Abstract
The humoral immune response to Borrelia burgdorferi during persistent infection is critical to both protective and disease-resolving immunity. This study examined the role of B cells in the absence of T cells during these events, using mice with selected immune dysfunctions. At 6 weeks postinfection, an interval at which arthritis resolves in immunocompetent mice, arthritis severity was equivalent among immunocompetent mice, αβ+-T-cell-deficient mice, and mice lacking both αβ+ and γδ+ T cells. Arthritis severity was worse in SCID mice, which lack T and B lymphocytes. Carditis regressed in immunocompetent mice and those lacking both αβ+ and γδ+ T cells but remained active in mice lacking only αβ+ T cells and in SCID mice. Mice lacking only αβ+ T cells and those lacking both αβ+ and γδ+ T cells generated immunoglobulin M (IgM) and IgG3 B. burgdorferi-reactive antibodies. Sera from infected immunocompetent mice, mice lacking only αβ+ T cells, and mice lacking both αβ+ and γδ+ T cells passively protected naive mice against challenge inoculation with B. burgdorferi. However, only sera from infected immunocompetent mice, but not sera from infected T-cell-deficient mice, were able to resolve arthritis when passively transferred to actively infected SCID mice. These data demonstrate that B-cell activation during a T-cell-independent response may be critical for resolution of arthritis and carditis and that protective antibodies are generated during this response.
Lyme disease, due to infection with Borrelia burgdorferi, is manifested by a variety of symptoms, but Lyme disease patients frequently develop arthritis and carditis, which inexplicably undergo spontaneous resolution with bouts of recurrence over the course of years (37). Despite persistent infection, Lyme disease patients appear to mount strong immune responses against B. burgdorferi (32), and passive transfer of human patient serum will protect naive laboratory mice against challenge inoculation (17).
Although not all features parallel human Lyme disease, the mouse model for Lyme disease offers incisive insight into host-pathogen interactions, particularly the nuances of host immunity. Immunocompetent mice develop a consistent pattern of events following infection with B. burgdorferi. Within several days, local invasion and spirochetemia result in the evolution of arthritis and carditis, which peak within 2 to 3 weeks and then undergo immune-mediated resolution with bouts of recurrence over the course of 1 or more years of persistent infection (1, 7, 10). Infection of severe combined immune deficient (SCID) mice results in persistent active carditis and progressively destructive arthritis, underscoring the importance of acquired immunity in modifying these events (11). Passive transfer of small amounts of serum from infected immunocompetent mice (immune serum) protects naive mice against high-dose syringe challenge (5, 9), and passive transfer of immune serum to actively infected SCID mice with progressing arthritis induces arthritis resolution but not carditis resolution (6, 9). These observations prove the importance of humoral immune responses, but not T-cell-mediated responses, in both protective and arthritis-resolving immunity in the mouse model of Lyme disease. They also suggest that the pathogenesis and immunoregulatory events important for resolution of arthritis and carditis differ.
These previous findings are based on experiments examining the role of antibodies generated against both T-cell-dependent (TD) and T-cell-independent (TI) antigens in regulating infection and resolving disease. Recent experiments suggest that TI immune responses against B. burgdorferi may be critical for protective immunity and disease resolution. CD40 ligand knockout (KO) mice infected with B. burgdorferi develop protective antibody responses and resolve arthritis (16). Normally, this ligand is expressed on activated CD4+ T cells and interacts with CD40 on B cells and monocytes/macrophages (30). CD40 ligand stimulation of B cells results in their activation and appears to be a necessary signal for TD humoral responses.
Additional data indicating the involvement of TI responses against B. burgdorferi were obtained using B. burgdorferi-infected major histocompatibility complex (MHC) class II-deficient mice (15). Although resolution of carditis is delayed, protective immunity is evoked and arthritis resolution occurs in MHC class II-deficient mice, implying that the arthritis-resolving immune response is distinct from the response required for carditis resolution. These findings, and parallel results obtained with B. burgdorferi-infected mice treated with a monoclonal antibody that inhibits the B7-CD28 costimulatory pathway (31), also suggest either that T cells are not necessary for protective and arthritis-resolving antibody responses to B. burgdorferi or that T cells may regulate the immune response to some TI antigens via nonclassical pathways. Apparently, under some circumstances TI antigens have been shown to directly stimulate T cells (13, 33, 40, 42).
The above-mentioned experiments suggest, but do not definitively demonstrate, that infection of mice with B. burgdorferi elicits TI immune responses that are critical for protective immunity and arthritis resolution. Additionally, the influence of αβ+ T cells and γδ+ T cells on these TI responses have not been evaluated. Therefore, the present studies were performed to definitively examine TI immune responses as they relate to protective and disease-resolving immunity in the mouse model for Lyme disease. In these studies, we demonstrate that TI immune responses are critical for protective, arthritis-resolving, and carditis-resolving immunity, but they appear to be independent phenomena.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free adult C3H/HeSnSmn (C3H), C3H/HeSnSmn-scid (C3H-scid), C57BL/6J (B6), B6-Tcrβtm1Mom129 (B6-Tcrβ KO), B6-Tcrβtm1Mom129Tcrδtm1Mom129 (B6-TcrβTcrδ KO), and B6-Prkdcscid/SzJ (B6-scid) mice, 3 to 5 weeks of age, were purchased from The Jackson Laboratory (Bar Harbor, Maine). Pregnant Swiss outbred Crl:CD-1(ICR) (CD-1) mice were obtained from Harlan Sprague-Dawley Inc. (Indianapolis, Ind.).
B. burgdorferi cultivation and inoculations.
A low-passage clonal strain of B. burgdorferi (cN40), with previously verified infectivity and pathogenicity, was used for all experiments (7). For each experiment, a frozen aliquot of B. burgdorferi was thawed and expanded at 33°C in modified Barbour-Stoenner-Kelley (BSK II) medium (3). Spirochetes were grown to mid-log phase, assessed for viability, and then counted by dark-field microscopy using a Petroff-Hauser bacterial counting chamber. Spirochetes (104) in 0.1 ml of BSK II medium were injected intradermally above the shoulders. To confirm infection, sera from mice were tested by enzyme-linked immunosorbent assay (ELISA) for antibodies reactive with B. burgdorferi lysates and recombinant N40-decorin binding protein A (DbpA) (14), and tissues (urinary bladder, spleen, blood, and inoculation sites) were cultured in BSK II medium. After 2 weeks, the presence of spirochetes was assessed by dark-field microscopy.
Flow cytometry.
