Abstract
Background
Management of drug–drug interactions (DDIs) for ensitrelvir, a novel 3-chymotrypsin-like protease inhibitor of SARS-CoV-2 infection is crucial. A previous clinical DDI study of ensitrelvir with midazolam, a clinical index cytochrome P450 (CYP) 3A substrate, demonstrated that ensitrelvir given for 5 days orally with a loading/maintenance dose of 750/250 mg acted as a strong CYP3A inhibitor.
Objectives
The objectives of this study were to investigate the effect of ensitrelvir on the pharmacokinetics of CYP3A substrates, dexamethasone, prednisolone and midazolam, and to assess the pharmacokinetics, safety, and tolerability of ensitrelvir following multiple-dose administration of ensitrelvir.
Methods
This was a Phase 1, multicenter, single-arm, open-label study in healthy Japanese adult participants. The effects of multiple doses of ensitrelvir in the fasted state on the pharmacokinetics of dexamethasone, prednisolone, and midazolam were investigated. Ensitrelvir was administered from Day 1 through Day 5, with a loading/maintenance dose of 750/250 mg for the dexamethasone and prednisolone cohorts whereas 375/125 mg for the midazolam cohort. Either dexamethasone, prednisolone, or midazolam was administered alone (Day − 2) or in combination with ensitrelvir (Day 5) in each of the cohorts. Additionally, dexamethasone or prednisolone was administered on Days 9 and 14. The pharmacokinetic parameters of ensitrelvir, dexamethasone, prednisolone, and midazolam were calculated based on their plasma concentration data with non-compartmental analysis. In safety assessments, the nature, frequency, and severity of treatment-emergent adverse events were evaluated and recorded.
Results
The area under the concentration-time curve (AUC) ratio of dexamethasone on Day 5 was 3.47-fold compared with the corresponding values for dexamethasone alone on Day − 2 and the effect diminished over time after the last dose of ensitrelvir. No clinically meaningful effect was observed for prednisolone. The AUC ratio of midazolam was 6.77-fold with ensitrelvir 375/125 mg suggesting ensitrelvir at 375/125 mg strongly inhibits CYP3A similar to that at 750/250 mg. No new safety signals with ensitrelvir were reported during the study.
Conclusion
The inhibitory effect for CYP3A was confirmed after the last dose of ensitrelvir, and the effect diminished over time. In addition, ensitrelvir at 375/125 mg showed CYP3A inhibitory potential similar to that at 750/250 mg. These findings can be used as a clinical recommendation for prescribing ensitrelvir with regard to concomitant medications.
Clinical Trial Registration
Japan Registry of Clinical Trials identifier: jRCT2031210202.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40261-023-01265-8.
Key Points
| The 5-day administration of ensitrelvir 750/250 mg increased the exposure of dexamethasone when co-administered with ensitrelvir on Day 5 and the effect diminished over time. No meaningful effect of ensitrelvir on the pharmacokinetics of prednisolone was confirmed. |
| The AUC ratio of midazolam was 6.77-fold on Day 5 when ensitrelvir was given at a 375/125-mg dose to assess its effect on the pharmacokinetics of midazolam (given on Days − 2 and 5), suggesting that ensitrelvir at 375/125 mg strongly inhibited cytochrome P450 3A. |
Introduction
Coronavirus Disease 2019 (COVID-19), an infectious respiratory disease, is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and was declared a global pandemic by the World Health Organization (WHO) in March 2020 [1]. As of December 2022, SARS-CoV-2 has resulted in 6.65 million deaths worldwide [2]. Ensitrelvir fumaric acid (hereafter ensitrelvir and also known as S-217622), discovered by Shionogi & Co., Ltd., is a novel oral inhibitor of 3C-like protease of SARS-CoV-2, which is essential for its viral replication [3]. A clinical study in healthy adults showed that the once-daily oral dose of ensitrelvir suspension in the treatment of COVID-19 was well tolerated and exhibited a favorable pharmacokinetic profile, including a long half-life [4]. In addition, ensitrelvir demonstrated a favorable antiviral efficacy with an acceptable safety profile in Phase 2a and 2b parts of the randomized Phase 2/3 study where multiple doses of ensitrelvir were administered: 375 mg as the loading dose on Day 1 followed by 125 mg as the maintenance dose on Days 2–5 (375/125 mg) and 750 mg as the loading dose on Day 1 followed by 250 mg as the maintenance dose on Days 2–5 (750/250 mg) [5, 6]. Moreover, the Ministry of Health, Labor and Welfare (MHLW) in Japan gave emergency regulatory approval to ensitrelvir tablet 375/125 mg once daily for 5 days, for the indication of SARS-CoV-2 infection [7].
Drug–drug interactions (DDIs) occur because of alterations in expression and activity of drug metabolizing enzymes, which consequently modify the pharmacokinetic properties of the therapeutic drugs used [8, 9]. Drug–drug interactions are an increasing burden for patients infected with COVID-19 [10, 11]. Cytochrome P450 (CYP) 3A is a hepatic mono-oxygenase enzyme that catalyzes the metabolism of substrates in a reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent manner [12]. Some findings have demonstrated that up to 40% of all drug metabolisms are mediated by CYP3A enzymes [12]. In an earlier study investigating the DDI potential of ensitrelvir with midazolam, which is a clinical index substrate of CYP 3A [13], administration of ensitrelvir 750/250 mg resulted in an increase in the plasma exposure of midazolam by 8.80-fold [4]. Since ensitrelvir will be administered to a large population, including patients who are already under some medications, clinical DDI studies are needed to provide more information about the DDIs of ensitrelvir with CYP3A substrates. COVID-19 treatment guidelines suggest the use of systemic corticosteroids such as dexamethasone and prednisolone, which are also CYP3A substrates [14, 15], in patients who are hospitalized with COVID-19 and require oxygen supplementation [16, 17]. Therefore, it is important to assess the DDI potential of ensitrelvir with dexamethasone and prednisolone. Moreover, as ensitrelvir shows a long half-life (t1/2,z = 42.2–48.1 h) [4], it is also important to evaluate the DDI after the last administration of ensitrelvir, since corticosteroids may be administered in patients whose conditions worsen after ensitrelvir treatment [18].
