Abstract
The effect of O2 and CO2 on expression of toxic shock syndrome toxin 1 (TSST-1) by Staphylococcus aureus was investigated under controlled growth conditions with continuous-culture techniques. To stimulate TSST-1 production, air and anaerobic gas were premixed before delivery to the culture vessel. At a growth rate—or mass doubling time (td)—of 3 h, production of specific TSST-1 (expressed as micrograms per milligram of cell dry weight) was 5.9-fold greater at an O2 concentration of 4% than under anaerobic conditions. Increasing the O2 concentration to 11% did not result in a significant increase (P > 0.05) in the rate of toxin production over that during growth in 4% O2 but did result in a significant increase (4.9-fold; P < 0.001) in the rate of toxin production over that during anaerobic growth. At a td of 9 h, addition of 3.5% O2 resulted in a 7.6-fold increase in specific TSST-1 production. When room air was sparged through a culture growing at a td of 9 h, TSST-1 production increased significantly (by 3.4-fold) over that during anaerobic growth. When a growth environment of 4% O2–remainder N2 was studied, no increase in TSST-1 production was observed; this was also the case with 8% O2 at gas-flow rates of 0.1, 0.2, and 0.4 liters/min. In all experiments, production of biomass (expressed as milligrams of cell dry weight per milliliter) increased, indicating that O2 was metabolized by S. aureus. Addition of CO2 to the gas mix (4% O2, 10% CO2, 86% N2) resulted in a 5.1- to 6.8-fold increase in TSST-1 production over that during anaerobic growth and a 3.6-fold increase over that during growth in an environment of 4% O2–remainder N2. The agr mutant strain tested produced 6.1-fold more specific TSST-1 in a growth environment of 4% O2–10% CO2–86% N2 than during anaerobic growth. These data suggest that in this system, O2 alone does not trigger production of TSST-1; rather, both CO2 and O2 are required.
Staphylococcal toxic shock syndrome (TSS) is a relatively rare condition (0.06 cases per 100,000 people [2]) caused by one or more potent exotoxins produced by some strains of Staphylococcus aureus (4, 14, 32; P. M. Schlievert, Letter, Lancet i:1149–1150, 1986). TSS is associated with a constellation of symptoms, including fever, rash, diarrhea, and the inability to maintain proper hemostasis. Severe cases often progress to multiple-organ involvement and desquamation of the skin over the entire body; some cases end in death (31). Of the 40% of TSS cases associated with menstruation (2), almost all are caused by TSS toxin 1 (TSST-1) (4, 14, 32; Schlievert, Letter, Lancet i:1149–1150, 1986).
The association between menstrual TSS and tampon use has provoked considerable discussion regarding the possible role of tampons in menstrual TSS. Epidemiologic studies identified tampon brand and style as the most important risk factors for development of menstrual TSS (8, 21, 26, 28). Although some high-absorbency tampons appeared to increase the relative risk of TSS, epidemiologic studies did not resolve the issue of which specific tampon characteristic was associated with this increased risk. The possibilities cited included increased absorbency, increased total surface area, chemical composition, and increased oxygen delivery via the tampon itself. In addition, alteration of the vaginal microflora during tampon use has received considerable attention. Previous in vivo studies from this laboratory indicated that tampons, regardless of fiber composition, neither alter the normal vaginal microflora during use nor provide a nidus for preferential growth of TSST-1-producing strains of S. aureus or other members of the vaginal microflora (18, 19, 20).
The concentrations of O2 and CO2, two components important to microbial growth, have been measured in the vagina before and after tampon insertion. In the undisturbed vaginal environment, O2 and CO2 levels (partial pressure) ranged from 0 to 3 mm Hg (0 to 0.5%) and from 50 to 60 mm Hg (6.5 to 7.8%), respectively (33). Immediately after tampon insertion, O2 and CO2 levels were 130 to 140 mm Hg (20.8 to 22.4%) and 10 to 15 mm Hg (1.3 to 2.0%), respectively. Later after insertion, the CO2 and O2 partial pressures approached preinsertion values at different rates: CO2 levels increased to preinsertion values within 20 to 90 min, while O2 concentrations did not fall to preinsertion levels within the 8-h observation period, remaining at >20 mm Hg or 3.2%. These observations suggest that substantial amounts of available O2 are present within the vagina for an extended period after tampon insertion. However, since the apparatus used for these measurements also resulted in the maintenance of a greater-than-normal total volume within the vagina, it was difficult to attribute the experimental observations to tampon insertion with any certainty, and the period of elevated O2 concentrations in the vaginas of test subjects may have been prolonged.