Splenocytes and lymph node cells from uninfected and infected mice were analyzed by flow cytometry to confirm the phenotype of cells populating mutant mice. Inguinal, superficial and deep cervical, axillary, brachial, mesenteric, and periaortic lymph nodes as well as the spleen were removed, and single-cell suspension were prepared. Cells (106) were incubated with anti-mouse CD32/CD16 (PharMingen, San Diego, Calif.) to block Fcγ II/III-mediated nonspecific antibody binding. Three minutes later, 30 μl of staining buffer (phosphate-buffered saline supplemented with 5% fetal calf serum and 0.2% sodium azide) or fluorochrome-conjugated antibodies specific for mouse surface molecules were added to the cell suspensions. Commercial fluorochrome-conjugated monoclonal antibodies (PharMingen) to the following surface molecules were used to determine the phenotype of B and T cells: (i) CD19, expressed throughout B-cell development but not on plasma cells; (ii) CD22, expressed on B cells throughout development, including plasma cells; (iii) CD5, expressed on a subset of B cells; (iv) CD4, expressed on thymocytes, a subset of mature T lymphocytes, and macrophages; (v) CD8, expressed on most thymocytes, a subpopulation of mature T lymphocytes, intestinal intraepithelial lymphocytes, and lymphokine-activated T cells; (vi) CD3, expressed on thymocytes and T cells; and (vii) antibodies to rat MHC antigens as an isotype control for immunoglobulin G1 (IgG1) and IgG2b antibodies. After incubation of cells for 30 min at 4°C, the cells were washed thoroughly, fixed, and resuspended for analysis on a Becton-Dickenson analyzer. Each histogram represented analysis of 104 cells.
Isotypic analysis of B. burgdorferi-specific antibodies.
Antibody capture ELISAs were used to determine IgM and IgG concentrations as well as the subclass of B. burgdorferi lysate- and DbpA-reactive antibodies in the sera of infected mice. Immunoglobulin titers were determined by comparing serial dilutions of immune sera generated in immunocompetent mice to sera collected from immunodeficient mice. Briefly, ELISA plates (Nunc ImmunoMaxi-Sorp plates) were coated with 1 μg of B. burgdorferi lysate or DpbA per ml in carbonate buffer and incubated overnight at 4°C. After washing plates and blocking nonspecific binding with 1% bovine serum albumin, serial dilutions of sera were titrated in the plates, which were incubated overnight at 4°C. The plates were washed again and then incubated with class- and subclass-specific alkaline phosphatase-conjugated rat or rabbit antisera specific for mouse IgM, IgG, IgG1, IgG2a, IgG2b, or IgG3 (Zymed Lab Inc., South San Francisco, Calif.). After a final wash, wells were incubated with 1 mg of alkaline phosphatase substrate (Sigma, St. Louis, Mo.) per ml for color development. Absorbance was read with an ELISA reader (Molecular Devices, Sunnyvale, Calif.) at a test wavelength of 405 nm and a reference wavelength of 650 nm. Titration curves were generated using Molecular Devices software. The mean absorbance of duplicate experimental samples, as well as the mean absorbance and standard deviation for a minimum of six wells containing uninfected normal mouse serum, was calculated. B. burgdorferi-specific antibodies were considered to be present when absorbance exceeded three standard deviations of the mean titer of control (uninfected) mouse serum.
Histology.
Rear limbs and hearts were fixed in neutral-buffered formalin (pH 7.2), bones were demineralized, and then tissue sections were stained with hematoxylin-eosin by standard histotechniques. The prevalence of arthritis among the four joints (knees and tibiotarsi) examined in each mouse was recorded. Values for arthritis severity are the mean scores from the most inflamed tibiotarsal joint of individual mice in each group ± the standard deviation, assessed on a scale of 0 (negative) to 3 (severe), as described previously (6). Sagittal sections through the heart, including the aortic valve, were examined for active or inactive inflammation and scored as active or inactive, as described previously (1). In brief, carditis was scored as active when there was evidence of transmural aortitis above the aortic valve, with inflammation of the connective tissue at the base of the heart. Carditis was scored as inactive when only lymphocytic, plasmacytic, or histiocytic infiltrates were present in the adventitia of vessels at the base of the heart.
Passive immunization for assessing protective activity in immune sera.
To compare the protective capacities of immune sera generated against B. burgdorferi by B cells in the presence and absence of T cells, sera were collected from B6 mice, B6-nude mice, B6-Tcrβ KO mice, and B6-TcrβTcrδ KO mice at 6 weeks postinfection (p.i.). Both the status of infection and antibody titer to B. burgdorferi lysate antigens were determined prior to pooling sera. Outbred 1-week-old CD-1 mouse pups (five pups per group) were immunized by intraperitoneal injection with 0.5 ml of a 1:10 dilution of 90-day immune serum from C3H mice (positive control), undiluted normal B6 mouse serum (negative control), or undiluted immune sera from the above-mentioned groups 18 h before challenge with B. burgdorferi. Previous studies have shown that this dose of 90-day immune serum is sufficient to protect infant outbred CD-1 pups challenged with as many as 107 spirochetes, and as little as 1 μl of immune serum provides protection against a challenge with 104 spirochetes (5). Three weeks after challenge, infection was assayed by culture and ELISA.
Passive immunization for assessing arthritis-resolving activity in immune sera.
The same pools of immune sera used in the protection assay were used to evaluate their arthritis-resolving capacity. Spirochetes (104) were injected into groups of five C3H-scid mice, and then 100 μl of 90-day immune sera from C3H mice (positive control), normal mouse sera (negative control), or immune sera from immunocompetent or T-cell-deficient mice infected for 6 weeks was injected intraperitoneally at 12, 16, 20, and 24 days p.i., as described previously (6). Mice were killed at 28 days p.i. and evaluated for infection by culture and ELISA. Tissues were microscopically examined for arthritis prevalence, arthritis severity, and carditis.
Statistical analysis.
Arthritis prevalence and severity are expressed as mean ± standard deviation. A Wilcoxon rank-sum test (two-tail probability) was used to evaluate differences in arthritis severity between control and experimental groups of mice.
Western blotting.
Nitrocellulose membrane strips containing fractionated proteins of B. burgdorferi were obtained from MRL Diagnostics (Cypress, Calif.). Blots were blocked with a 4% milk–phosphate-buffered saline solution containing 0.05% Tween 20, and then reactivity with immune sera (obtained from mice infected for 8 weeks) was tested. Immune serum from infected B6 mice was diluted 1:400, whereas immune serum from T-cell-deficient mice and serum from uninfected mice (negative control) were diluted 1:30. Nitrocellulose strips were incubated overnight at 4°C with the appropriate dilution of immune sera, washed thoroughly, and probed with an affinity-purified goat anti-mouse IgG (heavy plus light chains) conjugated to alkaline phosphatase (Sigma). Bands were visualized with a substrate solution containing 5-bromo-4-chloro-3-indolylphosphate and Nitro Blue Tetrazolium.
RESULTS
Outcome of infection in T-cell-deficient mice.
We had previously observed that infected athymic nude mice mounted an antibody response against B. burgdorferi, their immune sera protected naive mice from challenge, and their arthritis underwent resolution (unpublished data). These observations suggest that B. burgdorferi may elicit a TI humoral immune response that is important for protective immunity and arthritis resolution. However, a definitive conclusion could not be drawn because nude mice contain relatively small numbers of functional CD4+ and CD8+ T cells, which increase in number with age (22). Even though this small population of T cells may be inadequate to help TD responses, they may be adequate to mediate and help what appears to be a TI response. Definitive experiments examining the ability of B. burgdorferi to elicit a TI response can now be conducted using genetically manipulated KO mice. Mice rendered T cell deficient by disruption of the Tcrβ gene or by a double mutation involving the Tcrβ and Tcrδ chains allow assessment of the role of B and/or T cells in B. burgdorferi pathogenesis, as well as a means to evaluate the protective capacity and/or arthritis modulating capability of antibodies generated against B. burgdorferi in the absence of T cell help.
