ABSTRACT
Cutaneous leishmaniasis (CL), caused by an obligate intracellular protozoan parasite from the genus Leishmania, imposing a significant burden on underdeveloped countries especially those located in the Middle East. Four electronic databases were searched to evaluate the prevalence of CL in the Middle East. The random effects model (95% confidence intervals (CI)) were applied to determine the overall and subgroup pooled prevalence. Heterogeneity was assessed by Cochran’s Q test and I2 statistics. Among 2424 peer-reviewed papers, 37 datasets from 34 studies were included in the current meta-analysis. 285560 individuals were assessed across 9 Middle Eastern countries. The pooled prevalence of CL was estimated at 12% (95% CI 9-15 %; 10718/285560). The highest prevalence rate was observed in Syria (39%, 37-42%), and the lowest one was found in Iraq and Lebanon (0%, 0-1%). The prevalence of CL in studies that applied LST assays had the highest rate (48%, 17-80%). The infection rate in males was similar to females (7%, 4-10%). The prevalence of infection in individuals living in urban areas was higher than in rural areas (14%, 10-19%). The prevalence of CL in the age group 0-15 years was higher than in individuals 16-40 and >40 years (9%, 6-13%). Most of the lesions were found on the face, and single lesions were more prevalent than two and three ones. In conclusion, the occurrence of CL was considerable in Middle Eastern countries. Therefore, more efforts should be made to precisely report the CL in this region for developing appropriate preventive and controlling strategies.
KEYWORDS: Cutaneous leismaniasis, prevalence, Middle East
1. Introduction
Leishmaniasis is one of the neglected tropical diseases, according to the World Health Organization (WHO) report [1]. This disease is caused by an obligate protozoan parasite, which belongs to the genus Leishmania. It commonly occurs through the bite of sand flies, which inject metacyclic promastigotes (infective stage of Leishmania) into the skin [2]. Almost 90 species of sand flies are known to be vectors of Leishmania parasites, with the two mainly implicated vector species being Phlebotomus and Lutzomyia [2,3].
According to the WHO, almost 102 countries/areas are endemic for Leishmania infections [4]. This disease appears in four major clinical forms, including visceral leishmaniasis (VL, kala-azar), cutaneous leishmaniasis (CL), post-kala-azar dermal leishmaniasis (PKDL), and mucocutaneous leishmaniasis [4,5]. Globally, 70 countries are endemic for CL, and nearly 10 countries (Afghanistan, Algeria, Brazil, Colombia, Iran, Iraq, Libya, Pakistan, the Syrian Arab Republic, and Tunisia) have reported about 87% of new CL cases. The annual incidence of CL was estimated at 600,000 to 1,000,000 new cases [4,6]. It is also commonly found in two main forms in the Middle East, namely, zoonotic CL (ZCL) caused by L. major and anthroponotic CL (ACL) caused by L. tropica [2,7].
Considering the emergence of lesions, ACL is the so-called dry type, which is mainly transmitted among humans in urban areas. On the other hand, in ZCL, as the wet type of lesions, rodents are usually reservoir hosts in rural regions [2,8]. The active phase of CL is characterized by lesions that advance from papules and nodules to plaques and ulcers. Another phase of CL is inactive or scaring lesions which commonly develop following an active phase [9]. These lesions are generally found on the face, hands, legs, and to a lesser extent, in other parts of the body. According to epidemiological studies, approximately 50% of lesions occur on the face [9]. Since facial scars can persist for a long time, they are considered social stigmas with psychological consequences, including depression, seclusion, and decrease of quality of life (QOL) [10,11].
The facial scars due to CL can lead to the loss of job opportunities and also a decrease in self-confidence to participate in social activities, especially in women and children [12]. In recent years, several factors, including environmental conditions, climate changes, lack of urbanization management, war and migration, use of agricultural lands for residential purposes, and changes in vector populations, are associated with a considerable increase in leishmaniasis [13]. In recent years, some countries in the Middle East, such as Syria and Lebanon, have faced a war crisis, which caused an influx of refugees to the border areas of neighboring countries. With the migration of people from endemic areas to non-endemic areas and vice versa, the prevalence of CL increased significantly. To implement control programs and increase the level of public health, knowledge of the latest occurrence rate of this disease can be helpful in the region. Therefore, a systematic review and meta-analysis study of existing data on the prevalence of cutaneous leishmaniasis in the Middle East was performed.
2. Material and methods
2.1. Search scheme and selection criteria eligibility
We conducted a systematic review and meta-analysis study according to instructions from the Predefined Protocol Items for Systematic Reviews and Meta-Analyses (PRISMA) statements [14].
A comprehensive literature search was conducted from English language databases (PubMed, Web of Science, Scopus, and Google Scholar) up to the 1st June 2021. An additional literature search was performed through the references of key studies. The main search terms were applied alone or in combination, as follows: “Cutaneous Leishmaniasesˮ, “old world Leishmaniasisˮ, “Leishmania infectionˮ, “diffuse cutaneous leishmaniasisˮ, “prevalenceˮ, “epidemiology, “incidenceˮ, “Frequencyˮ and each country which located in the Middle East region (Bahrain, Cyprus, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Oman, Palestine, Qatar, Saudi Arabia, Syria, Turkey, United Arab Emirates, and Yemen) was also included.
In the next step, duplicate publications were removed, and the initial title and abstract were screened by two independent researchers according to inclusion and exclusion criteria (T.G.F. and M.K.). Only studies conducted on human subjects without time limitations, all population-based, cross-sectional, and cohort peer-reviewed original observational studies reporting the prevalence of cutaneous leishmaniasis in the Middle East, studies that applied different diagnostic methods such as skin examination, serological and molecular techniques for evaluation of the prevalence of CL were considered for inclusion. Studies which assessed the frequency of cutaneous leishmaniasis on suspected individuals/patients were not eligible for inclusion. In addition, studies that were carried out on non-human subjects, duplicate articles, non-English language papers, experimental research, conference papers, reviews, case reports, case series, and letters or correspondences were excluded from the current work.
