottke, R. et al. | If surgery is indicated the operative risks should be borne in mind, but advanced age should not be the crucial factor in decision-making.The quality of life is equal in both groups (operative vs. conservative) | 32 | retrospective study | |||
| 21 | 2021 | Lee, J.H. et al. | Clinical Outcomes in Older Patients Aged over 75 Years Who Underwent Early Surgical Treatment for Pyogenic Vertebral Osteomyelitis | 439 | retrospective study | IV | Low | *** |
| 22 | 2021 | Lackermair, S. et al. | Distribution of Underlying Causative Organisms, Patient Age, and Survival in Spontaneous spondylodiscitis with Special Focus on Elderly Patients | 51 | retrospective study | IV | Very low | |
| 23 | 2020 | Alas, H. et al | Comparative outcomes of operative relative to medical management of spondylodiscitis accounting for frailty status at presentation | 116 | retrospective study | IV | Low | ** |
| 24 | 2018 | Shah, K. et al | Role of Frailty Scoring in the Assessment of Perioperative Mortality in Surgical Management of Tuberculous Spondylodiscitis in the Elderly | 26 | retrospective study | IV | Very low | * |
| 25 | 2018 | Dubost, J.J. et al | Primary infectious spondylodiscitis in 51 patients over 75 years old: A comparative study | 152 | retrospective study | IV | Low | ** |
| 26 | 2017 | Courjon, J. et al | Pyogenic vertebral osteomyelitis of the elderly: Characteristics and outcomes | 351 | post-hoc analysis | III | moderate | *** |
| 27 | 2018 | Aguilar-Company, J. et al | Native vertebral osteomyelitis in aged patients: distinctive features. An observational cohort study | 247 | retrospective study | IV | Low | ** |
| 28 | 2017 | Shetty, A.P. et al | Tubercular spondylodiscitis in elderly is a more severe disease: a report of 66 consecutive patients | 66 | retrospective study | IV | low | * |
| 29 | 2017 | Amadoru, S. et al | Spinal infections in older people: an analysis of demographics, presenting features, microbiology and outcomes | 53 | retrospective study | IV | very low | * |
| 30 | 2016 | Kothari, M.K. et al | Surgical Management in Elderly Patients with Tuberculous Spondylodiscitis: Ten Year Mortality Audit Study | 20 | retrospective study | IV | very low | |
| 31 | 2016 | Kothari, M.K. et al | Short to Mid-Term Term Surgical Outcome Study with Posterior Only Approach on Tuberculous Spondylodiscitis in an Elderly Population | 16 | retrospective study | IV | very low | * |
Diagnosis
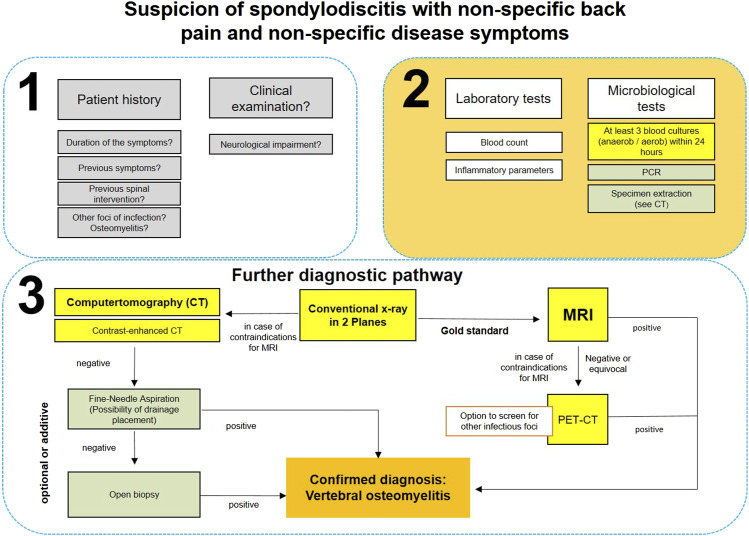
The search criteria used in this review showed only very few references for specific diagnostics especially in elderly patients (>65 years). Considering the current guidelines, the diagnostic workflow is identical in all age groups (>18 years). Therefore, the authors also implemented essential components from the guidelines of the USA 2015 (IDSA) and Germany 202012,13 and present a diagnostic algorithm for suspected spondylodiscitis (Figure 2).
Figure 2.
Diagnostic algorithm.