Drug–drug interaction potential of ensitrelvir with CYP3A substrates needs to be evaluated in clinical DDI studies in order to ensure the appropriate use of ensitrelvir and comedications. Therefore, we conducted a Phase 1 study to evaluate the DDI potential of ensitrelvir co-administered with CYP3A substrates in healthy adults. The aims of this Phase 1 study were (i) to investigate the effect of ensitrelvir 750/250 mg on the pharmacokinetics of dexamethasone or prednisolone; (ii) to investigate the effect of ensitrelvir 375/125 mg on the pharmacokinetics of midazolam; and (iii) to evaluate the pharmacokinetics, safety, and tolerability of ensitrelvir following oral multiple-dose administration of ensitrelvir tablet for 5 days in healthy Japanese adult participants. The results reported here are from part of the completed study (jRCT2031210202). The remaining data of this study will be presented separately.
Methods
Study Design
A Phase 1, multicenter, single-arm, open-label study in healthy Japanese adult participants consisted of 2 parts. Part 1 consisted of 2 cohorts (dexamethasone cohort and prednisolone cohort); in these cohorts, the effects of multiple doses (once daily for 5 days) of ensitrelvir tablet 750/250 mg administered orally in the fasted state on the pharmacokinetics of dexamethasone and prednisolone were investigated. Dexamethasone (1-mg tablet) or prednisolone (10-mg tablet) was administered on Days − 2, 5 (co-administered with ensitrelvir), 9 (5th day after the last ensitrelvir dose), and 14 (10th day after the last ensitrelvir dose) in the fasted state. Part 2 consisted of 1 cohort (midazolam cohort); in this cohort, the effects of multiple doses (once daily for 5 days) of ensitrelvir tablet 375/125 mg administered orally in the fasted state on the pharmacokinetics of midazolam were investigated. Midazolam (2 mg/mL syrup) was administered at 2 mg in the fasted state alone on Day − 2 and concomitantly with ensitrelvir on Day 5.
Eligible participants were Japanese healthy adult male participants aged 20–55 years at the time of signing the informed consent form (ICF), whose body mass index (BMI) ranged ≥ 18.5 and ≤ 30.0, and who took no other medicines apart from the study medicines.
Ethical Compliance
The study (jRCT2031210202) was conducted in compliance with the protocol, the Declaration of Helsinki [19] and Council for International Organizations of Medical Sciences International Ethical Guidelines [20], the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice Guidelines [21], and other applicable laws and regulations. It was also approved by the concerned Institutional Review Board. All participants gave their written informed consent for participation in the study.
Pharmacokinetic Assessments
Blood samples were collected and analyzed at several time points to evaluate the pharmacokinetics and determine the plasma concentration of ensitrelvir, dexamethasone, prednisolone, and midazolam. For plasma ensitrelvir concentration measurements in DDI studies with dexamethasone or prednisolone, blood samples were collected pre-dose (0 h) and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, and 12 h post-dose on Day 1; pre-dose (0 h) on Day 2; and pre-dose (0 h) and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 24, 96, 120, 216, 240, and 312 h post-dose on Day 5. For plasma dexamethasone and prednisolone concentration measurements, blood samples were collected pre-dose (0 h) and 0.5, 1, 2, 3, 4, 5, 6, 8, 12, and 24 h post-dose on Days − 2, 5, 9, and 14. For plasma ensitrelvir concentration measurements in the DDI study with midazolam, blood samples were collected pre-dose (0 h) and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, and 12 h post-dose on Day 1; pre-dose (0 h) on Days 2, 3, and 4; pre-dose (0 h) and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, and 24 h post-dose on Day 5. For midazolam, blood samples were collected pre-dose (0 h) and 0.25, 0.5, 1, 2, 3, 4, 5, 6, 8, 12, and 24 h post-dose on Day −2; and pre-dose (0 h) and 0.25, 0.5, 1, 2, 3, 4, 5, 6, 8, 12, 24, 36, and 48 h post-dose on Day 5.
The pharmacokinetic parameters of ensitrelvir, dexamethasone, prednisolone, and midazolam were calculated based on the plasma concentration data of ensitrelvir, dexamethasone, prednisolone, and midazolam with non-compartmental analysis. Pharmacokinetic analyses were performed using WinNonlin (version 6.2.1 for Part 1 and version 8.3.3 for Part 2, Certara USA Inc., Princeton, NJ, USA). The assessments of pharmacokinetic parameters included maximum plasma concentration (Cmax), time to maximum plasma concentration (Tmax), area under the plasma concentration-time curve (AUC0–last, AUC0-inf, and AUC0–τ), elimination rate constant (λz), terminal elimination half-life (t1/2,z) and mean residence time (MRT). Additional details of the bioanalytical procedures and the inhibitory kinetics of ensitrelvir with CYP enzymes are provided in Supplementary Tables 2 to 4, and Supplementary Figure 2.
Safety and Tolerability Analyses
Safety assessments included the analysis of all treatment-emergent adverse events (TEAEs), classified by System Organ Class and Preferred Term of MedDRA version 24.0. The nature, frequency, and severity of TEAEs were evaluated and recorded.
Statistical Methods for Pharmacokinetics Analyses
The statistical analyses for pharmacokinetic parameters were performed using SAS (version 9.4). For summarizing the quantitative variables, mean (SD) and median (minimum–maximum) values were reported. For qualitative variables, the number (%) data were reported. Analysis of variance (ANOVA) was used to assess the following: effects of multiple-dose administration of ensitrelvir once daily for 5 days on the pharmacokinetics of dexamethasone and prednisolone as measured through Cmax, AUC0–last, AUC0–inf, and t1/2,z of dexamethasone and prednisolone on Days − 2, 5, 9, and 14 and on the pharmacokinetics of midazolam as measured through Cmax, AUC0–last, AUC0–inf, t1/2,z, λz and MRT on Days − 2 and 5. Geometric least square (GLS) mean ratios and 90% confidence intervals (CIs) were calculated for dexamethasone, prednisolone, and midazolam for the subsequent days (Days 5, 9, and 14, as applicable) with respect to the GLS values on Day − 2. When the 90% CIs for Cmax, AUC0–last, and AUC0–inf ratio are completely contained within the range of 0.80–1.25, then ensitrelvir is considered not to have a clinically meaningful effect on the pharmacokinetics of the comedication drugs.
Results
Study Participants and Baseline Demographics
A total of 42 healthy Japanese adults participated in the study. Of these, 28 participated in part 1 (14 in each cohort) and 14 in part 2. The mean (SD) age ranged from 31.9 (5.1) to 36.0 (7.4) years and the mean (SD) BMI ranged from 21.51 (2.24) to 23.29 (1.82) kg/m2 across the cohorts. The demographic and baseline characteristics of these participants are summarized in Supplementary Table 1. There were no withdrawals and all participants completed the study.