We therefore compared the course of B. burgdorferi infection in B6 immunocompetent mice with that in B6 mice bearing B cells but lacking specific subpopulations of functional T lymphocytes, including B6-Tcrβ KO mice (which lack functional αβ+ T cells) and B6-TcrβTcrδ KO mice (which lack both αβ+ and γδ+ T cells). Each experiment included B6-scid mice, which are devoid of T cells and B cells. Infection of B6-scid mice served as a control, to confirm previous studies demonstrating that acquired immunity is necessary for disease resolution (11). Thus, the experimental design utilized B6 mice, having a Lyme disease-resistant genotype (1, 4, 41), and congenic gene-disrupted experimental groups. B. burgdorferi was injected into groups of five adult B6, B6-Tcrβ KO, B6-TcrβTcrδ KO, and B6-scid mice, and then infectious status and disease severity were determined at 2 and 6 weeks.
As expected for this disease-resistant genotype, infected B6 mice developed a low prevalence of mild arthritis (mean arthritis severity of 0.2 ± 0.5), but all had active carditis at 2 weeks p.i. (Table 1). Similarly, arthritis was absent in B6-Tcrβ KO mice and B6-TcrβTcrδ KO mice, whereas B6-scid mice developed a high prevalence of severe arthritis (mean arthritis severity of 2.2 ± 0.3) (Table 1). All mice in all groups developed mild carditis. At 6 weeks p.i., arthritis severity was mild or absent in B6, B6-Tcrβ KO, and B6-TcrβTcrδ KO mice, while arthritis severity was worse in B6-scid mice (Table 1). Carditis was inactive in B6 mice and B6-TcrβTcrδ KO mice but remained active in B6-Tcrβ KO and B6-scid mice (Table 1). All mice in all groups failed to clear infection. Thus, B6-Tcrβ KO and B6-TcrβTcrδ KO mice infected with B. burgdorferi developed mild arthritis, comparable to that observed in control immunocompetent B6 mice, but B6-scid mice developed progressively severe arthritis.
TABLE 1.
Comparison of arthritis and carditis among B. burgdorferi-infected immunocompetent and immunodeficient micea
| wk p.i. | Mouse strain | Arthritis (mean ± SD)
|
Carditis (no./total)
|
Significanceb | ||
|---|---|---|---|---|---|---|
| Severity | Prevalence | Active | Inactive | |||
| 2 | B6 | 0.2 ± 0.45 | 0.2 ± 0.5 | 5/5 | ||
| B6-SCID | 3.5 ± 0.6 | 2.2 ± 0.3 | 5/5 | P ≤ 0.015 | ||
| B6-Tcrβ KO | 0 ± 0 | 0 ± 0 | 5/5 | NSc | ||
| B6-TcrβTcrδ KO | 0 ± 0 | 0 ± 0 | 5/5 | NS | ||
| 6 | B6 | 0 ± 0 | 0 ± 0 | 5/5 | ||
| B6-SCID | 3.0 ± 0.8 | 3.0 ± 0 | 5/5 | P ≤ 0.015 | ||
| B6-Tcrβ KO | 0.2 ± 0.5 | 0.2 ± 0.5 | 5/5 | NS | ||
| B6-TcrβTcrδ KO | 0.6 ± 0.6 | 0.3 ± 0.3 | 1/5 | 4/5 | NS | |
Groups of five mice were infected with B. burgdorferi and necropsied 2 or 6 weeks later. Based on culture and serology all mice were infected. Severity of tibiotarsal joint arthritis was graded on a scale of 0 (negative) to 3 (severe), as described in Materials and Methods. Inflammation of knee joints was scored as positive or negative, whereas carditis was scored as active or inactive. Data are representative of those from three experiments.
The significance of arthritis severity between control B6 mice and experimental groups was determined by the Wilcoxon rank-sum test (two-tail probability).
NS, not significant.
Notably, both B6-Tcrβ KO and B6-TcrβTcrδ KO mice developed carditis at 2 weeks; however, carditis was resolving in B6-TcrβTcrδ KO mice, but not in B6-Tcrβ KO mice, at 6 weeks. Although not objectively scored, carditis was more severe in B6-Tcrβ KO mice than in B6 and B6-TcrβTcrδ KO mice. These results demonstrated that B. burgdorferi-reactive B cells, in the absence of T-cell help, played a role in arthritis resolution. T cells expressing the γδ chain of the T-cell receptor (TCR), and possibly those expressing the αβ chain of the TCR, potentiated carditis severity and prevented carditis resolution. Thus, carditis resolution may be regulated by a different mechanism or influenced by a different repertoire of antibodies than arthritis resolution.
Phenotypic analysis of lymphocytes from immunocompetent and T-cell-deficient mice.
Because the above-described studies strongly suggested that B cells were necessary and sufficient for resolution of disease, lymph node cells and splenocytes from infected B6 and mutant B6 mice were phenotyped by flow cytometry. As originally reported by Mombaerts et al. (25), Rag-1-deficient mice could be described as nonleaky because neither mature B cells (μ, CD19, and CD22) nor T cells (CD3, CD4, CD8, αβ+ TCR, and γδ+ TCR) were detected prior to infection (data not shown) or at 8 weeks p.i. (Table 2). Similarly, both uninfected and infected TcrβTcrδ KO mice lacked αβ+ and γδ+ T cells, and, as if compensating for the loss of T cells, the percentage of B cells in the lymph nodes of these mice almost tripled compared to those in B6 mice (Table 2). Tcrβ KO mice lacked αβ+ T cells, but approximately 4% of the peripheral lymphocytes were CD4− CD8− γδ+ T cells, and the percentage of B cells in the lymph nodes of these mice approximately doubled compared with those in B6 mice (Table 2). Thus, unlike Tcrα KO mice, which have been considered leaky due to the presence of αβ+ T cells (24), the mutant mice (Tcrβ KO, TcrβTcrδ KO, and Rag-1 KO mice) used for these experiments were considered nonleaky. Thus, these phenotypic experiments confirmed that TcrβTcrδ KO mice lacked T cells and supported pathogenesis studies demonstrating that B cells were sufficient for resolution of arthritis.
TABLE 2.
Phenotypic analysis of lymphocytes from wild-type and mutant B6 micea
| Mouse strain | Cell origin | CD19 CD22 | CD19 IgM | CD19 CD5 | CD5 IgM | CD3 CD4 | CD3 CD8 | CD3 δTCR |
|---|---|---|---|---|---|---|---|---|
| B6 | Lymph node | 28.1 | 15.7 | 2.6 | 2.2 | 30.1 | 20.4 | 1.1 |
| Spleen | 58.3 | 29.5 | 4.7 | 3.2 | 15.9 | 8.1 | 2.5 | |
| B6-Tcrβ KO | Lymph node | 54.1 | 40.8 | 3.3 | 3 | 0.3* | 0.3* | 3.9 |
| Spleen | 65.6 | 56.6 | 6.11 | 4.22 | 0.3* | 0.5* | 4.8 | |
| B6-TcrβTcrδ KO | Lymph node | 76.9 | 61.4 | 3.6 | 3.1 | 0.3* | 0.2* | 0.2 |
| Spleen | 74.6 | 58.9 | 4.8 | 4.6 | 0.4* | 0.6* | 0.4 | |
| B6-Rag-1 KO | Lymph node | 0.2* | 0.2* | 0.2* | 0.05* | 0.3* | 0.2* | 0.2 |
| Spleen | 0.4* | 0.4* | 0.4* | 0.06* | 0.2* | 0.4* | 0.4 |
Spleen and lymph node cells from mice were stained for flow cytometric analysis, as described in Materials and Methods. Values are expressed as the percentage of each subset within the population. B cells were identified with monoclonal antibodies reactive with CD19, CD22, CD5, and IgM, whereas T cells were characterized with monoclonal antibodies reactive with CD3, CD4, CD8, and the δ chain of the TCR. *, value were not significantly greater than that obtained with isotype control.