2.2. Data extraction and study quality assessment
The full-text of all selected papers was reviewed for inclusion by two independent authors. Then, data was extracted from selected studies and included in an Excel sheet. After that, variables were taken out from eligible studies that included the followings: the first author’s last name, publication year, operation year, implementation country, mean age or age range of the studied population, gender, diagnostic methods, location (urban/rural), total sample size and number of infected subjects. Any dissimilarity in data extraction was resolved with the contribution of the third author. The quality assessment (risk of bias) was evaluated using the Joanna Briggs Institute Critical Appraisal Tool (JBI) for prevalence studies. According to the JBI protocol, nine questions were answered for each study, and they were categorized based on the total score to show poor quality (high risk of bias), 0–3 points; moderate quality (moderate risk of bias), 4–6 points; and high quality (low risk of bias), 7–9 points [15].
2.3. Data synthesis and statistical analysis
All statistical analysis was performed by Stata version 15 (College Station, Texas 77,845 USA), and the significance level was considered as P- value <0.05.
In the current meta-analysis, for evaluation of the pooled (weighted) proportion of cutaneous leishmaniasis, a random-effects model was used, and the I2 statistic was considered to measure the discrepancy in the prevalence estimates across the studies. Subgroup analyses were applied to find the sources of heterogeneity. Subgroup analysis was performed based on several variables, including each Middle Eastern country, study period, type of diagnostic methods, sample size, gender, age, location (urban/rural), Leishmania identified species.
3. Results
3.1. Main characteristics of the studies
The study selection process according to the PRISMA statements is shown in Figure 1. The preliminary search resulted in 2424 studies. Subsequently, duplicate papers and unrelated studies were removed according to the title and abstract. Finally, 212 full texts of eligible articles were assessed, from which 37 datasets were included in the quantitative analysis based on the inclusion and exclusion criteria. Also, 6 articles were found through searching the references of relevant studies included in the quantitative analysis (Figure 1).
Figure 1.
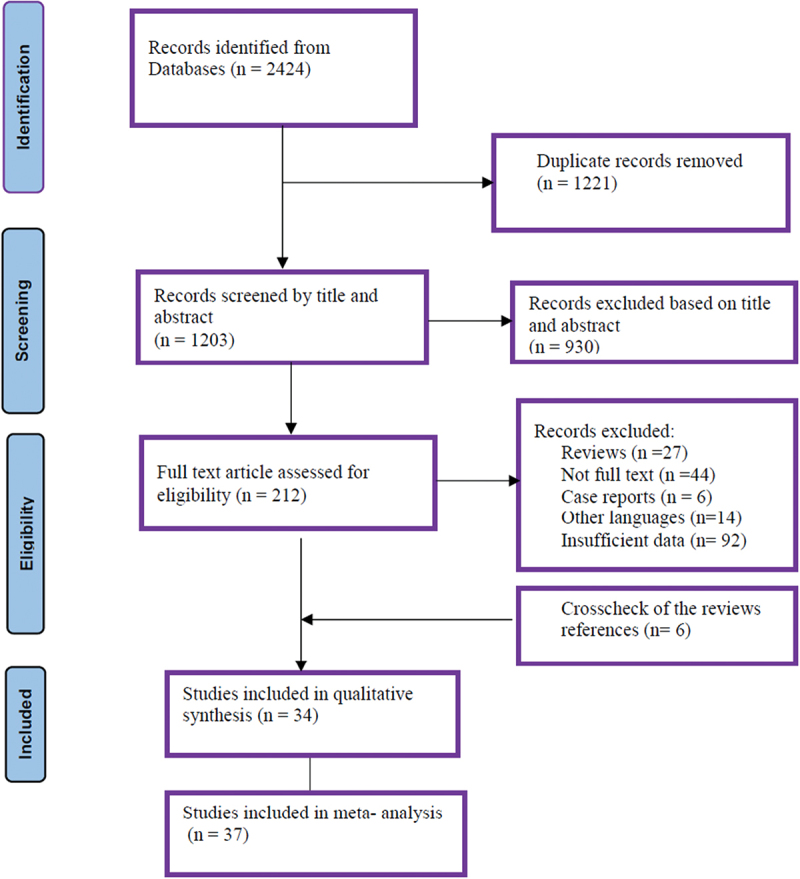
PRISMA flow chart of study identification and selection.
Out of 37 articles, 31, 4, and 2 studies evaluated CL infection by microscopy examination, leishmanin skin test (LST), and serological methods, respectively. Two studies used microscopy and LST methods [16,17]. Two other studies reported the CL prevalence based on the LST technique (Table 1) [23,34]. Totally, 37 datasets from 34 studies were included in the current meta-analysis. These studies illustrated data on 285,560 individuals from the 37 datasets across 9 countries of Middle Eastern regions as follow: 22 datasets for Iran (164595 individuals), 2 for Saudi Arabia (9253 individuals), 3 for Yemen (2855 individuals), 3 for Jordan (1897 individuals), 2 for Syria (1106 individuals), 1 for Lebanon (81486 individuals), 1 for Iraq (23778 individuals), 1 for Egypt (141 individuals), and 1 for Palestine (190 individuals) (Table 1).
Table 1.
