Abstract
Simple Summary
Broilers provide nutrition for people all around the world, and their muscle and fat contribute major roles. However, little is known about the relationships between the kruppel-like factor (KLF) and the development of muscle and fat. In recent years, studies have shown that KLFs are involved in many physiological processes such as cell development, adipocyte differentiation and neurodevelopment. In our study, we aim to learn the functions KLFs play in the muscle and fat development of chickens. Various bioinformatics analyses were used to illustrate the genomic information of KLFs. A qPCR was performed to show the relative expression level of KLFs. In addition, we collected RNA-seq data and we found that KLFs have different expression levels in the same tissues at different points in time. This study helps explore the regulation mechanism of KLFs in skeletal muscle and fat, and provides a theoretical basis for broiler breeding.
Abstract
The kruppel-like factor (KLF) gene family is a group of transcription factors containing highly conserved zinc-finger motifs, which play a crucial role in cell proliferation and differentiation. Chicken has been widely used as a model animal for analyzing gene function, however, little is known about the function of the KLF gene family in chickens. In this study, we performed genome-wide studies of chicken KLF genes and analyzed their biological and expression characteristics. We identified 13 KLF genes from chickens. Our phylogenetic, motif, and conserved domain analyses indicate that the KLF gene family has remained conserved through evolution. Synteny analysis showed the collinear relationship among KLFs, which indicated that they had related biomolecular functions. Interaction network analysis revealed that KLFs worked with 20 genes in biological processes. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis showed that KLF2 was involved in Apelin and Forkhead Box O (FOXO) signaling pathways. Moreover, qPCR showed that 13 KLF genes were expressed in the nine selected tissues and displayed various gene expression patterns in chickens. RNA-seq showed that KLF3 and KLF10 genes were differentially expressed in the normal and high-fat diet fed groups, and KLF4, KLF5, KLF6, KLF7, KLF9, KLF12, and KLF13 genes were differentially expressed between undifferentiated and differentiated chicken preadipocytes. Besides, RNA-seq also showed that KLF genes displayed different expression patterns in muscle at 11 and 16 embryonic days old, and in 1-day-old chickens. These results indicated that the KLF genes were involved in the development of muscle and fat in chickens. Our findings provide some valuable reference points for the subsequent study of the function of KLF genes.
Keywords: chicken, KLF, genome-wide identification, expression patterns, muscle, fat
1. Introduction
Chicken meat is a good source of protein, with lower fat and cholesterol than other kinds of meats [1]. Ensuring the stability of chicken production is crucial in promoting socio-economic development in the face of population growth. Biological and pathological processes and other genetic factors are the main limiting factors affecting chicken growth and development [2]. In recent years, Molecular breeding for synchronized improvement of both meat yield and quality has become a popular topic of research on chicken [3]. Skeletal muscle plays an important role in locomotion, metabolism, and meat production in farm animals [4]. Many scholars have attempted to use the method of selective breeding to improve the chicken muscle growth rate [5,6,7]. A previous study showed that KLFs could control Fibroblast Growth Factor Receptor 1 (FGFR1) gene expression to regulate myoblast proliferation and differentiation [8].
KLF genes are characterized by three conserved zinc fingers in the DNA-binding domain, wherein the binding efficiency and transcription regulation can be affected by mutations [9]. Recent studies have revealed that KLF genes play diverse and essential roles in controlling metabolism at the cellular, tissue, and systemic levels. Up to now, 18 KLF genes have been identified in humans, named KLF1 to KLF18. Moreover, KLF genes play various roles in different species such as mice, humans, pigs, etc. For example, KLF1 acts as an essential transcription factor for erythroid development in mice [10]. KLF1 is also proven to promote the proliferation, migration, and invasion of human lens epithelial cells by enhancing the expression of ZBTB7A (Zinc Finger and BTB Domain Containing 7A) gene [11]. Moreover, KLF1 is involved in the growth and metabolism of pig muscle [12].
KLF2 was found to regulate osteoblast differentiation by targeting Runx2 in mice and humans [13]. KLF3 is associated with the growth and development of muscle and adipose tissue in cattle [14]. KLF6 is targeted by miR-152 to inhibit bovine myoblast proliferation [15]. KLF4, KLF5, KLF6, KLF7, KLF10, and KLF15 are involved in the growth and development of mouse skeletal muscle [16,17,18,19,20,21]. The overexpression of KLF9 in the mouse liver markedly increased blood glucose levels and impaired glucose tolerance [22]. KLF12 was proven to regulate mouse Natural Killer cell proliferation [23]. KLF8, KLF11, and KLF13 were demonstrated to be involved in human diseases [24,25,26]. Yang et al. [27] discovered that KLF14 deletion resulted in increased fat mass in female mice and decreased fat mass in male mice. Knockout of KLF16 could reduce oxidative stress and inflammation in mice hearts [28]. Wang et al. [29] found that KLF17 may be critical for early mouse embryonic development. Up to now, no report was found regarding the function of KLF18 in any animal.
The KLF gene family has been systematically identified in several animals, including pigs, mice, and cattle. However, the KLF gene family in chickens has not been thoroughly researched. In this study, we determined KLFs in chickens by building a HMM model and running a BLAST according to the available genome information. Phylogenetic analysis, gene structures analysis, motif, and conserved domain analysis were performed to understand the evolution and structural features of KLFs. Synteny analysis and interaction network analysis were performed to show collinear relationships and biological functions of KLF genes in chickens. Additionally, we used the Go enrichment and KEGG pathway analysis to explore the biological characteristics of KLF gene family. RNA-seq data was collected to show various gene expression patterns of the KLF genes in chickens. Furthermore, the expression patterns of KLFs in chickens were systematically investigated by qPCR analysis. This study would provide the fundamental basis for further functional studies of KLF genes.
2. Materials and Methods
2.1. Ethics Statement
This study is fully compliant with the codes made by the Chinese Ministry of Agriculture. The animal experiments performed in the study were all evaluated and approved by the Animal Ethics Committee of Yangzhou University (202103298).
2.2. Genome-Wide Identification of KLF Family Members in Chickens
The protein sequences of the KLF genes from Homo Sapiens (H. sapiens), Mus musculus (M. musculus), and Sus scrofa (S. scrofa) were retrieved and downloaded from the National Center for Biotechnology (NCBI) database. Then these sequences were compared with the protein sequences of Gallus gallus (G. gallus) using BLASTp with the expected value (E-value) of 1e − 10 [30]. The KLF protein sequences were used as a query template to construct multiple alignment models using hidden Markov model software (HMM) to identify putative KLF genes in chickens using the presence of the zf-H2C2 domain [31].
2.3. Protein Alignment, Phylogenetic Analysis, and Chromosome Location Analysis of KLF Genes
To understand the evolutionary relationship of the KLF genes from these four species, multiple sequence alignments of identified KLF genes were performed using MUSCLE analysis [32]. The neighbor-joining (NJ) tree was constructed by Molecular Evolutionary Genetics Analysis (MEGA) 7.0 software with pairwise deletion of bootstrap value 1000 [33]. Conserved domains of KLF proteins were identified using CDD method in Batch software [34]. The SMART software also detected those domains found by a HMM model to confirm the final results [35]. The chromosomal locations of these KLF genes were derived from the genome annotation files downloaded from the Ensembl database [36]. Furthermore, the chromosome location of the KLF genes was displayed by TBtools [37].
2.4. Motif Identification and Conserved Domain Analysis of KLF Genes
The motifs of the KLF protein domain were identified using the Multiple EM (Expectation Maximization) for Motif Elicitation (MEME) program using the default parameters [38]. The number of motifs was set to 15. The minimum motif and maximum motif lengths were 6 and 50, respectively. The conserved domains of KLFs were searched in the Conserved Domain Database and displayed with TBtools [37].
2.5. Synteny Analysis and Interaction Network Analysis of KLF Genes in Chickens
The syntenic relationships between the KLF genes and gene density of KLF genes were analyzed by using TBtools. The interaction networks of chicken KLF genes were identified using the STRING database and the predicted interaction network was optimized by Cytoscape software [39,40].
2.6. GO Enrichment Analysis and KEGG Pathway Analysis of KLF Genes in Chicken
Gene Ontology enrichment analysis was used to identify the putative biological processes, cellular components, and molecular functions of KLF genes in chickens [41]. KEGG enrichment analysis was performed by KOBAS software to identify the functions of KLF genes in signaling pathways [42].
