Abstract
We have previously shown that Salmonella enterica serovar Typhimurium expressing the hagB hemagglutinin gene from Porphyromonas gingivalis can induce primary and recall immune responses in serum and secretions in mice; however, the longevity of memory induced by oral Salmonella carriers has not been adequately demonstrated. In this study, we examined the capacity of mice to mount a recall response 52 weeks after primary immunization. Recall responses were seen in serum immunoglobulin G (IgG) and IgA following boosting at week 52, and in most cases, they were equal to or greater than the primary responses. Significant mucosal IgA recall responses in saliva and vaginal wash were also detected following boosting at week 52. In addition, there was a considerable residual response in secretions at week 51, prior to boosting. These results indicate that oral Salmonella vectors can induce long-term memory to recombinant HagB and are particularly effective at inducing long-lasting mucosal responses as well as at inducing the capacity for mucosal recall responses.
The mucosae serve as portals of entry for many pathogens. Because of our growing understanding of pathogenic mechanisms and host-pathogen relationships, there is increased interest in stimulating mucosal immunity as a first line of defense against colonization and establishment of disease. In order to render potential vaccine antigens immunogenic, a variety of approaches have been taken to stimulate effective mucosal immunity. These approaches include mucosal adjuvants and nonliving and live delivery systems (7, 12, 18). Avirulent Salmonella enterica serovar Typhimurium expressing foreign gene products has been used as a delivery system for a number of vaccine antigens (4). Live, avirulent Salmonella induces a diverse response including both mucosal and systemic immunity. One of the historical problems with mucosal responses to oral vaccines has been the lack of long-term mucosal memory.
The hagB gene codes for a hemagglutinin from the periodontopathogen Porphyromonas gingivalis and is a potential virulence factor (15, 19). We have previously shown that mice immunized intragastrically with Salmonella serovar Typhimurium expressing the hagB gene exhibit a vigorous serum immunoglobulin G (IgG) and IgA response to purified, recombinant HagB as well as a significant mucosal IgA response in saliva, gut secretions, and vaginal washes (5). The primary response peaks around 5 or 6 weeks after primary immunization. When mice are boosted at 14 weeks, a more rapid and intense recall response in serum and secretions is seen (16). The objectives of this study were to examine the Salmonella delivery system in terms of the duration of the immune response and to determine the long-term ability to mount a systemic and mucosal recall response.
Bacterial strains, plasmids, media, and culture conditions.
Salmonella serovar Typhimurium χ4072, an SR-11 derivative (pStSR100− gyrA1816 Δcya-1 Δcrp-1 ΔasdA1 Δ[zhf-4::Tn10]), and plasmid pYA292 (10) were provided by Roy Curtiss III (Washington University, St. Louis, Mo.). The vaccine strain χ4072/pDMD1 was constructed by electroporation with plasmid pDMD1, which expresses the hagB gene of P. gingivalis, as previously described (5). Strains were routinely grown at 37°C in Luria-Bertani (LB) medium (23). Cultures were maintained at −80°C as glycerol stocks.
Purification of HagB.
Histidine-tagged HagB was purified using the QIA Express system (Qiagen Inc., Valencia, Calif.). A Tru9I-XbaI fragment of a hagB clone (carried on p18AX1) was subcloned into the expression vector pQE31. The recombinant plasmid was designated pQE31-TX1. Positive subclones were selected on colony blots by using absorbed antiserum to HagB (6). Cultures (500 ml) were grown with aeration at 37°C in LB broth to an A600 of 0.8 and then induced with 1 mM isopropyl β-d-thiogalactoside for 5 h. The cells were lysed for 1.5 h at room temperature in 6 M guanidine-HCl–0.1 M NaH2PO4–0.01 M Tris (pH 8.0) (buffer A). The supernatant was mixed with 8 ml of Ni-nitrilotriacetic acid resin for 1.5 h. The resin was loaded into a 1.6-cm-diameter column and washed with 10 column volumes of buffer A, followed by 5 column volumes of 8 M urea–0.1 M NaH2PO4–0.01 M Tris (pH 8.0) (buffer B). The column was then washed with buffer B adjusted to pH 6.3 until the A280 was <0.01. Attempts to refold eluted HagB by gradual dialysis were unsuccessful and resulted in precipitation of the protein. Refolding was accomplished while HagB was bound to the column. The column was equilibrated with refolding buffer (0.5 M NaCl, 10 mM Tris, 20% glycerol [pH 7.4]) containing 6 M urea. The column was then washed with a linear gradient of 6 M to 0 M urea at a flow rate of 12 ml/h over a period of 1.5 h. The histidine-tagged HagB was eluted with 250 mM imidazole and dialyzed against phosphate-buffered saline. The purified HagB appeared as a single band in sodium dodecyl sulfate-polyacrylamide gel electrophoresis and reacted with HagB antiserum on Western blots (not shown). Routine yields were 8 to 10 mg per 500-ml culture.