Main characteristics of included studies reporting prevalence of cutaneous leishmaniasis in the Middle East.
| First author/year/Reference | Study period | Country | specimen | Location | Gender | Age | Methods | Sample Size | Case | Active lesion | Scar lesion | PCR+ | Species |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aflatoonian et al 2013 [18] | 2010 | Iran | Skin scrap | rural | both | 1–96 | Microscopy/PCR | 5544 | 67 | NA | NA | 15 | L. tropica |
| Akhavan et al 2007a [19] | 2003 | Iran | Skin scrap | rural | both | 1>->25 | microscopy | 2441 | 280 | 27 | 253 | NA | L. major |
| Akhavan et al 2007b [19] | 2003 | Iran | Skin scrap | rural | both | 6-12 | microscopy | 1662 | 263 | 19 | 244 | NA | L. major |
| Al-Jawabreh et al 2003 [20] | 1994–99 | Palestine | Serum | rural | NA | NA | Serology | 190 | 50 | NA | NA | NA | NA |
| Alkulaibi et al 2019a [16] | 2015 | Yemen | Skin scrap | rural | both | 16->45 | microscopy | 1165 | 215 | 189 | 26 | NA | NA |
| Alkulaibi et al 2019b [16] | 2015 | Yemen | NA | rural | both | 16->45 | LST | 1165 | 215 | NA | NA | NA | NA |
| AlSamarai et al 2009 [21] | 2004-05 | Iraq | Skin scrap | urban | both | 1-60 | microscopy | 23778 | 107 | NA | NA | NA | NA |
| Amin et al 2012 [22] | 2009 | Saudi Arabia | Skin scrap | both | both | 15- ≥50 | microscopy | 1824 | 1313 | NA | NA | NA | NA |
| Arbaji et al 1993 [23] | 1991 | Jordan | NA | rural | NA | 3->9 | LST | 399 | 350 | NA | NA | NA | NA |
| Asgari Nezhad et al 2012 [24] | 2009 | Iran | Skin scrap | urban | both | 5-19 | Microscopy/PCR | 7555 | 767 | 32 | 735 | NA | L. major |
| Ashford et al 1993a [17] | 1990-91 | Syria | NA | rural | NA | <5-10 | LST | 259 | 160 | NA | NA | NA | NA |
| Ashford et al 1993a [17] | 1990-91 | Syria | NA | rural | NA | <5-10 | Microscopy | 847 | 278 | NA | NA | NA | NA |
| Askari et al 2018 [25] | 2015-16 | Iran | Skin scrap | both | both | 1-83 | Microscopy/PCR | 46799 | 160 | 160 | 0 | 160 | L. major |
| Asmaa et al 2017 [26] | 2012-13 | Yemen | Skin scrap | Rural | both | 1-60 | Microscopy | 525 | 99 | NA | NA | NA | NA |
| Dye et al 1989 [27] | 1982-83 | Saudi Arabia | NA | Rural | NA | 0-60 | Microscopy | 7411 | 474 | 202 | 272 | NA | NA |
| Emami et al 2009 [28] | 2000-02 | Iran | Skin scrap | Rural | both | 0-≥25 | Microscopy/PCR | 3277 | 144 | 57 | 87 | 28 | L. major |
| Faris et al 1988 [29] | 1967-73 | Egypt | Skin scrap | Rural | both | <10- >21 | Microscopy | 141 | 6 | NA | NA | NA | NA |
| Fazaeli et al 2009 [30] | 2005–2006 | Iran | Skin scrap | urban | both | <10- >40 | Microscopy | 762 | 102 | NA | NA | NA | L. Major |
| Hamzavi rt al 2018 [31] | 2104-15 | Iran | Skin scrap | urban | both | 0- >80 | Microscopy | 5277 | 254 | 60 | 194 | NA | NA |
| Hanafi Majd et al 2006 [32] | 2005 | Iran | Skin scrap | Rural | both | NA | Microscopy/PCR | 1392 | 129 | 14 | 115 | 129 | L. major |
| Jafari et al 2020 [33] | 2015-16 | Iran | Skin scrap | Rural | both | 0-≥25 | Microscopy/PCR | 914 | 15 | 1 | 14 | 5 | L. major |
| Kamhawi et al 1995 [34] | 1994 | Jordan | NA | Rural | both | 5>->40 | LST | 626 | 146 | NA | 56 | NA | NA |
| Khosravi et al 2013 [35] | 2011-12 | Iran | Skin scrap | Rural | both | ≤10- >50 | Microscopy/PCR | 18308 | 869 | 30 | 839 | 29 | L. tropica/major |
| Kolivand et al 2015 [36] | 2013-14 | Iran | Skin scrap | both | both | 7-12 | Microscopy/PCR | 4800 | 31 | 15 | 16 | 15 | L. major |
| Mirzaei et al 2012 [37] | 2008 | Iran | Skin scrap | both | both | 10-40 | Microscopy/PCR | 3516 | 188 | 26 | 162 | L. tropica | |
| Nuwayri-Salti et al 2000 [38] | 1993-97 | Lebanon | Skin scrap | both | both | <20- >50 | Microscopy | 81486 | 224 | 124 | 100 | NA | NA |
| Obaidat and Roess, 2019 [39] | 2015–2016 | Jordan | Serum | both | both | <30->50 | Serology | 872 | 22 | NA | NA | NA | NA |
| Razavinasab et al 2019 [40] | 2015-16 | Iran | Skin scrap | Rural | both | <6- >40 | Microscopy/PCR | 11021 | 58 | NA | NA | 50 | L. tropica |
| Razmjou et al 2009 [41] | 2004-05 | Iran | Skin scrap | Rural | both | <10- ≥10 | Microscopy/PCR | 1000 | 232 | 70 | 162 | 27 | L. major |
| Sharifi et al 2010 [42] | 1994–06 | Iran | Skin scrap | urban | both | 6-18 | Microscopy/PCR | 22 838 | 523 | 523 | 0 | 9 | L. tropica |
| Sharifi et al 2011 [43] | NA | Iran | Skin scrap | urban | both | ≤10- >40 | Microscopy | 3884 | 204 | 69 | 135 | 26 | L. tropica |
| Talari et al 2006 [44] | 2001-03 | Iran | Skin scrap | urban | both | 6-15 | Microscopy | 1625 | 117 | 49 | 68 | NA | L. major |
| Yaghoobi-Ershadi et al 2001 [45] | 1997-98 | Iran | Skin scrap | both | both | 7-15 | Microscopy | 1960 | 48 | 18 | 30 | NA | L. major |
| Yaghoobi-Ershadi et al 2003 [46] | 2001–2002 | Iran | Skin scrap | Rural | both | 0-≥25 | Microscopy | 807 | 108 | 24 | 84 | NA | L. major |
| Yaghoobi-Ershadi et al 2004 [47] | 2001–2002 | Iran | Skin scrap | Rural | NA | 0->25 | Microscopy | 3024 | 1663 | 743 | 919 | NA | NA |
| Zahraei-Ramazani et al 2007 [48] | NA | Iran | Skin scrap | Rural | NA | 6- >11 | Microscopy/PCR | 16380 | 734 | 685 | 49 | NA | NA |
3.2. Overall prevalence of cutaneous leishmaniasis in the Middle East
The random effects pooled prevalence of CL in the Middle East was 12% (95% CI 9–15 %; 10718/285560) with a very high heterogeneity (Q = 25403.63, df = 35, I2 = 99.86%, P < 0.001). According to Middle Eastern regions, Syria (39%, 37–42%) has the highest prevalence of CL, and the lowest prevalence rate was found in Iraq and Lebanon (0%, 0–0%). The Pooled prevalence in other Middle Eastern countries was as follows: Jordan (34%,0–86%), Palestine (26%, 21–33%), Yemen (19%,19–20%), Saudi Arabia (16%15–16%), Iran (7%, 4–10%), and Egypt (4%, 2–9%) (Table 2) (Figure 2). Figure 3 shows a Geographic Information System (GIS) map summarizing the prevalence of cutaneous leishmaniasis in individual countries in Middle Eastern region.