In vitro studies indicate that a number of environmental factors affect production of TSST-1, including magnesium concentration, oxygen concentration, growth rate, temperature, and pH (9, 13, 15, 16, 27, 29, 30, 34). However, it is still unclear whether any single factor affects TSST-1 production under controlled growth conditions that allow single variables to be altered. Most previous studies have used batch culture systems in which the growth rate, nutrient levels, and metabolite concentrations change during incubation. In such systems, alteration of one factor results in concomitant changes in other factors associated with growth. But some studies have used the continuous-culture system, which allows the researcher to separate and define parameters that are interdependent during batch culture growth, such as growth rate, nutrient and product concentrations, and cell density. During continuous culturing, fresh medium is added to a culture at a fixed rate, and cells and medium are removed at a rate that maintains a constant culture volume. If, in addition, other growth environment parameters are held constant, the culture will reach a steady state at which there is no net change in production rates of metabolites or biomass. Once steady-state growth has been achieved, the growth rate of the organism becomes independent of the concentration of nutrients. Continuous culture can be viewed as a technique for prolonging the exponential growth phase of a batch culture and for producing a population of cells growing indefinitely in an unchanging environment. Taylor and Holland used this type of system to evaluate the effects of various environmental factors on TSST-1 production; however, because they did not maintain a constant growth rate, it is unclear whether the effects noted for their experiments were due to changes in the environmental factors tested or were due to changes in the growth rate (29, 30). The purpose of the present study was to determine the effects of O2 and CO2 on production of TSST-1 in a controlled growth environment that included a constant growth rate.
MATERIALS AND METHODS
Bacterial strain, strain maintenance, and culture medium.
S. aureus MN8 (provided by Patrick Schlievert, University of Minnesota, Minneapolis) is a TSST-1-producing strain isolated from a patient with menstrual TSS. S. aureus RN8846 is an agr mutant strain generated from a TSST-1-producing parent, strain RN8465 (agr+), also isolated from a patient with menstrual TSS (provided by Richard Novick, New York University, New York, N.Y.). Strains were kept frozen at −80°C as multiple 1-ml aliquots from a 24-h batch culture with brain heart infusion broth (Difco Laboratories, Detroit, Mich.) and cysteine (0.05%, wt/vol) as the growth medium. In experimental studies, brain heart infusion broth served as the nutrient medium.
Continuous-culture growth system.
A 1.5-liter fermentor with a 1-liter working volume (BioFlo; New Brunswick Scientific, Edison, N.J.) was used for all experiments. Culture conditions were maintained at 37°C, and a pH of 7.1 ± 0.1 was maintained by addition of sterile 1 N NaOH with the use of a pH meter-controller (Cole Parmer Instrument Company, Vernon, Ill.). A constant mixing rate of 600 rpm was used. Gas was vigorously bubbled through the culture medium and the vessel head space. The oxidation-reduction potential of the medium in the growth vessel was continuously monitored with an Eh probe (Ingold, Wilmington, Mass.) and a pH-Eh meter (Radiometer, Westlake, Ohio). The growth rate, or mass doubling time (td), was adjusted by altering the medium inflow rate via a peristaltic pump. Inflow rates necessary to obtain tds of 3 and 9 h were determined by the formulas td = (ln 2)/μ and μ = D = F/V for steady-state cultures, where μ is the specific growth rate (h−1), D is the dilution rate (h−1), F is the medium inflow rate (liters/h), and V is the culture volume (liters). For tds of 3 and 9 h, D was 0.23 and 0.08 h−1 and F was 0.23 and 0.08 liters/h, respectively.
Studies were initiated after the culture reached a steady state either under anaerobic conditions or with a growth environment of 4% O2–remainder N2. At least one sample was obtained for analysis of cell dry weight (CDW) and TSST-1 levels before the growth environment was changed. If not otherwise indicated, all data were collected after a new steady state had been reached (at least five cell doublings after the growth environment was altered). Oxygen was delivered to the growth vessel continuously as a mixture of air and anaerobic gas mix (10% H2, 10% CO2, 80% N2), an O2-N2 analyzed gas mix, or an O2-CO2-N2 analyzed gas mix.
In experiments for which air and anaerobic gas were mixed to achieve a desired O2 concentration, a two-stage fermentor system was employed. Anaerobic mix plus controlled amounts of room air were sparged through sterile water in the first of two fermentation vessels. The gas outflow from the first-stage vessel was connected to the gas inflow line for the second fermentation vessel, which contained growth medium and organisms. The mixing rates for both vessels—and thus the degree of O2 saturation—were the same. A dissolved O2 probe and meter (Cole Parmer) were used to determine the dissolved O2 concentration produced by the gas mix being delivered to the second fermentor vessel. Samples (7 ml) were removed directly from the growth vessel of the second fermentor with a sidearm sampling port and were processed for confirmation of culture purity and measurement of CDW and TSST-1 concentrations.