Isotypic characterization of antibodies generated by B6-Tcrβ KO, B6-TcrβTcrδ KO, and B6 mice.
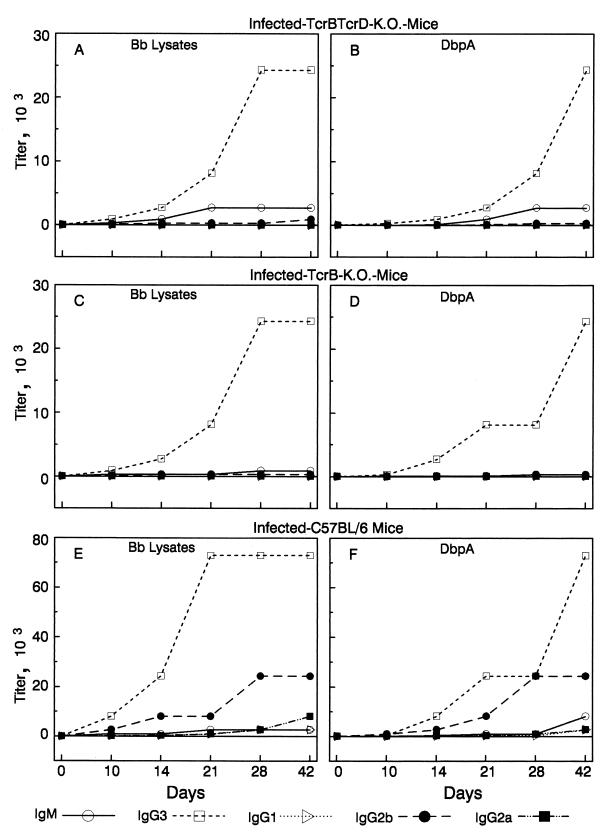
Because T-cell-deficient mice resolved arthritis and a primary effector function of B cells is antibody production, we next determined if infected mice lacking αβ+ T cells or those lacking both αβ+ T cells and γδ+ T cells produced antibodies against B. burgdorferi lysates (Fig. 1). Pooled sera from the same groups of mice that were used for the pathogenesis studies described above were obtained by eyebleeding mice at 0, 5, 10, 14, 21, 28, and 42 days after inoculation, and then sera were titrated and isotyped by ELISA. Antibodies reactive with B. burgdorferi lysates were not detected in the sera of any mouse at day 5. The predominant isotype of antibody produced during infection by both infected B6-TcrβTcrδ KO mice and infected B6-Tcrβ KO mice was IgG3, which peaked by day 28 with a titer of 1:24,300 (Fig. 1A and C). Although low concentrations of IgM (1:2,700), IgG1 (1:100), and IgG2b (1:300) antibodies reactive to B. burgdorferi lysates were detected (by day 42) in the sera of T-cell-deficient mice, IgG2a antibodies reactive with B. burgdorferi lysates were never detected, suggesting that IgG2a synthesis was dependent upon αβ+-T-cell help. The highest ELISA titers of immune sera from B6 mice against B. burgdorferi lysates were detected on day 21. IgG3 (1:72,900) and IgG2b (1:24,300) antibodies dominated this humoral response, with various levels of all immunoglobulin subclasses (peak IgM, 1:2,700; IgG1, 1:2,700; and IgG2a, 1:8,100) detected in the sera of infected B6 mice (Fig. 1E). Even though the antibody response of T-cell-deficient mice was approximately 3 to 10 times lower than that of immunocompetent control B6 mice, T-cell-deficient mice resolved arthritis.
FIG. 1.
Anti-B. burgdorferi and anti-DbpA IgM, IgG3, IgG1, IgG2b, and IgG2a titers in the sera of B6-TcrβTcrδ KO mice (A and B), B6-Tcrβ KO mice (C and D), or B6 mice (E and F) infected with B. burgdorferi. At each time point, sera were collected from five mice in each of the three groups. Sera from mice in each group were pooled, then the endpoint titers of immunoglobulins bound to plates coated with B. burgdorferi (Bb) lysates or recombinant DbpA were determined as described in Materials and Methods. Sera from uninfected mice did not react with B. burgdorferi lysates or recombinant DbpA.
As shown in Fig. 1B, D, and F, antibodies in immune sera of infected B6-TcrβTcrδ KO, B6-Tcrβ KO, and B6 mice, but not sera from uninfected mice, also reacted with recombinant DbpA. We specifically assessed antibody reactivity to DbpA because DbpA elicits a prominent antibody response during B. burgdorferi infection and immunization with recombinant DbpA induces protective immunity (14). As with B. burgdorferi lysates, T-cell-deficient mice produced only IgM and IgG3 DbpA-reactive antibodies, whereas B6 mice produced antibodies of all isotypes against this antigen (Fig. 1B, D, and F). As expected, lower antibody titers were detected in serum samples of T-cell-deficient mice than in those of immunocompetent B6 control mice. These results indicated that mice lacking both αβ+ and γδ+ T cells responded to infection by producing a number of B. burgdorferi-reactive antibodies, and arthritis resolution was correlated with the production of these antibodies. T cells expressing the γδ+ chain of the TCR did not appear to enhance this antibody response; their presence did not promote antibody class switching or increase antibody titers.
Immune serum from T-cell-deficient mice affords protective immunity.
Next, we compared the abilities of immune sera from infected T-cell-deficient mice and immunocompetent mice to confer protection against challenge with B. burgdorferi. For this purpose, groups of five CD-1 pups were passively immunized with immune sera obtained from infected B6-Tcrβ KO, B6-TcrβTcrδ KO, B6, and C3H mice or with normal sera from uninfected B6 mice. The groups treated with 90-day immune sera from C3H mice and normal mouse sera served as positive and negative controls, respectively (5, 9). Based upon cultures, mice treated with immune sera from infected T-cell-deficient mice, B6 mice, or C3H mice were protected from infection (Table 3). In contrast to the results obtained with immune sera, all pups immunized with normal mouse serum became infected. These results indicated that mice bearing B cells but lacking αβ+ T cells, as well as those lacking both αβ+ and γδ+ T cells, generated TI antibodies against B. burgdorferi that conferred protective immunity.
TABLE 3.