Table 2.
Sub-group analysis of potential factors influencing the prevalence of Cutaneous Leishmaniasis in Middle Eastern region.
| Potential factors | Number of datasets | Prevalence (CI 95%) | X2 | I2 |
|---|---|---|---|---|
| Middle East countries | ||||
| Egypt | 1 | 4(2–9) | NA | NA |
| Iran | 24 | 7(4–10) | 15123.01 | 99.85 |
| Iraq | 1 | 0(0–1) | NA | NA |
| Jordon | 3 | 34(0–86) | NA | NA |
| Lebanon | 1 | 0(0–0) | NA | NA |
| Palestine | 1 | 26(21–33) | NA | NA |
| Saudi Arabia | 2 | 16(15–16) | NA | NA |
| Syria | 2 | 39(37–42) | NA | NA |
| Yemen | 3 | 19(17–20) | NA | NA |
| Location | ||||
| Urban | 7 | 10(5–11) | NA | NA |
| Rural | 20 | 14(10–19) | 5037.79 | 99.62 |
| Gender | ||||
| Female | 18 | 7(4–10) | 6024.63 | 99.70 |
| Male | 18 | 7(4–10) | 5811.95 | 99.69 |
| Age | ||||
| 0-15 | 20 | 9(6–13) | 3224.71 | 99.38 |
| 16-40 | 16 | 6(4–9) | 1930.03 | 99.17 |
| >40 | 9 | 4(3–5) | 965.58 | 99.07 |
| Type of lesion | ||||
| Active | 24 | 48(28–69) | 8166.88 | 99.74 |
| Scar | 22 | 62(42–81) | 6534.16 | 99.71 |
| No. Lesion | ||||
| Single | 12 | 57(49–66) | 360.61 | 96.7 |
| Two | 12 | 15(6–26) | 1034.39 | 98.84 |
| Three/more | 12 | 23(13–34) | 808.86 | 98.52 |
| Site of lesion | ||||
| Face | 18 | 39(19–50) | 1143.51 | 98.43 |
| Hand | 18 | 30(23–40) | 910.85 | 98.13 |
| Leg | 18 | 13(10–17) | 281.50 | 93.61 |
| Other parts | 14 | 12(6–21) | 903.72 | 98.45 |
| Sample size | ||||
| ≤200 | 5 | 9(4–17) | 75.44 | 94.70 |
| 201-1500 | 13 | 20(16–40) | 6628.59 | 99.79 |
| >1501 | 20 | 7(4–11) | 21516.44 | 99.92 |
| Study period | ||||
| < 2009 | 22 | 4(1–30) | 3324.01 | 99.09 |
| 2010–2015 | 12 | 6(2–8) | NA | NA |
| ≥2016 | 3 | 0(0–1) | NA | NA |
| Methods | ||||
| Microscopy | 32 | 12(8–16) | 31217.69 | 99.9 |
| serology | 3 | 8(7–9) | NA | NA |
| LST | 4 | 48(17–80) | 1391.64 | 99.63 |
| Leishmaniaspecies | ||||
| L.tropica | 6 | 3(1–7) | 2188.13 | 99.82 |
| L. major | 12 | 7(3–12) | 4247.43 | 99.74 |
| L. tropica/major | 4 | 10(2–12) | 2364.27 | 99.87 |
| Quality assessment | ||||
| Low | 5 | 16(10–29) | 4296.62 | 99.88 |
| Moderate | 32 | 15(10–20) | 27300.18 | 99.89 |
| High | 1 | 1(0–1) | NA | NA |
Figure 2.