Biomass measurements.
Biomass measurements were calculated from CDW determinations. A 5-ml sample collected from the growth vessel was fixed with a final formaldehyde concentration of 4% (vol/vol), mixed, and allowed to sit overnight at room temperature. Cells were pelleted by centrifugation (1,625 × g; 10 min) and washed twice with sterile filtered water (0.22-μm-pore-size membrane). After the final centrifugation, the supernatant was decanted and the pelleted cells were resuspended in the residual liquid. The entire cell suspension was then transferred to a preweighed 0.22-μm-pore-size polycarbonate filter (Poretics, Livermore, Calif.). The tube was washed four times with sterile filtered water (500 μl each time). Each wash was transferred to the same 0.22-μm-pore-size polycarbonate filter. The filter was folded in half (to help retain the biomass during drying) and dried at 60°C for 24 to 48 h. Filters were reweighed with an analytic balance (Perkin-Elmer, Norwalk, Conn.) by the method of O'Toole, which compensates for moisture absorbed during weighing (22). Cell biomass values are reported as the difference between the weights of the dried filter before and after the addition of the sample and are expressed as CDW (milligrams of biomass per milliliter of culture).
TSST-1 determinations.
TSST-1 concentrations (milligrams per milliliter of culture) were determined in the laboratory of Jeffrey Parsonnet (Dartmouth Medical School, Dartmouth, Mass.), except for experiments using strain RN8846, for which they were determined at the Channing Laboratory. In both cases, a competitive enzyme-linked immunosorbent assay (1) was used, and concentrations were normalized using the corresponding CDW. Values were expressed as specific TSST-1 (micrograms per milligram of CDW).
Statistics.
The amounts of CDW and specific TSST-1 produced under different environmental conditions were compared by one-way analysis of variance in the Tukey-Kramer multiple-comparison test with the use of Instat software (version 2.0 for Macintosh; Graphpad Software, Inc., San Diego, Calif.).
RESULTS
Atmospheric O2.
When O2 was delivered as a mixture of air and anaerobic gas mix to a culture growing at a td of 3 h, specific TSST-1 increased by 5.9-fold—i.e., from 2.6 ± 0.1 μg of TSST-1/mg of CDW in anaerobic conditions to 15.3 ± 2.5 μg/mg at an approximate atmospheric O2 concentration of 4% (P < 0.001) (Table 1, experiment 1a). Biomass production increased 1.4-fold, which was determined not to be a significant increase (P > 0.05). At 11% atmospheric O2, there was a significant 4.9-fold increase in the amount of specific TSST-1 per unit of biomass and a 2-fold increase in biomass over that produced under anaerobic conditions (P < 0.001) (Table 1, experiment 1b). Increasing the atmospheric O2 concentration from 4 to 11% did not result in significantly more specific TSST-1 or biomass (P > 0.05). At a slower growth rate (td of 9 h), specific TSST-1 increased by 7.6-fold and biomass by 2.0-fold from anaerobic conditions to an approximate atmospheric O2 concentration of 3.5% (Table 1, experiment 2). Delivery of 100% room air resulted in a significant, 3.4-fold increase in specific TSST-1 production (P < 0.05) and a 4.8-fold increase in biomass (P < 0.001) from anaerobic conditions to 21% atmospheric O2 (Table 1, experiment 3).
TABLE 1.