Anamnesis and Laboratory Findings
In addition to radiological findings, the path to diagnosis is based on clinical, laboratory and microbiological results. It is not uncommon for this to cause a delay of 2-12 weeks between diagnosis and treatment initiation. 10 The aim is therefore to reduce the time until the start of therapy by means of adequate diagnostics. Beside an extended clinical examination, a complete medical history based on the known red flags is essential (Table 2). 14
Table 2.
Anamnestic red flags for vertebral osteomyelitis.
| B symptoms | Severe nocturnal pain |
|---|---|
| Past bacterial infection | Previous spinal infiltration treatment |
| Intravenous drug abuse | Immunosuppression |
| Underlying malignancy | Long-term stay abroad |
| Origin of the patient from a country with high incidence of tuberculosis | Haemodialysis |
In all patients with existing back pain and suspected infectious origin, laboratory diagnostics are the basis for further diagnostics. A specific parameter proving the diagnosis of vertebral osteomyelitis does not exist. Laboratory tests includes leukocyte and C-reactive protein (CRP) counts. In literature, the blood sedimentation rate (ESR) is often mentioned as a parameter that is easy to determine, but it is non-specific, especially in the elderly.15,16 Amadoru et al showed that older patients with spinal infections present later, with higher markers of inflammation and fewer typical symptoms and signs of infection, leading to poorer outcomes. 17 Leukocytosis is not necessarily present, whereas increased CRP is seen in 90%–98% of the cases.10,18 In case of clinical suspicion of a vertebral osteomyelitis, at least three blood culture pairs (aerobic/anaerobic) within 24 hours are obtained. The pathogen can be identified in 25%–59% of blood cultures, whereas a pathogen detection rate of as much as 70% is described in patients not previously treated with antibiotics.10,19
Imaging Diagnostics and Findings
With increasing age, the likelihood of the presence of coexisting spinal pathologies also increases. Thus, the ability to distinguish clear radiographic signs of spondylodiscitis from other disease-related changes such as degenerative and inflammatory diseases and neoplasms of the spine becomes even more difficult.
Plain radiographs of the relevant spinal segment are the first-line imaging study in patients with unclear spinal symptoms. A negative x-ray does not rule out vertebral osteomyelitis but is nevertheless important to assess disease progression. Erosions and changes on the end plates of the corresponding segment being visible after weeks or months, depending on the pathogen virulence, the patient’s immune status or the clinical course (acute/chronic). However, there is no evidence in the literature showing destructive inflammatory processes to be more progressive or different in elderly patients than in young patients. 20
Magnetic resonance imaging (MRI) is due to its very high sensitivity and specificity the golden standard in imaging studies to detect vertebral osteomyelitis, whereby adding a contrast agent also enables a distinction to be made between findings suspicious for vertebral osteomyelitis, degeneration (Modic type I), or neoplasia.21,22 With the help of MRI, both the extent of infection and possible skip lesions at other spinal levels can be shown. However, MRI often cannot be used in elderly patients with pacemakers or other electronic devices.
The computed tomography (CT) is primarily used for preoperative planning in order to be able to better evaluate the osseous situation, especially in osteoporotic bone. Depending on the system used, the preoperative performance of a thin-slice spiral CT can even be a prerequisite for the use of intraoperative navigation. 23 In patients with MRI contraindications (non-MRI compatible pacemakers, other patient-specific factors), CT is the alternative diagnostic tool. By the use of CT, fine-needle biopsy or abscess drain placement is simplified. 24
Fluorine-18 fluorodeoxyglucose positron emission tomography/CT (18F-FDG-PET- CT) is a well-known technique in oncology and infectious diseases and seems to play an increasingly important role in the diagnosis of vertebral osteomyelitis when MRI and CT are inconclusive or even in the first two weeks following disease onset. 25 It represents a good alternative particularly in the case of contraindications to contrast-enhanced MRI/CT (eg, kidney failure), especially in the elderly. However, PET-CT’s lack of specificity to differentiate between neoplasia, spondylodiscitis, and post-traumatic bone marrow edema represents a drawback.26,27 Thus, its combination with MRI is recommended. Apart from the high costs, the use of PET/CT remains an individual decision, even if it makes sense to shorten the time to diagnosis, especially in geriatric patients. Diabetes mellitus is a typical disease of old age and must be taken into account. PET-CT examinations in diabetic patients with a sugar level of more than 150 mg/dl influence the quality of the results, and in severe cases the PET-CT cannot be performed at all.
