Abstract
Anaerobically grown Neisseria gonorrhoeae has previously been shown to have elevated serum resistance in the absence of exogenous CMP-N-acetylneuraminic acid or detectable sialylation. We hypothesized that the anaerobically induced gonococcal outer membrane protein AniA might have a role in this phenomenon, as it is the only known gonococcal protein that is absent under aerobic conditions. An N. gonorrhoeae F62 derivative, RUG7035, in which aniA is under control of the tac promoter, was used to examine the effect of AniA expression on serum resistance. In this study, we found that expression of AniA enhanced the serum resistance of N. gonorrhoeae and may account for these earlier observations.
The complement cascade is a major factor in controlling neisserial infections. Individuals with complement deficiencies have a significantly higher incidence of gonococcal and meningococcal infections than the general population (reviewed in reference 14). Gonococcal sensitivity to complement is strain dependent, and serum-resistant strains are more often isolated from complicated infections. Serum resistance is directly correlated to development of disseminated gonococcal infection (DGI) (4).
Frangipane and Rest reported that anaerobically grown Neisseria gonorrhoeae strain F62 was less sensitive to the killing action of normal human sera in both the presence and absence of exogenous CMP-N-acetylneuraminic acid (5). They showed that anaerobically grown N. gonorrhoeae expressed more of the lipooligosaccharide acceptor molecule for sialylation (12) by the gonococcal sialyltransferase (10) and that anaerobic growth and sialylation act synergistically to allow the gonococci a higher level of serum resistance (5). However, the authors could only speculate as to why the anaerobically grown gonococci were resistant after growth without CMP-N-acetylneuraminic acid and suggested that it was the result of a protein present only on anaerobic outer membranes.
As AniA is the only known outer membrane protein that is present during anaerobic growth but absent during aerobic growth (3), we were interested in determining if it might have a role in this observed phenomenon. In N. gonorrhoeae strain RUG7035, a derivative of strain F62, the native aniA promoter has been replaced with the tac promoter (6), which allows for AniA expression in N. gonorrhoeae grown under aerobic conditions (Fig. 1). Thus, differences in the serum resistance between aerobically grown RUG7035 and parental F62 could be attributed directly to the expression of AniA.
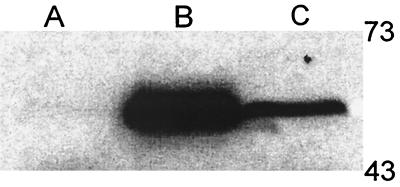
FIG. 1.
SDS-PAGE and Western analysis of constitutive AniA expression in N. gonorrhoeae RUG7035. Cell lysates from F62 grown aerobically (lane A) and anaerobically (lane B) and from RUG7035 (ani[Con]) grown aerobically (lane C) were separated by SDS-PAGE and transferred to nitrocellulose for Western analysis with a monoclonal anti-AniA antibody. AniA is normally present only in wild-type F62 when grown anaerobically; however, placing aniA under control of the tac promoter results in constitutive aerobic expression (lane C). The level of expression in RUG7035 is lower than in the wild-type anaerobic F62, which corresponds to the differences in promoter strength in N. gonorrhoeae (unpublished observations).
N. gonorrhoeae strains F62 and RUG7035 were grown in broth culture to mid-log phase, after which 107 bacteria were incubated with various dilutions of normal human serum (Sigma Chemical Corp., St. Louis, Mo.) in RPMI medium (Life Technologies, Rockville, Md.). The suspensions were incubated at 37°C for 30 min in 16- by 150-mm culture tubes with moderate shaking to maintain aerobic conditions. After incubation, suspensions were appropriately diluted in GC broth to determine viability, and serum resistance was measured as percent survival, determined as specified in the legend to Fig. 2.