Production of specific TSST-1 and biomass by S. aureus growing in continuous culture in response to different concentrations of O2 and CO2 and different gas flow and growth rates
| Exp no.a | td (h)b | O2 (%) | CO2 (%) | N2 (%) | H2 (%) | O2 source | Gas flow rate (LPM) | Production of:
|
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomass [mg of CDW/ml of culture (SD)]
|
Specific TSST-1 [μg of TSST-1/mg of CDW (SD)]
|
||||||||||
| Anaerobic conditions | Oxygenated conditions | Anaerobic conditions | Oxygenated conditions | ||||||||
| 1a | 3 | 4 | 8 | 80 | 8 | Air | NDc | 0.30 (0.02) | 0.42 (0.01) | 2.6 (0.1) | 15.3 (2.5) |
| 1b | 3 | 11 | 5 | 79 | 5 | Air | ND | 0.30 (0.02) | 0.59 (0.06) | 2.6 (0.1) | 12.1 (1.8) |
| 2 | 9 | 3.5 | 8.5 | 80 | 8 | Air | 0.1 | 0.33 (0.01) | 0.66 (ND) | 1.2 (0.1) | 9.1 (ND) |
| 3 | 9 | 21 | <1 | 78 | Td | Air | 0.2 | 0.42 (0.02) | 2.00 (0.12) | 1.1 (0.2) | 3.8 (0.6) |
| 4 | 9 | 4 | 0 | 96 | 0 | Analyzed gas | 0.1 | 0.36 (0.08) | 0.86 (0.01) | 1.5 (0.3) | 0.8 (0.1) |
| 5 | 9 | 8 | 0 | 92 | 0 | Analyzed gas | 0.1 | 0.48 (ND) | 0.76 (0.05) | 2.2 (ND) | 1.9 (0.2) |
| 6a | 9 | 8 | 0 | 92 | 0 | Analyzed gas | 0.2 | 0.40 (0.02) | 1.32 (0.02) | 1.2 (0.2) | 0.3 (0.0) |
| 6b | 9 | 8 | 0 | 92 | 0 | Analyzed gas | 0.4 | 0.40 (0.02) | 1.83 (0.05) | 1.2 (0.2) | 0.4 (0.0) |
| 7 | 9 | 4 | 10 | 86 | 0 | Analyzed gas | 0.2 | 0.35 (0.02) | 1.04 (0.04) | 0.82 (0.07) | 1.34 (0.04) |
| 8 | 9 | 4 | 10 | 86 | 0 | Analyzed gas | 0.2 | 0.37 (0.01) | 0.95 (0.04) | 0.28 (0.04) | 1.72 (0.50) |
Exp, experiment. Experiments 1 to 6b, strain MN8; experiment 7, strain RN8465; experiment 8, strain RN8846.
Frs for 3 and 9 h tds were 230 and 80 ml/h, respectively.
ND, not determined.
T, trace levels.
Analyzed gas mix of O2-N2.
At a 9-h td and an O2 concentration of 4% (remainder N2), there was no significant change in specific TSST-1 levels (1.9-fold decrease; P > 0.05), while biomass increased 2.4-fold (P < 0.001) (Table 1, experiment 4). Likewise, there was no significant change in specific TSST-1 production when an 8% O2–92% N2 gas mix and increasing gas flow rates were used (Table 1, experiments 5, 6a, and 6b). Biomass production did increase 1.6-, 3.3-, and 4.6-fold for gas flow rates of 0.1, 0.2, and 0.4 liters/min (LPM), respectively (P < 0.001, except for experiment 5, for which the significance could not be calculated). There was a significant linear relationship between increasing biomass and increasing flow rate of the 8% O2–92% N2 gas mix (P < 0.0001). After the linear trend was accounted for, the difference among these values was still significant (P < 0.01).
Analyzed gas mix of O2-CO2-N2.
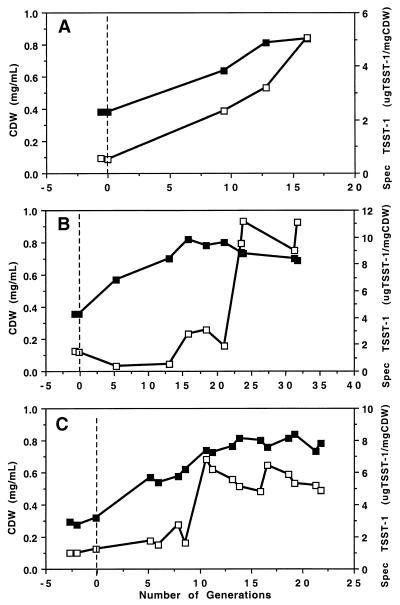
In a gaseous environment of 4% O2–10% CO2–86% N2, the amounts of specific TSST-1 expression and biomass production were determined (Fig. 1). In all three experiments, there was a significant increase (P < 0.01) in biomass (by 2.0- to 2.6-fold) as well as in specific TSST-1 expression (by 5.1- to 6.8-fold) following exposure to the O2-CO2-N2 analyzed gas mix. However, maximum biomass levels were not reached for 15 generations after the switch from anaerobic gas to the O2-CO2-N2 mix. For specific TSST-1 expression, the lag before significant levels of expression were reached varied with the experiment; for the experiments depicted in Fig. 1A, B, and C, it was 9.4, 23.6, and 10.6 generations, respectively. The amount of biomass produced was the same for all three experiments (0.71 to 0.77 mg/ml), while the mean level of specific TSST-1 expression was not (3.5 ± 1.4, 10.2 ± 1.1, and 5.6 ± 0.7 μg/mg for Fig. 1A, B, and C, respectively). When an agr mutant strain was tested, there was a significant increase in both biomass (2.6-fold; P < 0.001) and specific TSST-1 expression (6.1-fold; P < 0.01) by 6.8 generations following exposure to the O2-CO2-N2 analyzed gas mix (Table 1, experiment 8). In a similar experiment using the parent agr+ strain (RN8465), there was an increase in both biomass (2.6-fold; P < 0.001) and specific TSST-1 expression (1.6-fold; P > 0.05).