Mouse immunization and sample collection.
Female BALB/c, VAF/Plus mice, 6 to 8 weeks of age (Charles River, Wilmington, Mass.), were housed in the Infectious Disease Isolation Unit at the University of Florida Animal Resource Center and given food and water ad libitum. Groups of six mice were immunized with Salmonella serovar Typhimurium strain χ4072/pDMD1. The strain was grown as a static culture in LB broth overnight at 37°C, diluted 1/20 in fresh LB broth, grown for ca. 4 h at 37°C to an optical density at 600 nm of 0.8, after which the culture was centrifuged and resuspended in sterile 0.1 M NaHCO3 to a density of 1010 CFU/ml. The food supply was removed and the bedding was changed 4 h prior to immunization. Mice were immunized by gastric intubation with 109 cells (0.1 ml of 1010 cells/ml) in three doses on days 1, 3, and 5 of week 0. Boosting was carried out in the same manner.
Group I was immunized at week 0 and boosted at week 52. Week 52 was chosen to represent long-term memory since it equals approximately one-half the lifespan of a BALB/c mouse (8). Group II was immunized at week 0 and boosted at week 14 as part of a study on timing of boosting (16a) and then boosted at week 52 to assess long-term recall. Serum and saliva samples and vaginal washes were collected for evaluation of specific antibody directed against the hemagglutinin, as previously described (5, 16).
Immunoassay methods.
Samples were assayed for IgG and IgA antibody to HagB on microwell plates as described previously (5) using an enzyme-linked immunosorbent assay coated with purified HagB protein. The salivary IgA anti-HagB antibody levels were normalized to amylase activity levels, and the antibody levels in vaginal washes were normalized to the total IgA to account for variable dilution encountered in secretions. The amylase activity was determined using a colorimetric enzyme assay (3).
Anti-HagB responses in serum.
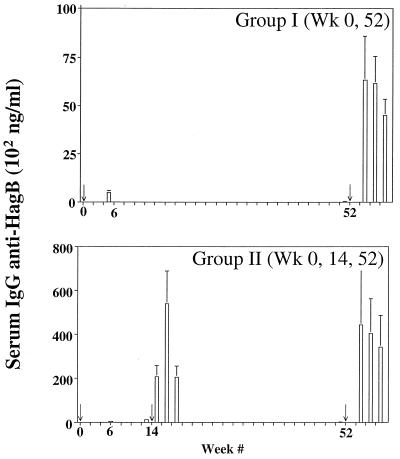
Mice immunized at week 0 and week 52 (Fig. 1) showed a low but measurable residual serum IgG response at week 51, just prior to boost, and a recall response at weeks 55, 57, and 59. Mice in group II, which were also boosted at week 14, showed a strong IgG recall response after the first boost and recall responses of up to ca. 1,000 ng/ml following the boost at week 52. Even though they did not exceed the peak responses seen at the earlier boost at week 14, the levels were higher than the week 6 levels and much higher than the 1-year recall levels seen in group I mice. With serum IgA (Fig. 2), antibody levels in group I mice following the boost at week 52 were higher than those for IgG. Much higher recall levels were seen in mice of group II than in mice of group I, although in this case the recall levels were comparable to those measured following the boost at week 14. Detectable levels of anti-HagB were measured at week 51 prior to boost in both groups, and they were low compared to the recall levels.