2.7. RNA-seq Results Analysis of the G. gallus KLF Genes
The first RNA-Seq data of 40 samples of testis, liver, lung, brain, kidney, intestine, muscle, and heart were obtained from the GEO DataSets and its GEO accession was GSE133401. The second RNA-Seq data derived from 12 samples of livers and abdominal fat in dwarf broilers were also obtained from the GEO DataSets and its GEO accession was GSE129840. The broilers began to be fed with a normal or a high-fat diet at 1 week old and they were executed to get livers and abdominal fat for extracting total RNAs at 8 weeks old [43]. The heatmap was made based on the sequencing data by R software [44].
The RNA-seq data of thigh muscle in Xinghua chicken was collected from GEO dataset and its GEO accession was GSE91060. Chicken leg muscles of three different developmental stages (11-day-old embryos, 16-day-old embryos, and 1-day post-hatch) were used to assess lncRNA and mRNA expression during chicken skeletal muscle development [45]. The sequencing data of preadipocytes that had not differentiated and six-day differentiated preadipocytes were obtained with this study.
2.8. Animals, Tissue Collections, RNA Extraction, and Quantitative Real-Time PCR (qPCR)
Heying Black chickens used in this study were provided by Jiangsu Heying Poultry Group Co., Ltd. (Sheyang, China). After artificial insemination, the eggs were collected and hatched at 37 °C and 60% humidity. The experiment group contained four male chickens and they were euthanized at 18 embryonic days (the sex was determined by the development of the gonad). Thigh muscle, pectoral muscle, heart, lung, glandular stomach, hypothalamus, liver, spleen and abdominal fat were collected. Subsequently, these tissues were snap-frozen in liquid nitrogen, and stored at −80 °C for RNA extraction.
Total RNA samples were extracted with TRIzol. The quality, concentration, and integrity of RNA samples were tested by spectrophotometer (Thermo, Waltham, MA, USA). Then 1 ug RNA of each sample was reverse-transcribed to cDNA by using HiScript QRT SuperMix for qPCR (+gDNA wiper) (Vazyme, Nanjing, China). The forward primers and reverse primers of KLFs were designed by Primer Premier 5.0 (Table S1). These primers were synthesized by Sangon (Shanghai, China). The qPCR experiments were conducted on Applied Biosystems 7500 (ABI, Los Angeles, California, USA) using SYBR qPCR Master Mix (Vazyme, Nanjing, China), and β-actin was used as housekeeping gene [46], with three technical repetitions for each sample. The 2−ΔΔCT method was used to calculate the relative expression of KLFs [47].
2.9. Statistics Analysis
Statistical analysis was performed by using SPSS18.0 software (SPSS Inc., Chicago, IL, USA). A one-way ANOVA was used for multiple-group comparison analysis. Duncan’s multiple range test was used to determine significance. Unpaired Student’s t-test was used for a two-group comparison analysis. The data were considered statistically significant when p less than 0.05.
3. Results
3.1. Identification and Phylogenetic Analysis of KLF Genes
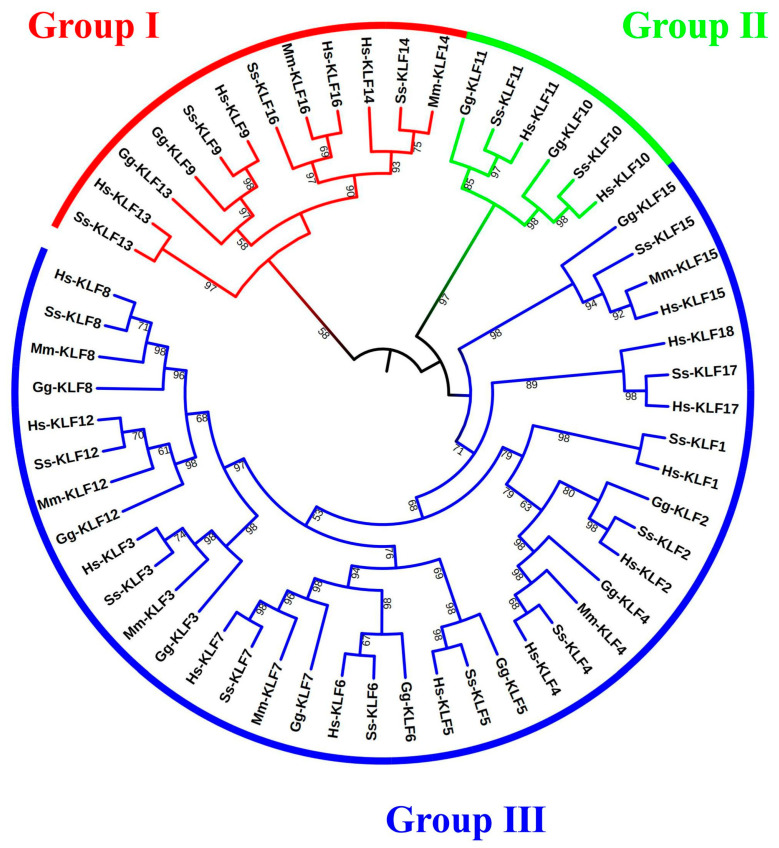
Based on the presence of the zf-H2C2 domain, we obtained 56 KLF genes in four species, including 18 H. sapiens genes, 8 M. musculus genes, 17 S. scrofa genes, and 13 G. gallus genes, respectively. To explore the evolutionary relationship among the members of the KLF families, an NJ tree based on protein sequences of these 56 genes from four species was constructed (Figure 1). Based on the alignment of the sequences and the phylogenetic analysis, 56 genes were divided into three groups, named groups I to III. Group I contained 12 KLFs, group II contained 6 KLFs, and group III contained 38 KLFs. We saw from the phylogenetic tree that Ss-KLF13, Hs-KLF13, Gg-KLF13, Gg-KLF9, Ss-KLF9, Hs-KLF9, Ss-KLF16, Mm-KLF16, Hs-KLF16, Hs-KLF14, Ss-KLF14, and Mm-KLF14 are clustered into Group I. Gg-KLF11, Ss-KLF11, Hs-KLF11, Gg-KLF10, Ss-KLF10, and Hs-KLF10 are clustered into Group II, and the remaining 38 KLFs are clustered into Group III.
Figure 1.
Phylogenetic analysis of KLF proteins in G. gallus, M. musculus, S. scrofa, and H. sapiens. The sequences of those 56 proteins were used to construct a neighbor-joining (NJ) tree. The tree was divided into three groups (I–III). “H. sapiens” was abbreviated to “Hs”. “M. musculus” was abbreviated to “Mm”. “S. scrofa” was abbreviated to “Ss”. “G. gallus” was abbreviated to “Gg”.
3.2. Chromosomal Locations of G. gallus KLF Genes
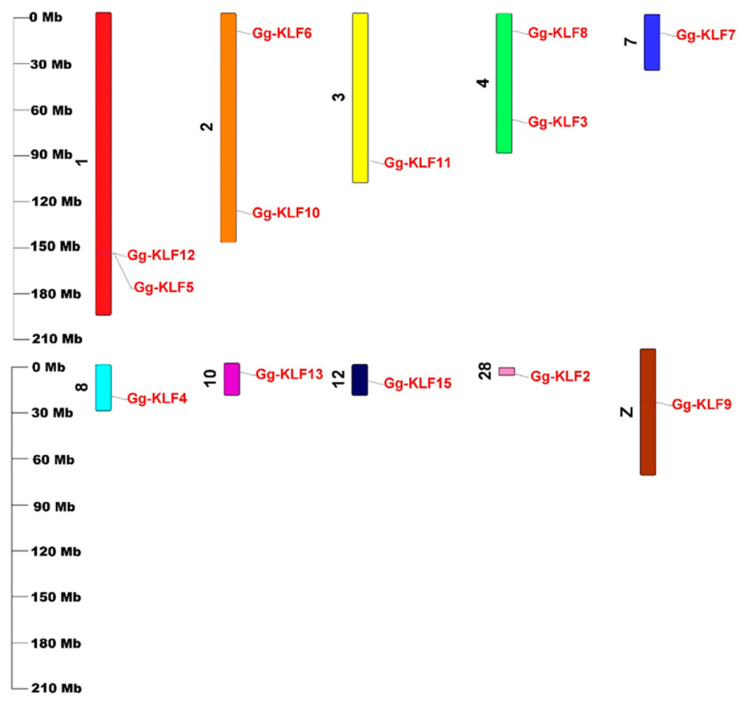
The 13 KLF genes were distributed unevenly on ten G. gallus chromosomes (Figure 2). The accession numbers of the gene sequence, protein sequence, and coding sequence of the 13 KLF genes and their genomic locations were listed in Table 1. Chromosome 1 contained two genes, Gg-KLF5 and Gg-KLF12. Chromosome 2 also had two genes, Gg-KLF6 and Gg-KLF10, but these two genes were at different positions on the same chromosome. Like chromosome 2, chromosome 4 contained two genes, Gg-KLF3 and Gg-KLF8. The remaining seven genes were distributed on the other seven chromosomes, meaning each had one KLF gene. Gg-KLF11, Gg-KLF7, Gg-KLF13, Gg-KLF4, Gg-KLF2, Gg-KLF15, and Gg-KLF9 were found on chromosome 3, chromosome 7, chromosome 10, chromosome 8, chromosome 28, chromosome 12, and chromosome Z, respectively.