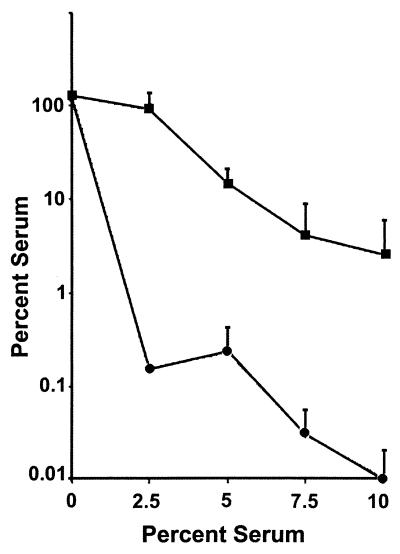
FIG. 2.
Percent survival from killing by normal human serum. N. gonorrhoeae RUG7035 (ani[Con]) (squares) and F62 (circles) were grown aerobically in broth until mid-log phase, after which they were assayed for resistance to killing by normal human serum as described in the text. Percent survival is expressed (average CFU of serum-treated N. gonorrhoeae at 30 min/average CFU of untreated N. gonorrhoeae at 0 min) × 100. Values are averages of three independent determinations. Error bars represent standard error of the mean.
Overall, increases in the concentration of serum resulted in a decreased survival of N. gonorrhoeae in the assay; however, N. gonorrhoeae constitutively expressing AniA showed a more than 100-fold-higher level of percent survival at all serum dilutions than the parental control F62 (Fig. 2). Controls for both strains in which the serum was heat inactivated at 56°C for 30 min prior to the complement killing assay did not differ from the 0% serum replicates (data not shown). Finally, our laboratory has recently shown that AniA contains a functional nitrite reductase domain (J. A. Cardinale and V. L. Clark, submitted for publication). Addition of 10 μM nitrite to the medium during incubation with serum did not enhance the ability of RUG7035 to survive serum killing, indicating that enzymatic reduction of nitrite and the production of nitric oxide was not the mechanism by which the gonococci became serum resistant.
There are a number of gonococcal proteins which have been shown to bind complement regulatory proteins: pilin will bind membrane cofactor protein (7); porin protein 1A binds factor H (11); and Opa binds heparin (1), which will then bind factor H (9). None of these mechanisms could account for the observed AniA-dependent protection, as nonpiliated, Opa− variants were used in the study, and F62, the control and parental strain of RUG7035, expresses porin protein 1B (13), which does not bind factor H (11).
Anti-AniA antibody was detected in women with local infection, DGI, and pelvic inflammatory disease (2). Studies have not been performed with men, as generally males have a poor antibody response to gonococcal antigens. A critical factor in determining whether or not a particular gonococcal strain will cause DGI is that it can become serum resistant (4). Anti-AniA antibody in the sera of infected women indicated that AniA is expressed in vivo and thus would allow N. gonorrhoeae to become more serum resistant. Women infected with N. gonorrhoeae have a significantly higher incidence of DGI than men (8), possibly due in part to anaerobic growth leading to increased serum resistance both by an increase in sialylation of lipooligosaccharide and by expression of AniA. Additionally, these data suggest that the infection environment may be more anaerobic in women than in men. The level of serum resistance due to AniA expression may be even greater in vivo, as AniA under control of the tac promoter is expressed at a lower level than under its own promoter during anaerobiosis (Fig. 1).
Serum protection due to AniA expression was either a direct result of AniA presence in the outer membrane or the result of a secondary protein induced in response to AniA expression. Previous studies by Clark et al. suggested that all outer membranes proteins detectable by either one- or two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis, with the exception of AniA, are present to some degree in N. gonorrhoeae grown under aerobic conditions (3). Therefore, the enhanced serum resistance of RUG7035 was most likely a direct effect of AniA presence. There are a number of possible mechanisms whereby AniA might accomplish this; however, the serum protection does not appear to depend on enzymatic activity of AniA, as addition of nitrite to the complement killing assays did not enhance protection. An answer to explain the AniA-dependent protection may lie in the proline-rich repeat region of AniA. Proline-rich repeat regions are involved in many types of protein-protein interactions. It is possible that the repeat region binds serum complement regulatory factor H directly, or that it binds a serum protein such as heparin which will act as a bridge to bind factor H. Studies are under way to construct a third isogenic strain which will constitutively express a truncated version of AniA. This will allow assessment of the role of the repeat region in protection from killing by normal human serum.