Material Acquisition: Puncture, Biopsy, Open Procedure
Material acquisition is the base for histopathological investigation and the start of potential antibiotic therapy. It can be obtained by CT-guided fine-needle biopsy or surgically removed. Pathogen detection is 19%–30% when using CT-guided fine-needle biopsy due to the small amount of tissue available, whereas detection can be achieved in 41% using histopathological methods [16]. A combination of local sampling and blood cultures therefore maximizes the likelihood of pathogen identification. According to recent systematic reviews, a pathogen can only be isolated in 33-60% of cases in geriatric patients.28,29 In addition to adequate abscess drainage, the diagnostic value of CT-guided fine-needle biopsy must certainly be questioned. Yang et al also suggest that percutaneous endoscopic discectomy and drainage yield higher bacterial recovery rates than CT-guided spinal biopsy. 30 In any case, open sampling for histopathological and microbiological examination is more reliable, so that positive pathogen detection is possible in up to 68-93% of the cases. 31
In the case of negative cultural results from the first biopsy and further clinical suspicion of spondylodiscitis, extended molecular biological tests (polymerase chain reaction) can be used to further identify the pathogen, especially in patients who have already been treated with antibiotics. Species-specific PCR (eg for S. aureus and Mycobacterium tuberculosis) can further increase sensitivity.3,32 However, the importance of nucleic acid-based detection methods (NAAT) in the diagnosis of spondylodiscitis has not yet been conclusively clarified. Individual studies provided indications of an increase in the sensitivity of pathogen detection, especially in connection with pathogens that are difficult to cultivate, when conventional culture-based methods were combined with NAAT.32,47 Choi et al were able to detect a total of 53.3% cases of spondylodiscitis using species-specific PCR, while only 28.9% could be detected using 16S rRNA-PCR. 32 This was also shown by a large retrospective study in which 275 of 427 diagnoses (62.9%) could only be recorded by a species-specific PCR and not by a 16S rRNA-PCR. 48 In contrast to the culture results, however, no statement can be made with the PCR on the antibiotic sensitivity of the pathogen. For the reasons mentioned above, NAAT is currently not part of routine diagnostics and is therefore reserved for rare diagnostic-scientific questions. (Figure 2)
Non-Operative Treatment
Non-operative treatment is mainly based on pathogen-specific antimicrobial treatment. Some authors recommend spinal bracing, but evidence therefore is missing.
Three studies compared younger and elderly patients.17,33,34 Older patients seem to suffer more often from concomitant endocarditis, neoplasms, and chronic inflammatory disease.33,34 Regarding isolated pathogens, a decrease of Staphylococcus aureus and increase of Gram-negative pathogens was observed17,33-35 and a higher rate of streptococci 34 and enterococci. 35 Kim performed a retrospective study including 586 patients analyzing the pathogen distribution. Although Staphylococcus aureus was the most common pathogen it was more common in patients <60 years (53.7%; 60-75 years: 43.4%); >75 years: 32.5%) while Gram-negative bacteria were more common in older patients (≥60 years: 30.9%; <60 years: 14.7%; 60-75 years: 22%) as enterococci (≥60 years: 6,5%; <60 years: 0,7%; 60-75 years: 4%). 35
In the elderly a higher mortality, treatment failure, rate of adverse events, longer hospital length of stay and lower quality of life was determined.17,18,33,34,36 Hutchinson reported that Staphylococcus aureus and Streptococcus species were the most common isolated pathogens. Intravenous therapy was performed for 36 days and in total 79.5 days. Patients had a mortality of 27%. 18
Five studies compared conservative and surgical treatment.37-41 Conservatively patients were older, suffered from more comorbidities, and displayed a higher failure rate with increased 30-day mortality.37-39 Noteworthy, 90-day mortality and 1-year mortality were not significantly different.39,41 Furthermore, older patients had longer durations for their length of hospital stay, time to CRP normalization and intravenous antibiotic treatment but initially less high laboratory parameters and less common presence of abscesses.37,39,41 Quality of life and pain intensity did not differ between conservatively and surgically treated patients.38,40 Despite Staphylococcus aureus is still the most frequent pathogen in the elderly the rate of Gram-negative pathogen related spondylodiscitis seems to increase and should be considered especially in empirical antimicrobial therapy.33,35 Due to the high failure and 30-day mortality rate in the elderly as well as comorbidities conservative treatment should be considered critically especially regarding associated infections and their therapy.33,39 Patients with spondylodiscitis and epidural abscess formation display a high failure rate and should be treated surgically. 37 No study examined if a longer treatment duration might be indicated for older patients to improve the outcome.