Figure 2.
Chromosomal location of KLF genes on ten chromosomes in chickens.
Table 1.
KLF gene family information in chickens (G. gallus).
| Gene Name | Gene ID | Protein ID | CDS ID | Chromosome | Gene Position | |
|---|---|---|---|---|---|---|
| Start | End | |||||
| Gg-KLF2 | ENSGALG00000003939 | ENSGALP00000006265 | ENSGALT00000006275 | Chr28 | 4484818 | 4487291 |
| Gg-KLF3 | ENSGALG00000041717 | ENSGALP00000053488 | ENSGALT00000090511 | Chr4 | 69533898 | 69558360 |
| Gg-KLF4 | ENSGALG00000047208 | ENSGALP00000065832 | ENSGALT00000097875 | Chr8 | 20767770 | 20771114 |
| Gg-KLF5 | ENSGALG00000016927 | ENSGALP00000086539 | ENSGALT00000124066 | Chr1 | 157437529 | 157456371 |
| Gg-KLF6 | ENSGALG00000033178 | ENSGALP00000049438 | ENSGALT00000073174 | Chr2 | 11821968 | 11830999 |
| Gg-KLF7 | ENSGALG00000008501 | ENSGALP00000013833 | ENSGALT00000013848 | Chr7 | 12266259 | 12323221 |
| Gg-KLF8 | ENSGALG00000037361 | ENSGALP00000068386 | ENSGALT00000099301 | Chr4 | 11462278 | 11469482 |
| Gg-KLF9 | ENSGALG00000027374 | ENSGALP00000043265 | ENSGALT00000043393 | chrZ | 35032072 | 35045242 |
| Gg-KLF10 | ENSGALG00000037913 | ENSGALP00000055499 | ENSGALT00000059886 | Chr2 | 129099993 | 129104922 |
| Gg-KLF11 | ENSGALG00000016440 | ENSGALP00000075856 | ENSGALT00000118896 | Chr3 | 96486867 | 96495981 |
| Gg-KLF12 | ENSGALG00000016926 | ENSGALP00000067131 | ENSGALT00000099648 | Chr1 | 156971380 | 157226168 |
| Gg-KLF13 | ENSGALG00000026590 | ENSGALP00000081341 | ENSGALT00000120340 | Chr10 | 6022200 | 6048656 |
| Gg-KLF15 | ENSGALG00000036133 | ENSGALP00000094959 | ENSGALT00000124262 | Chr12 | 11023377 | 11040828 |
3.3. Motif Identification and Conserved Domain Analysis
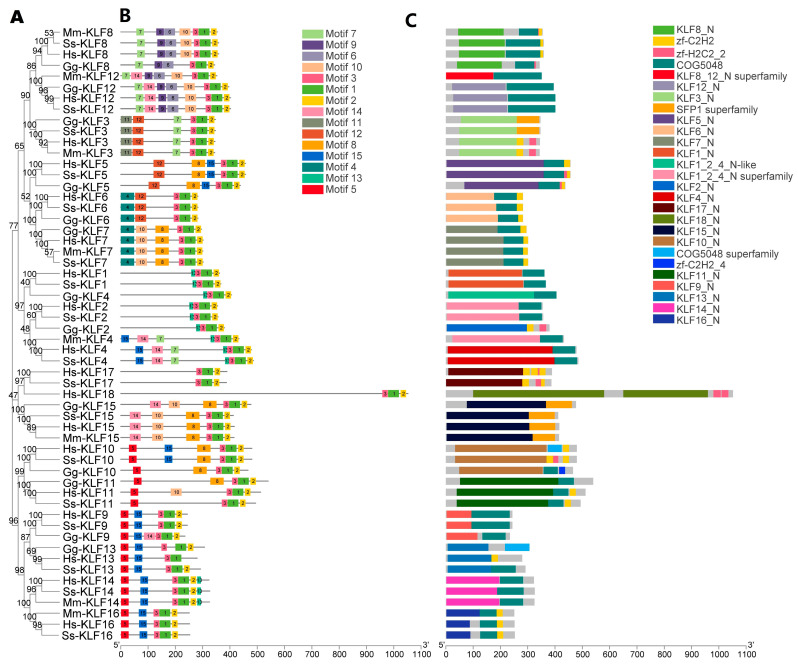
The phylogenetic analysis (Figure 3A) was combined with gene structures and motif analysis to indicate the relationship among KLF genes of H. sapiens, M. musculus, S. scrofa, and G. gallus. Using the HMMER program, we discovered that the KLF genes have 15 motifs. Motif 1 and motif 2 were present in all 56 KLF genes (Figure 3B). The most closely related genes in the same subfamilies shared common motif compositions, which may be indicative of similar functions. The conserved domain analysis illustrated that 27 conserved domains could be found in these 56 genes (Figure 3C). The genes clustered in the same group shared similar conserved domains.
Figure 3.
Phylogenetic relationships, Motif identification, and gene structure analysis. (A) Phylogenetic relationships of the 56 KLF genes identified in G. gallus, S. scrofa, M. musculus, and H. sapiens using the NJ method. (B) Motif analysis of the 56 KLF genes identified in G. gallus, S. scrofa, M. musculus, and H. sapiens. Colored boxes indicate conserved motifs and gray lines represent non-conserved sequences. (C) Conserved domain analysis of the 56 KLF genes identified in G. gallus, S. scrofa, M. musculus, and H. sapiens.
3.4. Synteny Analysis and Interaction Network Analysis
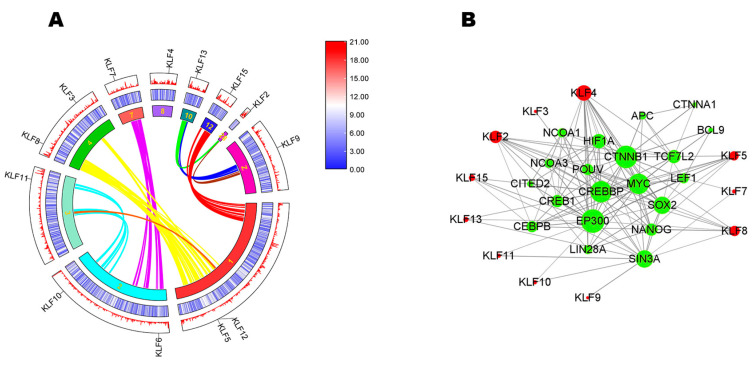
In Figure 4A, the middle and outermost circles showed low gene density of all 13 KLFs in chicken. According to curves of different colors, we found that KLF2 had a collinear relationship with KLF13. KLF3 and KLF8 had collinear relationships with KLF5 and KLF12. KLF6 had a collinear relationship with KLF7. KLF10 had a collinear relationship with KLF11. Other KLFs didn’t have collinear relationships.
Figure 4.
Synteny analysis and interaction network analysis. (A) The synteny analysis of KLF family in chicken. The innermost circle is composed of 10 chromosomes. The middle and outermost circles show gene density through a heatmap and line. The colors red, white, and blue represent high, medium, and low gene density, respectively. Gene names are shown at different locations of chromosomes. Curves of different colors represent the collinear relationship among different chromosomes and some of the KLFs had collinear relationships with each other. (B) Interaction network analysis. The interaction network shows the interaction relationship among KLFs and other genes.
To better understand the biological function and the regulatory network of KLFs, their protein-protein interactions (PPI) were predicted (Figure 4B). The results showed that 11 KLFs had putative interaction relationships and 20 corresponding functional genes were found.
3.5. GO Enrichment Analysis and KEGG Pathway Analysis of KLF Genes in Chicken
Figure 5A shows nine KLF proteins located at the nucleoplasm, nuclear lumen, membrane-enclosed lumen, organelle lumen, intracellular organelle lumen, and nuclear part, respectively, which were the top six GO Enrichment categories (Table S2). Figure 5B illustrates that 11 KLFs took part in biological processes like the regulation of transcription by RNA polymerase II, transcription by RNA polymerase II, regulation of gene expression, and so on (Table S3). According to the KEGG pathway analysis, we found only KLF2 was involved in Apelin signaling pathway and FOXO signaling pathway (Figure S1) [48]. Using KEGG, the 12 putative KLF proteins were not assigned to any signaling pathway.