Pharmacokinetics
Pharmacokinetics of Ensitrelvir in DDI Study
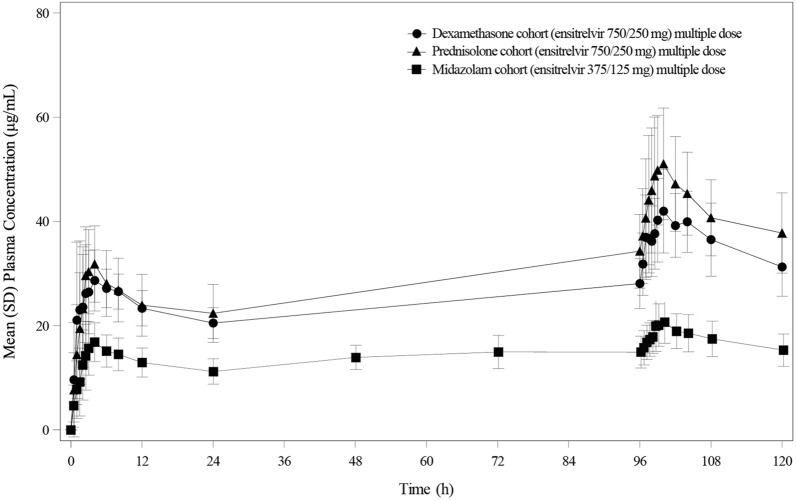
The comparison of plasma concentration–time profiles of ensitrelvir until 24 hours from last dose of ensitrelvir between the three cohorts following multiple-dose administration of ensitrelvir at 750/250 mg and 375/125 mg is shown in Fig. 1 and the plasma concentration-time profiles until 312 hours from last dose of ensitrelvir at 750/250 mg are shown in Supplementary Figure 1. The pharmacokinetic parameters were listed in Table 1. The plasma exposures of ensitrelvir in dexamethasone cohort were similar to those in prednisolone cohort. The medians of Tmax of ensitrelvir in dexamethasone, prednisolone and midazolam cohorts were 2.50 and 4.00, 3.00 and 4.00, and 3.00 and 4.00 h on Days 1 and 5, respectively.
Fig. 1.
Pharmacokinetic profile of multiple-dose administration of ensitrelvir. Comparison of mean (SD) plasma concentration profile of ensitrelvir (until 120 hours after the initial administration of ensitrelvir) following multiple dose administration of ensitrelvir, once daily for 5 days in the fasted state in healthy Japanese adult participants. Filled circle dexamethasone cohort (ensitrelvir 750/250 mg); filled triangle prednisolone cohort (ensitrelvir 750/250 mg); filled square midazolam cohort (ensitrelvir 375/125 mg)
Table 1.
Pharmacokinetic parameters for multiple-dose administration of ensitrelvir in the fasted state
| Parameters | Ensitrelvir 750/250 mg (dexamethasone cohort) (N = 14) | Ensitrelvir 750/250 mg (prednisolone cohort) (N = 14) | Ensitrelvir 375/125 mg (midazolam cohort) (N = 14) | |||
|---|---|---|---|---|---|---|
| Day 1 | Day 5 | Day 1 | Day 5 | Day 1 | Day 5 | |
| Cmax (μg/mL) | 32.4 (20.0) | 43.9 (14.7) | 33.1 (21.8) | 52.3 (20.2) | 18.1 (24.7) | 21.9 (17.4) |
| Tmax (h) | 2.50 (1.00, 8.00) | 4.00 (1.00, 8.00) | 3.00 (2.00, 4.00) | 4.00 (1.50, 6.00) | 4.00 (1.50, 4.00) | 3.00 (2.00, 12.00) |
| AUC0–τ (μg·h/mL) | 545.2 (16.3) | 852.8 (16.6) | 553.7 (23.6) | 997.3 (18.4) | 306.3 (25.2) | 424.5 (18.8) |
Geometric means (percentage coefficient of variation) are presented, except for Tmax, for which medians (minimum, maximum) are presented. 750/250 mg, multiple once-daily doses with 750 mg as the loading dose on Day 1 and 250 mg as the maintenance dose on Days 2–5; 375/125 mg, multiple once-daily doses with 375 mg as the loading dose on Day 1 and 125 mg as the maintenance dose on Days 2–5
AUC0–τ area under the concentration-time curve over the dosing interval τ (i.e., 24 h), CI confidence interval, Cmax maximum plasma concentration, N number of participants, Tmax time to maximum plasma concentration
The Effect of Ensitrelvir on the Pharmacokinetics of Dexamethasone
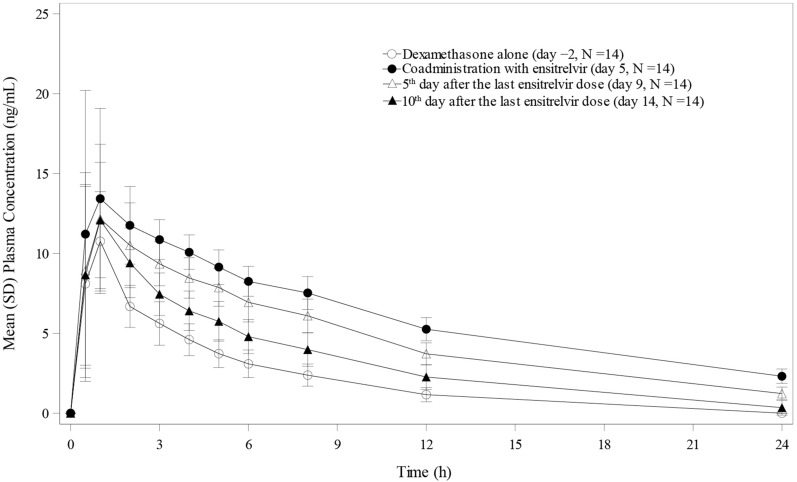
The plasma concentration-time profiles of dexamethasone following single-dose administration of dexamethasone 1 mg alone on Day − 2, concomitantly with ensitrelvir 750/250 mg on Day 5, and alone again on Days 9 and 14 are shown in Fig. 2. When dexamethasone was co-administered with ensitrelvir 250 mg on Day 5, the GLS mean ratios for Cmax, AUC0–last, and AUC0–inf were 1.47-, 3.18-, and 3.47-fold, respectively, compared to the corresponding values for dexamethasone alone on Day − 2 (Table 2). The Cmax, AUC0–last, and AUC0–inf of dexamethasone following single-dose administration of dexamethasone on Day 9 were 1.24-, 2.45-, and 2.38-fold, respectively, and those on Day 14 were 1.17-, 1.56-, and 1.58-fold, respectively, compared to dexamethasone alone on Day − 2, suggesting that the effect of ensitrelvir 750/250 mg on the pharmacokinetics of dexamethasone diminished over time on Day 14.