Protective capacity of sera from infected immunocompetent and immunodeficient micea
| Immune serum treatment | Arthritis (mean ± SD)
|
Carditis (no. positive/ total) | Culture (no. positive/ total) | |
|---|---|---|---|---|
| Prevalence | Severity | |||
| B6 normal | 2.4 ± 0.5 | 1.9 ± 0.4 | 5/5 | 5/5 |
| C3H | 0 ± 0 | 0 ± 0b | 0/5 | 0/5 |
| B6 | 0 ± 0 | 0 ± 0b | 0/5 | 0/5 |
| B6-Tcrβ KO | 0 ± 0 | 0 ± 0b | 0/5 | 0/5 |
| B6-TcrβTcrδ KO | 0 ± 0 | 0 ± 0b | 0/5 | 0/5 |
Groups of five CD-1 mouse pups were treated with B6 normal mouse serum or immune sera from the indicated experimental groups 18 h before challenge with B. burgdorferi, as described in Materials and Methods. Tibiotarsal joint arthritis severity was assessed on a scale of 0 to 3, with 3 being the most severe. Inflammation of knee joints and hearts was scored as positive or negative. Significance was determined by the Wilcoxon rank-sum test (two-tail probability).
P ≤ 0.015 compared with arthritis severity of control groups treated with B6 normal mouse serum.
Passive administration of immune sera from B6-Tcrβ or B6-TcrβTcrδ KO mice does not resolve B. burgdorferi-induced arthritis in C3H-scid mice.
In order to evaluate the arthritis-resolving capacity of antibodies generated against B. burgdorferi during a TI response, groups of five C3H-scid mice were infected with B. burgdorferi, allowed to develop arthritis, and then treated with immune sera from B. burgdorferi-infected B6-Tcrβ KO, B6-TcrβTcrδ KO, or B6 mice. C3H-scid mice were utilized because they have an arthritis-susceptible genotype (11), and passive antibody-induced arthritis resolution has been optimized in C3H-scid mice (6, 9). As a negative control, one group of mice received sera from uninfected naive B6 mice, and as a positive control, one group was treated with immune sera from C3H mice that had been infected for 90 days. As expected, passive administration of immune sera from infected B6 or C3H mice to actively infected SCID mice induced arthritis resolution and decreased the number of arthritic joints (Table 4) compared to those in SCID mice treated with B6 normal sera. However, this treatment did not influence active carditis (Table 4). Unexpectedly, passive transfer of immune sera from B6-Tcrβ KO or B6-TcrβTcrδ KO mice did not resolve arthritis in infected SCID mice. The number of arthritic joints and severity of arthritis were comparable to those observed in the group treated with normal mouse serum (Table 4). Sera from infected C3H-scid mice that had been treated with immune sera from infected B6-Tcrβ KO or infected B6-TcrβTcrδ KO mice, but not mice treated with naive mouse sera, contained detectable antibodies to B. burgdorferi lysates, suggesting that a sufficient amount of serum had been injected. All mice, regardless of treatment, had carditis and were culture positive for B. burgdorferi. These results indicated that antibodies in the sera of B. burgdorferi-infected T-cell-deficient mice were unable to resolve arthritis, even though T-cell-deficient mice that bear B cells were able to resolve arthritis.
TABLE 4.
Arthritis-resolving capacity of sera from infected immunocompetent and immunodeficient micea
| Immune serum treatment | Arthritis (mean ± SD)
|
Carditis (no./total) | Significanceb | |
|---|---|---|---|---|
| Prevalence | Severity | |||
| B6 (normal) | 4.0 ± 0 | 2.8 ± 0.5 | 5/5 | |
| C3H | 2.0 ± 0 | 1.3 ± 0.5 | 5/5 | P ≤ 0.015 |
| B6 | 1.8 ± 0.5 | 0.9 ± 0.2 | 5/5 | P ≤ 0.015 |
| B6-Tcrβ KO | 4.0 ± 0 | 2.5 ± 0.5 | 5/5 | NSc |
| B6-TcrβTcrδ KO | 3.8 ± 0 | 2.3 ± 0.5 | 5/5 | NS |
Groups of five C3H-scid mice were infected with B. burgdorferi and then treated with normal mouse serum or immune sera from immunocompetent, T-cell-deficient, or immunodeficient mice, as described in Materials and Methods. Severity of tibiotarsal joint arthritis was graded on a scale of 0 (negative) to 3 (severe). Inflammation of knee joints and hearts was scored as positive or negative.
The significance of arthritis severity was determined by the Wilcoxon rank-sum test (two-tail probability).
NS, not significant (P > 0.05).
Reactivity of B. burgdorferi immune serum lysates revealed by immunoblotting.
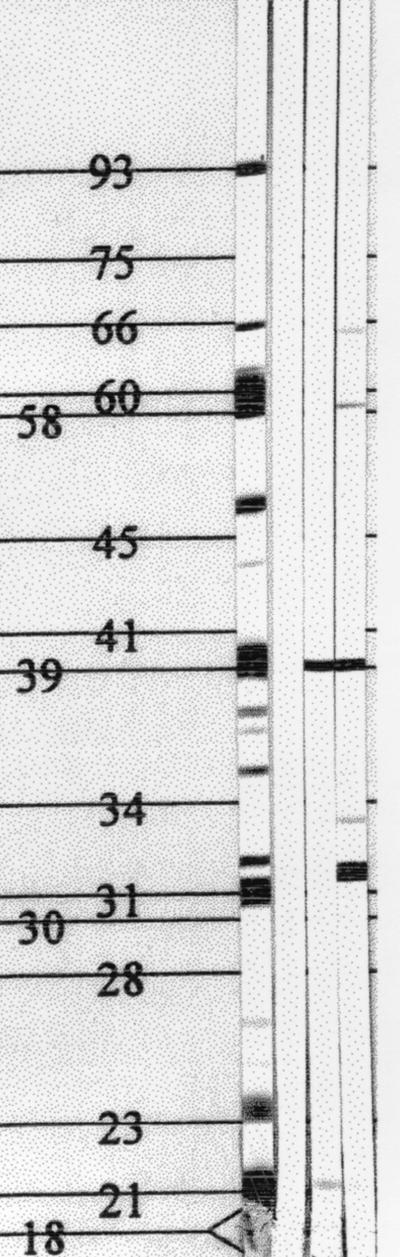
Because immune sera from both B6-Tcrβ KO and B6-TcrβTcrδ KO mice provided protection against a subsequent homologous challenge, the antibody repertoires of immune sera from different mutant mice were characterized using immunoblots. Serum immunoglobulins produced by infected immunocompetent B6 mice, infected T-cell-deficient mice (B6-TcrβTcrδ KO mice), and infected mice lacking αβ+ T cells but bearing γδ+ T cells (B6-Tcrβ KO mice) were compared. All immune sera, which were collected 8 weeks p.i., reacted strongly with a 39-kDa protein (presumably BmpA), but immune sera collected from B6-Tcrβ KO mice reacted weakly to a limited number of additional proteins (21 and 58 kDa) (Fig. 2). Interestingly, immune sera from B6-TcrβTcrδ KO mice reacted with a diverse number of proteins having molecular masses of 21, 32, 34, 39, 58, and 66 kDa (Fig. 2). Antibodies in the sera of naive B6 mice did not react with any protein, whereas immune sera from B6 mice reacted with the characteristic Borrelia proteins: 93, 66, 61, 60, 59, 58, 41 (presumed to be flagellin), 39 (presumed to be BmpA), 34 (presumed to be OspB), 31 (presumed to be OspA), 23 (presumed to be OspC), and 22 (presumed to be DbpA) kDa (Fig. 2). The limited reactivity pattern of immune sera from infected B6-Tcrβ KO may be useful for identifying proteins capable of eliciting protective immunity and suggests that γδ+ T cells suppressed or inhibited the response of B cells to some TI antigens expressed by B. burgdorferi.