Figure 5.
Go enrichment analysis of KLF genes in chicken. (A) The top 25 Go enrichment analysis of the cellular components of KLF genes in chicken; (B) The top 25 Go enrichment analysis of biological processes of KLF genes in chicken.
3.6. Expression Profile Analysis of G. gallus KLF Genes
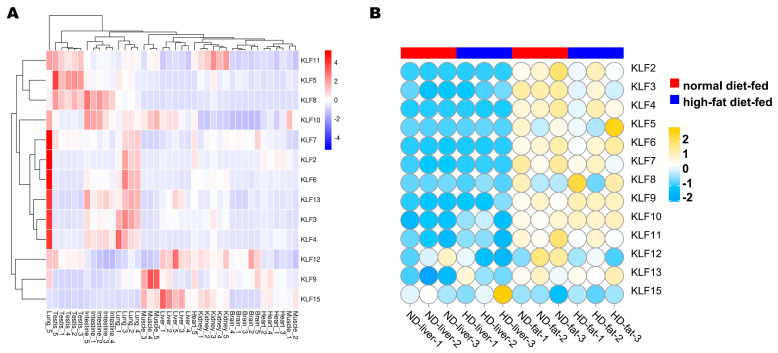
The KLF genes can be classified into four groups (Figure 6A) according to their differential expression patterns in tissues. Moreover, the eight chicken tissue types can also be clustered into eight main clades based on the expression patterns of all 13 genes. It could be seen from the expression profile that KLF2, KLF3, KLF4, KLF6, KLF7, and KLF13 were expressed highly in the lungs. Further, KLF9 was expressed highly in muscle. In Figure 6B, the expression levels of KLF genes were significantly higher in fat than liver, except for KLF15. The expression level of KLF3 was significantly higher in the normal diet-fed group than the high-fat diet-fed group, while the expression level of KLF10 was significantly lower in the normal diet-fed group than the high-fat diet-fed group.
Figure 6.
Expression patterns of the KLF gene family in chickens. (A) mRNA profiles of eight kinds of tissues from five adult roosters: testis, liver, lung, brain, kidney, intestine, muscle, and heart. Colors red, white, blue represent high expression, medium expression and low expression levels, respectively. (B) KLF genes in livers and abdominal fats between normal- and high-fat diet-fed dwarf broilers. The colors yellow, white, and blue represent high expression, medium expression, and low expression levels, respectively.
3.7. QPCR Results of the G. gallus KLF Genes
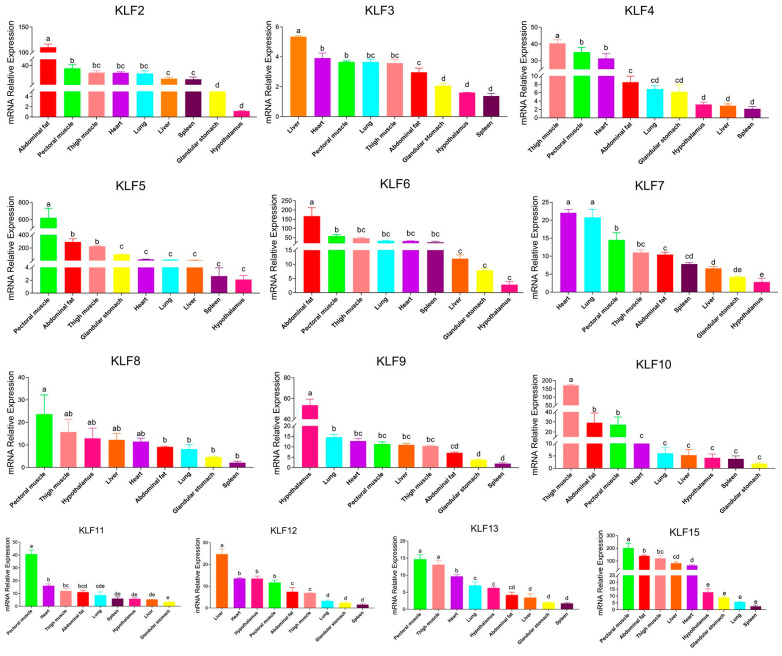
QPCR data was normalized with the same and unique normalizer gene, β-actin. The qPCR results showed that KLF genes had quite different expression patterns (Figure 7). The expression levels of KLF2, KLF5, KLF6, KLF10 and KLF15 were highest in thigh muscle, pectoral muscle and abdominal fat. KLF4, KLF8, and KLF13 were most highly expressed in thigh muscle and pectoral muscle. We also discovered that the expression level of KLF3 was highest in liver, followed by heart, pectoral muscle, lungs, thigh muscle, abdominal fat and other organs. The expression levels of KLF7 were highest in the hearts and lungs, while the expression level of KLF9 was highest in the hypothalamus. KLF11 expressed highest in pectoral muscle but much lower in other organs. Like KLF3, the expression level of KLF12 was highest in liver.
Figure 7.
qPCR expression analysis of KLF genes in thigh muscle, pectoral muscle, heart, lung, glandular stomach, hypothalamus, liver, spleen and abdominal fat of four male chickens at 18 embryonic days old. Letters a, b, c, d, e and other lowercase letters denote a significant difference exists.
3.8. RNA-seq Result Analysis of the G. gallus KLF Genes
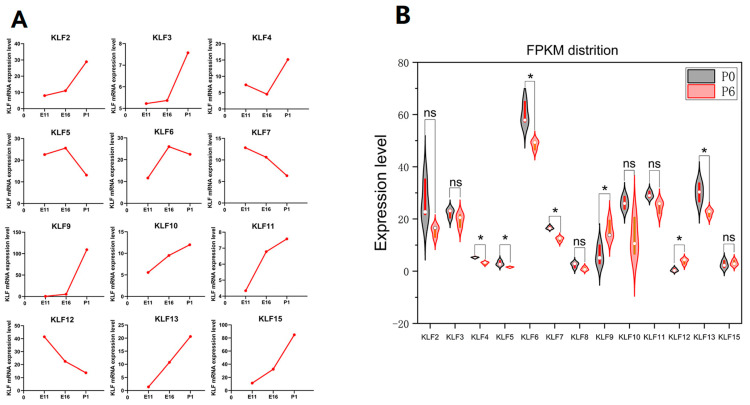
The expression level of KLF2, KLF3, KLF9, KLF10, KLF11, KLF13 and KLF15 increased in samples from 11 embryonic-day-old to 1-day-old chicken (Figure 8A). The expression level of KLF4 decreased between samples from 11 embryonic-day-old to 16 embryonic-day-old chicken, but it increased quickly from 16-embryonic-day-old to 1-day-old chicken. Contrary to KLF4, the expression levels of KLF5 and KLF6 rose between samples from 11 embryonic-day-old to 16 embryonic-day-old chickens, but it decreased quickly from 16-embryonic-day-old to 1-day-old chickens. The expression levels of KLF7 and KLF12 decreased between samples from 11 embryonic-day-old to 1-day-old chickens. As to KLF8, it was not included in the RNA-seq data and the expression of KLF8 was absent. As Figure 8B showed, the expression levels of KLF9 and KLF12 increased significantly in differentiated preadipocytes, while the expression levels of KLF4, KLF5, KLF6, KLF7, and KLF13 all decreased significantly after preadipocyte differentiation.
Figure 8.
RNA-seq results analysis of the G. gallus KLF Genes. (A) mRNA relative expression levels of 12 KLF genes in leg muscles of Xinghua chickens at 11 and 16 embryonic days and 1 day old. (B) KLF mRNA relative expression levels in preadipocytes which had not differentiated and six-day-differentiated preadipocytes. P0 referred to preadipocytes which had not differentiated. P6 referred to six-day-differentiated preadipocytes. “ns” represents no significance. “*” represents p < 0.05.