Operative Treatment
If conservative treatment fails, surgical options must also be considered in geriatric patients. 42 However, especially in this collective, surgical measures have their own morbidity and mortality risks.38,42 The complication rate in the surgically treated geriatric group of Sobottke et al was about twice as high compared to the conservative group.43,44
With regard to the surgical treatment strategy, the same paradigm applies to geriatric patients as to younger ones. The work of Kothari et al shows good short and medium-term postoperative results through a pure posterior treatment with regard to correction of the sagittal deformity and improvement of preoperative neurological deficits. 43 In particular, the use of a pedicle screw/rod system was the best way to reconstruct the sagittal profile. Non-unions or recurrences did not occur in the collective. 43 The results of Dreimann et al, who treated patients with thoracic spondylodiscitis via a purely dorsal approach using 360-degree stabilization and correction of the kyphosis, are comparable. 45 Neither access-related nor pulmonary complications were observed. There was also no recurrence of the infection. The VAS was reduced from 8.8 preoperatively to 3.2, the sagittal profile could be significantly improved with a reduction of the thoracic kyphosis from 20° preoperatively to 7° after the operation. The results of Hempelmann et al also showed good clinical results in their geriatric patient collective (median age 72 years) after posterior stabilization and interbody fusion with iliac crest bone graft. 42
However, pure decompression has also proven to be a sufficient treatment option in selected cases. Thus, Park et al compared collectives with debridement alone (median age 65 years) and debridement and instrumentation (median age 67 years). 46 Clinical outcomes were comparable between groups, including the rate of infection-related deaths (2.1% vs 0%; P = .52), primary failure (1.1% vs 5.1%; P = .30) and frequency of recurrence (4.8% vs 6.8%; P = .72). Nevertheless, debridement alone should only be reserved for isolated epidural abscesses. In all other cases, additive stabilization makes sense and is necessary. Increased recurrence rates are not to be expected with sufficient antibiotic therapy. 46
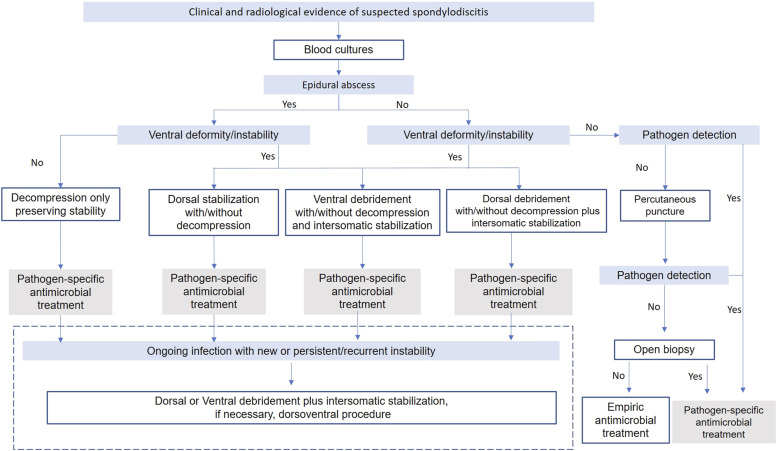
In summary, a dorsal approach in treatment of spondylodiscitis in the area of the lumbar and thoracic spine has proven itself in geriatric patients comparable to younger ones.42,44,45 However, as far as the ventral extent of destruction, the surgeon’s experience and the patient’s conditions allows, the operation should be expanded to include a one-stage ventral treatment, especially concerning spondylodiscitis in the cervical spine. The argument for a one- or two-stage ventro-dorsal surgical procedure is based on the theory that both stabilization and direct focus rehabilitation are possible (Figure3).
Figure 3.
Treatment algorithm.
Limitation
This is the first systematic literature review of the outcome and treatment of spondylodiscitis in elderly patients >65 years of age. The cutoff from 65 years of age may certainly create a bias. To provide an evidence-based assessment of the literature on this topic, this review was conducted in accordance with the PRISMA statement and used the GRADE approach. We limited our search to articles published worldwide in English and German language. This may have led to selection bias in our literature review. In addition, life expectancies vary across countries, so this may also be a source of bias. However, we set as the focus of our search to review the outcomes of both conservative and surgical treatment of pyogenic spondylodiscitis. Therefore, this review contains a very heterogeneous study population. Furthermore, this heterogeneity is reflected in the individual study populations of the included articles.