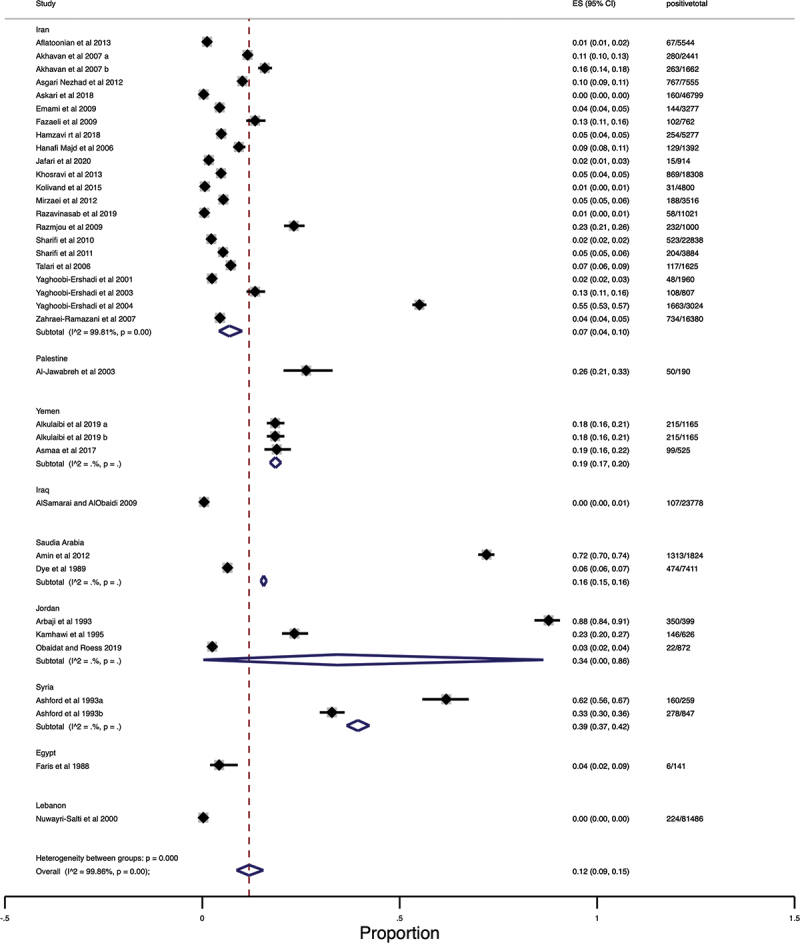
Forest Plot of the prevalence of cutaneous leishmaniasis in the Middle East.
Figure 3.
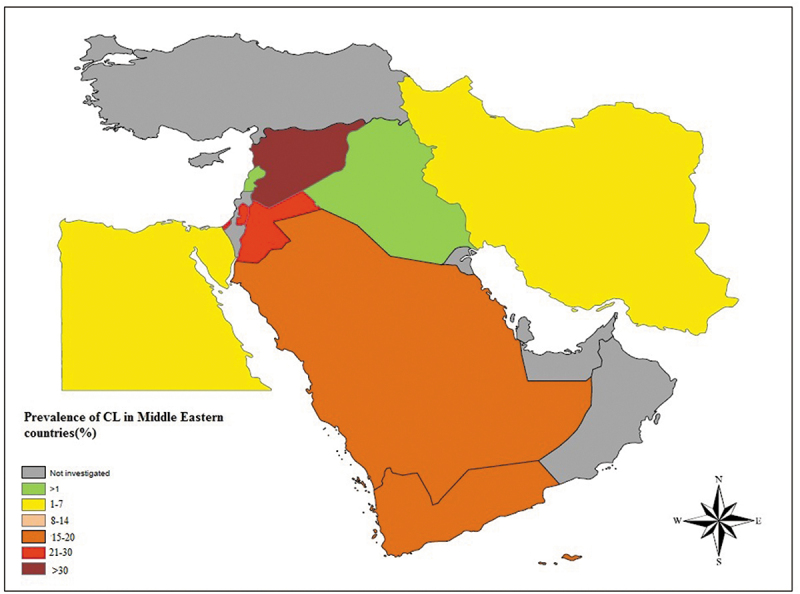
Prevalence of cutaneous leishmaniasis in Middle Eastern countries using geographic information system (GIS).
3.3. Prevalence of CL infection according to study characteristics
In subgroup analysis, we observed high heterogeneity in the prevalence rate among study characteristics. According to the diagnostic methods, the prevalence of CL in studies that applied LST assay had the highest rate (35, 10–68%). Regarding gender, the prevalence of infection in males was similar to that in females (7%, 4–10%). The pooled prevalence of infection in individuals living in rural areas was higher than in urban areas with no significant variations (14%, 10–19%) (P > 0.05). The prevalence of CL in the age group 0–15 years was higher than (9%, 6–13%) in individuals 16–40 and >40 years. The number of scar lesions was higher than active lesions (62%, 42–81%) with significant differences (P < 0.05). Most of the lesions were observed on the face, with a prevalence rate of 39% (19–50%). Single lesions were more prevalent than two or three lesions (57%, 49–66%). The details of pooled prevalence of CL infection obtained from subgroups analysis are shown in Table 2.
3.4. Sensitivity analysis
A sensitivity analysis was performed, in which a particular study was removed from the meta-analysis at each time to calculate the stability of the results. The obtained results did not show any considerable changes in the heterogeneity of the studies (P < 0.0001) (Figure S1).
3.5. Quality assessment
Quality assessment of the included studies for the prevalence of CL was estimated and summarized in Supplementary Table S1. Analysis of the risk of bias showed that the prevalence rates of CL in studies with low (16%, 10–29%) and moderate (15%,10–20%) risks of bias were considerable (Table 2).
4. Discussion
The results of this systematic review and meta-analysis indicated that the occurrence of CL was considerable in the Middle East at 12% (95% CI, 9–15 %). These studies illustrated data on 285,560 individuals across 9 Middle Eastern countries. Based on the findings, this prevalence rate may be underestimated due to misdiagnosis and insufficient reporting strategies in more than half of the endemic countries. It should be noted that some Middle Eastern countries, such as Syria, have faced war crisis in recent years. These war-torn countries face problems, such as migration and the influx of refugees to the borders of other countries. Turkey and Jordan were the most affected countries in this region [49].