4. Discussion
It is known that KLFs are a family of transcription factors characterized by zinc-finger structures at the C-terminal end, and they play critical roles in cell proliferation, differentiation, and migration [49]. Using BLAST and HMM, we identified 56 KLF genes from four species and then a total of 13 KLF genes with specific conserved-domain zf-H2C2 genes were identified in G. gallus. The NJ tree was constructed with 56 gene sequences to compare chicken KLF genes with sequences from the three other species. The results demonstrated that the 56 KLF genes were divided into three groups. Comparative analysis suggested that chickens, mice, and humans have similar numbers of gene families [50]. Gene structure is an important factor in the evolution of gene families [51]. We found that the KLF gene structure of chickens is quite similar to that of humans, pigs, and mice. Motif analysis revealed that KLFs clustered in groups based on the similarity of their subdomains. The results of conserved-domain analysis showed that KLF genes are relatively conserved among these species. Among 20 genes predicted by interaction network analysis, NCOA3 was found to participate in regulating porcine skeletal muscle satellite cell proliferation [52]. Furthermore, differential expression of NCOA3 was proven to be regulated by miR-17-5p in pig intramuscular and subcutaneous adipose tissues [53].
Excessive fat deposition is considered an undesirable factor which affects feed efficiency, meat production costs, meat quality, and consumer health [54]. Hence, studies have been done to explore the relationship between KLF genes and fat deposition. A previous experiment proved that Gg-KLF2 was highly expressed in abdominal adipose tissue, and its transcripts fluctuated during adipose tissue development [55]. Our research showed that the relative expression level of KLF2 was highest in abdominal fat in male chickens. Genetic variants in the promoter region of the KLF3 gene were associated with fat deposition in Qinchuan cattle [56]. KLF3 was also demonstrated to be negatively correlated with intramuscular fat content in the longissimus dorsi muscle of Erhualian pigs [57]. The expression profile analysis found that KLF3 might play important roles in the fat development process. In our research, the expression level of KLF3 in abdominal fat was higher than that in the glandular stomach, hypothalamus, and spleen. However, there was no significant difference between the expression level of KLF3 in differentiated and undifferentiated preadipocytes. Sometimes, KLF genes work together to produce a marked effect. Guo et al. found the KLF15 gene was overexpressed through adenoviral vector (Ad-KLF15) in bovine adipocytes, and the expression level of the KLF3 gene was increased, which indicated that KLF15 promoted the transcription of the KLF3 gene in bovine adipocytes [58]. Our synteny analysis showed that KLF3 had collinear relationships with KLF5 and KLF12, while interaction network analysis showed that 11 KLF genes had interaction relationships, including KLF3 and KLF15. Besides, our research showed that KLF15 was expressed at relatively high levels in abdominal fat. KLF4 was proven to be one of potential therapeutic targets for obesity-induced cardiac injuries [59]. There was a significant difference between the expression level of KLF4 in differentiated and undifferentiated preadipocytes in our research. Cardiomyocyte-specific KLF5 knockout mice were found to have accelerated diet-induced obesity associated with increased white adipose tissue [60]. A qPCR showed that the expression level of KLF5 was second highest in abdominal fat. The expression levels of KLF5 decreased significantly after preadipocyte differentiation.
Iqbal et al. discovered that KLF6 regulated fat synthesis in bovine mammary epithelial cells (BMECs) by targeting the PPARA, the PPARG pathway, and other fat marker genes [61]. Our research showed that KLF6 expression was highest in abdominal fat. In our study, a significant difference was found between undifferentiated and differentiated adipocytes. RNA-seq result revealed that the down-regulation of the KLF6 gene significantly up-regulated the genes that regulate adipogenesis, differentiation and regulation of adipocytes, and the homeostasis of bovine adipocytes, which proved that KLF6 has a role in regulating lipid metabolism in bovine adipocytes [62]. KLF7 was revealed to modulate the differentiation and proliferation of chicken preadipocytes [63]. We also found the expression level of KLF7 significantly decreased after differentiation. KLF8 was targeted by miR-10a-5p to inhibit the differentiation of goat intramuscular preadipocytes [64]. In our study, the expression level of KLF8 decreased after their differentiation in chicken preadipocytes. KLF9 was found to inhibit chicken intramuscular preadipocyte differentiation [65]. However, the expression level of KLF9 increased significantly after chicken preadipocyte differentiation. KLF10, KLF11, KLF12, and KLF13 function as positive organizers by a variety of different mechanisms, such as crosstalk with C/EBP and PPARγ, to regulate adipogenesis and associated pathways in cattle [66]. The expression profile analysis revealed that KLF10 might play important roles in the fat development process. Our research showed that the expression of KLF10 in abdominal fat was the second-most abundant of all the tissues tested, while no significant difference was detected between the expression level of KLF10 in differentiated and un-differentiated preadipocytes. There was also no significant difference between the expression level of KLF11 in differentiated and undifferentiated preadipocytes. However, we found the expression level of KLF12 increased significantly and the expression level of KLF13 decreased significantly in chicken preadipocytes.
KLF genes play not only important roles in fat deposition but also have great effects on skeletal muscle development. Manoharan et al. [67] identified KLF2 as a key regulator of myeloid cell functions in mouse skeletal muscle regeneration. In our study, KLF2 expression level increased in samples from 11 embryonic-day-old chicks to 1-day-old chicks. Moreover, KLF2 was found to be involved in the FOXO signaling pathway. This FOXO signaling pathway played an important role in the pathogenesis of skeletal muscle atrophy by regulating E3 ubiquitin ligases and some autophagy factors [68]. This indicated that KLF2 played an important role in chickens’ skeletal muscle development. Zhang et al. [69] confirmed that miR-21-5p promoted the proliferation and differentiation of skeletal muscle satellite cells (SMSCs) by targeting KLF3. The increasing expression level of KLF3 in 1-day-old chicks might prove the function of KLF3 for promoting muscle development. The KLF4 gene was found to significantly promote the proliferation and differentiation of chicken primary myoblasts (CPMs) [70]. The expression level of KLF4 was extremely high in chicken thigh muscle and pectoral muscle in samples at 18 embryonic days old in our research. A study suggested that KLF5 may regulate the atrophy of chicken skeletal muscle through the Wnt/β-catenin signaling pathway [71], while KLF5 expression levels decreased when chickens were hatched in our study. These results meant that KLF5 displayed a vital role in the development of chicken muscle.
KLF6 was confirmed as a new potential target gene of miR-148a-3p, and miR-148a-3p impeded bovine myoblast cell proliferation and promoted apoptosis through the posttranscriptional down-regulation of KLF6 [72]. This conclusion corresponded with the downtrend of KLF6 expression level in samples from 16 embryonic-day-old to 1-day-old chicks in our study. KLF6 was also found to play a crucial role in regulating cell differentiation, proliferation, and muscle development, so it could be used as a potential candidate marker gene for the improvement of the Qinchuan cattle breed [73]. There is no report on the relationship between muscle development and KLF7 or KLF8. In our research, the results in Figure 8A also showed that KLF7 and KLF8 might have a relationship with the development of muscle. RNA-Seq exploration of meat quality in Spanish goats illustrated that KLF9 was associated with the effects of stress on skeletal muscle proteins [74]. We also found that KLF9 expression levels were very high in the thigh muscle of one-day-old chicken. KLF10 was known to control numerous genes in many cell types that are involved in various key biological processes like differentiation, proliferation, and apoptosis [75]. KLF10 was expressed highly in the thigh muscle of Heying Black chickens and the expression level increased in samples from 11-embryonic-day-old to 1-day-old chicken, indicating that KLF10 was involved in chicken muscle proliferation and differentiation. Furthermore, the sequencing result was used to show that KLF11 was related to muscle development [76]. In our experiment, we also found the expression level of KLF11 was high in pectoral muscle and that KLF11 was also related to chicken muscle development. According to the RNA-seq results from the embryonic days samples and 1-day-old samples (Figure 8A), KLF12 and KLF13 might have the opposite effect on muscle development in chickens. KLF15 was found to be a key regulator of branched-chain amino acid metabolism in the skeletal muscle of fish (tilapia) [77]. Both the RNA-seq and qPCR results showed that the expression level of KLF15 was high in muscle, and its expression increased in samples from 11 embryonic-day-old to 1-day-old chicken, which indicated that KLF15 was involved in the development of chicken muscle.
5. Conclusions
In this study, we identified 13 KLF genes in G. gallus. Phylogenetic analysis showed that the KLF genes could be divided into three groups. Chromosomal locations showed that the 13 Gg-KLFs were distributed on ten different chromosomes. Motif analysis and conserved domain analysis showed KLFs shared similar conserved domains in four species. Synteny analysis and interaction network analysis illustrated the collinear relationships and the relationships among KLFs and 20 corresponding genes. KEGG pathway analysis predicted that KLF2 was involved in the Apelin-signaling pathway and the FOXO-signaling pathway. Our expression profile analysis revealed that the KLF genes displayed diverse expression patterns in chickens. This study provides some evidence that the KLF gene family plays a critical role in the development of chicken muscle and fat, which may give some suggestions for future research in the KLF gene family.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13091429/s1, Figure S1: Apelin signaling pathway and FOXO signaling pathway; Table S1: The forward primers and reverse primers of KLFs designed by Primer Premier 5.0; Table S2: The Go enrichment analysis of the cellular components of KLF genes in chicken; Table S3: The Go enrichment analysis of biological processes of KLF genes in chicken.