FIG. 1.
Production of specific TSST-1 and CDW by S. aureus MN8 growing in continuous culture at a generation time of 9 h in response to a switch in the gaseous environment from being anaerobic to containing O2 and CO2. Graphs A, B, and C show data from three separate experiments. The dashed lines mark the switch in gas components from 10% H2–10% CO2–80% N2 (anaerobic gas mixture) to 4% O2–10% CO2–86% N2. Filled squares, CDW; open squares, specific TSST-1.
Switch from O2-N2 analyzed gas mix to O2-CO2-N2 analyzed gas mix.
When the growth conditions were switched from O2 to O2 and CO2, specific TSST-1 increased by 3.6-fold—i.e., from 4.8 ± 0.02 μg/mg of CDW in a 4% O2–96% N2 gaseous environment to 15.3 ± 2.5 μg/mg in 4% O2–10% CO2–86% N2 (P < 0.001). Biomass production did not significantly change. In a 4% O2–96% N2 gaseous environment, a CDW of 0.76 ± 0.02 mg/ml was produced; the switch to a gaseous environment of 4% O2–10% CO2–86% N2 produced a CDW of 0.71 ± 0.02 mg/ml.
DISCUSSION
There are a number of ways air can be introduced into the vaginal environment, including through the tampon insertion process and as trapped air within the tampon. Our original aim was to determine how inclusion of O2 introduced by tampon insertion affects S. aureus TSST-1 production by investigating whether O2 as part of the gaseous environment of a continuous culture growth system resulted in increased specific TSST-1 production.
Isolation and study of the effect of a single environmental variable, such as the concentration of dissolved O2, on batch culture growth is difficult unless other variables are adequately controlled. An alternative to traditional batch culture methods is the use of the continuous-culture method, in which organisms are cultivated in a stable growth environment at a set growth rate. The growth rate can be changed without changing the amount of biomass in the system. The amounts of cell biomass produced during anaerobic growth were not statistically different (P > 0.05) between experiments at two growth rates (Table 1 and Fig. 1). These results demonstrate the separation between the growth rate and the biomass yield that can be achieved if continuous-culture methods are used. This type of system permits the study of factors that affect interactions of microfloral components or, as in the case of S. aureus TSST-1, production of virulence factors.
S. aureus MN8, growing at a td of 3 h, did not produce significantly more specific TSST-1 when the level of O2 (from air) was increased from 4 to 11%. When room air flowed through the system at 0.2 LPM, there was a significant increase in biomass compared to growth under anaerobic conditions and to oxygenated growth in experiments 1a, 1b, and 2, while TSST-1 production decreased in comparison to production at lower levels of O2 (Table 1, experiment 3). When the growth rate was decreased (td, 9 h), the fold increase in specific TSST-1 was similar to that noted during growth at a td of 3 h (Table 1, experiments 1a, 1b, and 2). These findings suggest that increased O2 does not result in increased TSST-1 production and that the growth rate alone is not a factor in TSST-1 production.
In an attempt to more accurately study the effect of oxygen on TSST-1 production, an analyzed gas mix containing only O2 and N2 was used. Oxygen alone did not stimulate specific TSST-1 production but did stimulate a significant increase in biomass (2.39-fold at 4% O2; Table 1, experiment 4). In light of the biomass increase (a result indicating O2 metabolism), the lack of an increase in specific TSST-1 production cannot be attributed to insufficient O2 transfer. In addition, increasing volumetric gas flow rates of 8% O2 gas mix were associated with a significant trend of increasing biomass (Table 1, experiments 5, 6a, and 6b). These data indicate that oxygen alone, while capable of promoting increased biomass, does not trigger increased production of TSST-1 in this system. Hypothesizing that CO2 may be necessary for TSST-1 production in our system, we repeated the previous experiments, adding CO2 to the analyzed gas mix. Significant increases in specific TSST-1 indicated the importance of CO2 in TSST-1 production (Fig. 1).
In our studies, we normalized TSST-1 concentrations using CDW. Biomass was used as the denominator for all experiments to eliminate (i) the inherent variability of determinations of optical density due to the changes in the sizes of individual cells under different growth conditions, (ii) the poor sensitivity of optical methods for detecting small changes in the total number of cells, and (iii) the use of the viable cell count method, which does not differentiate between colonies formed by single and multiple bacterial cells. Only biomass adequately defines the cell mass producing TSST-1 under different growth conditions.