FIG. 2.
Infected T-cell-deficient mice generate antibodies reactive with B. burgdorferi proteins. Nitrocellulose strips containing fractionated B. burgdorferi proteins were blotted with immune sera collected at 8 weeks p.i. from immunocompetent B6 mice (lane 1), Tcrβ KO mice (lane 3), and TcrβTcrδ KO mice (lane 4) and probed with alkaline phosphatase-conjugated goat anti-mouse IgG (heavy plus light chains). Lane 2, negative control, which was blotted with normal mouse serum, as described in Materials and Methods. Numbers indicate molecular weights in thousands.
DISCUSSION
It is now clear that TI antigenic determinants expressed by a wide variety of bacterial and viral microbes, such as Brucella abortis (36), Haemophilus influenzae (2), Streptococcus pneumoniae (19), vesicular stomatitis virus (23), rotavirus (18, 28), and polyomavirus (38), can stimulate B cells to proliferate and differentiate into antibody-producing cells in the absence of T cells. This study, using B. burgdorferi, demonstrated that (i) infection stimulated the production of B. burgdorferi-reactive IgM and IgG3 antibodies in the absence of T-cell help, (ii) sera from infected T-cell-deficient mice afforded passive protection against a homologous challenge, (iii) T-cell-deficient mice infected with B. burgdorferi resolved arthritis, (iv) B cells were both necessary and sufficient to resolve arthritis and carditis, and (v) sera from infected T-cell-deficient mice were unable to mediate resolution of arthritis or carditis.
Our initial observation that B. burgdorferi-infected athymic nude mice develop protective antibodies and are able to resolve arthritis and carditis suggests that B cells and the antibodies they produce against B. burgdorferi-associated antigens play a critical role in the pathogenesis of Lyme disease. Although data using CD40 ligand KO and MHC class II-deficient mice (15, 16) further support this hypothesis, we systematically examined the role of B cells and the antibodies they produce during infection. When infected with B. burgdorferi, both immunocompetent B6 mice and mice bearing B cells but lacking both αβ+ and γδ+ T cells generated protective antibodies and resolved arthritis and carditis. On the other hand, mice lacking αβ+ T cells but bearing γδ+ T and B cells generated protective antibodies and resolved arthritis, but carditis remained active. As expected, SCID mice did not produce protective antibodies, and their arthritis and carditis were progressive and persisted, respectively. Similarly, arthritis and carditis were progressive and persistent in B. burgdorferi-infected B-cell-deficient mice that bear T cells, (C57BL/6-Igh-6tmiCgn129 mice) (unpublished observation). These results demonstrate that the TI response to B. burgdorferi is important for resolution of both arthritis and carditis. Importantly, γδ+ T cells did not appear to potentiate the TI response to B. burgdorferi. Instead, B6-Tcrβ KO mice produced antibodies with a lower titer and more limited antigenic repertoire, as indicated by ELISA and immunoblotting results, and this dampened antibody response may be related to the inability of these mice to resolve carditis. Thus, γδ+ T cells either directly or indirectly (by regulating B cells) appear to potentiate cardiac disease severity in mice.
Because B cells were necessary for protective immunity and appeared to be necessary for arthritis and carditis resolution, we characterized the antibody response to infection. Interestingly, significant IgM and IgG3 titers were generated in T-cell-deficient mice, demonstrating that infection induced class switching in the absence of conventional αβ+ and γδ+ T-cell help. However, the data also indicated that T lymphocytes influenced the distribution of isotypes produced. This influence was seen predominantly with respect to IgG2a, an isotype that was not produced by T-cell-deficient mice. This isotypic difference between immunocompetent mice and T-cell-deficient mice probably reflects indirect and direct regulatory effects of T-cell-derived lymphokines.
When investigating the response kinetics of the various isotypes in immunocompetent mice and T-cell-deficient mice, several observations were made. First, with respect to immunocompetent mice, peak IgM and IgG3 titers were generally seen earlier than in T-cell-deficient mice. This suggested that T cells may have influenced the rate of switching from IgM to IgG. In terms of the magnitude of the immunoglobulin response, both the titer and isotypes were affected by T cells. Importantly, although the concentration of IgG antibodies was significantly lower in sera of B-cell-bearing, T-cell-deficient mice than in those of immunocompetent mice, sera from infected T-cell-deficient mice provided protection against challenge with B. burgdorferi, suggesting that adequate concentrations of protective antibodies were contained within immune sera. Additionally, even though the presence of T cells hastened class switching, they did not cause a pronounced fall in antibody titer after peak production had been reached, suggesting the absence of a TD regulatory event and potentially reflecting the presence of persisting antigen. Antigen persistence is a characteristic trait of most if not all TI antigens, probably reflecting their complex nature and resistance to degradation, as well as their tendency to be associated with persistent infection (20), which are all characteristics of B. burgdorferi infection (7).
The paradoxical observation that infected T-cell-deficient mice bearing B cells resolved both arthritis and carditis, whereas passively transferred immune sera from infected T-cell-deficient mice to infected SCID mice did not mediate arthritis resolution, highlights potential differences between the properties of cells and antibodies. A restricted interpretation of these results suggests that B. burgdorferi-reactive B cells may play a direct role in arthritis resolution or that their interaction with other cells in the joint microenvironment is necessary to resolve arthritis. It is unlikely that this finding is due to an insufficient concentration of antibodies being administered, because significant IgM and IgG antibody titers against B. burgdorferi lysates and DbpA were detected in the sera of infected SCID mice treated with immune sera but were not detected in sera of mice treated with naive sera from uninfected mice. Admittedly, definitive proof that an adequate concentration of the appropriate antibody was administered depends on identifying the antigen or antigens unique to arthritis.
Alternatively, we would hypothesize that both epitope specificity and isotype contribute to the efficacy of antibody-mediated resolution of arthritis. First, IgM, due to its pentameric structure, may not diffuse adequately from the vessels into the joint, whereas B cells could easily infiltrate perivascular tissue. Moreover, many antibody effector functions are mediated by the Fc portion of the immunoglobulin. For example, in mice, IgG2a, IgG2b, and IgG3 fix complement, but IgG1 does not. Conversely, both mononuclear phagocytes and neutrophils express receptors for the Fc portions of IgG molecules, which mediate phagocytosis. Thus, elimination of bacteria and resolution of arthritis by IgG1 and IgM antibodies would involve a complement-independent pathway. At this time, the mechanisms of protection and resolution of arthritis are unknown, and thus, it is difficult to predict the optimal isotype. Future experiments will be required to address these questions and to identify the accessory cells or factors mediating B-cell differentiation, maturation, and isotype switching during TI responses against B. burgdorferi.
The current paradigm maintains that antibody class switching is an event associated with T-cell help, and it is generally absent in traditionally defined TI responses. The presence of IgG3 B. burgdorferi-reactive antibodies in sera of T-cell-deficient mice is intriguing, given that type 2 TI antigens tend to elicit antibodies with IgM and IgG3 isotypes (34). B. burgdorferi expresses a number of type 2 glycosylated molecules and polymeric proteins that could contribute to the induction of a TI antibody response. Therefore, the very nature of B. burgdorferi is probably, at least partially, responsible for its ability to trigger TI antibody responses and isotype switching.