Author Contributions
X.L. wrote the original manuscript. Q.W., J.Z. and G.Z. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal experiments performed in the study were all evaluated and approved by the Animal Ethics Committee of Yangzhou University (202103298).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was jointly supported by National Natural Science Foundation of China (32272856), the New Agricultural Breeds Creation Project in Jiangsu Province (PZCZ201730), the China Agriculture Research System (CARS-41), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Katemala S., Molee A., Thumanu K., Yongsawatdigul J. Meat quality and Raman spectroscopic characterization of Korat hybrid chicken obtained from various rearing periods. Poult. Sci. 2021;100:1248–1261. doi: 10.1016/j.psj.2020.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu S., Chang Y., Wu G., Zhang W., Man C. Potential role of miR-155-5p in fat deposition and skeletal muscle development of chicken. Biosci. Rep. 2020;40:BSR20193796. doi: 10.1042/BSR20193796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian W., Wang Z., Wang D., Zhi Y., Dong J., Jiang R., Han R., Li Z., Kang X., Li H., et al. Chromatin Interaction Responds to Breast Muscle Development and Intramuscular Fat Deposition Between Chinese Indigenous Chicken and Fast-Growing Broiler. Front. Cell. Dev. Biol. 2021;9:782268. doi: 10.3389/fcell.2021.782268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen X., Wei Y., You G., Liu W., Amevor F.K., Zhang Y., He H., Ma M., Zhang Y., Li D., et al. Circular PPP1R13B RNA Promotes Chicken Skeletal Muscle Satellite Cell Proliferation and Differentiation via Targeting miR-9-5p. Animals. 2021;11:2396. doi: 10.3390/ani11082396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng J., Wang L., Wang S., Chen R., Zhang T., Ma H., Lu H., Yuan G. Transcriptomic analysis of thigh muscle of Lueyang black-bone chicken in free-range and caged feeding. Anim. Biotechnol. 2021:1–11. doi: 10.1080/10495398.2021.1993235. [DOI] [PubMed] [Google Scholar]
- 6.Zhu M., Wang M., Shao Y., Nan Y., Blair H.T., Morris S.T., Zhao Z., Zhang H. Characterization of muscle development and gene expression in early embryos of chicken, quail, and their hybrids. Gene. 2021;768:145319. doi: 10.1016/j.gene.2020.145319. [DOI] [PubMed] [Google Scholar]
- 7.Shen X., Cui C., Tang S., Han S., Zhang Y., Xia L., Tan B., Ma M., Kang H., Yu J., et al. MyoG-enhanced circGPD2 regulates chicken skeletal muscle development by targeting miR-203a. Int. J. Biol. Macromol. 2022;222:2212–2224. doi: 10.1016/j.ijbiomac.2022.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Parakati R., DiMario J.X. Repression of myoblast proliferation and fibroblast growth factor receptor 1 promoter activity by KLF10 protein. J. Biol. Chem. 2013;288:13876–13884. doi: 10.1074/jbc.M113.457648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira K.K.S., de Morais Gomes E.R., de Lima Filho J.L., Castelletti C.H.M., Martins D.B.G. Bioinformatics analysis of non-synonymous variants in the KLF genes related to cardiac diseases. Gene. 2018;650:68–76. doi: 10.1016/j.gene.2018.01.085. [DOI] [PubMed] [Google Scholar]
- 10.Cantú I., van de Werken H.J.G., Gillemans N., Stadhouders R., Heshusius S., Maas A., Esteghamat F., Ozgur Z., van IJcken W.F.J., Grosveld F., et al. The mouse KLF1 Nan variant impairs nuclear condensation and erythroid maturation. PLoS ONE. 2019;14:e0208659. doi: 10.1371/journal.pone.0208659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi G., Yang F. Krüppel-like factor 1 (KLF1) promoted the proliferation, migration and invasion of human lens epithelial cells by enhancing the expression of Zinc Finger and BTB Domain Containing 7A (ZBTB7A) and activating Wnt/β-catenin pathway. Bioengineered. 2021;12:4374–4384. doi: 10.1080/21655979.2021.1953901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayuso M., Fernández A., Núñez Y., Benítez R., Isabel B., Fernández A.I., Rey A.I., González-Bulnes A., Medrano J.F., Cánovas Á., et al. Developmental Stage, Muscle and Genetic Type Modify Muscle Transcriptome in Pigs: Effects on Gene Expression and Regulatory Factors Involved in Growth and Metabolism. PLoS ONE. 2016;11:e0167858. doi: 10.1371/journal.pone.0167858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song C., Fang X., Yang Z., Wang Q., Meng F., Chen Y., Chen J., Zhao B., Wang Y., Fang X., et al. miR-152 Regulates Bovine Myoblast Proliferation by Targeting KLF6. Animals. 2021;11:3001. doi: 10.3390/ani11103001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa M., Geng F.S., Humphreys D.T., Kristianto E., Sheng D.Z., Hui S.P., Zhang Y., Sugimoto K., Nakayama M., Zheng D., et al. Krüppel-like factor 1 is a core cardiomyogenic trigger in zebrafish. Science. 2021;372:201–205. doi: 10.1126/science.abe2762. [DOI] [PubMed] [Google Scholar]
- 15.Xu J.W., Zheng L., Li L.J., Yao Y.F., Hua H., Yang S.Z., Wen Y.F., Song C.C., Cao X.K., Liu K.P., et al. Novel copy number variation of the KLF3 gene is associated with growth traits in beef cattle. Gene. 2019;680:99–104. doi: 10.1016/j.gene.2018.08.040. [DOI] [PubMed] [Google Scholar]
- 16.Hou Z., Wang Z., Tao Y., Bai J., Yu B., Shen J., Sun H., Xiao L., Xu Y., Zhou J., et al. KLF2 regulates osteoblast differentiation by targeting of Runx2. Lab. Investig. 2019;99:271–280. doi: 10.1038/s41374-018-0149-x. [DOI] [PubMed] [Google Scholar]
- 17.de Lázaro I., Yilmazer A., Nam Y., Qubisi S., Razak F.M.A., Degens H., Cossu G., Kostarelos K. Non-viral, Tumor-free Induction of Transient Cell Reprogramming in Mouse Skeletal Muscle to Enhance Tissue Regeneration. Mol. Ther. 2019;27:59–75. doi: 10.1016/j.ymthe.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi S., Manabe I., Suzuki Y., Relaix F., Oishi Y. Klf5 regulates muscle differentiation by directly targeting muscle-specific genes in cooperation with MyoD in mice. Elife. 2016;5:e17462–e17484. doi: 10.7554/eLife.17462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dionyssiou M.G., Salma J., Bevzyuk M., Wales S., Zakharyan L., McDermott J.C. Krüppel-like factor 6 (KLF6) promotes cell proliferation in skeletal myoblasts in response to TGFβ/Smad3 signaling. Skelet. Muscle. 2013;3:7. doi: 10.1186/2044-5040-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X., Shen Q.W., Wang J., Zhang Z., Feng F., Chen T., Zhang Y., Wei H., Li Z., Wang X., et al. KLF7 Regulates Satellite Cell Quiescence in Response to Extracellular Signaling. Stem. Cells. 2016;34:1310–1320. doi: 10.1002/stem.2346. [DOI] [PubMed] [Google Scholar]
- 21.Fan L., Sweet D.R., Fan E.K., Prosdocimo D.A., Madera A., Jiang Z., Padmanabhan R., Haldar S.M., Vinayachandran V., Jain M.K. Transcription factors KLF15 and PPARδ cooperatively orchestrate genome-wide regulation of lipid metabolism in skeletal muscle. J. Biol. Chem. 2022;298:101926. doi: 10.1016/j.jbc.2022.101926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui A., Fan H., Zhang Y., Zhang Y., Niu D., Liu S., Liu Q., Ma W., Shen Z., Shen L., et al. Dexamethasone-induced Krüppel-like factor 9 expression promotes hepatic gluconeogenesis and hyperglycemia. J. Clin. Investig. 2019;129:2266–2278. doi: 10.1172/JCI66062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam V.C., Folkersen L., Aguilar O.A., Lanier L.L. KLF12 Regulates Mouse NK Cell Proliferation. J. Immunol. 2019;203:981–989. doi: 10.4049/jimmunol.1900396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cherukunnath A., Davargaon R.S., Ashraf R., Kamdar U., Srivastava A.K., Tripathi P.P., Chatterjee N., Kumar S. KLF8 is activated by TGF-β1 via Smad2 and contributes to ovarian cancer progression. J. Cell. Biochem. 2022;123:921–934. doi: 10.1002/jcb.30235. [DOI] [PubMed] [Google Scholar]