The observation that both CO2 and O2 are required for increased TSST-1 production is interesting. Since increasing the concentration of O2 alone causes increases in biomass, the role of CO2 in toxin production remains enigmatic. CO2 may serve as a carbon donor in some basic process associated with toxin expression or may act as a regulatory stimulus for toxin expression. It is important to note the lag in stimulation of specific TSST-1 production when an analyzed gas mixture of 4% O2–10% CO2–86% N2 was used (Fig. 1). This lag in toxin production was not observed in other studies in which specific TSST-1 values increased or with strains RN8846 and RN8465. Within five generations after the addition of air to an anaerobic culture or CO2 to a culture in an O2-N2 gaseous environment, the culture had already reached a new steady state and TSST-1 concentrations were maximal. Additional studies are under way to determine the cause of the lag in TSST-1 expression in this system.
The effect of CO2 on TSST-1 production contrasts with that of O2 and CO2 on expression of other S. aureus virulence factors. In addition to exotoxins, such as TSST-1, S. aureus produces surface structures, such as teichoic acid and protein A, as well as several capsular polysaccharides (CPs), including CP5 (1). It has been shown that O2 enhances CP5 production during exponential growth in batch culture (7, 11, 24) and that enhanced CP5 production is not due to respiratory activity alone (5). In batch culture studies in which increasing concentrations of CO2 were mixed with air, CO2 levels as low as 1% inhibited expression of CP5 relative to expression in the presence of air alone, which has a CO2 concentration of 0.1% (10). The authors noted that preliminary results from studies investigating the effects of CO2 on CP8 expression were similar. In contrast, increased CO2 levels (5%) did not affect the expression of protein A or teichoic acid. The effect of CO2 alone on CP5 production was confirmed in studies using an O2-N2 gas mix. A 5% CO2–20% O2–75% N2 gas mix inhibited CP5 expression relative to that measured for a gas mix without CO2 (20% O2, 80% N2). In our studies, the positive effect of CO2 on TSST-1 production was confirmed in a similar investigation. These experiments clearly demonstrate the importance of both O2 and CO2 in some S. aureus metabolic functions.
The human microflora encounters a variety of growth environments characterized by changing temperatures, nutrient concentrations, and gaseous atmospheres. These organisms have developed a number of regulatory responses by which to adapt to these changes. The mechanisms involved in the regulation of TSST-1 expression remain unclear. At least two global regulatory systems control expression of surface protein and exoprotein in S. aureus (including the expression of TSST-1): the regulatory loci agr and sar (3, 17, 23, 25). Our data demonstrate that a combination of O2 and CO2—but not O2 alone—results in increased TSST-1 expression. The agr system regulates expression of some genes in S. aureus in response to the presence of O2; an example is the positive regulation of CP5 expression (6). Preliminary results indicate that the agr system is not affected by changing CO2 concentrations (10). The agr system also includes a cell-density-dependent regulatory mode that involves an octapeptide pheromone (12). Our studies show that the agr system is unlikely to be regulating TSST-1 production in this system, at least through a quorum-sensing signal. Specific TSST-1 production increased significantly while biomass remained unchanged when the gaseous environment of the culture was switched from 4% O2 to 4% O2–10% CO2. In addition, when the biomass increased in response to the addition of O2, specific TSST-1 production did not (Table 1, experiments 4 to 6b). Stimulation of specific TSST-1 production is not cell density dependent. Results from studies using an agr mutant, strain RN8846, (Table 1, experiment 8) also support the conclusion that the agr system is not regulating expression of TSST-1. Whether one of these systems or an as yet undescribed system regulates expression of TSST-1 is unclear. The nature of the relationship of TSST-1 production to CO2 and O2 will be the focus of additional research.
ACKNOWLEDGMENTS
This research was funded by grants from Tambrands, Procter and Gamble.
We thank Paul Modern for technical assistance in performing TSST-1 determinations.