In terms of risk of bias, each included study was graded using the Newcastle-Ottawa scale (Table 1). The small sample sizes of most studies as well as the low level of evidence and missing prospective studies limit these recommendations.
Conclusion
The incidence of spondylodiscitis increases by age with a peak at 75 years or older. The 1-year mortality is up to 20%. In conventional radiographs of the affected spinal segment, radiological signs of destruction are only seen in advanced stages of disease. If there is a medical history and clinical suspicion of spondylodiscitis, MRI with contrast agent of the of the spine is the Gold Standard for diagnostics. Geriatric patients show initially lower inflammatory parameters, have a longer time of hospitality, and take longer until inflammatory parameters normalize. The most frequent pathogen is Staphylococcus aureus. With increasing age, the rate of Gram-negative pathogens increases. Due to the high failure and mortality rate in the elderly patients, conservative treatment should be considered critically especially regarding associated infections and their therapy.
The main therapeutic goal in the treatment of spondylodiscitis is to achieve a recovery to improve the patient’s quality of life through mobility and pain relief. For this purpose, first the causative pathogens must be identified and thus a specific antibiotic therapy must be initiated.
Conservative and operative treatment must take the concomitant diseases into account. Patients with spinal instability, immobilizing pain, epidural abscess, and newly emerged neurological deficits should be considered for operative treatment. However, in all surgical procedures the presence of an osteoporotic geriatric bone structure should be expected.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was organized and financially supported by Deutsche Gesellschaft für Orthopädie und Unfallchirurgie e.V. (DGOU).
ORCID iDs
Nicolas Heinz von der Hoeh https://orcid.org/0000-0003-1928-5686
Max J. Scheyerer https://orcid.org/0000-0003-1392-3990
References
- 1.Santoro A, Bientinesi E, Monti D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Res Rev. 2021;71:101422. [DOI] [PubMed] [Google Scholar]
- 2.Lian J, Yue Y, Yu W, Zhang Y. Immunosenescence: A key player in cancer development. J Hematol Oncol. 2020;13(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: Update on diagnosis and management. J Antimicrob Chemother. 2010;65(suppl 3):iii11-iii24. [DOI] [PubMed] [Google Scholar]
- 4.Kehrer M, Pedersen C, Jensen TG, Lassen AT. Increasing incidence of pyogenic spondylodiscitis: A 14-year population-based study. J Infect. 2014;68(4):313-320. [DOI] [PubMed] [Google Scholar]
- 5.Conan Y, Laurent E, Belin Y, et al. Large increase of vertebral osteomyelitis in France: A 2010-2019 cross-sectional study. Epidemiol Infect. 2021;149:e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Digby JM, Kersley JB. Pyogenic non-tuberculous spinal infection: An analysis of thirty cases. J Bone Joint Surg Br. 1979;61(1):47-55. [DOI] [PubMed] [Google Scholar]
- 7.Krogsgaard MR, Wagn P, Bengtsson J. Epidemiology of acute vertebral osteomyelitis in Denmark: 137 cases in Denmark 1978-1982, compared to cases reported to the national patient register 1991-1993. Acta Orthop Scand. 1998;69(5):513-517. [DOI] [PubMed] [Google Scholar]
- 8.Malawski SK, Lukawski S. Pyogenic infection of the spine. Clin Orthop Relat Res. 1991;272:58-66. [PubMed] [Google Scholar]
- 9.Sapico FL, Montgomerie JZ. Pyogenic vertebral osteomyelitis: Report of nine cases and review of the literature. Rev Infect Dis. 1979;1(5):754-776. [DOI] [PubMed] [Google Scholar]
- 10.Cheung WY, Luk KD. Pyogenic spondylitis. Int Orthop. 2012;36(2):397-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Bmj. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berbari EF, Kanj SS, Kowalski TJ, et al. Executive summary: 2015 infectious diseases society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61(6):859-863. [DOI] [PubMed] [Google Scholar]