- 25.Zhao G., Chang Z., Zhao Y., Guo Y., Lu H., Liang W., Rom O., Wang H., Sun J., Zhu T., et al. KLF11 protects against abdominal aortic aneurysm through inhibition of endothelial cell dysfunction. JCI Insight. 2021;6:e141673. doi: 10.1172/jci.insight.141673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao W., Jiao Y., Zhou Y., Luo X. KLF13 suppresses the proliferation and growth of colorectal cancer cells through transcriptionally inhibiting HMGCS1-mediated cholesterol biosynthesis. Cell Biosci. 2020;10:76. doi: 10.1186/s13578-020-00440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xin Y., Su P., Liu Y., Gu S., An X., Zhang X., Yan J., Guo Y., Zhou J., Yang G. Knock out hepatic Krüppel-like factor 16 (KLF16) improve myocardial damage and promoted myocardial protection of myocardial ischemia-reperfusion via anti-oxidative and anti-inflammation effects by TFAM/PPARβ signal passage. Bioengineered. 2021;12:10219–10231. doi: 10.1080/21655979.2021.1982302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S.H., Hao J., Zhang C., Duan F.F., Chiu Y.T., Shi M., Huang X., Yang J., Cao H., Wang Y. KLF17 promotes human naive pluripotency through repressing MAPK3 and ZIC2. Sci. China Life Sci. 2022;65:1985–1997. doi: 10.1007/s11427-021-2076-x. [DOI] [PubMed] [Google Scholar]
- 29.Kammoun M., Pouletaut P., Morandat S., Subramaniam M., Hawse J.R., Bensamoun S.F. Krüppel-like factor 10 regulates the contractile properties of skeletal muscle fibers in mice. Muscle Nerve. 2021;64:765–769. doi: 10.1002/mus.27412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emilia Q., Groover K., Clark J., Le T., Burrowes B., Liu M. Complete Genome Sequence of Stenotrophomonas maltophilia Siphophage Suso. Microbiol. Resour. Announc. 2022;11:e0011722. doi: 10.1128/mra.00117-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y., Xu L., Zou Q., Lin C. prPred-DRLF: Plant R protein predictor using deep representation learning features. Proteomics. 2022;22:e2100161. doi: 10.1002/pmic.202100161. [DOI] [PubMed] [Google Scholar]
- 32.Randhawa G.S., Hill K.A., Kari L. ML-DSP: Machine Learning with Digital Signal Processing for ultrafast, accurate, and scalable genome classification at all taxonomic levels. BMC Genom. 2019;20:267. doi: 10.1186/s12864-019-5571-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu X.Y., Zhang Q.F., Jiang D.D., Liu Y.F., Chen B., Yang S.P., Shao Z.T., Jiang H., Wang J., Fang Y.H., et al. Complete mitogenomes and phylogenetic relationships of Haemaphysalis nepalensis and Haemaphysalis yeni. Front. Vet. Sci. 2022;9:1007631. doi: 10.3389/fvets.2022.1007631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J., Chitsaz F., Derbyshire M.K., Gonzales N.R., Gwadz M., Lu S., Marchler G.H., Song J.S., Thanki N., Yamashita R.A., et al. The conserved domain database in 2023. Nucleic Acids Res. 2023;51:D384–D388. doi: 10.1093/nar/gkac1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perkel J.M. Smart software untangles gene regulation in cells. Nature. 2022;609:428–431. doi: 10.1038/d41586-022-02826-1. [DOI] [PubMed] [Google Scholar]
- 36.Martin F.J., Amode M.R., Aneja A., Austine-Orimoloye O., Azov A.G., Barnes I., Becker A., Bennett R., Berry A., Bhai J., et al. Ensembl 2023. Nucleic Acids Res. 2023;51:D933–D941. doi: 10.1093/nar/gkac958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., He Y., Xia R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Ngo V., Wang M., Wang W. Finding de novo methylated DNA motifs. Bioinformatics. 2019;35:3287–3293. doi: 10.1093/bioinformatics/btz079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doncheva N.T., Morris J.H., Holze H., Kirsch R., Nastou K.C., Cuesta-Astroz Y., Rattei T., Szklarczyk D., von Mering C., Jensen L.J. Cytoscape stringApp 2.0: Analysis and Visualization of Heterogeneous Biological Networks. J. Proteome Res. 2023;22:637–646. doi: 10.1021/acs.jproteome.2c00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szklarczyk D., Gable A.L., Nastou K.C., Lyon D., Kirsch R., Pyysalo S., Doncheva N.T., Legeay M., Fang T., Bork P., et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49:D605–D612. doi: 10.1093/nar/gkaa1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu S., Yu G., Lu X., Domeniconi C., Guo M. Isoform function prediction by Gene Ontology embedding. Bioinformatics. 2022;38:4581–4588. doi: 10.1093/bioinformatics/btac576. [DOI] [PubMed] [Google Scholar]
- 42.Bu D., Luo H., Huo P., Wang Z., Zhang S., He Z., Wu Y., Zhao L., Liu J., Guo J., et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021;49:W317–W325. doi: 10.1093/nar/gkab447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J., Ren X., Li L., Lu S., Chen T., Tan L., Liu M., Luo Q., Liang S., Nie Q., et al. Integrative Analyses of mRNA Expression Profile Reveal the Involvement of IGF2BP1 in Chicken Adipogenesis. Int. J. Mol. Sci. 2019;20:2923. doi: 10.3390/ijms20122923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang A., Lehnert K., You L., Snell R.G. ICARUS, an interactive web server for single cell RNA-seq analysis. Nucleic Acids Res. 2022;50:W427–W433. doi: 10.1093/nar/gkac322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Z., Ouyang H., Zheng M., Cai B., Han P., Abdalla B.A., Nie Q., Zhang X. Integrated Analysis of Long Non-coding RNAs (LncRNAs) and mRNA Expression Profiles Reveals the Potential Role of LncRNAs in Skeletal Muscle Development of the Chicken. Front. Physiol. 2017;7:687. doi: 10.3389/fphys.2016.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang G., Zhang J., Wu P., Ling X., Wang Q., Zhou K., Li P., Zhang L., Ye H., Zhang Q., et al. Transcriptome Sequencing Analysis of circRNA in Skeletal Muscle between Fast- and Slow-Growing Chickens at Embryonic Stages. Animals. 2022;12:3166. doi: 10.3390/ani12223166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khumpeerawat P., Duangjinda M., Phasuk Y. Factors affecting gene expression associated with the skin color of black-bone chicken in Thailand. Poult. Sci. 2021;100:101440. doi: 10.1016/j.psj.2021.101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanehisa M., Furumichi M., Sato Y., Kawashima M., Ishiguro-Watanabe M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023;51:D587–D592. doi: 10.1093/nar/gkac963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y., Zhao X., Xu M., Chen M. Krüppel-like factors in glycolipid metabolic diseases. Mol. Biol. Rep. 2022;49:8145–8152. doi: 10.1007/s11033-022-07565-0. [DOI] [PubMed] [Google Scholar]
- 50.Yang G., Lu H., Wang L., Zhao J., Zeng W., Zhang T. Genome-Wide Identification and Transcriptional Expression of the METTL21C Gene Family in Chicken. Genes. 2019;10:628. doi: 10.3390/genes10080628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lei P., Wei X., Gao R., Huo F., Nie X., Tong W., Song W. Genome-wide identification of PYL gene family in wheat: Evolution, expression and 3D structure analysis. Genomics. 2021;113:854–866. doi: 10.1016/j.ygeno.2020.12.017. [DOI] [PubMed] [Google Scholar]