FIG. 1.
Serum IgG anti-HagB levels following oral immunization and boosting (arrows) with Salmonella serovar Typhimurium χ4072/pDMD1. Error bars, standard errors of the means.
FIG. 2.
Serum IgA anti-HagB levels following oral immunization and boosting (arrows) with Salmonella serovar Typhimurium χ4072/pDMD1. Error bars, standard errors of the means.
Anti-HagB responses in secretions.
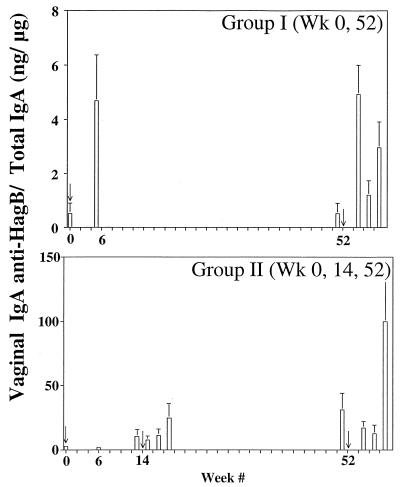
Recall responses in vaginal washes of single-immunized mice (Fig. 3) were comparable to primary responses, while in mice immunized both at week 0 and week 14 (group II), there were significant residual levels at week 51 as well as higher recall responses following boosting at week 52.
FIG. 3.
Vaginal wash IgA anti-HagB levels following oral immunization and boosting (arrows) with Salmonella serovar Typhimurium χ4072/pDMD1. Error bars, standard errors of the means.
Evidence of the longevity of mucosal memory is most dramatically evident from salivary responses (Fig. 4). In mice of group I, residual levels were detected at week 51, prior to boosting, with subsequent recall responses which far exceeded the primary response. Likewise, in mice boosted at week 14 (group II), residual levels of IgA anti-HagB remained at week 51, and significant recall responses were seen following the boost.
FIG. 4.
Salivary IgA anti-HagB levels following oral immunization and boosting (arrows) with Salmonella serovar Typhimurium χ4072/pDMD1. Error bars, standard errors of the means.
Poor long-term memory with respect to secretory IgA has historically been a problem with oral immunization aimed at inducing mucosal responses. Early attempts to induce mucosal responses using orally administered, soluble proteins resulted in poor immune responses, no memory induction, and, often, oral tolerance. Various strategies including encapsulation in nonliving carriers and use of oral adjuvants have been employed to increase the immunogenicity of orally delivered antigens (12, 18).
One of the most effective oral adjuvants for the induction of mucosal responses is cholera toxin (CT) (7). Lycke and Holmgren have shown that oral immunization with CT induces memory B cells in the gut lamina propria to CT itself, which can be detected after 2 years (17). Hajishengallis et al. (11) showed that oral immunization with the saliva-binding region of Streptococcus mutans AgI/II genetically fused to a cholera toxin A2/B construct with CT as an adjuvant resulted in serum and salivary responses in mice which persisted to approximately 1 year; however, no recall responses were obtained following boosting at 1 year.
Fifty-two weeks correspond to nearly half the mean life span of a BALB/c mouse (8). In our experiments, while serum IgG responses after week 52 do not equal peak responses at week 14, they still reach significant levels, particularly with respect to single-immunized animals. Recall responses are seen in serum IgA, however, perhaps reflecting the mucosal inductive route. Even within the mucosal compartment, there appears to be a subcompartmentalization with respect to the optimum site of induction for various effector sites. This subcompartmentalization may be due in part to differential expression of tissue-specific adhesins on cells arising from different inductive sites (20). We have consistently detected lower levels of responses in vaginal washes than in saliva with the Salmonella system. This may reflect the fact that the oral route may not be optimal for inducing vaginal responses, where intranasal immunization appears to be superior (13, 14). Nevertheless, the capacity to mount a modest recall response is seen in the vaginal compartment.