- 13.(AWMF) Ad. WMF . Diagnostik und therapie der spondylodiszitis. 2020. https://www.awmf.org/leitlinien/detail/ll/151-001.html [Google Scholar]
- 14.Casser HR, Seddigh S, Rauschmann M. Acute lumbar back pain. Dtsch Arztebl Int. 2016;113(13):223-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon SH, Chung SK, Kim KJ, Kim HJ, Jin YJ, Kim HB. Pyogenic vertebral osteomyelitis: Identification of microorganism and laboratory markers used to predict clinical outcome. Eur Spine J. 2010;19(4):575-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carragee EJ, Kim D, van der Vlugt T, Vittum D. The clinical use of erythrocyte sedimentation rate in pyogenic vertebral osteomyelitis. Spine Phila Pa. 1976;22(18):2089-2093. [DOI] [PubMed] [Google Scholar]
- 17.Amadoru S, Lim K, Tacey M, Aboltins C. Spinal infections in older people: An analysis of demographics, presenting features, microbiology and outcomes. Intern Med J. 2017;47(2):182-188. [DOI] [PubMed] [Google Scholar]
- 18.Hutchinson C, Hanger C, Wilkinson T, Sainsbury R, Pithie A. Spontaneous spinal infections in older people. Intern Med J. 2009;39(12):845-848. [DOI] [PubMed] [Google Scholar]
- 19.Nolla JM, Ariza J, Gómez-Vaquero C, et al. Spontaneous pyogenic vertebral osteomyelitis in nondrug users. Semin Arthritis Rheum. 2002;31(4):271-278. [DOI] [PubMed] [Google Scholar]
- 20.Sans N, Faruch M, Lapègue F, Ponsot A, Chiavassa H, Railhac JJ. Infections of the spinal column--spondylodiscitis. Diagn Interv Imaging. 2012;93(6):520-529. [DOI] [PubMed] [Google Scholar]
- 21.Diehn FE. Imaging of spine infection. Radiol Clin North Am. 2012;50(4):777-798. [DOI] [PubMed] [Google Scholar]
- 22.Sharif HS. Role of MR imaging in the management of spinal infections. AJR Am J Roentgenol. 1992;158(6):1333-1345. [DOI] [PubMed] [Google Scholar]
- 23.Zheng G, Nolte LP. Computer-assisted orthopedic surgery: Current state and future perspective. Front Surg. 2015;2:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enoch DA, Cargill JS, Laing R, Herbert S, Corrah TW, Brown NM. Value of CT-guided biopsy in the diagnosis of septic discitis. J Clin Pathol. 2008;61(6):750-753. [DOI] [PubMed] [Google Scholar]
- 25.Skanjeti A, Penna D, Douroukas A, et al. PET in the clinical work-up of patients with spondylodiscitis: A new tool for the clinician? Q J Nucl Med Mol Imaging. 2012;56(6):569-576. [PubMed] [Google Scholar]
- 26.Zhuang H, Sam JW, Chacko TK, et al. Rapid normalization of osseous FDG uptake following traumatic or surgical fractures. Eur J Nucl Med Mol Imaging. 2003;30(8):1096-1103. [DOI] [PubMed] [Google Scholar]
- 27.Schmitz A, Risse JH, Grünwald F, Gassel F, Biersack HJ, Schmitt O. Fluorine-18 fluorodeoxyglucose positron emission tomography findings in spondylodiscitis: Preliminary results. Eur Spine J. 2001;10(6):534-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasalak Ö, Adams HJA, Jutte PC, et al. Culture yield of repeat percutaneous image-guided biopsy after a negative initial biopsy in suspected spondylodiscitis: A systematic review. Skeletal Radiol. 2018;47(10):1327-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sertic M, Parkes L, Mattiassi S, Pritzker K, Gardam M, Murphy K. The efficacy of computed tomography-guided percutaneous Spine biopsies in determining a causative organism in cases of suspected infection: A systematic review. Can Assoc Radiol J. 2019;70(1):96-103. [DOI] [PubMed] [Google Scholar]
- 30.Yang SC, Fu TS, Chen LH, Chen WJ, Tu YK. Identifying pathogens of spondylodiscitis: Percutaneous endoscopy or CT-guided biopsy. Clin Orthop Relat Res. 2008;466(12):3086-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fleege C, Wichelhaus TA, Rauschmann M. [Systemic and local antibiotic therapy of conservative and operative treatment of spondylodiscitis]. Orthopade. 2012;41(9):727-735. [DOI] [PubMed] [Google Scholar]
- 32.Choi SH, Sung H, Kim SH, et al. Usefulness of a direct 16S rRNA gene PCR assay of percutaneous biopsies or aspirates for etiological diagnosis of vertebral osteomyelitis. Diagn Microbiol Infect Dis. 2014;78(1):75-78. [DOI] [PubMed] [Google Scholar]