- 52.Liu D., Li J., Hao W., Lin X., Xia J., Zhu J., Yang S., Yang X. Chimeric RNA TNNI2-ACTA1-V1 Regulates Cell Proliferation by Regulating the Expression of NCOA3. Front. Vet. Sci. 2022;9:895190. doi: 10.3389/fvets.2022.895190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han H., Gu S., Chu W., Sun W., Wei W., Dang X., Tian Y., Liu K., Chen J. miR-17-5p Regulates Differential Expression of NCOA3 in Pig Intramuscular and Subcutaneous Adipose Tissue. Lipids. 2017;52:939–949. doi: 10.1007/s11745-017-4288-4. [DOI] [PubMed] [Google Scholar]
- 54.Nematbakhsh S., Pei Pei C., Selamat J., Nordin N., Idris L.H., Abdull Razis A.F. Molecular Regulation of Lipogenesis, Adipogenesis and Fat Deposition in Chicken. Genes. 2021;12:414. doi: 10.3390/genes12030414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Z.W., Rong E.G., Shi M.X., Wu C.Y., Sun B., Wang Y.X., Wang N., Li H. Expression and functional analysis of Krüppel-like factor 2 in chicken adipose tissue. J. Anim. Sci. 2014;92:4797–4805. doi: 10.2527/jas.2014-7997. [DOI] [PubMed] [Google Scholar]
- 56.Guo H., Raza S.H.A., Schreurs N.M., Khan R., Wei D., Wang L., Zhang S., Zhang L., Wu S., Ullah I., et al. Genetic variants in the promoter region of the KLF3 gene associated with fat deposition in Qinchuan cattle. Gene. 2018;672:50–55. doi: 10.1016/j.gene.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 57.Liu H., Wei W., Lin W., Yu W., Luo W., Niu Y., Zhang L., Chen J. miR-32-5p Regulates Lipid Accumulation in Intramuscular Fat of Erhualian Pigs by Suppressing KLF3. Lipids. 2021;56:279–287. doi: 10.1002/lipd.12294. [DOI] [PubMed] [Google Scholar]
- 58.Guo H., Khan R., Raza S.H.A., Ning Y., Wei D., Wu S., Hosseini S.M., Ullah I., Garcia M.D., Zan L. KLF15 promotes transcription of KLF3 gene in bovine adipocytes. Gene. 2018;659:77–83. doi: 10.1016/j.gene.2018.03.049. [DOI] [PubMed] [Google Scholar]
- 59.Ding L., Li S., Wang F., Xu J., Li S., Wang B., Kou J., Wang Y., Cao W. Berberine improves dietary-induced cardiac remodeling by upregulating Kruppel-like factor 4-dependent mitochondrial function. Biol. Chem. 2021;402:795–803. doi: 10.1515/hsz-2020-0267. [DOI] [PubMed] [Google Scholar]
- 60.Pol C.J., Pollak N.M., Jurczak M.J., Zacharia E., Karagiannides I., Kyriazis I.D., Ntziachristos P., Scerbo D.A., Brown B.R., Aifantis I., et al. Cardiac myocyte KLF5 regulates body weight via alteration of cardiac FGF21. Biochim. Biophys Acta Mol. Basis. Dis. 2019;1865:2125–2137. doi: 10.1016/j.bbadis.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iqbal A., Yu H., Jiang P., Zhao Z. Deciphering the Key Regulatory Roles of KLF6 and Bta-miR-148a on Milk Fat Metabolism in Bovine Mammary Epithelial Cells. Genes. 2022;13:1828. doi: 10.3390/genes13101828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raza S.H.A., Khan R., Cheng G., Long F., Bing S., Easa A.A., Schreurs N.M., Pant S.D., Zhang W., Li A., et al. RNA-Seq reveals the potential molecular mechanisms of bovine KLF6 gene in the regulation of adipogenesis. Int. J. Biol. Macromol. 2022;195:198–206. doi: 10.1016/j.ijbiomac.2021.11.202. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Z., Wang H., Sun Y., Li H., Wang N. Klf7 modulates the differentiation and proliferation of chicken preadipocyte. Acta Biochim. Biophys Sin. 2013;45:280–288. doi: 10.1093/abbs/gmt010. [DOI] [PubMed] [Google Scholar]
- 64.Xu Q., Wang Y., Li X., Du Y., Li Y., Zhu J., Lin Y. miR-10a-5p Inhibits the Differentiation of Goat Intramuscular Preadipocytes by Targeting KLF8 in Goats. Front. Mol. Biosci. 2021;8:700078. doi: 10.3389/fmolb.2021.700078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun G.R., Zhang M., Sun J.W., Li F., Ma X.F., Li W.T., Han R.L., Li Z.J., Jiang R.R., Li G.X., et al. Krüppel-like factor KLF9 inhibits chicken intramuscular preadipocyte differentiation. Br. Poult. Sci. 2019;60:790–797. doi: 10.1080/00071668.2019.1657229. [DOI] [PubMed] [Google Scholar]
- 66.Raza S.H.A., Pant S.D., Wani A.K., Mohamed H.H., Khalifa N.E., Almohaimeed H.M., Alshanwani A.R., Assiri R., Aggad W.S., Noreldin A.E., et al. Krüppel-like factors family regulation of adipogenic markers genes in bovine cattle adipogenesis. Mol. Cell. Probes. 2022;65:101850. doi: 10.1016/j.mcp.2022.101850. [DOI] [PubMed] [Google Scholar]
- 67.Manoharan P., Song T., Radzyukevich T.L., Sadayappan S., Lingrel J.B., Heiny J.A. KLF2 in Myeloid Lineage Cells Regulates the Innate Immune Response during Skeletal Muscle Injury and Regeneration. iScience. 2019;17:334–346. doi: 10.1016/j.isci.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen K., Gao P., Li Z., Dai A., Yang M., Chen S., Su J., Deng Z., Li L. Forkhead Box O Signaling Pathway in Skeletal Muscle Atrophy. Am. J. Pathol. 2022;192:1648–1657. doi: 10.1016/j.ajpath.2022.09.003. [DOI] [PubMed] [Google Scholar]
- 69.Zhang D., Ran J., Li J., Yu C., Cui Z., Amevor F.K., Wang Y., Jiang X., Qiu M., Du H., et al. miR-21-5p Regulates the Proliferation and Differentiation of Skeletal Muscle Satellite Cells by Targeting KLF3 in Chicken. Genes. 2021;12:814. doi: 10.3390/genes12060814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang G., Chen F., Wu P., Li T., He M., Yin X., Shi H., Duan Y., Zhang T., Wang J., et al. MicroRNA-7 Targets the KLF4 Gene to Regulate the Proliferation and Differentiation of Chicken Primary Myoblasts. Front. Genet. 2020;11:842. doi: 10.3389/fgene.2020.00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang D.H., Yin H.D., Li J.J., Wang Y., Yang C.W., Jiang X.S., Du H.R., Liu Y.P. KLF5 regulates chicken skeletal muscle atrophy via the canonical Wnt/β-catenin signaling pathway. Exp. Anim. 2020;69:430–440. doi: 10.1538/expanim.20-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song C., Yang J., Jiang R., Yang Z., Li H., Huang Y., Lan X., Lei C., Ma Y., Qi X., et al. miR-148a-3p regulates proliferation and apoptosis of bovine muscle cells by targeting KLF6. J. Cell. Physiol. 2019;234:15742–15750. doi: 10.1002/jcp.28232. [DOI] [PubMed] [Google Scholar]
- 73.Raza S.H.A., Khan R., Schreurs N.M., Guo H., Gui L.S., Mei C., Zan L. Expression of the bovine KLF6 gene polymorphisms and their association with carcass and body measures in Qinchuan cattle (Bos Taurus) Genomics. 2020;112:423–431. doi: 10.1016/j.ygeno.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 74.Naldurtiker A., Batchu P., Kouakou B., Terrill T.H., Shaik A., Kannan G. RNA-Seq exploration of the influence of stress on meat quality in Spanish goats. Sci. Rep. 2022;12:20573. doi: 10.1038/s41598-022-23269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baroukh N., Canteleux N., Lefèvre A., Dupuy C., Martias C., Presset A., Subramaniam M., Hawse J.R., Emond P., Pouletaut P., et al. Serum and Soleus Metabolomics Signature of Klf10 Knockout Mice to Identify Potential Biomarkers. Metabolites. 2022;12:556. doi: 10.3390/metabo12060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang H., Liu D., Zhang H., Fan W., Hu J., Xu Y., Guo Z., Huang W., Hou S., Zhou Z. Genome-wide association studies demonstrate the genes associated with perimysial thickness in ducks. Anim. Genet. 2023:1–12. doi: 10.1111/age.13297. [DOI] [PubMed] [Google Scholar]
- 77.Li H., An X., Bao L., Li Y., Pan Y., He J., Liu L., Zhu X., Zhang J., Cheng J., et al. MiR-125a-3p-KLF15-BCAA Regulates the Skeletal Muscle Branched-Chain Amino Acid Metabolism in Nile Tilapia (Oreochromis niloticus) During Starvation. Front. Genet. 2020;11:852. doi: 10.3389/fgene.2020.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.