According to the results of the current study, the pooled estimates for the prevalence of CL were (39%, 37–42 %), (34%, 0–86%) and (26%, 21–33%) in Syria, Jordan, and Palestine, respectively. In a study by Al-Salem et al. in 2016, it was found that only two regions of Aleppo and Damascus in Syria were endemic for CL until 1960, while with the outbreak of a war, a significant increase was reported in the number of CL cases [50]. As mentioned above, Turkey is one of the countries affected by the crisis of the Syrian war. Currently, 4.4 % of Turkey’s population is allocated to Syrian refugees. Some provinces bordering Syria, such as Sanlurfa and Gaziantep, have reported CL more often since 2011 [51]. The study, which was performed in Gaziantep in southeast Turkey revealed that 81.1% (900/1110) of suspected individuals were positive for CL, of which 93.8% (845/900) were Syrian refugees, and 6.2% (55/900) were Turkish citizens [52]. Besides, in a study conducted between 2009 and 2015 in the border towns of Turkey, the frequency of CL among suspected patients was estimated 46.4%; in this study and 66% of the patients were Syrian refugees [53]. Moreover, an investigation performed in Lebanon confirmed that all CL patients were Syrian refugees [49]. All studies in Turkey were accomplished on suspected CL individuals, and the target population did not precisely represent the general population since they were not eligible for analysis in the present study.
The results of the current study revealed that the prevalence rate of CL increased by the year 2015 (Table 2). As mentioned above, a possible explanation for this finding is inefficient surveillance and control programs in some countries due to instabilities in the Middle East. The prevalence of CL was higher in the age group of 0–15 years compared to the age group of >15- >40 years. The higher infection rate in younger people may be associated with a weak immune system for protecting against sand-fly bites. The higher infection rate in younger people may be due to the immune system’s inefficiency in inhibiting the parasite’s propagation [26,53,54].
Based on the findings, the prevalence rate of scar lesions was higher than active lesions (62%, 42–81%). The number of active and scar lesions in urban areas was more than in rural areas. Regarding the number of lesions, single lesions were more prevalent than two or three concurrent lesions in this study. The possible reason may be related to the variations in the nutritional behaviors of sand flies and blood feeding from multiple hosts [26,55]. Concerning the lesion site, the face was the most common site of involvement (39%, 19–50%), followed by the hands and legs. It is well documented that CL lesions usually develop on uncovered body parts and are more exposed to sand-fly bites. The present results were in agreement with some previous studies [8,53,55]. One of the consequences of facial lesions is the permanent scar with lifelong stigma for individuals [8,56]. In a recent investigation by Bennis et al. in 2018, CL lesions were a source of psychological problems, stigmatization, and decreased QOL in patients, especially women and young girls [56]. Several studies evaluating QOL in patients with CL scars concluded that CL had considerable effects on QOL and different aspects of life, such as social activities, education, and job opportunities [57,58]. The pooled prevalence of L. major and mixed infection with L. tropica/major were high in the Middle East, which is in agreement with a previous study [59].
4.1. Limitations
There are some limitations to the present study. First, the epidemiological data were not available for several Middle Eastern countries. Second, the full-text of some potentially eligible papers was not available in this systematic review. Third, some potentially relevant studies were not in English, and consequently, they were excluded from the analysis. Fourth, many studies had only assessed the frequency of CL in suspected patients and were not eligible for inclusion. Therefore, selection bias can occur due to the exclusion of some relevant data.
5. Conclusion
According to this systematic review, the pooled prevalence of CL is considerable in the Middle East. Since different factors, including environmental and weather changes, war and migration, and changes in vector populations, influence the burden of CL in this region, awareness of the disease prevalence can help us implement more appropriate control and preventive programs.
Supplementary Material
Funding Statement
The work was supported by the Babol University of Medical Sciences [140012913].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20477724.2022.2133452.
References
- [1].Galgamuwa LS, Dharmaratne SD, Iddawela D.. Leishmaniasis in Sri Lanka: spatial distribution and seasonal variations from 2009 to 2016. Parasites Vectors. 2018;11:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Reithinger R, Dujardin J-C, Louzir H, et al. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–596. [DOI] [PubMed] [Google Scholar]
- [3].Killick-Kendrick R. The biology and control of Phlebotomine sand flies. Clin Dermatol. 1999;17:279–289. [DOI] [PubMed] [Google Scholar]
- [4].World Health Organization . Leishmaniasis in high-burden countries: an epidemiological update based on data reported in 2014. Weekly Epidemiol Res. 2016;91:287–296. [PubMed] [Google Scholar]
- [5].Ready PD. Leishmaniasis emergence in Europe. Eurosurveillance. 2010;15(10):19505. [PubMed] [Google Scholar]
- [6].World Health Organization . Leishmaniasis. [cited 2021 Des 1]. Available from: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis
- [7].Rafati S, Modabber F. Cutaneous Leishmaniasis in Middle East and North Africa. In Neglected Tropical Diseases - Middle East and North Africa. Neglected Tropical Diseases. 2014;117–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bilgic-Temel A, Murrell DF, Uzun S. Cutaneous leishmaniasis: a neglected disfiguring disease for women. Int J Women Dermatol. 2019;5:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bailey F, Mondragon-Shem K, Hotez P, et al. A new perspective on cutaneous leishmaniasis—implications for global prevalence and burden of disease estimates. PLoS Negl Trop Dis. 2017;11(8):e0005739-e0005739. DOI: 10.1371/journal.pntd.0005739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Du R, Hotez PJ, Al-Salem WS, et al. Old World Cutaneous Leishmaniasis and Refugee Crises in the Middle East and North Africa. PLoS Negl Trop Dis. 2016;10:e0004545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bailey F, Mondragon-Shem K, Haines LR, et al. Cutaneous leishmaniasis and co-morbid major depressive disorder: a systematic review with burden estimates. PLoS Negl Trop Dis. 2019;13:e0007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kassi M, Kassi M, Afghan AK, et al. Marring leishmaniasis: the stigmatization and the impact of cutaneous leishmaniasis in Pakistan and Afghanistan. PLoS Negl Trop Dis. 2008;2:e259-e259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Feiz-Haddad M-H, Kassiri H, Kasiri N, et al. Prevalence and epidemiologic profile of acute cutaneous leishmaniasis in an endemic focus, Southwestern Iran. J Acute Dis. 2015;4(4):292–297. DOI: 10.1016/j.joad.2015.06.007 [DOI] [Google Scholar]
- [14].Munn Z, Moola S, Riitano D, et al. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. [cited 2021 Des 1]. Available from : https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- [16].Alkulaibi MM, Suleiman AM, Khalil EAG, et al. Prevalence of Cutaneous Leishmaniasis in Western Highlands in Yemen. J Trop Med. 2019;2019:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ashford RW, Rioux J-A, Jalouk L, et al. Evidence for a long-term increase in the incidence of Leishmania tropica in Aleppo, Syria. Trans R Soc Trop Med Hyg. 1993;87(3):247–249. DOI: 10.1016/0035-9203(93)90111-3 [DOI] [PubMed] [Google Scholar]
- [18].Aflatoonian MR, Sharifi I, Poursmaelian S, et al. The Emergence of Anthroponotic Cutaneous Leishmaniasis Following the Earthquake in Southern Villages of Bam District, Southeastern Iran, 2010. J Arthropod Borne Dis. 2013;7:8–14. [PMC free article] [PubMed] [Google Scholar]
- [19].Akhavan AA, Yaghoobi-Ershadi MR, Hasibi F, et al. Emergence of Cutaneous Leishmaniasis due to Leishmania major in a New Focus of Southern Iran. Iran J Arthropod Borne Dis. 2007;1:1–8. [Google Scholar]
- [20].Al-Jawabreh A, Barghuthy F, Schnur LF, et al. Epidemiology of cutaneous leishmaniasis in the endemic area of Jericho, Palestine. East Mediterr Health J. 2003;9:805–815. [PubMed] [Google Scholar]
- [21].AlSamarai A, AlObaidi H. Cutaneous leishmaniasis in Iraq. J Infect Developing Countries. 2009;3:123–129. [DOI] [PubMed] [Google Scholar]
- [22].Amin TT, Kaliyadan F, Al-Ajyan MI, et al. Public awareness and attitudes towards cutaneous leishmaniasis in an endemic region in Saudi Arabia. J Eur Acad Dermatol Venereol. 2011;26:1544–1551. [DOI] [PubMed] [Google Scholar]
- [23].Arbaji AK, Gradoni L, Gramiccia M. Leishmanin skin test survey in a focus of high endemicity of Leishmania major in Jordan. Acta Trop. 1993;54:77–79. [DOI] [PubMed] [Google Scholar]
- [24].Asgari Nezhad H, Mirzaie M, Sharifi I, et al. The prevalence of cutaneous leishmaniasis in school children in southwestern Iran, 2009. Comp Clini Pathol. 2012;21:1065–1069. [Google Scholar]
- [25].Askari A, Sharifi I, Aflatoonian MR, et al. A newly emerged focus of zoonotic cutaneous leishmaniasis in South-western Iran. Microbial Pathogen. 2018;121:363–368. [DOI] [PubMed] [Google Scholar]
- [26].Asmaa Q, Al-Shamerii S, Al-Tag M, et al. Parasitological and biochemical studies on cutaneous leishmaniasis in Shara’b District, Taiz, Yemen. Ann Clin Microbiol Antimicrob. 2017;16(1):16. DOI: 10.1186/s12941-017-0224-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dye C, Killick-Kendrick R, Ben Ismail R, et al. Zoonotic cutaneous leishmaniasis in Saudi Arabia: results of a preliminary epidemiological survey in Al-Ahsa oasis. Trans R Soc Trop Med Hyg. 1989;83:493–498. [DOI] [PubMed] [Google Scholar]
- [28].Emami MM, Yazdi M, Nitforoushzadeh M. Emergence of cutaneous leishmaniasis due to Leishmania major in a new focus of central Iran. Trans R Soc Trop Med Hyg. 2009;103:1257–1262. [DOI] [PubMed] [Google Scholar]
- [29].Faris R, Feinsod FM, Morsy TA, et al. Human cutaneous leishmaniasis in two communities in eastern Sinai, Egypt. Eur J Epidemiol. 1988;4:45–48. [DOI] [PubMed] [Google Scholar]
- [30].Fazaeli A, Fouladi B, Sharifi I. Emergence of cutaneous leishmaniasis in a border area at south-east of Iran: an epidemiological survey. J Vector Borne Disease. 2009;46:36–42. [PubMed] [Google Scholar]
- [31].Hamzavi Y, Nazari N, Khademi N, et al. Cutaneous leishmaniasis in Qasr-e Shirin, aborder area in the west of Iran. Vet World. 2018;11:1692–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hanafi Majd AA, Yaghoubi Ershadi MR, Zamani GH, et al. Epidemiologic aspects of cutaneous leishmaniasis in Hajiabad, Hormozgan, Iran (2003). Hormozgan Med J. 2006;10:63–70. [Google Scholar]