Fig. 2.
Plasma concentration profiles of dexamethasone following single-dose administration of dexamethasone alone (Day − 2), when co-administered with ensitrelvir 750/250 mg on Day 5, and then alone on Days 9 and 14 after the treatment of ensitrelvir
Table 2.
Statistical analysis of the effect of multiple-dose administration of ensitrelvir on the pharmacokinetics of dexamethasone following single-dose administration of dexamethasone (1 mg) alone, when co-administered with ensitrelvir (750/250 mg)a on Day 5, and then alone on Days 9 and 14
| Parameters | Dexamethasone alone (Day − 2) | Dexamethasone with ensitrelvir (Day 5) | 5th day after the last ensitrelvir dose (Day 9) | 10th day after the last ensitrelvir dose (Day 14) | Dexamethasone with ensitrelvir/dexamethasone alone (Day 5/Day − 2) |
5th day after the last ensitrelvir dose/dexamethasone alone (Day 9/Day − 2) | 10th day after the last ensitrelvir dose/dexamethasone alone (Day 14/Day − 2) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | GLS mean | N | GLS mean | N | GLS mean | N | GLS mean | GLS mean ratio (90% CI) | GLS mean ratio (90% CI) | GLS mean ratio (90% CI) | |
| Cmax (ng/mL) | 14 | 11.4 | 14 | 16.9 | 14 | 14.2 | 14 | 13.4 | 1.4737 (1.3037–1.6660) | 1.2376 (1.0948–1.3991) | 1.1725 (1.0372–1.3255) |
| AUC0–last (ng·h/mL) | 14 | 45.21 | 14 | 143.9 | 14 | 110.7 | 14 | 70.56 | 3.1840 (2.9607–3.4240) | 2.4480 (2.2764–2.6326) | 1.5608 (1.4514–1.6785) |
| AUC0–inf (ng·h/mL) | 13 | 51.73 | 11 | 179.3 | 14 | 123.0 | 11 | 81.70 | 3.4666 (3.2318–3.7184) | 2.3769 (2.2263–2.5377) | 1.5792 (1.4704–1.6960) |
| λz (1/h) | 14 | 0.1716 | 14 | 0.0738 | 14 | 0.1000 | 14 | 0.1289 | 0.4304 (0.4105–0.4512) | 0.5830 (0.5561–0.6113) | 0.7514 (0.7166–0.7878) |
| t1/2,z (h) | 14 | 4.04 | 14 | 9.39 | 14 | 6.93 | 14 | 5.38 | 2.3235 (2.2161–2.4361) | 1.7152 (1.6360–1.7984) | 1.3309 (1.2694–1.3954) |
| MRT (h) | 13 | 5.80 | 11 | 13.9 | 14 | 10.4 | 11 | 7.72 | 2.4006 (2.2526–2.5584) | 1.7949 (1.6913–1.9048) | 1.3298 (1.2465–1.4187) |
AUC0-last area under the concentration-time curve to the last measurable concentration, AUC0-inf area under the concentration-time curve extrapolated to infinity, CI confidence interval, Cmax maximum plasma concentration, GLS geometric least square, MRT mean residence time, N number of participants, λz terminal elimination rate constant, t1/2z terminal elimination half-life
a750/250 mg, multiple once-daily doses with 750 mg as the loading dose on Day 1 and 250 mg as the maintenance dose on Days 2–5
The Effect of Ensitrelvir on the Pharmacokinetics of Prednisolone
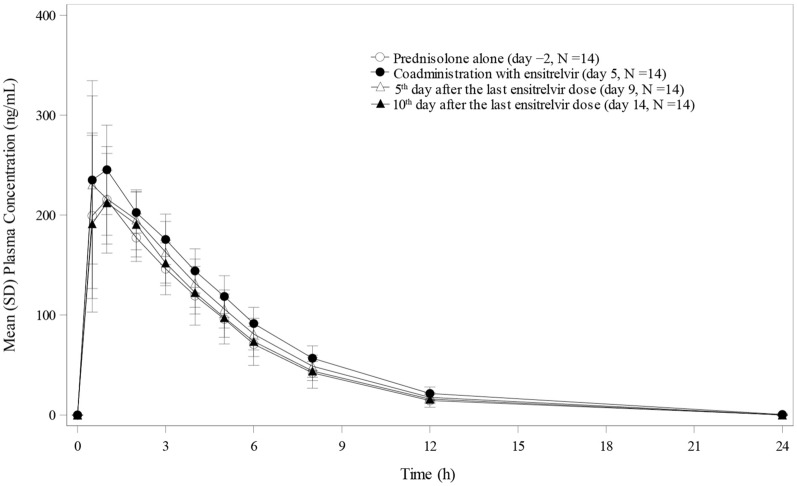
The plasma concentration-time profiles of prednisolone following single-dose administration of prednisolone 10 mg alone on Day − 2, concomitantly, with ensitrelvir 750/250 mg on Day 5 and alone again on Days 9 and 14, are displayed in Fig. 3. When prednisolone was co-administered with ensitrelvir 250 mg on Day 5, the GLS mean ratios for Cmax, AUC0–last, and AUC0–inf were 1.11-, 1.24-, and 1.25-fold, respectively, compared to the corresponding values for prednisolone alone on Day − 2 (Table 3). The 90% CIs of the Cmax on Day 5 and those of Cmax, AUC0–last, and AUC0–inf on Days 9 and 14 were contained within 0.8000 and 1.2500, suggesting no clinically meaningful effect of ensitrelvir 750/250 mg on the pharmacokinetics of prednisolone.
Fig. 3.
Plasma concentration profiles of prednisolone following single-dose administration of prednisolone alone (Day − 2), when co-administered with ensitrelvir 750/250 mg on Day 5, and then alone on Days 9 and 14 after the treatment of ensitrelvir
Table 3.