What is most remarkable is the persistence and recall capacity reflected in salivary IgA responses, both in single-immunized animals and animals boosted at week 14. The Salmonella delivery system appears to be more capable of inducing long-term memory for salivary responses to foreign antigens, compared to the CTB subunit (11, 22, 25).
Some studies have raised concerns that repeated use of Salmonella vectors would not be possible due to immunity to the vector itself (1, 9, 21), while others report that prior exposure leads to enhancement of subsequent responses (2, 24). Our results from short-term recall experiments (16) and the present study support the efficacy of repeated use of Salmonella vectors for induction of long-term mucosal immunity.
Acknowledgments
We thank Roy Curtiss III and Sandra Kelly for providing bacterial strains and plasmids and Jeffrey D. Hillman for his helpful advice.
This study was supported by Public Health Service grants DE-10963 and DE-07496 and by Training Grant DE-07200 from the National Institute of Dental and Craniofacial Research.
REFERENCES
- 1.Attridge S R, Davies R, LaBrooy J T. Oral delivery of foreign antigens by attenuated Salmonella: consequences of prior exposure to the vector strain. Vaccine. 1997;15:155–162. doi: 10.1016/s0264-410x(96)00158-2. [DOI] [PubMed] [Google Scholar]
- 2.Bao J X, Clements J D. Prior immunologic experience potentiates the subsequent antibody response when Salmonella strains are used as vaccine carriers. Infect Immun. 1991;59:3841–3845. doi: 10.1128/iai.59.10.3841-3845.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernfeld P. Amylases α and β. Methods Enzymol. 1955;1:149–158. [Google Scholar]
- 4.Cárdenas L, Clements J D. Oral immunization using live attenuated Salmonella spp. as carriers of foreign antigens. Clin Microbiol Rev. 1992;5:328–342. doi: 10.1128/cmr.5.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dusek D M, Progulske-Fox A, Brown T A. Systemic and mucosal immune responses in mice orally immunized with an avirulent Salmonella typhimurium expressing a cloned Porphyromonas gingivalis hemagglutinin. Infect Immun. 1994;62:1652–1657. doi: 10.1128/iai.62.5.1652-1657.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dusek D M, Progulske-Fox A, Whitlock J, Brown T A. Isolation and characterization of a cloned Porphyromonas gingivalis hemagglutinin from an avirulent strain of Salmonella typhimurium. Infect Immun. 1993;61:940–946. doi: 10.1128/iai.61.3.940-946.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elson C O, Dertzbaugh M T. Mucosal adjuvants. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. San Diego, Calif: Academic Press, Inc.; 1999. pp. 817–838. [Google Scholar]
- 8.Finch C E. Longevity, senescence, and the genome. Chicago, Ill: University of Chicago Press; 1990. [Google Scholar]
- 9.Forrest B D. Impairment of immunogenicity of Salmonella typhi Ty21a due to preexisting cross-reacting intestinal antibodies. In: Mestecky J, Russell M W, Jackson S, Michalek S M, editors. Advances in mucosal immunology. 371B. New York, N.Y: Plenum Press; 1995. pp. 1649–1652. [Google Scholar]
- 10.Galán J E, Nakayama K, Curtiss R., III Cloning and characterization of the asd gene of Salmonella typhimurium: use in stable maintenance of recombinant plasmids in Salmonella vaccine strains. Gene. 1990;94:29–35. doi: 10.1016/0378-1119(90)90464-3. [DOI] [PubMed] [Google Scholar]
- 11.Hajishengallis G, Michalek S M, Russell M W. Persistence of serum and salivary antibody responses after oral immunization with a bacterial protein antigen genetically linked to the A2/B subunits of cholera toxin. Infect Immun. 1996;64:665–667. doi: 10.1128/iai.64.2.665-667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hantman M J, Hohmann E L, Murphy C G, Knipe D M, Miller S I. Antigen delivery systems: development of recombinant live vaccines using viral or bacterial vectors. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. San Diego, Calif: Academic Press, Inc.; 1999. pp. 779–791. [Google Scholar]