- 33.Aguilar-Company J, Pigrau C, Fernández-Hidalgo N, et al. Native vertebral osteomyelitis in aged patients: Distinctive features. An observational cohort study. Infection. 2018;46(5):679-686. [DOI] [PubMed] [Google Scholar]
- 34.Courjon J, Lemaignen A, Ghout I, et al. Pyogenic vertebral osteomyelitis of the elderly: Characteristics and outcomes. PLoS One. 2017;12(12):e0188470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim DY, Kim UJ, Yu Y, et al. Microbial etiology of pyogenic vertebral osteomyelitis according to patient characteristics. Open Forum Infect Dis. 2020;7(6):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernard L, Dinh A, Ghout I, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: An open-label, non-inferiority, randomised, controlled trial. Lancet. 2015;385(9971):875-882. [DOI] [PubMed] [Google Scholar]
- 37.Uchida K, Nakajima H, Yayama T, et al. Epidural abscess associated with pyogenic spondylodiscitis of the lumbar spine; evaluation of a new MRI staging classification and imaging findings as indicators of surgical management: A retrospective study of 37 patients. Arch Orthop Trauma Surg. 2010;130(1):111-118. [DOI] [PubMed] [Google Scholar]
- 38.Sobottke R, Röllinghoff M, Zarghooni K, et al. Spondylodiscitis in the elderly patient: Clinical mid-term results and quality of life. Arch Orthop Trauma Surg. 2010;130(9):1083-1091. [DOI] [PubMed] [Google Scholar]
- 39.Alas H, Fernando H, Baker JF, et al. Comparative outcomes of operative relative to medical management of spondylodiscitis accounting for frailty status at presentation. J Clin Neurosci. 2020;75:134-138. [DOI] [PubMed] [Google Scholar]
- 40.Shetty AP, Viswanathan VK, Kanna RM, Shanmuganathan R. Tubercular spondylodiscitis in elderly is a more severe disease: A report of 66 consecutive patients. Eur Spine J. 2017;26(12):3178-3186. [DOI] [PubMed] [Google Scholar]
- 41.Lee JH, Kim J, Kim TH. Clinical outcomes in older patients aged over 75 years who underwent early surgical treatment for pyogenic vertebral osteomyelitis. J Clin Med. 2021;10(22):5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hempelmann RG, Mater E, Schön R. Septic hematogenous lumbar spondylodiscitis in elderly patients with multiple risk factors: Efficacy of posterior stabilization and interbody fusion with iliac crest bone graft. Eur Spine J. 2010;19(10):1720-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kothari M, Shah K, Tikoo A, Nene A. Short to mid-term term surgical outcome study with posterior only approach on tuberculous spondylodiscitis in an elderly population. Asian Spine J. 2016;10(2):258-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kothari MK, Shah KC, Tikoo A, Nene AM. Surgical management in elderly patients with tuberculous spondylodiscitis: Ten year mortality audit study. Asian Spine J. 2016;10(5):915-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dreimann M, Viezens L, Hoffmann M, Eicker SO. Retrospective feasibility analysis of modified posterior partial vertebrectomy with 360-degree decompression in destructive thoracic spondylodiscitis. Acta Neurochir. 2015;157(9):1611-1618. [DOI] [PubMed] [Google Scholar]
- 46.Park KH, Cho OH, Lee YM, et al. Therapeutic outcomes of hematogenous vertebral osteomyelitis with instrumented surgery. Clin Infect Dis. 2015;60(9):1330-1338. [DOI] [PubMed] [Google Scholar]
- 47.Colmenero JD, Morata P, Ruiz-Mesa JD, et al. Multiplex real-time polymerase chain reaction: A practical approach for rapid diagnosis of tuberculous and brucellar vertebral osteomyelitis. Spine (Phila Pa. 1976;35(24):E1392-E1396. [DOI] [PubMed] [Google Scholar]
- 48.Morel AS, Dubourg G, Prudent E, et al. Complementarity between targeted real-time specific PCR and conventional broad- range 16S rDNA PCR in the syndrome-driven diagnosis of infectious diseases. Eur J Clin Microbiol Infect Dis. 2015;34(3):561-570. [DOI] [PubMed] [Google Scholar]