Statistical analysis of the effect of ensitrelvir on the pharmacokinetics of prednisolone following single-dose administration of prednisolone (10 mg) alone, when co-administered with ensitrelvir (750/250 mg)a on Day 5, and then alone on Days 9 and 14
| Parameters | Prednisolone alone (Day − 2) | Prednisolone with ensitrelvir (Day 5) | 5th day after the last ensitrelvir dose (Day 9) | 10th day after the last ensitrelvir dose (Day 14) | Prednisolone with ensitrelvir/ prednisolone alone (Day 5/Day − 2) | 5th day after the last ensitrelvir dose/prednisolone alone (Day 9/Day − 2) | 10th day after the last ensitrelvir dose/prednisolone alone (Day 14/Day − 2) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | GLS mean | N | GLS mean | N | GLS mean | N | GLS mean | GLS mean ratio (90% CI) | GLS mean ratio (90% CI) | GLS mean ratio (90% CI) | |
| Cmax (ng/mL) | 14 | 243 | 14 | 270 | 14 | 266 | 14 | 241 | 1.1141 (1.0035–1.2369) | 1.0953 (0.9865–1.2160) | 0.9927 (0.8941–1.1021) |
| AUC0–last (ng·h/mL) | 14 | 1042 | 14 | 1293 | 14 | 1161 | 14 | 1070 | 1.2408 (1.2073–1.2752) | 1.1142 (1.0842–1.1451) | 1.0267 (0.9990–1.0552) |
| AUC0–inf (ng·h/mL) | 14 | 1088 | 14 | 1358 | 14 | 1223 | 14 | 1131 | 1.2476 (1.2176–1.2784) | 1.1243 (1.0973–1.1521) | 1.0396 (1.0146–1.0653) |
| λz (1/h) | 14 | 0.2722 | 14 | 0.2439 | 14 | 0.2553 | 14 | 0.2598 | 0.8960 (0.8699–0.9230) | 0.9381 (0.9107–0.9663) | 0.9547 (0.9269–0.9834) |
| t1/2,z (h) | 14 | 2.55 | 14 | 2.84 | 14 | 2.71 | 14 | 2.67 | 1.1160 (1.0835–1.1496) | 1.0660 (1.0349–1.0980) | 1.0474 (1.0169–1.0789) |
| MRT (h) | 14 | 4.26 | 14 | 4.71 | 1 | 4.50 | 14 | 4.44 | 1.1065 (1.0569–1.1584) | 1.0569 (1.0095–1.1065) | 1.0419 (0.9952–1.0908) |
AUC0–last area under the concentration–time curve to the last measurable concentration, AUC0–inf area under the concentration-time curve extrapolated to infinity, CI confidence interval, Cmax maximum plasma concentration, GLS geometric least square, MRT mean residence time, N number of participants, λz terminal elimination rate constant, t1/2z terminal elimination half-life
a750/250 mg, multiple once-daily doses with 750 mg as the loading dose on Day 1 and 250 mg as the maintenance dose on Days 2–5
The Effect of Ensitrelvir on the Pharmacokinetics of Midazolam
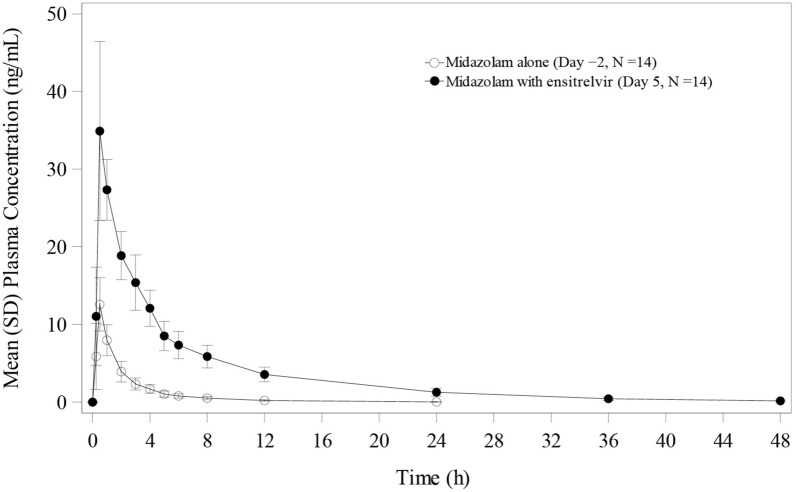
The plasma concentration-time profiles of midazolam following single-dose administration of midazolam 2 mg on Day − 2 and concomitantly with ensitrelvir 375/125 mg on Day 5 are shown in Fig. 4. Pharmacokinetics parameters for midazolam alone, when co-administered with multiple doses of the tablet formulation of 375/125 mg ensitrelvir to healthy Japanese adult participants, are listed in Table 4. When midazolam was co-administered with ensitrelvir 125 mg on Day 5, the GLS mean ratios for Cmax, AUC0–last, and AUC0–inf were 2.80-, 6.90-, and 6.77-fold, respectively, compared with the corresponding values for midazolam alone on Day − 2, suggesting that ensitrelvir is a strong CYP3A inhibitor with the dose regimen.
Fig. 4.
Plasma concentration profiles of midazolam following single-dose administration of midazolam alone (Day − 2) and when co-administered with ensitrelvir 375/125 mg on Day 5
Table 4.
Statistical analysis of the effect of ensitrelvir on the pharmacokinetics of midazolam following single-dose administration of midazolam (2 mg) alone and when co-administered with ensitrelvir (375/125 mg)a
| Parameters | Midazolam alone | Midazolam with ensitrelvir | Midazolam with ensitrelvir/midazolam alone | ||
|---|---|---|---|---|---|
| N | GLS mean (Day − 2) | N | GLS mean (Day 5) | GLS mean ratio (90% CI) (Day 5/Day − 2) | |
| Cmax (ng/mL) | 14 | 12.6 | 14 | 35.2 | 2.8012 (2.3798–3.2971) |
| AUC0–last (ng·h/mL) | 14 | 23.35 | 14 | 161.1 | 6.9011 (6.2722–7.5931) |
| AUC0–inf (ng·h/mL) | 14 | 24.08 | 14 | 163.0 | 6.7685 (6.1572–7.4404) |
| λz (1/h) | 14 | 0.2139 | 14 | 0.0940 | 0.4396 (0.3936–0.4909) |
| t1/2,z (h) | 14 | 3.24 | 14 | 7.37 | 2.2750 (2.0373–2.5406) |
| MRT (h) | 14 | 3.18 | 14 | 8.44 | 2.6506 (2.4623–2.8532) |
AUC0–last area under the concentration-time curve to the last measurable concentration, AUC0–inf area under the concentration-time curve extrapolated to infinity, CI confidence interval, Cmax maximum plasma concentration, GLS geometric least square, MRT mean residence time, N number of participants, λz terminal elimination rate constant, t1/2z terminal elimination half-life
a375/125 mg, multiple once-daily doses with 375 mg as the loading dose on Day 1 and 125 mg as the maintenance dose on Days 2–5
Safety and Tolerability
The major TEAEs witnessed for multiple-dose administrations of ensitrelvir in cohorts are presented in Tables 5 and 6. In the dexamethasone cohort, 9 of the 14 participants reported 13 TEAEs. Five participants reported decreased high-density lipoprotein levels, 4 participants reported increased blood triglyceride levels, and 1 participant each reported dizziness, headache, atrioventricular block, and papule. All TEAEs were reported by the investigator to be related to ensitrelvir but not to dexamethasone, except for 1 event each of atrioventricular block and increased blood triglycerides, which were not related to both drugs. In the prednisolone cohort, 14 TEAEs (decreased high-density lipoprotein levels considered to be related to ensitrelvir and not related to prednisolone) were reported in all of the 14 participants. In the midazolam cohort, 21 TEAEs were reported in 11 of the 14 participants. Among these, 9 participants reported somnolence, 8 reported decreased high-density lipoprotein levels, and 1 each reported diarrhea, increased blood triglyceride level, and arthropod sting. All 8 events of decreased high-density lipoprotein level, 2 events of somnolence, and 1 event of increased blood triglyceride level were reported by the investigator to be related to ensitrelvir, whereas the 8 events of somnolence and 1 event of diarrhea were related to midazolam. Except for the arthropod sting, all TEAEs were related to the study intervention. Most TEAEs observed were mild in severity and were resolved without treatment.