- 13.Hordnes K, Tynning T, Brown T A, Haneberg B, Jonsson R. Nasal immunization with group B streptococci can induce high levels of specific IgA antibodies in cervicovaginal secretions of mice. Vaccine. 1997;15:1244–1251. doi: 10.1016/s0264-410x(97)00021-2. [DOI] [PubMed] [Google Scholar]
- 14.Johansson E L, Rask C, Fredriksson M, Eriksson K, Czerkinsky C, Holmgren J. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect Immun. 1998;66:514–520. doi: 10.1128/iai.66.2.514-520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz J, Black K P, Michalek S M. Host responses to recombinant hemagglutinin B of Porphyromonas gingivalis in an experimental rat model. Infect Immun. 1999;67:4352–4359. doi: 10.1128/iai.67.9.4352-4359.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohler J J, Pathangey L, Brown T A. Oral immunization with recombinant Salmonella typhimurium expressing a cloned Porphyromonas gingivalis hemagglutinin: effect of boosting on mucosal, systemic and IgG subclass response. Oral Microbiol Immunol. 1998;13:81–88. doi: 10.1111/j.1399-302x.1998.tb00717.x. [DOI] [PubMed] [Google Scholar]
- 16a.Kohler J J, Panthagey L B, Gillespie S R, Brown T A. Effect of preexisting immunity to Salmonella on the immune response to recombinant Salmonella enterica serovar Typhimurium expressing a Porphyromonas gingivalis hemagglutinin. Infect Immun. 2000;68:3116–3120. doi: 10.1128/iai.68.6.3116-3120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lycke N, Holmgren J. Intestinal mucosal memory and presence of memory cells in lamina propria and Peyer's patches in mice 2 years after oral immunization with cholera toxin. Scand J Immunol. 1986;23:611–616. doi: 10.1111/j.1365-3083.1986.tb01995.x. [DOI] [PubMed] [Google Scholar]
- 18.Michalek S M, O'Hagan D T, Gould-Fogerite S, Rimmelzwaan G F, Osterhaus A D M E. Antigen delivery systems: nonliving microparticles, liposomes, cochleates and ISCOMS. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. San Diego, Calif: Academic Press, Inc.; 1999. pp. 759–778. [Google Scholar]
- 19.Progulske-Fox A, Tumwasorn S, Lepine G, Whitlock J, Savett D, Ferretti J J, Banas J A. The cloning, expression and sequence analysis of a second Porphyromonas gingivalis gene that codes for a protein involved in hemagglutination. Oral Microbiol Immunol. 1995;10:311–318. doi: 10.1111/j.1399-302x.1995.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 20.Quiding-Jabrink M, Nordstrom I, Granstrom G, Kilander A, Jertborn M, Butcher E C, Lazarovits A I, Holmgren J, Czerkinsky C. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations. A molecular basis for the compartmentalization of effector B cell responses. J Clin Investig. 1997;99:1281–1286. doi: 10.1172/JCI119286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts M, Bacon A, Li J, Chatfield S. Prior immunity to homologous and heterologous Salmonella serotypes suppresses local and systemic anti-fragment C antibody responses and protection from tetanus toxin in mice immunized with Salmonella strains expressing fragment C. Infect Immun. 1999;67:3810–3815. doi: 10.1128/iai.67.8.3810-3815.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell M W, Wu H Y. Distribution, persistence, and recall of serum and salivary antibody responses to peroral immunization with protein antigen I/II of Streptococcus mutans coupled to the cholera toxin B subunit. Infect Immun. 1991;59:4061–4070. doi: 10.1128/iai.59.11.4061-4070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 24.Whittle B L, Verma N K. The immune response to a B-cell epitope delivered by Salmonella is enhanced by prior immunological experience. Vaccine. 1997;15:1737–1740. doi: 10.1016/s0264-410x(97)00119-9. [DOI] [PubMed] [Google Scholar]
- 25.Wu H Y, Russell M W. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect Immun. 1993;61:314–322. doi: 10.1128/iai.61.1.314-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]