REFERENCES
- 1.Arvidson S. Extracellular enzymes from Staphylococcus aureus. In: Easmon C, Adlam C, editors. Staphylococci and staphylococcal infections. London, England: Academic Press; 1983. pp. 745–808. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Summary of notifiable diseases, United States, 1996. Morb Mortal Wkly Rep. 1996;45:1–87. [PubMed] [Google Scholar]
- 3.Cheung A L, Koomey J M, Butler C A, Projan S J, Fischetti V A. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci USA. 1992;89:6462–6466. doi: 10.1073/pnas.89.14.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crass B, Bergdoll M. Toxin involvement in toxic shock syndrome. J Infect Dis. 1986;153:918–926. doi: 10.1093/infdis/153.5.918. [DOI] [PubMed] [Google Scholar]
- 5.Dassy B, Fournier J M. Respiratory activity is essential for post-exponential-phase production of type 5 capsular polysaccharide by Staphylococcus aureus. Infect Immun. 1996;64:2408–2414. doi: 10.1128/iai.64.7.2408-2414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dassy B, Hogan T, Foster T J, Fournier J M. Involvement of the accessory gene regulator (agr) in expression of type 5 capsular polysaccharide by Staphylococcus aureus. J Gen Microbiol. 1993;139:1301–1306. doi: 10.1099/00221287-139-6-1301. [DOI] [PubMed] [Google Scholar]
- 7.Dassy B, Stringfellow W T, Lieb M, Fournier J M. Production of type 5 capsular polysaccharide by Staphylococcus aureus grown in semi-synthetic medium. J Gen Microbiol. 1991;137:1155–1162. doi: 10.1099/00221287-137-5-1155. [DOI] [PubMed] [Google Scholar]
- 8.Davis J P, Chesney P J, Wand P J, LaVenture M. Toxic-shock syndrome: epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med. 1980;303:1429–1435. doi: 10.1056/NEJM198012183032501. [DOI] [PubMed] [Google Scholar]
- 9.Fischetti V A, Chapman F, Kakani R, James J, Grun E, Zabriskie J B. Role of air in growth and production of toxic shock syndrome toxin 1 by Staphylococcus aureus in experimental cotton and rayon tampons. Rev Infect Dis. 1989;11:S176–S181. doi: 10.1093/clinids/11.supplement_1.s176. [DOI] [PubMed] [Google Scholar]
- 10.Herbert S, Worlitzsch D, Dassy B, Boutonnier A, Fournier J M, Bellon G, Dalhoff A, Doring G. Regulation of Staphylococcus aureus capsular polysaccharide type 5: CO2 inhibition in vitro and in vivo. J Infect Dis. 1997;176:431–438. doi: 10.1086/514061. [DOI] [PubMed] [Google Scholar]
- 11.Hochkeppel H K, Braun D G, Vischer W, Imm A, Sutter S, Staeubli U, Guggenheim R, Kaplan E L, Boutonnier A, Fournier J M. Serotyping and electron microscopy studies of Staphylococcus aureus clinical isolates with monoclonal antibodies to capsular polysaccharide types 5 and 8. J Clin Microbiol. 1987;25:526–530. doi: 10.1128/jcm.25.3.526-530.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji G Y, Beavis R C, Novick R P. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA. 1995;92:12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kass E H, Kendrick M I, Tsai Y C, Parsonnet J. Interaction of magnesium ion, oxygen tension, and temperature in the production of toxic-shock-syndrome toxin-1 by Staphylococcus aureus. J Infect Dis. 1987;155:812–815. doi: 10.1093/infdis/155.4.812. [DOI] [PubMed] [Google Scholar]
- 14.Lee V T, Chang A H, Chow A W. Detection of staphylococcal enterotoxin B among toxic shock syndrome (TSS)- and non-TSS-associated Staphylococcus aureus isolates. J Infect Dis. 1992;166:911–915. doi: 10.1093/infdis/166.4.911. [DOI] [PubMed] [Google Scholar]
- 15.Mills J T, Dodel A W, Kass E H. Regulation of staphylococcal toxic shock syndrome toxin-1 and total exoprotein production by magnesium ion. Infect Immun. 1986;53:663–670. doi: 10.1128/iai.53.3.663-670.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mills J T, Parsonnet J, Tsai Y-C, Kendrick M, Hickman R K, Kass E H. Control of production of toxic-shock-syndrome toxin-1 (TSST-1) by magnesium ion. J Infect Dis. 1985;151:1158–1161. doi: 10.1093/infdis/151.6.1158. [DOI] [PubMed] [Google Scholar]
- 17.Morfeldt E, Janzon L, Arvidson S, Lofdahl S. Cloning of a chromosomal locus (exp) which regulates the expression of several exoprotein genes in Staphylococcus aureus. Mol Gen Genet. 1988;211:435–440. doi: 10.1007/BF00425697. [DOI] [PubMed] [Google Scholar]