Acknowledgments
This work was supported by Public Health Service grant ROI AI11709 from the National Institutes of Health to V.L.C. J.A.C. was supported in part by a Public Health Service predoctoral training grant in microbial pathogenesis of bacteria and viruses (AI07362; 1998–1999).
REFERENCES
- 1.Chen T, Swanson J, Wilson J, Belland R J. Heparin protects Opa+Neisseria gonorrhoeae from the bactericidal action of normal human serum. Infect Immun. 1995;63:1790–1785. doi: 10.1128/iai.63.5.1790-1795.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark V L, Knapp J S, Thompson S, Klimpel K W. Presence of antibodies to the major anaerobically induced gonococcal outer membrane protein in sera from patients with gonococcal infections. Microb Pathog. 1988;5:381–390. doi: 10.1016/0882-4010(88)90038-1. [DOI] [PubMed] [Google Scholar]
- 3.Clark V L, Campbell L A, Palermo D A, Evans T M, Klimpel K W. Induction and repression of outer membrane proteins by anaerobic growth of Neisseria gonorrhoeae. Infect Immun. 1987;55:1359–1364. doi: 10.1128/iai.55.6.1359-1364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Densen P. Interaction of complement with Neisseria meningitidis and Neisseria gonorrhoeae. Clin Microbiol Rev. 1989;2(Suppl.):S11–S17. doi: 10.1128/cmr.2.suppl.s11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frangipane J V, Rest R F. Anaerobic growth and cytidine 5′-monophospho-N-acetylneuramic acid act synergistically to induce high level serum resistance in N. gonorrhoeae. Infect Immun. 1993;61:1657–1666. doi: 10.1128/iai.61.5.1657-1666.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Householder T C, Belli W A, Lissenden S, Cole J A, Clark V L. cis- and trans-acting elements involved in regulation of aniA, the gene encoding the major anaerobically induced outer membrane protein in Neisseria gonorrhoeae. J Bacteriol. 1999;181:541–551. doi: 10.1128/jb.181.2.541-551.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Källström H, Liszewski M K, Atkinson J P, Jonsson A-B. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol Microbiol. 1997;25:639–647. doi: 10.1046/j.1365-2958.1997.4841857.x. [DOI] [PubMed] [Google Scholar]
- 8.McNeeley S G., Jr Gonococcal infections in women. Sex Transm Dis. 1989;16:467–478. [PubMed] [Google Scholar]
- 9.Pangburn M K, Atkinson M A, Meri S. Localization of the heparin-binding site on complement factor H. J Biol Chem. 1991;266:16847–16852. [PubMed] [Google Scholar]
- 10.Parsons N J, Andrade J R C, Patel P V, Cole J A, Smith H. Sialylation of lipopolysaccharide and loss of absorption of bactericidal antibodies during conversion of gonococci to serum resistance by cytidine 5′-monophospho-N-acetyl-neuraminic acid. Microb Pathog. 1989;7:63–72. doi: 10.1016/0882-4010(89)90112-5. [DOI] [PubMed] [Google Scholar]
- 11.Ram S, McQuillen D P, Gulati S, Elkins C, Pangburn M K, Rice P A. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J Exp Med. 1998;188:671–680. doi: 10.1084/jem.188.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rest R F, Liu J, Talukdar R, Frangipane J V, Simon D. Interaction of pathogenic Neisseria with host defenses: what happens in vivo? Ann N Y Acad Sci. 1994;730:182–196. doi: 10.1111/j.1749-6632.1994.tb44248.x. [DOI] [PubMed] [Google Scholar]
- 13.Schneider H, Griffiss J M, Williams G D, Pier G B. Immunological basis of serum resistance of Neisseria gonorrhoeae. J Gen Microbiol. 1982;128:13–22. doi: 10.1099/00221287-128-1-13. [DOI] [PubMed] [Google Scholar]
- 14.Vogel U, Frosch M. Mechanisms of neisserial serum resistance. Mol Microbiol. 1999;32:1133–1139. doi: 10.1046/j.1365-2958.1999.01469.x. [DOI] [PubMed] [Google Scholar]