Table 5.
Treatment-emergent adverse events (TEAEs) following multiple-dose administration of ensitrelvir in Japanese healthy adult participants (part 1) in the fasted statea
| System Organ Classb Preferred term |
Ensitrelvir 750/250 mg + dexamethasone | Ensitrelvir 750/250 mg + prednisolone | ||||
|---|---|---|---|---|---|---|
| N = 14 | N = 14 | |||||
| N (%) | Events | Related to ensitrelvir | N (%) | Events | Related to ensitrelvir | |
| Participants with any TEAE | 9 (64.3) | 13 | 11 | 14 (100.0) | 14 | 14 |
| Nervous system disorders | 1 (7.1) | 2 | 2 | 0 | 0 | 0 |
| Dizziness | 1 (7.1) | 1 | 1 | 0 | 0 | 0 |
| Headache | 1 (7.1) | 1 | 1 | 0 | 0 | 0 |
| Cardiac disorders | 1 (7.1) | 1 | - | 0 | 0 | 0 |
| Atrioventricular block | 1 (7.1) | 1 | - | 0 | 0 | 0 |
| Skin and subcutaneous tissue disorders | 1 (7.1) | 1 | 1 | 0 | 0 | 0 |
| Papule | 1 (7.1) | 1 | 1 | 0 | 0 | 0 |
| Investigations | 7 (50.0) | 9 | 8 | 14 (100.0) | 14 | 14 |
| High-density lipoprotein decreased | 5 (35.7) | 5 | 5 | 14 (100.0) | 14 | 14 |
| Blood triglycerides increased | 4 (28.6) | 4 | 3 | 0 | 0 | 0 |
Events number of events, N number of participants, TEAE treatment-emergent adverse event
a750/250 mg, multiple once-daily doses with 750 mg as the loading dose on Day 1 and 250 mg as the maintenance dose on Days 2–5
bSystem Organ Class and Preferred Term of MedDRA ver. 24.0
Table 6.
Treatment-emergent adverse events (TEAEs) following multiple-dose administration of ensitrelvir in Japanese healthy adult participants (part 2) in the fasted statea
| System Organ Classb Preferred term |
Ensitrelvir 375/125 mg + midazolam | ||
|---|---|---|---|
| N = 14 | |||
| N (%) | Events | Related to ensitrelvir | |
| Participants with any TEAE | 11 (78.6) | 21 | 11 |
| Nervous system disorders | 9 (64.3) | 10 | 2 |
| Somnolence | 9 (64.3) | 10 | 2 |
| Gastrointestinal disorders | 1 (7.1) | 1 | – |
| Diarrhea | 1 (7.1) | 1 | – |
| Investigations | 8 (57.1) | 9 | 9 |
| High-density lipoprotein decreased | 8 (57.1) | 8 | 8 |
| Blood triglycerides increased | 1 (7.1) | 1 | 1 |
| Injury, poisoning and procedural complications | 1 (7.1) | 1 | – |
| Arthropod sting | 1 (7.1) | 1 | – |
Events number of events, N number of participants, TEAE treatment-emergent adverse event
a375/125 mg, multiple once-daily doses with 375 mg as the loading dose on Day 1 and 125 mg as the maintenance dose on Days 2–5
bSystem Organ Class and Preferred Term of MedDRA ver. 24.0
Discussion
Information about the DDI potential of a drug is essential for drug development and appropriate clinical use. As reported in the previous Phase 1 study assessing the DDI of ensitrelvir with midazolam, ensitrelvir suspension at a high dose regimen of 750/250 mg was a strong CYP3A inhibitor [4]. Additionally, in pre-clinical in vitro findings, ensitrelvir showed no reversible inhibitory effect on CYP3A4; instead, ensitrelvir demonstrated a time-dependent NADPH-mediated inhibition of CYP3A (Supplemental Table S-1 and S-2). Therefore, the DDI potential of ensitrelvir tablets at 750/250 mg dose with dexamethasone and prednisolone, the key drugs used in the treatment of COVID-19 and also substrates for CYP3A, was evaluated in part 1 of this study. In addition, for ensitrelvir tablets at 375/125 mg, an emergency approval dose in Japan, the DDI potential with CYP3A substrates was confirmed using midazolam in part 2 of this study. Whereas ensitrelvir 375/125 mg is a clinical dose under emergency approval, ensitrelvir 750/250 mg was a candidate dose in the Phase 2/3 study [5].
Co-administration of ensitrelvir with CYP3A substrates in the current studies was associated with minimal changes to the pharmacokinetics of ensitrelvir itself. Pharmacokinetic analyses indicated that prednisolone, dexamethasone, and midazolam impact the metabolic clearance of ensitrelvir. The time-course of the plasma concentration of ensitrelvir suggests that the impact of prednisolone and dexamethasone on ensitrelvir is similar, whereas midazolam, in comparison with both prednisolone and dexamethasone, may be associated with a reduction in the renal clearance of ensitrelvir. These changes to the pharmacokinetics of ensitrelvir were similar to those observed in studies that co-administered isavuconazole-prednisone [22] and isavuconazole-midazolam [23], where changes to the pharmacokinetics of isavuconazole itself were minimal.