- [33].Jafari R, Abdoli H, Arandian MH, et al. Emerging of Cutaneous Leishmaniasis due to Leishmania major in a New Focus in Esfahan Province, Central Iran. J Arthropod Borne Dis. 2020;14:134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kamhawi S, Abdel-Hafez SK, Arbagi A. A new focus of cutaneous leishmaniasis caused by Leishmania tropica in northern Jordan. Trans R Soc Trop Med Hyg. 1995;89:255–257. [DOI] [PubMed] [Google Scholar]
- [35].Khosravi A, Sharifi I, Dortaj E, et al. The present status of cutaneous leishmaniasis in a recently emerged focus in South-west of Kerman province, iran. Iran J Public Health. 2013;42:182–187. [PMC free article] [PubMed] [Google Scholar]
- [36].Kolivand M, Fallah M, Salehzadeh A, et al. An Epidemiological Study of Cutaneous Leishmaniasis Using Active Case Finding among Elementary School Students in Pakdasht, Southeast of Tehran, Iran 2013-2014. J Res Health Sci. 2015;15:104–108. [PubMed] [Google Scholar]
- [37].Mirzaei M, Sharifi I, Poursmaelian S. A new focus of anthroponotic cutaneous leishmaniasis and identification of parasite species by nested PCR in Jiroft, Iran. Comp Clini Pathol. 2012;21:1071–1075. [Google Scholar]
- [38].Nuwayri-Salti N, Baydoun E, El-Tawk R, et al. The epidemiology of leishmaniases in Lebanon. Trans R Soc Trop Med Hyg. 2000;94:164–166. [DOI] [PubMed] [Google Scholar]
- [39].Obaidat M, Roess A. Nationwide seroprevalence, spatial distribution and risk factors of Leishmania in Jordan. Asian Pac J Tropical Biomedicine. 2019;9:227–231. [Google Scholar]
- [40].Razavinasab SZ, Sharifi I, Aflatoonian MR, et al. Expansion of urban cutaneous leishmaniasis into rural areas of southeastern Iran: clinical, epidemiological and phylogenetic profiles explored using 7SL high resolution melting-PCR analysis. Transboundary Emerging Disease. 2019;66:1602–1610. [DOI] [PubMed] [Google Scholar]
- [41].Razmjou S, Hejazy H, Motazedian MH, et al. A new focus of zoonotic cutaneous leishmaniasis in Shiraz, Iran. Trans R Soc Trop Med Hyg. 2009;103:727–730. [DOI] [PubMed] [Google Scholar]
- [42].Sharifi I, Fekri AR, Aflatoonian MR, et al. Leishmaniasis recidivans among school children in Bam, South-east Iran, 1994-2006. Int J Dermatol. 2010;49:557–561. [DOI] [PubMed] [Google Scholar]
- [43].Sharifi I, Poursmaelian S, Aflatoonian MR, et al. Emergence of a new focus of anthroponotic cutaneous leishmaniasis due to Leishmania tropica in rural communities of Bam district after the earthquake, Iran. Trop Med Int Health. 2011;16:510–513. [DOI] [PubMed] [Google Scholar]
- [44].Talari SA, Talaei R, Shajari G, et al. Childhood cutaneous leishmaniasis: report of 117 cases from Iran. Korean J Parasitol. 2006;44:355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yaghoobi-Ershadi MR, Hanafi-Bojd AA, Akhavan AA, et al. Epidemiological study in a new focus of cutaneous leishmaniosis due to Leishmania major in Ardestan town, central Iran. Acta Trop. 2001;79:115–121. [DOI] [PubMed] [Google Scholar]
- [46].Yaghoobi-Ershadi MR, Akhavan AA, Zahraei-Ramazani AV, et al. Epidemiological study in a new focus of cutaneous leishmaniasis in the Islamic Republic of Iran. East Mediterr Health J. 2003;9(4):816–826. DOI: 10.26719/2003.9.4.816 [DOI] [PubMed] [Google Scholar]
- [47].Yaghoobi-Ershadi MR, Jafari R, Hanafi-Bojd AA. A new epidemic focus of zoonotic cutaneous leishmaniasis in central Iran. Ann Saudi Med. 2004;24:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zahraei-Ramazani AR, Yaghoobi-Ershadi MR, Mokhtari AR, et al. Anthroponotic Cutaneous Leishmaniasis in nonendemic quarters of a central city in Iran. Iran J Public Health. 2007;36:7–11. [Google Scholar]
- [49].Saroufim M, Charafeddine K, Issa G, et al. Ongoing epidemic of cutaneous leishmaniasis among Syrian refugees, Lebanon. Emerg Infect Dis. 2014;20:1712–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Al-Salem WS, Pigott DM, Subramaniam K, et al. Cutaneous Leishmaniasis and Conflict in Syria. Emerg Infect Dis. 2016;22(5):931–933. DOI: 10.3201/eid2205.160042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ergönül Ö, Tülek N, Kayı I, et al. Profiling infectious diseases in Turkey after the influx of 3.5 million Syrian refugees. Clin Microbiol Infect. 2020;26:307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Eroglu F, Ozgoztasi O. The increase in neglected cutaneous leishmaniasis in Gaziantep province of Turkey after mass human migration. Acta Trop. 2019;192:138–143. [DOI] [PubMed] [Google Scholar]
- [53].Özkeklikçi A, Karakuş M, Özbel Y, et al. The new situation of cutaneous leishmaniasis after Syrian civil war in Gaziantep city, Southeastern region of Turkey. Acta Trop. 2017;166:35–38. [DOI] [PubMed] [Google Scholar]
- [54].Volpedo G, Pacheco-Fernandez T, Holcomb EA, et al. Mechanisms of immunopathogenesis in cutaneous Leishmaniasis and post Kala-azar dermal Leishmaniasis (PKDL). Front Cell Infect Microbiol. 2021;11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sirekbasan S, Polat E. A Retrospective Study of Cutaneous and Visceral Leishmaniasis in Istanbul, Turkey. J Infect Developing Countries. 2021;15(04):595–598. [DOI] [PubMed] [Google Scholar]
- [56].Bennis I, De Brouwere V, Belrhiti Z, et al. Psychosocial burden of localised cutaneous Leishmaniasis: a scoping review. BMC Public Health. 2018;18:358-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vares B, Mohseni M, Heshmatkhah A, et al. Quality of life in patients with cutaneous leishmaniasis. Archive Iran Med. 2013;16:474–477. [PubMed] [Google Scholar]
- [58].Reithinger R, Aadil K, Kolaczinski J, et al. Social impact of leishmaniasis, Afghanistan. Emerg Infect Dis. 2005;11:634–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tabbabi A. Review of Leishmaniasis in the Middle East and North Africa. African Health Sci. 2019;19:1329–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