Additionally, production of IgG1, IgG2b, and IgG3 Borrelia burgdorferi-reactive antibodies by T-cell-deficient mice suggests that accessory cells (NK cells, macrophages, and dendritic cells) elicited isotype class switching via the cytokines they produced. Although T cells normally mediate this event, they can be replaced by accessory-cell-derived cytokines or lymphokines produced by T cells (26, 27, 29, 39). Cytokines that may have provided help to B cells during the course of B. burgdorferi infection include (i) interleukin-1 (IL-1), produced primarily by macrophages, endothelial, and epithelial cells, which mediates local inflammation and IL-6 synthesis; (ii) IL-5, produced by TH2 cells and activated mast cells, which promotes growth and differentiation of B cells into antibody-producing cells; (iii) IL-6, produced by a number of cells in response to IL-1 and tumor necrosis factor alpha, which (TNF), promotes expansion and maturation of B cells into antibody-producing cells; (iv) gamma interferon, produced by NK cells, which promotes isotype switching to IgG2a and IgG3; (v) IL-4, produced by mast cells, which promotes switching to IgGI; and (vi) transforming growth factor B, produced by B cells and macrophages, which promotes isotype switching to IgG2b (35). Limited data suggest that different accessory-cell-derived factors and mechanisms may mediate isotype switching to different microbes. As mentioned previously, γδ+ T cells do not appear to potentiate the response of B cells to B. burgdorferi. In contrast, neutralizing antibody responses to vesicular stomatitis virus infection require γδ+ T cells (23).
As is now apparent, several outcomes of Lyme borreliosis have been studied as indices of infection, including arthritis and carditis. Often these disease manifestations have been assumed to reflect equally the components of the immune response necessary for resolving disease. We are now beginning to document that several immunoregulatory processes affect differentially these disease manifestations, and it is becoming clear that arthritis and carditis resolution as well as protective immunity can be dissociated. For example, these and past studies demonstrated that immunocompetent mice resolved arthritis and carditis, and passively transferred immune sera from infected immunocompetent mice mediated resolution of arthritis but not carditis in infected SCID mice (6, 8). In this study, we expanded those findings by demonstrating that infected B6-Tcrβ KO and B6-TcrβTcrδ KO mice resolved arthritis, but only B6-TcrβTcrδ KO mice resolved carditis, and passive transfer of immune sera from infected B6-Tcrβ KO or infected B6-TcrβTcrδ KO to SCID mice, which had been infected 12 days before initiation of treatment, was not able to resolve arthritis or carditis. Thus, the least restrictive readout appeared to be protective immunity, because immune sera from immunocompetent mice, B6-Tcrβ KO mice, and B6-TcrβTcrδ KO mice conferred protection. Yet none of these readouts reflect the infectious status of the mouse, because regardless of disease severity, both immunocompetent and immunodeficient mice remain infected.
Despite the obvious TI response to B. burgdorferi, antibodies reactive with nonlipidated recombinant DbpA, as well as recombinant OspA, OspC, and P39 (unpublished observation), were detected in sera of T-cell-deficient mice. One possible explanation for this observation is that recombinant proteins generated using Escherichia coli were glycosylated, but this requires confirmation. Another explanation is the nonspecific nature of antibodies recognizing TI antigens, which may recognize multiple epitopes against unrelated molecules, usually with a low affinity (12). A more complex explanation involves several principles discovered during attempts to optimize vaccines using polysaccharide-protein conjugates. Although cross-linking of surface immunoglobulin receptors by TI antigens are important for B-cell triggering, both the antigenicity and immunogenicity of polysaccharides appear to be dependent upon the saccharide length and epitope density. Small oligosaccharides fail to stabilize conformational epitopes and do not generate high-affinity protective polysaccharide-specific antibodies, whereas intermediate-fragment conjugates may generate numerous stable conformational epitopes that are functional (21). Thus, the protein portion of the conjugated molecule not only may induce T-cell help but may help stabilize functional epitopes. Apparently, numerous conformational epitopes were stabilized by the lipidated and glycosylated proteins of B. burgdorferi, and these functional epitopes may have elicited antibodies recognizing the protein and saccharide portion of the molecule. Further studies are necessary to resolve this issue.
In conclusion, we have shown that B. burgdorferi infection stimulates an effective TI response that results in the production of antibodies, antibody class switching, the generation of protective antibodies, and the resolution of arthritis. Additionally, mice truly deficient in T cells (αβ+- and γδ+-T-cell deficient) are able to resolve carditis, whereas carditis remains active in mice bearing γδ+ T cells. Thus, the TI response against B. burgdorferi plays an important role in Lyme disease and host immunity.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI-26815 and AI-45253.
We appreciate the technical assistance of L. Adamson and W. Redmond. We thank S. Feng for providing recombinant DbpA.
REFERENCES
- 1.Armstrong A L, Barthold S W, Persing D H, Beck D S. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am J Trop Med Hyg. 1992;47:249–258. doi: 10.4269/ajtmh.1992.47.249. [DOI] [PubMed] [Google Scholar]
- 2.Baker P J. Immune responses to Haemophilus influenzae type B polysaccharide. T cell regulation of the antibody response to bacterial polysaccharide antigens: an examination of some general characteristics and their implications. J Infect Dis. 1992;165(Suppl. 1):S44–S48. doi: 10.1093/infdis/165-supplement_1-s44. [DOI] [PubMed] [Google Scholar]
- 3.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 4.Barthold S W, Beck D S, Hansen G M, Terwilliger G A, Moody K D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 5.Barthold S W, Bockenstedt L K. Passive immunizing activity of sera from mice infected with Borrelia burgdorferi. Infect Immun. 1993;61:4696–4702. doi: 10.1128/iai.61.11.4696-4702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barthold S W, deSouza M, Feng S. Serum-mediated resolution of Lyme arthritis in mice. Lab Invest. 1996;74:57–67. [PubMed] [Google Scholar]
- 7.Barthold S W, deSouza M S, Janotka J L, Smith A L, Persing D H. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:951–971. [PMC free article] [PubMed] [Google Scholar]
- 8.Barthold S W, Feng S, Bockenstedt L K, Fikrig E, Feen K. Protective and arthritis-resolving activity in sera of mice infected with Borrelia burgdorferi. Clin Infect Dis. 1995;25(Suppl. 1):S9–S17. doi: 10.1086/516166. [DOI] [PubMed] [Google Scholar]
- 9.Barthold S W, Feng S, Bockenstedt L K, Fikrig E, Feen K. Protective and arthritis-resolving activity in sera of mice infected with Borrelia burgdorferi. Clin Infect Dis. 1997;25:S9–S17. doi: 10.1086/516166. [DOI] [PubMed] [Google Scholar]