When dexamethasone was co-administered with ensitrelvir 750/250 mg on Day 5, ensitrelvir increased the exposures of dexamethasone due to the time-dependent inhibitory effect of ensitrelvir on CYP3A. Furthermore, the effect of drug interaction decreased over time as ensitrelvir was eliminated (Fig. 2). Moreover, the AUC of prednisolone changed slightly when it was co-administered with ensitrelvir 750/250 mg on Day 5. The CIs of Cmax on Day 5 and those of Cmax, AUC0–last, and AUC0–inf on Days 9 and 14 were confined within 0.8000 and 1.2500, indicating no clinically meaningful effect of 750/250 mg ensitrelvir on the pharmacokinetics of prednisolone. A previous study reported that when itraconazole, a strong inhibitor for CYP3A, was administered at a 200-mg dose, orally, once daily for 4 days, with dexamethasone 4.5 mg co-administered on Day 4, the AUC of dexamethasone was increased 3.7-fold [24]. Similarly, when prednisolone 20 mg was administered on Day 5, the AUC of prednisolone was increased 1.24-fold [25]. These findings of itraconazole reported by Varis et al. [24, 25] and the suggestion by Ohno et al. [26, 27] that the AUC ratio for CYP3A substrates would be estimated by the contribution ratio of CYP3A metabolism, supported that our findings could be explained by the differences in the contribution ratio of CYP3A for dexamethasone and prednisolone. As the interaction between 750/250 mg ensitrelvir and prednisolone was minor, the effect of ensitrelvir 375/125 mg on the CYP3A substrates, which have similar contribution ratio of CYP3A, would be similar to the results of our DDI study with prednisolone.
The DDI study for ensitrelvir with midazolam was conducted for the 375/125-mg dose, which was a low-dose regimen in the clinical Phase 2/3 study [5] and also an approval dose regimen in Japan [7]. When midazolam was co-administered with ensitrelvir 375/125 mg for 5 days, the AUC ratio on Day 5 increased to 6.77-fold compared with the corresponding values for midazolam alone on Day − 2, indicating strong inhibition of CYP3A by ensitrelvir 375/125 mg. The effect of ensitrelvir 375/125 mg on the pharmacokinetics of midazolam was similar to that with ensitrelvir 750/250 mg, especially the increased ratios of Cmax, which were almost the same between 375/125 mg (2.80-fold) and 750/250 mg (2.78-fold [4]). These results suggest that almost all of the CYP3A located in the gastrointestinal tract is already inhibited with the ensitrelvir 375/125 mg dose regimen. In addition, these results support that the AUC for dexamethasone and prednisolone when co-administered with ensitrelvir at 375/125 mg is expected to be similar to that with ensitrelvir 750/250 mg.
From these results, we found that the rank order of the AUC increase of these substrates with the inhibitor was in the order of midazolam > dexamethasone > prednisolone. These results suggest that the coadministration of ensitrelvir with CYP3A substrates resulted in inhibition of metabolism of all the substrates with a diverse drug-interaction profile ranging from mild to strong inhibition. In this series of studies, there were no serious TEAEs. Almost all of the TEAEs were resolved without treatment as they were mild in severity. The observed TEAEs for ensitrelvir were atrioventricular block, increased blood triglycerides, decreased high-density lipoprotein, and somnolence. The safety results show that ensitrelvir was well tolerated with no additional safety signal.
Conclusion
In summary, the effects of ensitrelvir on the pharmacokinetics of CYP3A substrates were evaluated. Ensitrelvir increased the exposure of dexamethasone when co-administered with ensitrelvir on Day 5 and the effect was diminished over time, and no meaningful effect of ensitrelvir on the pharmacokinetics of prednisolone was confirmed. The observed minor interaction between ensitrelvir and prednisolone is possibly of limited clinical implication. In addition, the study with midazolam found that ensitrelvir 375/125 mg is a strong CYP3A inhibitor as is ensitrelvir 750/250 mg. Therefore, medicines that are reported to have DDIs with other strong CYP3A inhibitors, such as itraconazole, should be co-administered with caution. Ensitrelvir at the clinical dose was well tolerated with no additional safety signal and can be co-administered following possible dose adjustments with several CYP3A substrates likely to be used in COVID-19 patients. These findings can be useful information as a clinical recommendation for prescribing ensitrelvir with regard to concomitant medications.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all the study participants and their families, investigators, and study teams. Medical writing support was provided by MedPro Clinical Research. The authors also thank Vidula Bhole, MD, MHSc, and Ivan D’Souza, MS, of MedPro Clinical Research, for providing medical writing support for this manuscript. The plasma concentrations of ensitrelvir and prednisolone were determined at Sumika Chemical Analysis Service, Ltd, dexamethasone was at Nemoto Science Co., Ltd, and midazolam was at Shin Nippon Biomedical Laboratories, Ltd.
Declarations
Funding
This study was funded by Shionogi & Co., Ltd. The funder of the study was involved in study design, data collection, data analysis, and data interpretation.
Conflict of interest
RS, TS, TF, AK, RK, and TM are employees of Shionogi & Co., Ltd. and Y.M. was an employee of Shionogi & Co., Ltd. at the time of this research.
Ethics approval
The study was approved by Council for International Organizations of Medical Sciences (CIOMS) International Ethical Guidelines and conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation (ICH) guidelines for Good Clinical Practice (GCP).
Consent to participate
All participants gave their written informed consent for participation in the study.
Consent for publication
Not applicable.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
Conceptualization, RS, TS, TF, AK, YM; methodology, RS, YM, RK, TM; formal analysis, RS; investigation, TS; writing original draft preparation, RS; writing review and editing, RS, TS, TF, AK, YM, RK, TM. All authors have read and agreed to the published version of the manuscript.
References
- 1.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. WHO coronavirus dashboard. 2022. https://covid19.who.int/. Accessed on 23 Dec 2022.
- 3.Unoh Y, Uehara S, Nakahara K, Nobori H, Yamatsu Y, Yamamoto S, Maruyama Y, Taoda Y, Kasamatsu K, Suto T, Kouki K, Nakahashi A, Kawashima S, Sanaki T, Toba S, Uemura K, Mizutare T, Ando S, Sasaki M, Orba Y, Sawa H, Sato A, Sato T, Kato T, Tachibana Y. Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19. J Med Chem. 2022;65:6499–6512. doi: 10.1021/acs.jmedchem.2c00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimizu R, Sonoyama T, Fukuhara T, Kuwata A, Matsuo Y, Kubota R. Safety, tolerability, and pharmacokinetics of the novel antiviral agent ensitrelvir fumaric acid, a SARS-CoV-2 3CL protease inhibitor, in healthy adults. Antimicrob Agents Chemother. 2022;66:e0063222. doi: 10.1128/aac.00632-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukae H, Yotsuyanagi H, Ohmagari N, Doi Y, Imamura T, Sonoyama T, Fukuhara T, Ichihashi G, Sanaki T, Baba K, Takeda Y, Tsuge Y, Uehara T. A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: Results of the phase 2a part. Antimicrob Agents Chemother. 2022;66:e0069722. doi: 10.1128/aac.00697-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukae H, Yotsuyanagi H, Ohmagari N, Doi Y, Sakaguchi H, Sonoyama T, Ichihashi G, Sanaki T, Baba K, Tsuge Y, Uehara T. Efficacy and safety of ensitrelvir in patients with mild-to-moderate COVID-19: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Press release on November 22, 2022. Xocova® (Ensitrelvir Fumaric Acid) tablets 125 mg approved in Japan for the treatment of SARS-CoV-2 infection, under the emergency regulatory approval system. https://www.shionogi.com/global/en/news/2022/11/e20221122.html. Accessed on 17 Dec 2022.