- 18.Onderdonk A B, Delaney M L, Zamarchi G R, Hirsch M L, Munoz A, Kass E H. Normal vaginal microflora during use of various forms of catamenial protection. Rev Infect Dis. 1989;11:S61–S67. doi: 10.1093/clinids/11.supplement_1.s61. [DOI] [PubMed] [Google Scholar]
- 19.Onderdonk A B, Zamarchi G R, Rodriguez M L, Hirsch M L, Munoz A, Kass E H. Qualitative assessment of vaginal microflora during use of tampons of various compositions. Appl Environ Microbiol. 1987;53:2779–2784. doi: 10.1128/aem.53.12.2779-2784.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onderdonk A B, Zamarchi G R, Rodriguez M L, Hirsch M L, Munoz A, Kass E H. Quantitative assessment of vaginal microflora during use of tampons of various compositions. Appl Environ Microbiol. 1987;53:2774–2778. doi: 10.1128/aem.53.12.2774-2778.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osterholm M T, Davis J P, Gibson R W, Mandel J S, Wintermeyer L A, Helms C M, Forfang J C, Rondeau J, Vergeront J M. Tri-state toxic-shock syndrome study. I. Epidemiologic findings. J Infect Dis. 1982;145:431–440. doi: 10.1093/infdis/145.4.431. [DOI] [PubMed] [Google Scholar]
- 22.O'Toole D K. Weighing technique for determining bacterial dry mass based on rate of moisture uptake. Appl Environ Microbiol. 1983;46:506–508. doi: 10.1128/aem.46.2.506-508.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng H-L, Novick R P, Kreiswirth B, Kornblum J, Schlievert P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol. 1988;170:4365–4372. doi: 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poutrel D M, Gilbert F B, Lebrun M. Effects of culture conditions on production of type 5 capsular polysaccharide by human and bovine Staphylococcus aureus strains. Clin Diagn Lab Immunol. 1995;2:166–171. doi: 10.1128/cdli.2.2.166-171.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Recsei P, Kreiswirth B, O'Reilly M, Schlievert P, Gruss A, Novick R P. Regulation of exoprotein gene expression in Staphylococcus aureus by agr. Mol Gen Genet. 1986;202:58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- 26.Schlech W F, Shands K N, Reingold A L, Dan B B, Schmid G P, Hargrett N T, Hightower A, Herwaldt L A, Neill M A, Band J D, Bennett J V. Risk factors for development of toxic shock syndrome: association with tampon brand. JAMA. 1982;248:835–839. [PubMed] [Google Scholar]
- 27.Schlievert P M, Blomster D A. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J Infect Dis. 1983;147:236–242. doi: 10.1093/infdis/147.2.236. [DOI] [PubMed] [Google Scholar]
- 28.Shands K N, Schmid G P, Dan B B, Blum D, Guidotti R J, Hargrett N T, Anderson R L, Hill D L, Broome C V, Band J D, Fraser D W. Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical features in 52 cases. N Engl J Med. 1980;303:1436–1442. doi: 10.1056/NEJM198012183032502. [DOI] [PubMed] [Google Scholar]
- 29.Taylor D, Holland K T. Effect of dilution rate and Mg2+ limitation on toxic shock syndrome toxin-1 production by Staphylococcus aureus grown in defined continuous culture. J Gen Microbiol. 1988;134:719–723. doi: 10.1099/00221287-134-3-719. [DOI] [PubMed] [Google Scholar]
- 30.Taylor D, Holland K T. Production of TSST-1 by Staphylococcus aureus under aerobic and anaerobic conditions and the effects of magnesium ion limitation. Rev Infect Dis. 1988;11:S151–S156. doi: 10.1093/clinids/11.supplement_1.s151. [DOI] [PubMed] [Google Scholar]
- 31.Todd J K, Kapral F A, Fishaut M, Welch T R. Toxic shock syndrome associated with phage group 1 staphylococci. Lancet. 1978;ii:1116–1118. doi: 10.1016/s0140-6736(78)92274-2. [DOI] [PubMed] [Google Scholar]
- 32.Vergeront J M, Stolz S J, Crass B A, Nelson D B, Davis J P, Bergdoll M S. Prevalence of serum antibody to staphylococcal enterotoxin F among Wisconsin residents: implications for toxic-shock syndrome. J Infect Dis. 1983;148:692–698. doi: 10.1093/infdis/148.4.692. [DOI] [PubMed] [Google Scholar]
- 33.Wagner G, Bohr L, Wagner P, Petersen L N. Tampon-induced changes in vaginal oxygen and carbon dioxide tensions. Am J Obstet Gynecol. 1984;148:147–150. doi: 10.1016/s0002-9378(84)80165-9. [DOI] [PubMed] [Google Scholar]
- 34.Wong A C L, Bergdoll M S. Effect of environmental conditions on production of toxic shock syndrome toxin 1 by Staphylococcus aureus. Infect Immun. 1990;58:1026–1029. doi: 10.1128/iai.58.4.1026-1029.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]