- 10.Barthold S W, Persing D H, Armstrong A L, Peeples R A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease following intradermal inoculation of mice. Am J Pathol. 1991;139:263–274. [PMC free article] [PubMed] [Google Scholar]
- 11.Barthold S W, Sidman C L, Smith A L. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am J Trop Med Hyg. 1992;47:605–613. doi: 10.4269/ajtmh.1992.47.605. [DOI] [PubMed] [Google Scholar]
- 12.Bondada S, Garg M. Thymus-independent antigens. In: Snow E C, editor. Handbook of B and T lymphocytes. San Diego, Calif: Academic Press, Inc.; 1994. pp. 343–370. [Google Scholar]
- 13.DeKruyff R, Clayberger C, Fay R, Cantor H. B cell activation: role of dendritic and T cell factors in the response to thymic-independent and -dependent antigens. J Immunol. 1985;134:2860–2866. [PubMed] [Google Scholar]
- 14.Feng S, Hodzic E, Stevenson B, Barthold S W. Humoral immunity to Borrelia burgdorferi N40 decorin binding proteins during infection of laboratory mice. Infect Immun. 1998;66:2827–2835. doi: 10.1128/iai.66.6.2827-2835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fikrig E, Barthold S W, Chen M, Chang C-H, Flavell R A. Protective antibodies develop, and murine Lyme arthritis regresses, in the absence of MHC class II and CD4+ T cells. J Immunol. 1997;159:5682–5686. [PubMed] [Google Scholar]
- 16.Fikrig E, Barthold S W, Chen M, Grewal I S, Craft J, Flavell R A. Protective antibodies in murine Lyme disease arise independently of CD40 ligand. J Immunol. 1996;157:1–3. [PubMed] [Google Scholar]
- 17.Fikrig W, Bockenstedt L K, Barthold S W, Chen M, Tao H, Ali-Salaam P, Telford III T R, Flavell R A. Sera from patients with chronic Lyme disease protect mice from Lyme borreliosis. J Infect Dis. 1994;169:568–574. doi: 10.1093/infdis/169.3.568. [DOI] [PubMed] [Google Scholar]
- 18.Franco M A, Greenberg H B. Immunity to rotavirus in T cell deficient mice. Virology. 1997;238:169–179. doi: 10.1006/viro.1997.8843. [DOI] [PubMed] [Google Scholar]
- 19.Gillespie S H. Aspects of pneumococcal infection including bacterial virulence, host response and vaccination. J Med Microbiol. 1989;28:237–248. doi: 10.1099/00222615-28-4-237. [DOI] [PubMed] [Google Scholar]
- 20.Humphrey J H. Tolerogenic or immunogenic activity of hapten-conjugated polysaccharides correlated with cellular localization. Eur J Immunol. 1981;11:212–220. doi: 10.1002/eji.1830110310. [DOI] [PubMed] [Google Scholar]
- 21.Jennings H. Further approaches for optimizing polysaccharide-protein conjugate vaccines for prevention of invasive bacterial disease. J Infect Dis. 1992;165:S156–S159. doi: 10.1093/infdis/165-supplement_1-s156. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald H R, Lees R K, Sordat B, Zaech P, Marryanski J L, Bron C. Age associated increase in expression of the T cell surface markers Thy1, Lyt1, and Lyt2 in congenitally thymic (nu-nu) mice: analysis by flow cytometry. J Immunol. 1981;126:865–870. [PubMed] [Google Scholar]
- 23.Maloy K J, Odermatt B, Hengartner H, Zinkernagel R M. Interferon γ-producing γδ T cell-dependent antibody isotype switching in the absence of germinal center formation during virus infection. Proc Natl Acad Sci USA. 1998;95:1160–1165. doi: 10.1073/pnas.95.3.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mombaerts P, Clarke A R, Rudnicki M A, Iacomini J, Itohara S, Lafaille J J, Wang L, Ichikawa Y, Jaenisch R, Hooper M L, Tonegawa S. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 25.Mombaerts P, Iacomini J, Johnson R S, Herrup K, Tonegawa S, Papaioannou V E. Rag-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 26.Mond J J, Lees A, Snapper C M. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 27.Mond J J, Vos Q, Lees A, Snapper C M. T cell independent antigens. Curr Opin Immunol. 1995;7:349–354. doi: 10.1016/0952-7915(95)80109-x. [DOI] [PubMed] [Google Scholar]
- 28.Moser C A, Cookinham S, Coffin S E, Clark H F, Offit P A. Relative importance of rotavirus-specific effector and memory B cells in protection against challenge. J Virol. 1998;72:1108–1114. doi: 10.1128/jvi.72.2.1108-1114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosier D E, Subbarao B. Thymus-independent antigens: complexity of B-lymphocyte activation revealed. Immunol Today. 1982;3:217–222. doi: 10.1016/0167-5699(82)90095-0. [DOI] [PubMed] [Google Scholar]
- 30.Roy M, Waldschmidt T, Aruffo A, Ledbetter J A, Noelle R J. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J Immunol. 1993;151:2497–2510. [PubMed] [Google Scholar]
- 31.Shanafelt M-C, Kang I, Barthold S W, Bockenstedt L K. Modulation of murine Lyme borreliosis by interruption of the B7/CD28 T-cell costimulatory pathway. Infect Immun. 1998;66:266–271. doi: 10.1128/iai.66.1.266-271.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sigal L H. Lyme disease: a review of aspects of its immunology and immunopathogenesis. Annu Rev Immunol. 1997;15:63–92. doi: 10.1146/annurev.immunol.15.1.63. [DOI] [PubMed] [Google Scholar]
- 33.Siliciano R F, Keeggan A D, Dintzis R Z, Dintzis H M, Shin H S. The interaction of nominal antigen with T cell antigen receptors. I. Specific binding of multivalent nominal antigen to cytolytic T cell clones. J Immunol. 1985;135:906–914. [PubMed] [Google Scholar]
- 34.Slack J, Der-Balian G P, Nahm M, Davie J M. Subclass restriction of murine antibodies. II. The IgG plaque-forming response to thymus-independent type 1 and type 2 antigens in normal mice and mice expressing an X-linked immunodeficiency. J Exp Med. 1980;151:853–862. doi: 10.1084/jem.151.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snapper C M, Mond J J. Towards a comprehensive view of immunoglobulin class switching. Immunol Today. 1993;14:15–17. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- 36.Spellman J M, Reed N D. Immune and mitogenic responses by BALB/c, CeH/HeJ, and nude mice to Brucella abortus bacterin and lipopolysaccharide. Infect Immun. 1979;24:371–378. doi: 10.1128/iai.24.2.371-378.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 38.Szomolanyi-Tsuda E, Welsh R M. T cell-independent antibody-mediated clearance of polyoma virus in T cell-deficient mice. J Exp Med. 1996;183:403–411. doi: 10.1084/jem.183.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szomolanyi-Tsuda E, Welsh R M. T-cell-independent antiviral antibody responses. Curr Opin Immunol. 1998;10:431–435. doi: 10.1016/s0952-7915(98)80117-9. [DOI] [PubMed] [Google Scholar]
- 40.Thomas D W, Solvay M J. Direct stimulation of T lymphocytes by antigen coated beads. J Immunol. 1986;137:419–421. [PubMed] [Google Scholar]
- 41.Ying M, PetriSeiler K, Eichwald E J, Weis J H, Teuscher C, Weis J J. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6 mice. Infect Immun. 1998;66:161–168. doi: 10.1128/iai.66.1.161-168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zisman E, Dayan M, Sela M, Mozes E. Ia-antigen-T-cell interactions for a thymus-independent antigen composed of D amino acids. Proc Natl Acad Sci USA. 1993;90:994–998. doi: 10.1073/pnas.90.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]