- 8.Jeong E, Nelson SD, Su Y, Malin B, Li L, Chen Y. Detecting drug–drug interactions between therapies for COVID-19 and concomitant medications through the FDA adverse event reporting system. Front Pharmacol. 2022;13:938552. doi: 10.3389/fphar.2022.938552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mann HJ. Drug-associated disease: cytochrome P450 interactions. Crit Care Clin. 2006;22(329–45):vii. doi: 10.1016/j.ccc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Cattaneo D, Pasina L, Maggioni AP, Oreni L, Conti F, Pezzati L, Casalini G, Bonazzetti C, Morena V, Ridolfo A, Antinori S, Gervasoni C. Drug–drug interactions and prescription appropriateness at hospital discharge: experience with COVID-19 patients. Drugs Aging. 2021;38:341–346. doi: 10.1007/s40266-021-00840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cattaneo D, Pasina L, Conti F, Giacomelli A, Oreni L, Pezzati L, Bonazzetti C, Piscaglia M, Carrozzo G, Antinori S, Gervasoni C. Risks of potential drug–drug interactions in COVID-19 patients treated with corticosteroids: a single-center experience. J Endocrinol Invest. 2021;44:2849–2851. doi: 10.1007/s40618-021-01604-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonnell AM, Dang CH. Basic review of the cytochrome P450 system. J Adv Pract Oncol. 2013;4(4):263–268. doi: 10.6004/jadpro.2013.4.4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FDA . Drug development and drug interactions. Table of substrates, inhibitors and inducers. Silver Spring: FDA; 2020. [Google Scholar]
- 14.Al Rihani SB, Deodhar M, Dow P, Turgeon J, Michaud V. Is dexamethasone a substrate, an inducer, or a substrate-inducer of CYP3As? Arch Pharm Pharmacol Res. 2020;2:APPR.MS.ID.000546. doi: 10.33552/APPR.2020.02.000546. [DOI] [Google Scholar]
- 15.Skauby RH, Gustavsen MT, Andersen AM, Bjerre A, Åsberg A, Midtvedt K, Vethe NT, Bergan S. Prednisolone and prednisone pharmacokinetics in adult renal transplant recipients. Ther Drug Monit. 2021;43:247–255. doi: 10.1097/FTD.0000000000000835. [DOI] [PubMed] [Google Scholar]
- 16.Bouadma L, Mekontso-Dessap A, Burdet C, Merdji H, Poissy J, Dupuis C, Guitton C, Schwebel C, Cohen Y, Bruel C, Marzouk M, Geri G, Cerf C, Mégarbane B, Garçon P, Kipnis E, Visseaux B, Beldjoudi N, Chevret S, Timsit JF, COVIDICUS Study Group High-dose dexamethasone and oxygen support strategies in intensive care unit patients with severe COVID-19 acute hypoxemic respiratory failure: the COVIDICUS Randomized Clinical Trial. JAMA Intern Med. 2022;182:906–916. doi: 10.1001/jamainternmed.2022.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NIH 2022. COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/corticosteroids/. Last updated: 31 May 2022.
- 18.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WMA Declaration of Helsinki—ethical principles for medical research involving human subjects. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Last accessed: 23 Sept 2022.
- 20.Council for International Organizations of Medical Sciences. International ethical guidelines for health-related research involving humans. Available from: https://cioms.ch/wp-content/uploads/2017/01/WEB-CIOMS-EthicalGuidelines.pdf. Last accessed: 23 Sept 2022.
- 21.ICH International Council for Harmonisation. Efficacy guidelines. Available from: https://www.ich.org/page/efficacy-guidelines. Last accessed: 23 Sept 2022.
- 22.Groll AH, Desai A, Han D, Howieson C, Kato K, Akhtar S, Kowalski D, Lademacher C, Lewis W, Pearlman H, Mandarino D, Yamazaki T, Townsend R. Pharmacokinetic assessment of drug–drug interactions of isavuconazole with the immunosuppressants cyclosporine, mycophenolic acid, prednisolone, sirolimus, and tacrolimus in healthy adults. Clin Pharmacol Drug Dev. 2017;6:76–85. doi: 10.1002/cpdd.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Townsend R, Dietz A, Hale C, Akhtar S, Kowalski D, Lademacher C, Lasseter K, Pearlman H, Rammelsberg D, Schmitt-Hoffmann A, Yamazaki T, Desai A. Pharmacokinetic evaluation of CYP3A4-mediated drug–drug interactions of isavuconazole with rifampin, ketoconazole, midazolam, and ethinyl estradiol/norethindrone in healthy adults. Clin Pharmacol Drug Dev. 2017;6:44–53. doi: 10.1002/cpdd.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varis T, Kivisto KT, Backman JT, Neuvonen PJ. The cytochrome P450 3A4 inhibitor itraconazole markedly increases the plasma concentrations of dexamethasone and enhances its adrenal-suppressant effect. Clin Pharmacol Ther. 2000;68:487–494. doi: 10.1067/mcp.2000.110772. [DOI] [PubMed] [Google Scholar]
- 25.Varis T, Kivisto KT, Neuvonen PJ. The effect of itraconazole on the pharmacokinetics and pharmacodynamics of oral prednisolone. Eur J Clin Pharmacol. 2000;56:57–60. doi: 10.1007/s002280050720. [DOI] [PubMed] [Google Scholar]
- 26.Ohno Y, Hisaka A, Suzuki H. General framework for the quantitative prediction of CYP3A4-mediated oral drug interactions based on the AUC increase by coadministration of standard drugs. Clin Pharmacokinet. 2007;46:681–696. doi: 10.2165/00003088-200746080-00005. [DOI] [PubMed] [Google Scholar]
- 27.Ohno Y, Hisaka A, Ueno M, Suzuki H. General framework for the prediction of oral drug interactions caused by CYP3A4 induction from in vivo information. Clin Pharmacokinet. 2008;47:669–680. doi: 10.2165/00003088-200847100-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.